Abstract
ACTH, the primary secretagogue for corticosteroid biosynthesis, binds to melanocortin 2 receptor (MC2R) and activates the signaling cascade leading to steroid biosynthesis in the adrenal cortex. Whereas MC2R regulation has been studied using mammalian models, little is known about the molecular mechanisms involved in ACTH signaling in nonmammalian vertebrates. A full-length cDNA encoding MC2R was sequenced from rainbow trout (Oncorhynchus mykiss) interrenal tissue (analogous to the adrenal cortex in mammals) and showed about 60 and about 44% amino acid sequence similarity to teleosts and humans, respectively. Phylogenetic analysis confirmed that MC2R from all species clustered together and was distant from other MCRs. Quantitative real-time PCR revealed a marked tissue-specific difference in MC2R mRNA abundance, with the highest levels observed in the interrenal tissue, ovary, and testis. Acute ACTH, but not α-MSH or [Nle4, d-Phe7]-MSH, stimulation resulted in a time- and dose-related elevation in MC2R mRNA abundance in the interrenal tissue. This corresponded with higher steroidogenic acute regulatory protein and cytochrome P450 side-chain cleavage enzyme gene expression as well as elevated cortisol production. An acute stressor transiently elevated plasma ACTH and cortisol levels at 1 h, and this was followed by a significant increase in MC2R mRNA abundance at 4 h after stressor exposure. Taken together, our results demonstrate that ACTH regulation of MC2R is highly conserved in vertebrates, whereas the tissue-specific distribution of this receptor transcript level leads us to propose a role for ACTH signaling in the stressor-mediated suppression of sex steroid levels in fish.
THE SYNTHESIS AND secretion of glucocorticoid hormone is under the control of the hypothalamus-pituitary-adrenal axis. ACTH, the proopiomelanocortin (POMC)-derived peptide from the pituitary gland, is the primary trophic hormone activating corticosteroid biosynthesis in the adrenal cortex (1). The sequence of events in the adrenal involves the binding of ACTH to melanocortin 2 receptor (MC2R), a G protein-coupled receptor belonging to the family of melanocortin receptors (MCRs), and activation of adenylate cyclase and cAMP signaling cascade, leading to the transport of steroid precursor cholesterol from the outer to inner mitochondrial membrane (2,3). Although the precise mechanism by which this cholesterol transport is accomplished is still uncertain, two proteins, the steroidogenic acute regulatory protein (StAR) and the peripheral-type benzodiazepine receptor, are thought to play a key role in this mitochondrial intermembrane shuttling (4,5). Corticosteroid synthesis from cholesterol involves a series of enzymatic steps, including cytochrome P450 family proteins, dehydrogenases and hydroxylases, whereas the rate-limiting step in this cascade is the conversion of cholesterol to pregnenolone, which is catalyzed by the cytochrome P450 side-chain cleavage (P450scc) enzyme (6).
In addition to ACTH, other POMC-derived peptides, including α-MSH, β-MSH, and γ-MSH activate MCRs (7). So far, five MCRs (MC1R-MC5R) have been characterized by molecular cloning in tetrapods, and they play a role in a variety of physiological processes, including skin pigmentation, steroidogenesis, appetite regulation, and inflammation (8). The characteristic feature of MCRs is the presence of seven-transmembrane domains, with a short extracellular and intracellular domains at the amino- and carboxyl-terminals, respectively (9,10). The MCRs are all functionally coupled to adenylate cyclase and their effects are mediated primarily by activating the cAMP-dependent signaling pathways (8).
As in mammals, corticosteroid biosynthesis and secretion in teleosts is also under the control of the hypothalamus-pituitary axis, but unlike mammals, fish lack a discrete adrenal gland. Instead, the corticosteroidogenic cells are distributed around the posterior cardinal vein in the anterior part of the kidney (interrenal tissue) and secrete cortisol, the principal glucocorticoid in teleosts (11,12). ACTH regulation of plasma cortisol levels in vivo, as well as in vitro, using interrenal tissue preparations and isolated cell suspension, is well established (11,13,14). Recent studies also confirmed ACTH-mediated molecular regulation of steroid biosynthetic pathway, including up-regulation of StAR and P450scc mRNA abundance in trout interrenal tissues (15,16,17,18). Whereas most studies in teleosts focused on ACTH stimulation of steroidogenic capacity and cortisol production, little is known about the role of MC2R, a key signaling molecule, in the stress response process.
MC2R was cloned and sequenced only recently in teleosts [Takifugu rubripes, Tetraodon nigroviridis (19); Danio rerio (20,21); Cyprinus carpio (22)], whereas no sequence information exists for salmonids. These studies confirmed ACTH binding and receptor activation using in vitro reporter assays (19) and also showed changes in MC2R gene expression in response to longer-term stressor exposure in vivo (22). However, no study has addressed MC2R expression patterns in response to an acute stressor exposure in fish. Because ACTH signaling is critical for the evolutionarily conserved cortisol response during stress adaptation, we hypothesized that the acute stressor-induced MC2R regulation seen in tetrapods is conserved in teleosts. To this end, using rainbow trout as a model, we cloned and sequenced the full-length MC2R cDNA from interrenal tissue and examined the acute regulation of this receptor in response to ACTH exposure in vitro and stressor exposure in vivo.
Materials and Methods
Chemicals
L-15 medium, porcine ACTH1–39, α-MSH, [Nle4, d-Phe7]-α-MSH trifluoroacetate salt (NDP-MSH), SHU9119 (acetyl-[Nle4, Asp5, d-2-Nal7, Lys10]-cyclo-α-MSH amide fragment 4–10), 2-phenoxyethanol, and plasmid preparation kit were obtained from Sigma-Aldrich (St. Louis, MO). RNeasy minikit, ribonuclease-free deoxyribonuclease, and Qiaquick gel extraction kit were purchased from QIAGEN (Mississauga, Ontario, Canada). Taq polymerase, first-strand cDNA synthesis kit, and DNA ladder were obtained from MBI Fermentas (Burlington, Ontario, Canada). SMART RACE cDNA amplification kit and iQ SYBR Green Supermix were purchased from CLONTECH Inc. (Palo Alto, CA) and Bio-Rad (Mississauga, Ontario, Canada), respectively.
Fish
Juvenile rainbow trout (Oncorhynchus mykiss, average body mass 150 g) were obtained from Humber Springs trout hatchery, Mono Mills, Ontario, Canada. Fish were acclimated in 2000-liter tanks with continuous running water at 13 C and 12-h light, 12-h dark photoperiod for 3 wk before the experiment. The fish were fed to satiety (three point sinking food; Martin Mills Inc., Elmira, Ontario, Canada) once daily for 5 d/wk. The experimental protocol was approved by the animal care committee, University of Waterloo, and is in accordance with the Canadian Council for Animal Care guidelines.
Acute stressor exposure in vivo
The details of the experimental protocol were published recently (16). Briefly, groups of eight fish were distributed in three tanks (100 liter capacity) and acclimated for 2 wk before the experiment. After 5 d of feeding, six fish (two fish each from three tanks) were sampled quickly and killed with an overdose of 2-phenoxyethanol (1:1000), and these were the unstressed (0 h) control fish. The remaining fish (six fish per tank from three tanks) were subjected to a standardized stress protocol of netting and chasing the fish for 5 min after which they were left undisturbed until sampling. For sampling, all fish in each tank was quickly netted at 1, 4, and 24 h after stressor exposure. Also, an additional four tanks (five fish per tank distributed in four tanks) were maintained exactly as mentioned above as unstressed controls and sampled at 0, 1, 4, and 24 h to take into consideration the effect of sampling time on the measured parameters. Fish were quickly bled by caudal puncture into heparinized tubes, and the plasma collected after centrifugation (6000 × g for 10 min) were stored frozen (−70 C) for later determination of plasma cortisol (see Ref. 16) and ACTH levels. Pieces of interrenal tissue were dissected and frozen immediately on dry ice for RNA extraction and MC2R mRNA quantification.
