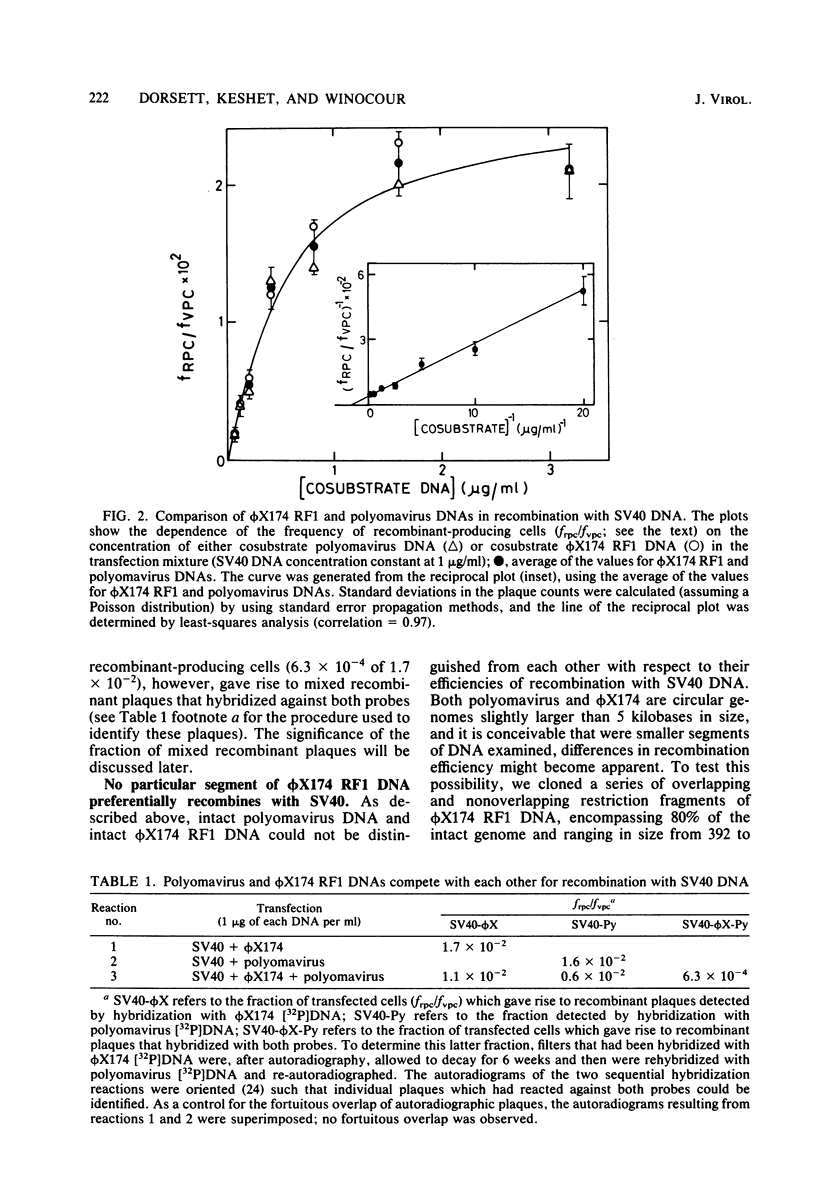
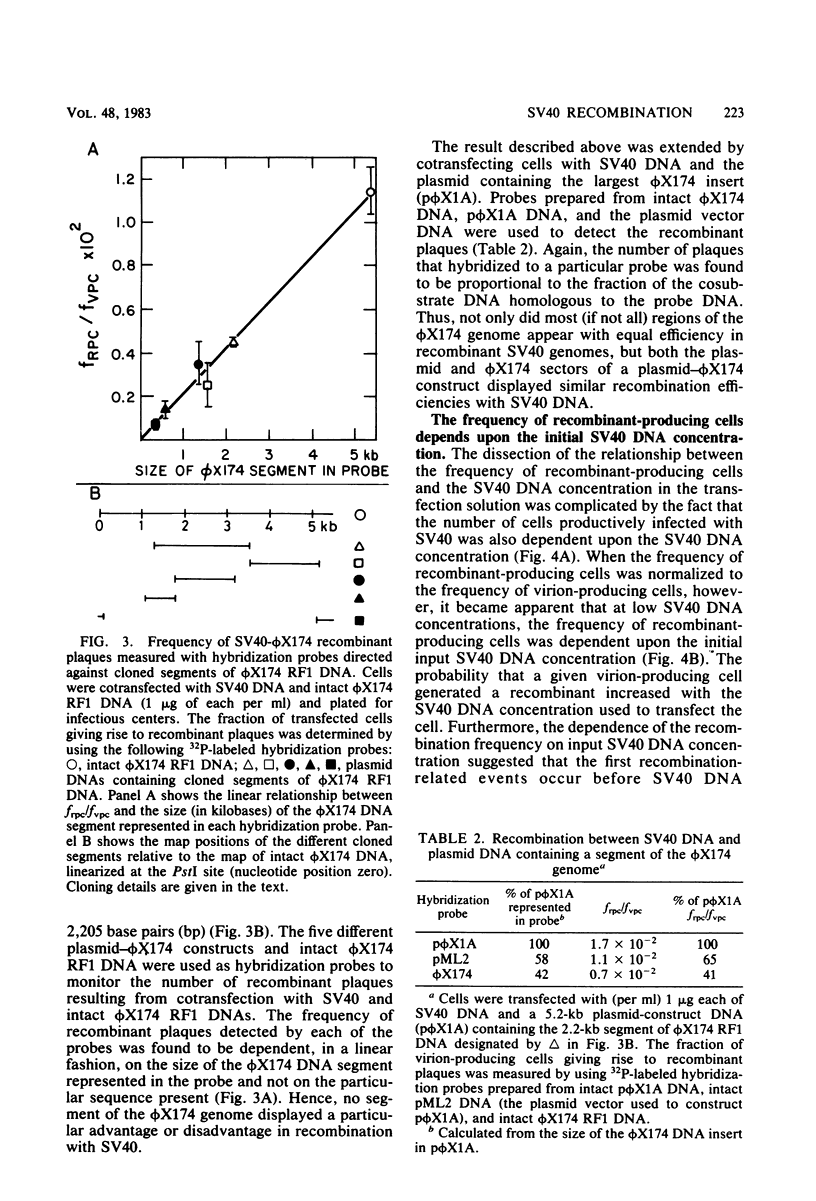
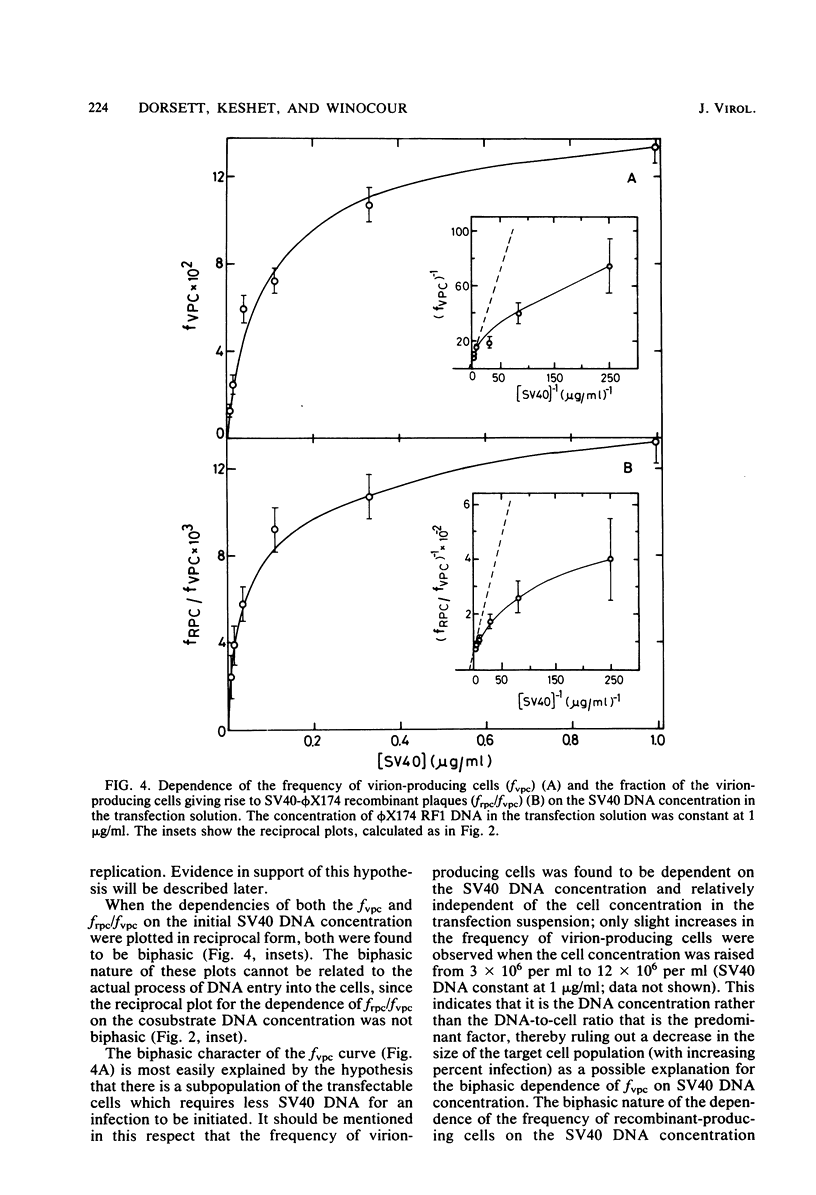
Abstract
We describe an infectious-center in situ plaque hybridization procedure which quantitates simian virus 40 (SV40) nonhomologous recombination in terms of the number of recombinant-producing cells in the DNA transfected cell population. Using this assay to measure the efficiency of recombination with SV40 DNA in permissive monkey BSC-1 cells, we found that: (i) over a range of DNA concentrations, polyomavirus DNA (which is partially homologous to SV40 DNA) cannot be distinguished from nonhomologous phi X174 RF1 DNA with respect to its ability to recombine with SV40 DNA; (ii) at defined DNA concentrations, polyomavirus and phi X174 RF1 DNA compete with each other for recombination with SV40 DNA; (iii) virtually all segments of the phi X174 genome recombine, apparently at random, with SV40 DNA; (iv) the frequency of recombinant-producing cells, among the