In vitro ACTH exposure
Interrenal tissue slices were stimulated in vitro with porcine ACTH and cortisol production rate measured exactly as described previously (23). Briefly, interrenal tissue dissected from juvenile trout was rinsed in ice-cold L-15 medium and finely minced to approximately 1 mm3. The incubation consisted of distributing the tissue slices from each fish equally into ten wells in a 24-well tissue culture plate (Corning Inc., Corning, NY). Tissue pieces were allowed to incubate with gentle shaking for 2 h at 13 C (equilibration period), after which the supernatant was replaced with fresh L-15 media and stimulated with trophic hormones. Time course studies to establish the effect of trophic hormone stimulation on MC2R mRNA levels in trout interrenal tissues were conducted using ACTH concentration of 1.3 μm over an 8-h time period. L-15 medium and tissue samples were collected at 0, 1, 2, 4, and 8 h for determining cortisol production and MC2R mRNA levels. Control incubations were carried out for the same time periods without ACTH in the medium. Based on the results obtained, concentration response studies were conducted by incubating interrenal tissue slices for 8 h in different concentrations of ACTH (0, 0.3, 0.6, 1.3, 2.6, 6.4, 12 μm), and the medium was collected for later determination of cortisol. Tissue slices were frozen at −80 C for total RNA extraction and cDNA synthesis. Tissue wet weight was recorded and cortisol production rate was calculated as nanograms per hour per milligram wet weight and values reflect the magnitude of increase in cortisol production compared with unstimulated group. All the in vitro experiments were repeated with at least four different fish.
In vitro exposure to MCR agonists (α-MSH, NDP-MSH) and antagonist (SHU9119)
Interrenal tissue slices were stimulated with either α-MSH or NDP-MSH (MC1R/MC4R agonists) to determine the role of these receptors in stimulating cortisol production. Interrenals were incubated for 3 h with various concentrations of α-MSH (10−6, 10−8, and 10−10 m) and NDP-MSH (10−7, 10−8, 10−9, and 10−10 m) and the media collected for measuring cortisol concentration. Also, tissue slices were incubated with SHU9119 (MC4R/MC3R antagonist; 10−6 and 10−8 m) either alone or in combination with ACTH (1.3 μm) to tease out the role of MC4R/MC3R in ACTH signaling. The protocols followed were exactly as described in the section above.
Tissue-specific MC2R expression
For determining the tissue-specific MC2R expression patterns, different tissue samples were collected from four male juvenile rainbow trout, except ovary samples, which were collected from female fish. The different tissues collected include, interrenal tissue, pituitary, hypothalamus, lens, skin, muscle, gills, testis, liver, posterior intestine, and ovary. Samples collected were stored at −80 C for later determination of MC2R mRNA abundance.
Plasma ACTH and medium cortisol analyses
ACTH and cortisol concentrations were measured using a commercially available ImmuChem 125I-labeled RIA kits (ICN Biomedicals, CA) following the manufacturer’s protocols. These kits were used previously for measuring hormones in trout plasma (23,24).
Isolation and sequencing of full-length rainbow trout MC2R gene
RNA isolation and first-strand cDNA synthesis.
Total RNA was isolated from interrenal tissue using RNeasy minikit following the manufacturer’s protocol and quantified by spectrophotometry at 260 nm. RNA integrity was determined by 1% agarose gel electrophoresis containing ethidium bromide. RNA was treated with deoxyribonuclease to avoid genomic contamination before cDNA synthesis. The first-strand cDNA was synthesized from 1 μg of total RNA following the manufacturer’s instructions. Briefly, total RNA was heat denatured (70 C) and cooled on ice. The sample was used in a 20-μl reverse transcriptase reaction using 0.5 μg oligodeoxythymidine primers and 1 mm each deoxynucleotide triphosphate, 20 U ribonuclease inhibitors, and 40 U Moloney murine leukemia virus reverse transcriptase. The reaction was incubated at 37 C for 1 h and stopped by heat inactivation at 70 C for 10 min.
PCR, cloning, and sequencing.
Primers sequences for PCR were designed based on the conserved regions of MC2R sequences in different species (Homo sapiens, D. rerio, T. rubripes, T. nigroviridis, C. carpio). The PCR was carried out with 1 μl of interrenal tissue cDNA as a template, 0.5 μm each of MC2R forward and reverse primer (Table 1), 10 mm deoxynucleotide triphosphates (dNTPs), 1.5 mm MgCl2 and 1 U Taq polymerase in a 25-μl total volume. The PCR conditions include initial denaturation for 3 min at 94 C, 40 cycles of 30 sec at 94 C, 30 sec at 60 C, and 30 sec at 72 C and final elongation for 5 min at 72 C. The resultant PCR product was subjected to 1.2% agarose gel electrophoresis and a single amplicon of approximately 225 bp was detected. The PCR product was gel extracted, ligated into a pGEM-Teasy cloning vector (Promega, Valencia, CA) and introduced into DH5α Escherichia coli cells. After LB-ampicillin selection, transformed cells were cultured and plasmids isolated using plasmid preparation kit. The isolated plasmid was sequenced using an Applied Biosystems 337 DNA sequencer (PerkinElmer, Foster City, CA) at York University Molecular Core facility.
Table 1.
List of primer sequences used to obtain full-length MC2R cDNA as well as mRNA abundance using qPCR
| Primer | Sequence (5′–3′) | Accession no. | Product size (bp) |
|---|---|---|---|
| MC2R forward | CACCATCTTCCACGCACTGC | ||
| MC2R reverse | CCTGGTGTGGGATCGAGCC | 225 | |
| 5′-RACE | AGCCAGCAGGAACATGTGGACGTA | 649 | |
| 3′-RACE | TCCACGCCCTGCGCTACCAC | ||
| 3′-Nested | CGGGGATCTGGGCGCTATGC | 836 | |
| Full MC2R forward | TGTGGACGTTGCTCTGACAC | ||
| Full MC2R reverse | GGGTCAAAAACTCTGGTGGA | ||
| MC2R forward (qPCR) | GAGAACCTGTTGGTGGTGGT | EU119870 | 105 |
| MC2R reverse (qPCR) | GAGGGAGGAGATGGTGTTGA | ||
| StAR forward | TGGGGAAGGTGTTTAAGCTG | AB047032 | 101 |
| StAR reverse | AGGGTTCCAGTCTCCCATCT | ||
| P450scc forward | GCTTCATCCAGTTGCAGTCA | S57305.1 | 140 |
| P450scc reverse | CAGGTCTGGGGAACACATCT | ||
| β-Actin forward | TGTCCCTGTATGCCTCTGGT | AF157514 | 121 |
| β-Actin reverse | AAGTCCAGACGGAGGATGG |
The 3′ and 5′-rapid amplification of cDNA ends (RACE).
To isolate 3′ and 5′ ends of the presumptive trout MC2R cDNA, RACE reactions were performed using the SMART RACE cDNA amplification kit. To this end, trout interrenal tissue total RNA was reverse transcribed to 5′- and 3′-RACE ready cDNA, according to the manufacturer’s instructions. Trout MC2R-specific primers (5′-RACE and 3′-RACE primers; Table 1), designed based on the sequence information of the presumed trout MC2R, were used in combination with a universal primer mix (UPM) for the 5′- and 3′-RACE, respectively. Touchdown PCR was performed using Advantage 2 PCR kit (CLONTECH). The reaction mix includes 2.5 μl of RACE-ready cDNA, 10 pmol of gene-specific primers, 30 pmol of UPM, 0.2 μm of dNTP, 1× Advantage 2 Taq polymerase and Advantage 2 PCR buffer in a final volume of 50 μl. The touchdown PCR protocol consisted of the following: 1) an initial denaturation of 1 min at 94 C; 2) five cycles of 30 sec at 94 C and 3 min at 72 C; 3) five cycles of 30 sec at 94 C, 30 sec at 70 C, and 3 min at 72 C; and 4) 30 cycles of 30 sec at 94 C, 30 sec at 68 C, and 3 min at 72 C. The PCR products were subjected to 1.2% agarose gel electrophoresis. 5′-RACE yielded a product of approximately 650 bp, which was subsequently gel extracted and sequenced as described above. The 3′-RACE reaction was followed by nested PCR using MC2R-specific nested primer (3′-nested primer; Table 1) in combination with the nested universal primer (NUP). The nested PCR consisted of 2.5 μl of 3′-RACE product, 10 pmol each of 3′-nested primer and NUP, 0.2 μm of dNTP, and 1× Advantage 2 Taq polymerase and Advantage 2 PCR buffer in a final volume of 50 μl. The reaction conditions include initial denaturation for 1 min at 94 C, followed by 25 cycles of 30 sec at 94 C, 30 sec at 68 C and 2 min at 72 C, and a final elongation for 3 min at 72 C. Both the UPM and NUP were supplied with the SMART RACE cDNA amplification kit. The PCR products were analyzed and sequenced as described above. To confirm that the sequences determined by 5′- and 3′-RACE were derived from the same transcripts, we attempted to amplify cDNAs containing the entire coding region by PCR with primers localized in the 5′- and 3′-untranslated regions (full-MC2R forward and full-MC2R reverse; Table 1).
Alignment and phylogenetic analysis
Alignment of the predicted full-length amino acid sequence of rainbow trout MC2R together with other known fish and tetrapod MC2Rs was generated with ClustalW 1.8 software (PHYLIP, Seattle, WA). Transmembrane domains were predicted using TMHMM server version 2, protein kinase phosphorylation sites were predicted using Netphos K 1.0 server, and serine/threonine/tyrosine phosphorylation sites were predicted using Netphos 2.0 server, provided by the center for biological sequence analysis, Technical University of Denmark (www.cbs.dtu.dk).
For phylogenetic analysis, trout MC2R was aligned together with all other known MCRs using ClustalW 1.8 software and analyzed using phylogenetic inferences using maximum likelihood, with JTT substitution model (25). Phylogenetic tree was built using TreeDyn software (26). The following receptor sequences (with their accession numbers) were retrieved from GenBank for alignment and phylogenetic analysis: MC1R, H. sapiens (Hsa) (NM_002386), Gallus gallus (Gga) (D78272), Takifugu rubripes (Tru) (AAO65549), Tetraodon nigroviridis (Tni) (AAQ55176), D. rerio (Dre) (NM_180970); MC2R, Hsa (NM_000529), Gga (NP_001026686), Tru (NP_001027936), Tni (AAQ55177), Dre (NP_851302), C. carpio (Cca) (CAE53845); MC3R, Hsa (XM_009545), Gga (AB017137), Dre (NM_180972), Squalus acanthias (Sac) (AAS66720); MC4R, Hsa (NM_005912), Gga (AB012211), Tru (AAO65551), Tni (AAQ55178), Dre (AY078989), Sac MC4 (AY169401), Carassius auratus (Cau) (CAD58853); Oncorhynchus mykiss (Omy) (AY534915); MC5R, Hsa (XM_008685), Gga (AB012868), Tru (AAO65552), Tni (AAQ55179), Dre (MC5a, AY078990; and MC5b, AY078991), Cau (CAE11349), Cca (CAH04351), Omy (AY534916), Sac(AY562212). In addition, MCRs from river lamprey, Lampetra fluviatilis (LflMCa, DQ213059; LflMCb, DQ213060) and Atlantic hagfish, Myxine glutinosa, (MglMCc, DQ213061) were also included in the analysis. Human cannabinoid 2 receptor (CAJ42137) was used to root the phylogenetic tree. A bootstrap consensus tree assessing the robustness of the nodes was made with 1000 bootstrap simulations.
Quantitative real-time PCR (qPCR)
Total RNA isolation and cDNA synthesis from various tissues was carried out as described above. Tissue-specific expression of MC2R was determined by qPCR from four different fish. PCR was performed to amplify the predicted products under the following conditions: initial denaturation for 2 min at 94 C and 40 cycles of 15 sec at 94 C, 30 sec at 60 C, and 30 sec at 72 C.
Construction of plasmid stocks
Plasmid stocks with target sequence inserts were constructed by amplifying the gene specific products using RT-PCR and subsequently cloning them using a vector system. Gene-specific products were amplified using the primers designed for qPCR. GenBank accession numbers and primer sequences for MC2R (MC2R forward, MC2R reverse), StAR, P450scc, and β-actin are given in Table 1.
Standard curve
Standard curves were generated using a 10× dilution series of the plasmid stock, ranging from 101 to 108 copies (starting quantity) per reaction. Each standard reaction mix contained 1 μl of standard, 4 pmol of each primer and SYBR green super mix (50 U/ml−1 of iTaq DNA polymerase, 40 mm of Tris-HCl (pH 8.4), 100 mm of KCl, 6 mm of MgCl2, 0.4 mm of each dNTP component (dATP, dGTP, dCTP, and deoxythymidine triphosphate), SYB Green I, 20 μm of fluorescein, and stabilizers) in a total volume of 25 μl. PCR was performed using iCycler iQ (Bio-Rad) under the following conditions: 2 min at 94 C followed by 40 cycles of 15 sec at 94 C, 30 sec at 60 C, and 30 sec at 72 C. PCR products were subjected to melt curve analysis to confirm the presence of a single amplicon. Negative controls either with no template or total RNA was carried out for each tissue. Master mixes, to reduce pipetting errors, were prepared at every stage for triplicate reactions (3 × 25 μl) for each standard. Background subtracted threshold cycle (CT) values were plotted against log of standard copy numbers to obtain standard curves. The PCR efficiency (E) was determined using the formula, E = [10−(1/slope) × 100] and it ranged from 97 to 100%.
Quantification of samples
One microliter cDNA was used as a template for every 25-μl reaction. For every test sample, qPCR was performed for the gene of interest and β-actin. The reaction components, PCR conditions and melt curve analysis were exactly same as the previous section. Background subtracted CT values were used to determine the absolute quantity of the mRNA based on the standard curve. β-Actin mRNA levels in interrenal tissue samples showed little change with treatments and used for the normalization of transcript abundance. MC2R transcript levels in different tissues are expressed as copy numbers per microgram RNA.
Statistical analysis
All statistical analyses were performed with SPSS version 14.0.1 (SPSS Inc., Chicago, IL), and data are shown as mean ± sem. The data were transformed (logarithmic), wherever necessary, for homogeneity of variance, but nontransformed values are shown in the figures. Two-way ANOVA followed by Bonferroni post hoc test was used to determine the effect of acute stressor on temporal plasma ACTH concentration and MC2R mRNA abundance. Repeated-measures ANOVA was used to determine temporal and concentration-related effect of ACTH, and MCR agonists and antagonist, on cortisol production and MC2R mRNA levels. One-way ANOVA was used to determine the MC2R mRNA abundance in different tissues, compared with interrenal tissue. A probability level of P ≤ 0.05 was considered significant.
Results
Cloning and sequencing of MC2R
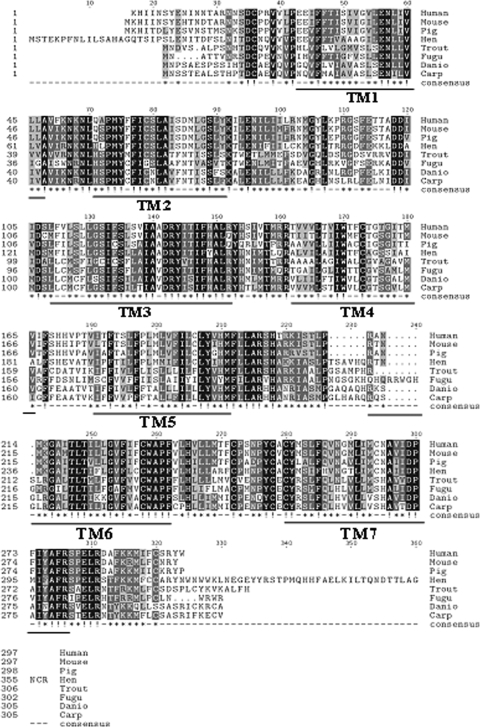
The full-length MC2R sequence is 1339 bp long, which contains an open reading frame encoding a protein of 305 amino acids (Fig. 1; GenBank accession no. EU119870). The overall amino acid sequence of trout MC2R is 61% identical with D. rerio and 58% identical with T. rubripes, T. nigroviridis, and C. carpio MC2R. In comparison with tetrapods, the amino acid identity is 46% to human, mouse, and pig, whereas it was 41% with chicken MC2R (Fig. 2). There is one potential protein kinase C (PKC) phosphorylation site in the intracellular domain (Thr136) and three potential phosphorylation sites each at tyrosine (Tyr53,122,248), serine (Ser69,87,91), and threonine residues (Thr124,137,284).
Figure 1.
Full-length nucleotide and deduced amino acid sequence of rainbow trout MC2R cDNA. The deduced amino acid is shown below the nucleotide sequence. The start codon is in bold and stop codon is indicated by an asterisk. The GenBank accession no. is EU119870.
Figure 2.
Multiple alignment of MC2R amino acid sequences of eight different vertebrate species. Dots indicate gaps that were introduced to achieve maximum identity. Identical amino acids are indicated in black boxes and conservative substitutions are in gray. The predicted seven transmembrane regions (TMs) are underlined in the bottom. The sequences aligned were retrieved from GenBank and have accession codes: Homo sapiens (human; NM_000529), Mus musculus (mouse; NM_008560), Gallus gallus (hen; AB009605), pig (Sus scrofa; AF450083.1); Oncorhynchus mykiss (trout; EU119870); Takifugu rubripes (fugu; NP_001027936), Danio rerio (zebrafish; NP_851302), and Cyprinus carpio (carp; CAE53845).
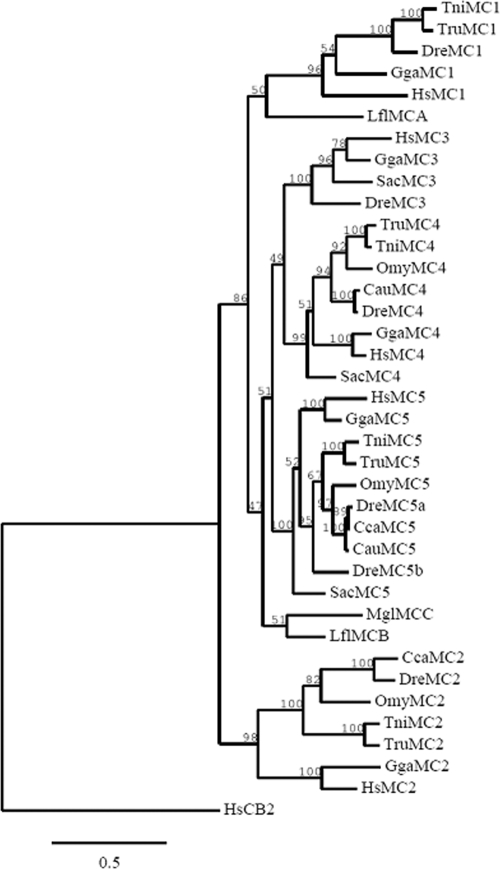
Phylogenetic analysis of the full-length amino acid sequence of trout MC2R, together with other species of fish and tetrapod MCRs using maximum likelihood method (Fig. 3), placed MC2Rs as the earliest branch among all the MCRs. All other MCRs are grouped together, with MC1R branching from the rest. For each member of the MCR family, all fish sequences are closely related compared with human and chicken sequences and are clustered in a separate clade. The topology of the MC2R clade shows trout MC2R sequence clustered together with C. carpio and D. rerio and separate from the modern fugu (T. rubripes and T. nigroviridis).
Figure 3.
Phylogenetic tree of MCR amino acid sequences built using maximum likelihood method (phylogenetic inferences using maximum likelihood). The accession numbers of the sequences are listed in Materials and Methods and human cannabinoid receptor was used as an outgroup. Numbers at branch nodes represent percentage of 1000 bootstrap simulations.
MC2R tissue distribution
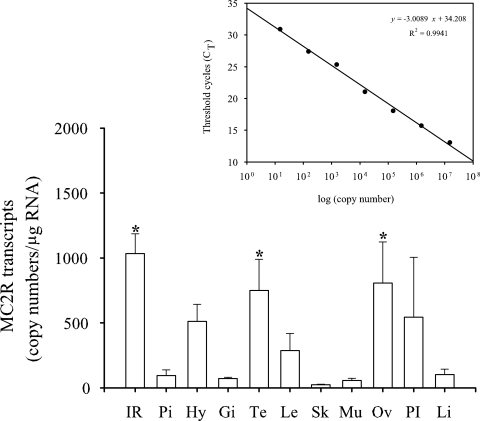
MC2R transcripts were detected in all tissues examined, including both steroidogenic and nonsteroidogenic tissues (Fig. 4). qPCR revealed a tissue-specific difference with the highest MC2R mRNA levels in the interrenal tissue, ovary, and testis, and these levels were significantly different from all other tissues tested, including hypothalamus, pituitary gland, posterior intestine, lens, gills, skin, muscle, and liver (Fig. 4). Melt curve analysis showed only a single peak confirming the absence of any nonspecific amplification. In addition, there was no product amplification with RNA as a template confirming the absence of any genomic contamination in the samples.
Figure 4.
Tissue-specific expression of MC2R in rainbow trout. Quantitative real-time PCR was used to determine MC2R mRNA levels in rainbow trout tissues. MC2R copy numbers in each tissue were calculated based on the standard curve generated by serial dilution of plasmids with insert sequences and expressed as copy number per microgram RNA. Standard curve was drawn by plotting CT values against the logarithm of their known initial copy numbers (inset figure). Bars with asterisk are not statistically significant from one another but significantly different from the rest (one-way ANOVA, P < 0.05); all values represent mean ± sem) (n = 4 fish). IR, Interrenal tissue; Pi, pituitary; Hy, hypothalamus; Gi, gills; Te, testis; Le, lens; Sk, skin; Mu, muscle; Ov, ovary; PI, posterior intestine; Li, liver.
Acute stressor exposure
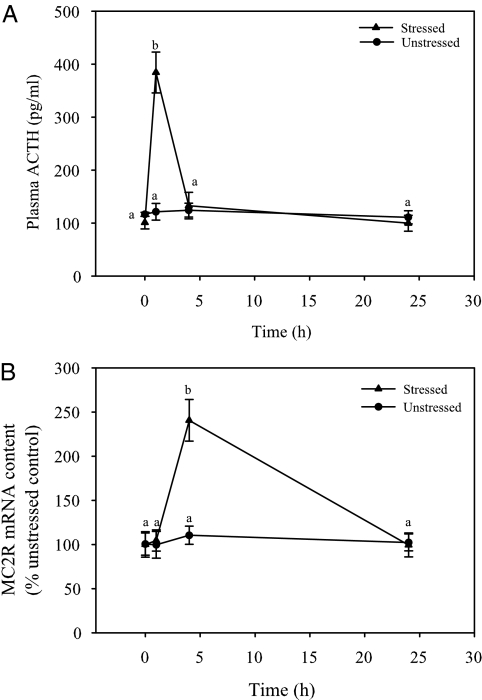
In the unstressed control fish, plasma cortisol (16) and ACTH levels did not change with time (Fig. 5A). Handling disturbance resulted in a significant transient elevation in plasma cortisol (16) and plasma ACTH levels at 1 h poststressor exposure, compared with the prestressor group, after which the levels declined and were not statistically different from the prestressor values at 4 and 24 h (Fig. 5A). Interrenal tissue MC2R mRNA content also showed no significant changes in the unstressed control fish over the 24-h period. An acute stressor significantly elevated interrenal tissue MC2R mRNA content at 4 h after stressor exposure but not at 1 or 24 h, compared with the prestressor group (Fig. 5B).
Figure 5.
Acute-stressor-induced elevation in plasma ACTH concentrations and MC2R mRNA levels in rainbow trout. Plasma ACTH level (A) and interrenal tissue MC2R mRNA abundance (B) in fish that were either unstressed (control) or subjected to a standardized handling stressor (5 min repeated netting and chasing). Plasma and interrenal tissue samples were collected at 0 (before stress), 1, 4, and 24 h after handling stressor. All values represent mean ± sem (n = 5–6 fish); time points with different letters are statistically significant (two-way ANOVA; Bonferroni post hoc test; P < 0.05).
ACTH-induced temporal changes
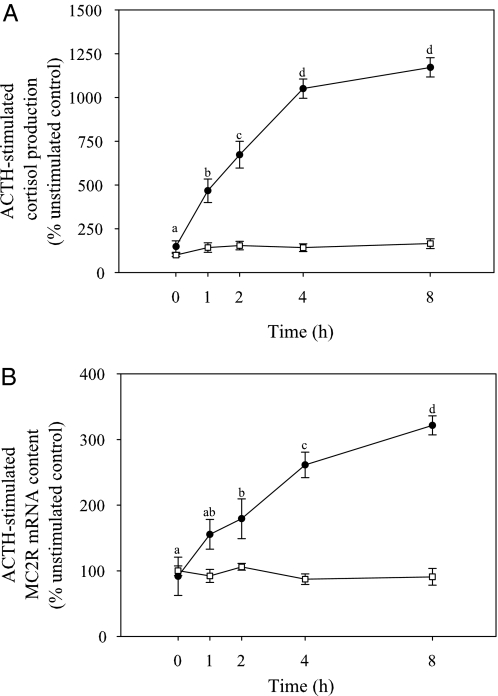
No significant differences in cortisol production and MC2R mRNA levels were observed between different time points in the unstimulated control group (Figs. 6, A and B). ACTH (1.3 μm) stimulation significantly increased cortisol production, compared with unstimulated groups at all time points. There was a significant time-related elevation in cortisol production over a 4-h period, whereas no significant difference in cortisol production was observed between 4 and 8 h after ACTH stimulation (Fig. 6A).
Figure 6.
Temporal changes in ACTH (1.3 μm)-induced cortisol production (A) and MC2R mRNA abundance (B) in rainbow trout interrenal tissue slices in vitro. Interrenals were incubated either with (•) or without ACTH (□) and sampled at 0, 1, 2, 4, and 8 h and L15 medium sampled for measuring cortisol concentration. Tissue slices were sampled for quantifying MC2R mRNA content. Both cortisol production and MC2R mRNA levels are expressed as percent unstimulated control; all values represent mean ± sem (n = 4–5 fish); sampling time with different letters are statistically significant (repeated measures ANOVA followed by Bonferonni post hoc test; P < 0.05).
MC2R mRNA content was also significantly elevated after ACTH (1.3 μm) stimulation, compared with unstimulated control at all the time points. Significant temporal change in MC2R transcripts was initially observed at 2 h after ACTH stimulation. Thereafter, a time-related increase in MC2R mRNA levels was observed at 4 and 8 h after ACTH stimulation (Fig. 6B).
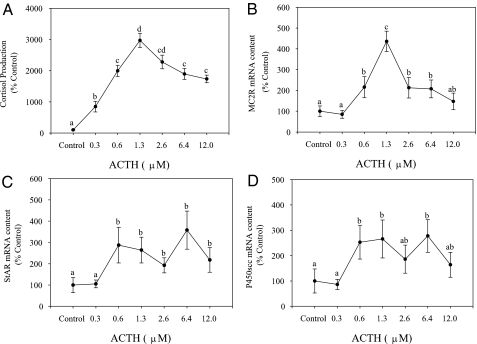
ACTH concentration-related changes
All concentrations of ACTH significantly elevated cortisol production, compared with the control group. Cortisol production increased in a concentration-related manner, peaking at 1.3 μm after which the levels dropped slightly but significantly with higher ACTH concentrations (Fig. 7A). MC2R mRNA abundance showed a concentration-related increase at 0.6 and 1.3 μm ACTH, compared with the unstimulated group (Fig. 7B). Thereafter MC2R transcript levels dropped significantly at higher ACTH concentrations but were significantly higher than the unstimulated group except at 0.3 and 12 μm ACTH (Fig. 7B). StAR and P450scc mRNA levels did not show a concentration-related response with ACTH stimulation. StAR mRNA level was significantly higher at ACTH concentrations of 0.6 μm and above, compared with the unstimulated control (Fig. 7C). P450scc mRNA level was significantly higher at ACTH concentrations of 0.6 and 6.4 μm but not at other concentrations, compared with the unstimulated control group (Fig. 7D).
Figure 7.
Effect of ACTH on steroidogenic gene expression and cortisol production. Interrenal tissue slices were incubated with either control (no ACTH) or varying concentrations of ACTH for 8 h and medium cortisol levels (A), and tissue mRNA levels of MC2R (B), StAR (C), and P450scc (D) determined by qPCR. Values are shown as percent control and represent mean ± sem (n = 4–5 fish); different letters denote statistically significant differences between different ACTH concentrations (repeated measures ANOVA, followed by Bonferonni post hoc test; P < 0.05).
Effect of MCR agonists and antagonist
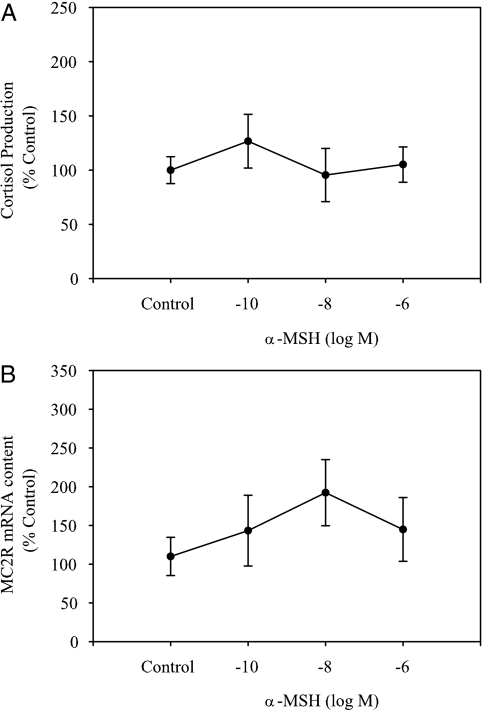
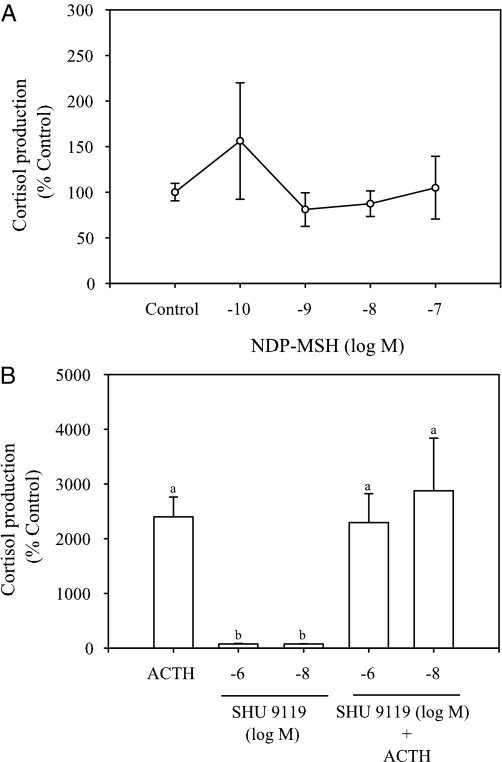
α-MSH stimulation (10−6 to 10−10 m) of interrenal tissue slices for 8 h did not significantly affect either cortisol production or MC2R mRNA abundance (Fig. 8, A and B). Similarly, NDP-MSH did not stimulate cortisol production at any of the concentrations tested (Fig. 9A). In addition, SHU9119 (10−6 and 10−8 m) did not modify ACTH-stimulated cortisol production in vitro (Fig. 9B).
Figure 8.
Effect of α-MSH on in vitro cortisol production (A) and MC2R levels (B) in rainbow trout interrenal tissue preparations. Interrenal tissue slices were incubated with either control (no α-MSH) or α-MSH (10−6 to 10−10 m) for 8 h, and both medium and tissue slices were sampled for determining cortisol production and MC2R mRNA levels, respectively. Values are shown as percent unstimulated control and represent mean ± sem (n = 4–5 fish); no significant differences were observed between different MSH concentrations (repeated measures ANOVA, followed by Bonferonni post hoc test; P < 0.05).
Figure 9.
Effect of NDP-MSH (A) and SHU9119 (B) on in vitro cortisol production in rainbow trout. Interrenal tissue slices were incubated with either control or different concentrations of NDP-MSH (10−7 to 10−10 m) for 3 h and cortisol production determined (A). Similarly, interrenal slices were treated with SHU 9119 (10−6 and 10−8 m) either alone or in combination with ACTH (1.3 μm) for 3 h and cortisol production determined (B). Cortisol production is expressed as percent control and values represent mean ± sem (n = 4–5 fish); no significant differences in cortisol production were observed with NDP-MSH, whereas bars with different letters were statistically significant (one-way ANOVA, followed by Bonferonni post hoc test; P < 0.05).
Discussion
MC2R was cloned and sequenced from a salmonid species, the rainbow trout, and the deduced amino acid sequence shows about 60% sequence similarity to other piscine MC2R and about 44% similarity to tetrapods. For the first time in teleosts, we demonstrate that ACTH up-regulates MC2R mRNA levels in interrenal tissue and this corresponded with enhanced steroidogenic capacity, including higher transcript levels of StAR and P450scc and elevated cortisol production. The positive regulation of MC2R expression by ACTH in vitro was also confirmed in vivo after an acute stressor exposure in trout. The MC2R regulation seen in trout is similar to that reported for tetrapods, suggesting that the stressor-induced ACTH signaling pathway is highly conserved in vertebrates. Together, MC2R regulation may be a key aspect of the evolutionarily conserved corticosteroid response that is essential for stress adaptation.
MC2R gene in trout
The trout MC2R amino acid sequence shows about 60% similarity with other teleosts MC2R (D. rerio, C. carpio, and T. rubripes) and approximately 44% similarity with tetrapod MC2R. The sequence similarity is even higher within the transmembrane regions (62–68% with other fish species and 46–50% with tetrapods), compared with the intra- and extracellular loops and the N- and C-terminal ends. Similar results were also seen with T. rubripes and D. rerio MC2Rs (19,27), and previous studies confirmed this conserved transmembrane regions as the ligand binding site (9,10). Another similarity that trout MC2R shares with tetrapod and piscine models is that the entire coding region is in a single exon, the only exception being T. rubripes MC2R that has a single intron (19). Trout also shares high degree (45–50%) of amino acid similarity with known MCRs, suggesting that this receptor family is highly conserved among vertebrates (28).
Phylogenetic analysis suggests that trout MC2R is an ortholog of tetrapod MC2R and that the ancestral MCR gene may have initially split sequentially into MC2R, MC1R, and the rest of the MCR family (MC3/MC4/MC5R) (19,28). This is supported by the observation that teleost MCRs preferentially bind ACTH peptides over other ligands (19,29,30), suggesting ACTH as a ligand for the ancestral MCR. Furthermore, only ACTH binds to MC2R but not other POMC-derived peptides in both mammalian and nonmammalian vertebrates, supporting the notion that this receptor evolved earlier than the other members of the MCR family during vertebrate evolution.
Compared with tetrapods, ray-finned (actinopterygian) fish possess an extra copy of genes due to whole-genome duplication that occurred deep in the ray-finned fish lineage (31). In addition, ancestors of salmonids underwent additional genome duplication, and therefore, for every gene copy in tetrapods, one might expect to find two copies in teleosts and up to four copies in salmonids (32). However, that is not the case for MC2R because only a singly copy has been reported in teleosts, including trout. Although teleosts lack additional copies of MC2R, molecular clock analysis revealed the presence of two copies of MC2R gene in fish lineage before the split of Fugu and zebrafish (19,30). Also, molecular clock analysis revealed that MC1R and MC2R evolve faster, compared with other MCRs, which could lead to either secondary loss of function or their divergence leading to new ligands and function for duplicated genes (30).
MC2R distribution in trout
The MC2R in mammals is predominantly expressed in the adrenal cortex, including zona fasciculata and glomerulosa regions, and this receptor activation by ACTH leads to corticosteroid biosynthesis (33,34). However, lower transcript levels, compared with the adrenals, have also been reported in other tissues, notably skin, adipose tissue, hypothalamus, pituitary, and liver (35,36,37). In teleosts, MC2R genes were primarily expressed in the interrenal tissue region, and low levels were also reported in the hypothalamus and pituitary (19,22), suggesting a role for this receptor in the functioning of hypothalamus-pituitary-adrenal axis among vertebrates. Using quantitative real-time PCR, we show for the first time that there is a clear tissue-specific distribution of MC2R mRNA abundance in rainbow trout. As in mammalian models, we did see lower MC2R transcript levels, compared with the interrenal tissue in extraadrenal tissues, including hypothalamus, pituitary, skin, liver, gills, lens, muscle, and the posterior intestine. Even though the functional significance of this extraadrenal expression is not known, recent studies suggest a possible link between ACTH signaling and fetal development (38,39), immunomodulation in human skin keratinocytes (35), and localized stress response in hair follicles (40). These results along with the tissue-specific MC2R mRNA abundance in the present study leads us to propose an extracorticosteroidogenic role for ACTH in teleosts, whereas the specific pathways activated and their implications in nonsteroidogenic tissues remains to be determined.
Interestingly, MC2R was abundantly expressed in the testis and gonads, similar to that seen in the interrenal tissue, leading to the hypothesis that ACTH may be playing a role in sex steroid modulation during stress. Whereas no study has addressed this possibility, recent studies points to an involvement of MC2R signaling in testosterone production in fetal testis (38,39). Also, we observed significant reduction in the acute gonadotropin-induced estradiol production by ACTH in zebrafish ovarian follicles in vitro (Alsop, D., J. Ings, and M. M. Vijayan, unpublished data). Based on these observations, we propose a direct role for ACTH signaling, in addition to a cortisol-mediated effect, in the stressor-mediated modulation of sex steroid production in fish (41,42,43,44).
MC2R regulation
Our results agree with previous observations from mammalian and fish studies that ACTH is the only endogenous agonist of MC2R, compared with other MCRs, which bind other POMC-derived melanocortin peptides (19,22,45). Whereas α-MSH, another POMC-derived peptide did show corticotropic activity in tilapia (46), we were unable to observe any significant effect of this peptide on either MC2R mRNA abundance or cortisol production in trout interrenal tissue in vitro. Indeed, α-MSH showed no significant binding to either Fugu or mammalian MC2R (19,47) and failed to induce cortisol production in common carp interrenals (22). We did not use a heterologous expression system to confirm ligand binding and activation of trout MC2R because of the differences in ambient temperature between the trout (∼11 C) and mammalian cell systems (37 C). However, NDP-MSH, an MC4R agonist, failed to stimulate cortisol production, despite the presence of MC4R in trout interrenals (48). This was further confirmed by the lack of SHU9119, an MC4R/MC3R antagonist, to modulate ACTH-induced cortisol production. Together, these results support MC2R activation by ACTH as the primary signaling pathway leading to corticosteroid biosynthesis in teleosts.
In mammals, autoregulation of MC2R by ACTH is well documented, but the results are equivocal (45,49,50,51). For instance, stimulation of mouse Y1 adrenocortical carcinoma cell lines with ACTH up-regulated MC2R transcripts in a time and dose-dependent fashion. Whereas MC2R mRNA abundance was higher within 4–7 h, maximum (6-fold) induction was attained only around 19–27 h after ACTH exposure (45). Similarly, ACTH-induced MC2R mRNA up-regulation was seen at 12 h in human adrenocortical cells (51), whereas in human H295 adrenocortical carcinoma cell lines and bovine adrenal fasciculate-reticularis cells, the maximal induction was observed only after 24 h (45,49). Based on these temporal differences, it is unclear whether there is desensitization and/or receptor regulation of MC2R after ACTH exposure.
Similar to mammalian cell models, we show for the first time a positive regulation of MC2R by ACTH stimulation in trout interrenal tissue in vitro. However, whereas changes in MC2R mRNA abundance were reported only after longer-term ACTH incubation in mammalian cell system, we observed MC2R transcript up-regulation with acute (2–4 h) ACTH stimulation in vitro. Whereas the reason for the temporal differences in gene expression is unknown, it may be either a species-specific effect or related to the cell system. For instance, unlike the mammalian studies, we used tissues from in vivo treatments for our in vitro incubations. This was further confirmed by MC2R gene expression in the interrenal tissue in vivo after a stressor exposure, which mimicked the response seen in vitro.
In teleosts, acute stressors elicit significant elevation in plasma ACTH and cortisol levels within 1 h of stimulation (11,52,53) and is in agreement with our results (16) (Fig. 2A). The rapid elevation in cortisol levels in response to an acute stressor is due to the positive trophic effects of ACTH, involving cAMP production and activation of steroidogenic enzyme system (14,50). This agrees with our previous findings in trout that the steroidogenic capacity is enhanced rapidly in response to ACTH stimulation, including significant elevation in cortisol production and up-regulation of steroidogenic enzyme machinery (StAR protein and P450scc) in trout interrenal tissues (16). Here we demonstrate for the first time that elevation in MC2R mRNA abundance at 4 h after stressor exposure follows the hormonal response in vivo. This was also the case in vitro in which ACTH-stimulated cortisol production was observed at 1 h, whereas MC2R increase occurred only at 2 h after stimulation. The lag time in MC2R expression in response to either acute ACTH stimulation in vitro or acute stressor exposure in vivo, unlike StAR and P450scc mRNA levels that increased at 1 h (16), leads us to propose additional molecular events, in addition to direct ACTH signaling, involved in MC2R transcript regulation.
For instance, mammalian studies points to a down-regulation of MC2R in response to ACTH stimulation as a mechanism of regulating stress signaling (54,55,56). Indeed, MC2R protein down-regulation was observed within minutes after ligand binding, and it involves uncoupling from the adenylate cyclase, followed by internalization (54). This is initiated by receptor phosphorylation by second messenger-regulated kinases such as protein kinase A or PKC, with subsequent binding of proteins referred to as arrestins, leading to down-regulation of the protein (56). This is generally followed by increased transcription of MC2R and new protein synthesis to replenish the receptors on the cell surface (56). Indeed, in bovine primary cultures of fasciculata cells, short-term ACTH treatment desensitized MC2R and cAMP response, and this was followed by an increase in the number of ACTH-binding sites (57) as well as MC2R mRNA abundance (49). Together, these results suggest an increased MC2R turnover as a key aspect of the stress adaptation process, including autoregulation of the receptor by ACTH, but the mechanism(s) involved are far from clear. Indeed, the recent discovery of MC2R accessory protein (58), and its role in the regulation of MC2R signaling (59,60) suggests additional mechanisms modulating receptor function. The presence of putative PKC and protein kinase A phosphorylation sites in trout MC2R, similar to that in mammals, suggests that the mechanism for receptor regulation is highly conserved in vertebrates, underscoring a key role for this receptor in stress adaptation. Specifically, we propose receptor cycling as a possible mechanism to maintain interrenal responsiveness to ACTH stimulation during subsequent stressor exposures, whereas the mechanism(s) of action remains to be elucidated.
Conclusions
In conclusion, MC2R was cloned and sequenced from a salmonid, the rainbow trout, and based on the sequence alignment, phylogenetic analysis, and tissue distribution can be categorized as ortholog of mammalian MC2R. ACTH stimulation, the primary ligand for MC2R, elevated MC2R mRNA abundance in trout interrenal tissue, supporting a highly conserved receptor autoregulation by its ligand in vertebrates. This MC2R up-regulation along with enhanced steroidogenic capacity observed in vitro was also evident in vivo in response to an acute stressor exposure, underpinning a critical role for MC2R dynamics in the evolutionarily conserved cortisol response essential for stress adaptation in vertebrates. A key finding was the tissue-specific distribution of MC2R transcripts in extraadrenal tissues, especially in the gonads of trout. Because acute stressor exposure has been linked to altered circulating sex steroid levels in teleosts, we hypothesize a role for ACTH signaling in the stressor-mediated reproductive dysfunction. Taken together, our results suggest a highly conserved ACTH signaling system in vertebrates, whereas tissue-specific distribution suggests an extraadrenal role for ACTH in teleosts.
Footnotes
This work was supported by the Natural Sciences and Engineering Research Council (NSERC), Canada, discovery grant (to M.M.V.), and postdoctoral fellowship (to N.A.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online June 5, 2008
Abbreviations: CT, Threshold cycle; dNTP, deoxynucleotide triphosphate; MCR, melanocortin receptor; NDP-MSH, [Nle4, d-Phe7]-α-MSH trifluoroacetate salt; NUP, nested universal primer; PKC, protein kinase C; POMC, proopiomelanocortin; P450scc, P450 side-chain cleavage; qPCR, quantitative real-time PCR; RACE, rapid amplification of cDNA ends; StAR, steroidogenic acute regulatory protein; UPM, universal primer mix.
References
- Sewer M, Waterman M 2003 ACTH modulation of transcription factors responsible for steroid hydroxylase gene expression in the adrenal cortex. Microsc Res Technol 61:300–307 [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Robbins LS, Mortrud MT, Cone RD 1992 The cloning of a family of genes that encode the melanocortin receptors. Science 257:1248–1251 [DOI] [PubMed] [Google Scholar]
- Penhoat A, Naville D, Begeot M 2001 The adrenocorticotrophic hormone receptor. Curr Opin Endocrinol Diabetes 8:112–117 [Google Scholar]
- Stocco DM 2000 The role of the StAR in steroidogenesis: challenges for the future. J Endocrinol 164:247–253 [DOI] [PubMed] [Google Scholar]
- Papadopoulos V 1993 Peripheral-type benzodiazepine/diazepam binding inhibitor receptor: biological role in steroidogenic cell function. Endocr Rev 14:222–240 [DOI] [PubMed] [Google Scholar]
- Payne AH, Hales DB 2004 Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev 25:947–970 [DOI] [PubMed] [Google Scholar]
- Abdel-Malek ZA 2001 Melanocortin receptors: their functions and regulation by physiological agonists and antagonists. Cell Mol Life Sci 58:434–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz I, Fong TM 2003 The melanocortin system. Am J Physiol 284:E468–E474 [DOI] [PubMed] [Google Scholar]
- Schiöth HB 2001 The physiological role of melanocortin receptors. Vitam Horm 63:195–232 [DOI] [PubMed] [Google Scholar]
- Schiöth HB, Haitina T, Ling MK, Ringholm A, Fredriksson R, Cerda- Reverter JM, Klovins J 2005 Evolutionary conservation of the structural, pharmacological, and genomic characteristics of the melanocortin receptor subtypes. Peptides 26:1886–1900 [DOI] [PubMed] [Google Scholar]
- Wendalaar Bonga SEW 1997 The stress response in fish. Physiol Rev 77:591–625 [DOI] [PubMed] [Google Scholar]
- Mommsen TP, Vijayan MM, Moon TW 1999 Cortisol in teleosts: dynamics, mechanisms of action, and metabolic regulation. Rev Fish Biol Fisher 9:211–268 [Google Scholar]
- Donaldson EM 1981 The pituitary-interrenal axis as indicator of stress in fish. In: Pickering AD, ed. Stress and fish. London: Academic Press; 11–47 [Google Scholar]
- Hontela A 2005 Stress and the hypothalamo-pituitary-interrenal axis: adrenal toxicology—effects of environmental pollutants on the structure and function of the HPI axis. In: Moon TW, Mommsen TP, eds. Biochemical and molecular biology of fishes. Vol 6. Environmental toxicology. Amsterdam: Elsevier; 331–363 [Google Scholar]
- Geslin M, Auperin B 2004 Relationship between changes in mRNAs of the genes encoding steroidogenic acute regulatory protein and P450 cholesterol side chain cleavage in head kidney and plasma levels of cortisol in response to different kinds of acute stress in the rainbow trout (Oncorhynchus mykiss). Gen Comp Endocrinol 135:70–80 [DOI] [PubMed] [Google Scholar]
- Aluru N, Vijayan MM 2006 Aryl hydrocarbon receptor activation impairs cortisol response to stress in rainbow trout by disrupting the rate-limiting steps in steroidogenesis. Endocrinology 147:1895–1903 [DOI] [PubMed] [Google Scholar]
- Gravel A, Vijayan MM 2006 Salicylate disrupts interrenal steroidogenesis and brain glucocorticoid receptor expression in rainbow trout. Toxicol Sci 93:41–49 [DOI] [PubMed] [Google Scholar]
- Hagen IJ, Kusakabe M, Young G 2006 Effects of ACTH and cAMP on steroidogenic acute regulatory protein and P450 11β-hydroxylase messenger RNAs in rainbow trout interrenal cells: relationship with in vitro cortisol production. Gen Comp Endocrinol 145:254–262 [DOI] [PubMed] [Google Scholar]
- Klovins J, Haitina T, Fridmanis D, Kilianova Z, Kapa I, Fredriksson R, Gallo-Payet N, Schiöth HB 2004 The melanocortin system in Fugu: determination of POMC/AGRP/MCR gene repertoire and synteny, as well as pharmacology and anatomical distribution of the MCRs. Mol Biol Evol 21:563–579 [DOI] [PubMed] [Google Scholar]
- Logan DW, Bryson-Richardson RJ, Pagan KE, Taylor MS, Currie PD, Jackson IJ 2003 The structure and evolution of the melanocortin and MCH receptors in fish and mammals. Genomics 81:184–191 [DOI] [PubMed] [Google Scholar]
- Logan DW, Bryson-Richardson RJ, Taylor MS, Currie P, Jackson IJ 2003 Sequence characterization of teleost fish melanocortin receptors. Ann NY Acad Sci 994:319–330 [DOI] [PubMed] [Google Scholar]
- Metz JR, Geven EJW, vanden Burg EH, Flik G 2005 ACTH, α-MSH and control of cortisol release: cloning, sequencing and functional expression of the melanocortin-2 and melanocortin-5 receptor in Cyprinus carpio. Am J Physiol 289:R814–R826 [DOI] [PubMed] [Google Scholar]
- Aluru N, Vijayan MM 2004 β-Naphthoflavone disrupts cortisol production and liver glucocorticoid responsiveness in rainbow trout. Aquat Toxicol 67:273–285 [DOI] [PubMed] [Google Scholar]
- Doyon C, Leclair J, Trudeau VL, Moon TW 2006 Corticotropin-releasing factor and neuropeptide Y mRNA levels are modified by glucocorticoids in rainbow trout, Oncorhynchus mykiss. Gen Comp Endocrinol 146:126–135 [DOI] [PubMed] [Google Scholar]
- Guindon S, Lethiec F, Duroux P, Gascuel O 2005 PHYML Online—a web server for fast maximum likelihood-based phylogenetic inference. Nucleic Acids Res 33:W557–W559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevenet F, Brun C, Banuls AL, Jacq B, Christen R 2006 TreeDyn: towards dynamic graphics and annotations for analyses of trees. BMC Bioinformatics 7:439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringholm A, Fredriksson R, Poliakova N, Yan YL, Postlethwait JH, Larhammar D, Schiöth HB 2002 One melanocortin 4 and two melanocortin 5 receptors from zebrafish show remarkable conservation in structure and pharmacology. J Neurochem 82:6–18 [DOI] [PubMed] [Google Scholar]
- Takahashi A, Kawauchi H 2006 Evolution of melanocortin systems in fish. Gen Comp Endocrinol 148:85–94 [DOI] [PubMed] [Google Scholar]
- Haitina T, Klovins J, Schiöth HB 2005 Pharmacological characterization of melanocortin receptors in fish suggests an important role for ACTH. Ann NY Acad Sci 1040:337–339 [DOI] [PubMed] [Google Scholar]
- Haitina T, Klovins J, Takahashi A, Löwgren M, Ringholm A, Enberg J, Kawauchi H, Larson ET, Fredriksson R, Schiöth HB 2007 Functional characterization of two melanocortin (MC) receptors in lamprey showing orthology to the MC1 and MC4 receptor subtypes. BMC Evol Biol 7:101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Van de Peer Y 2005 From 2R to 3R: evidence for a fish-specific genome duplication (FSGD). Bioessays 27:937–945 [DOI] [PubMed] [Google Scholar]
- Allendorf FW, Thorgaard GH 1984 Tetraploidy and the evolution of salmonid fishes. In: Turner BJ, ed. Evolutionary genetics of fishes. New York: Plenum Press Corp.; 1–53 [Google Scholar]
- Chhajlani V 1996 Distribution of cDNA for melanocortin receptor subtypes in human tissues. Biochem Mol Biol Int 38:73–80 [PubMed] [Google Scholar]
- Xia Y, Wikberg JE 1996 Localization of ACTH receptor mRNA by in situ hybridization in mouse adrenal gland. Cell Tissue Res 286:63–68 [DOI] [PubMed] [Google Scholar]
- Park HJ, Kim HJ, Lee JY, Cho BK, Gallo RL, Cho DH 2007 Adrenocorticotropin hormone stimulates interleukin-18 expression in human HaCaT keratinocytes. J Invest Dermatol 127:1210–1216 [DOI] [PubMed] [Google Scholar]
- Kijima H, Kubo M, Shimizu C, Ishizuka T, Takano K, Nagai S, Koike T 2004 Effects of hypophysectomy and in vivo administration of ACTH or dexamethasone on the level of ACTH receptor mRNA in adrenal glands and adipose tissues of mice. Endocr Regul 38:87–95 [PubMed] [Google Scholar]
- Nimura M, Udagawa J, Hatta T, Hashimoto R, Otani H 2006 Spatial and temporal patterns of expression of melanocortin type 2 and 5 receptors in the fetal mouse tissues and organs. Anat Embryol (Berl) 211:109–117 [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy PJ, Fleming LM, Jackson G, Hochgeschwender U, Reed P, Baker PJ 2003 Adrenocorticotropic hormone directly stimulates testosterone production by the fetal and neonatal mouse testis. Endocrinology 144:3279–3284 [DOI] [PubMed] [Google Scholar]
- Johnston H, King PJ, O'Shaughnessy PJ 2007 Effects of ACTH and expression of the melanocortin-2 receptor in the neonatal mouse testis. Reproduction 133:1181–1187 [DOI] [PubMed] [Google Scholar]
- Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, Straub RH, Paus R 2005 Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB J 19:1332–1340 [DOI] [PubMed] [Google Scholar]
- Carragher JF, Sumpter JP 1990 The effect of cortisol on the secretion of sex steroids from cultured ovarian follicles of rainbow trout. Gen Comp Endocrinol 77:403–407 [DOI] [PubMed] [Google Scholar]
- Campbell PM, Pottinger TG, Sumpter JP 1992 Stress reduces the quality of gametes produced by rainbow trout. Biol Reprod 47:1140–1150 [DOI] [PubMed] [Google Scholar]
- Lethimonier C, Flouriot G, Valotaire Y, Kah O, Ducouret B 2000 Transcriptional interference between glucocorticoid receptor and estradiol receptor mediates the inhibitory effect of cortisol on fish vitellogenesis. Biol Reprod 62:1763–1771 [DOI] [PubMed] [Google Scholar]
- Consten D, Keuning ED, Bogerd J, Zandbergen MA, Lambert JG, Komen J, Goos HJ 2002 Sex steroids and their involvement in the cortisol-induced inhibition of pubertal development in male common carp, Cyprinus carpio L. Biol Reprod 67:465–472 [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Bird IM, Rainey WE, Cone RD 1994 ACTH induces upregulation of ACTH receptor mRNA in mouse and human adrenocortical cell lines. Mol Cell Endocrinol 99:R17–R20 [DOI] [PubMed] [Google Scholar]
- Lamers AE, Flik G, Atsma W, Wendelaar Bonga SE 1992 A role for di-acetyl α-melanocyte-stimulating hormone in the control of cortisol release in the teleost Oreochromis mossambicus. J Endocrinol 135:285–292 [DOI] [PubMed] [Google Scholar]
- Schiöth HB, Chhajlani V, Muceniece R, Klusa V, Wikberg JE 1996 Major pharmacological distinction of the ACTH receptor from other melanocortin receptors. Life Sci 59:797–801 [DOI] [PubMed] [Google Scholar]
- Haitina T, Klovins J, Andersson J, Fredriksson R, Lagerström MC, Larhammar D, Larson ET, Schiöth HB 2004 Cloning, tissue distribution, pharmacology and three-dimensional modelling of melanocortin receptors 4 and 5 in rainbow trout suggest close evolutionary relationship of these subtypes. Biochem J 380:475–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penhoat A, Jaillard C, Saez JM 1994 Regulation of bovine adrenal cell corticotropin receptor mRNA levels by corticotropin (ACTH) and angiotensin-II (A-II). Mol Cell Endocrinol 103:R7–R10 [DOI] [PubMed] [Google Scholar]
- Le Roy C, Li JY, Stocco DM, Langlois D, Saez JM 2000 Regulation by adrenocorticotropin (ACTH), angiotensin II, transforming growth factor-β and insulin-like growth factor I of bovine adrenal cell steroidogenic capacity and expression of ACTH receptor, steroidogenic acute regulatory protein, cytochrome P450c17, and 3β-hydroxysteroid dehydrogenase. Endocrinology 141:1599–1607 [DOI] [PubMed] [Google Scholar]
- Lebrethon MC, Naville D, Begeot M, Saez JM 1994 Regulation of corticotropin receptor number and messenger RNA in cultured human adrenocortical cells by corticotropin and angiotensin II. J Clin Invest 93:1828–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton BA, Morgan JD, Vijayan MM 2002 Physiological and condition-related indicators of environmental stress in fish. In: Adams SM, ed. Biological indicators of stress in fish. 2nd ed. Bethesda, MD: American Fisheries Society; 111–148 [Google Scholar]
- Iwama GK, Alfonso LOB, Vijayan MM 2006 Stress in fish. In: Evans DH, Claiborne JB, eds. The physiology of fishes. 3rd ed. Boca Raton, FL: CRC Press; 319–342 [Google Scholar]
- Baig AH, Swords FM, Noon LA, King PJ, Hunyady L, Clark AJ 2001 Desensitization of the Y1 cell adrenocorticotropin receptor: evidence for a restricted heterologous mechanism implying a role for receptor-effector complexes. J Biol Chem 276:44792–44797 [DOI] [PubMed] [Google Scholar]
- Baig AH, Swords FM, Szaszák M, King PJ, Hunyady L, Clark AJ 2002 Agonist activated adrenocorticotropin receptor internalizes via a clathrin-mediated G protein receptor kinase dependent mechanism. Endocr Res 28:281–289 [DOI] [PubMed] [Google Scholar]
- Kilianova Z, Basora N, Kilian P, Payet MD, Gallo-Payet N 2006 Human melanocortin receptor 2 expression and functionality: effects of protein kinase A and protein kinase C on desensitization and internalization. Endocrinology 147:2325–2337 [DOI] [PubMed] [Google Scholar]
- Penhoat A, Jaillard C, Saez JM 1989 Corticotropin positively regulates its own receptors and cAMP response in cultured bovine adrenal cells. Proc Natl Acad Sci USA 86:4978–4981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Rached M, Gallo-Payet N 2007 Differential regulation of the human adrenocorticotropin receptor [melanocortin-2 receptor (MC2R)] by human MC2R accessory protein isoforms α and β in isogenic human embryonic kidney 293 cells. Mol Endocrinol 21:1656–1669 [DOI] [PubMed] [Google Scholar]
- Metherell LA, Chapple JP, Cooray S, David A, Becker C, Rüschendorf F, Naville D, Begeot M, Khoo B, Nürnberg P, Huebner A, Cheetham ME, Clark AJ 2005 Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nat Genet 37:166–170 [DOI] [PubMed] [Google Scholar]
- Sebag JA, Hinkle PM 2007 Melanocortin-2 receptor accessory protein MRAP forms antiparallel homodimers. Proc Natl Acad Sci USA 104:20244–20249 [DOI] [PMC free article] [PubMed] [Google Scholar]