successfully transfected (virion-producing) cells, depends upon the input SV40 DNA concentration in the transfection solution; and (v) replication-defective SV40 mutant DNAs compete with wild-type SV40 DNA for recombination with phi X174 RF1 DNA. From these observations, we conclude that the efficiency of recombination with SV40, in the system under study, is unaffected by nucleotide sequence homology and that a limiting stage in the recombination pathway occurs before SV40 DNA replication. Comparison of the dependency of recombination on initial SV40 DNA concentration with the dependency on initial phi X174 RF1 DNA concentration indicates that SV40 DNA sequences are a controlling factor in the nonhomologous recombination pathway.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Eisenberg S., Harbers B., Hours C., Denhardt D. T. The mechanism of replication of phiX174 DNA. XII. Non-random location of gaps in nascent phiX174 RF II DNA. J Mol Biol. 1975 Nov 25;99(1):107–123. doi: 10.1016/s0022-2836(75)80162-8. [DOI] [PubMed] [Google Scholar]
- Gluzman Y., Sambrook J. F., Frisque R. J. Expression of early genes of origin-defective mutants of simian virus 40. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3898–3902. doi: 10.1073/pnas.77.7.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., So M., McCarthy B. J. In vitro mutagenesis of a circular DNA molecule by using synthetic restriction sites. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6012–6016. doi: 10.1073/pnas.75.12.6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi S., Winocour E. Acquisition of sequences homologous to host deoxyribonucleic acid by closed circular simian virus 40 deoxyribonucleic acid. J Virol. 1972 Feb;9(2):309–316. doi: 10.1128/jvi.9.2.309-316.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L. E., Manos M. M., Gluzman Y. Sequence of the junction in adenovirus 2-SV40 hybrids: examples of illegitimate recombination. Nucleic Acids Res. 1982 Dec 20;10(24):8099–8112. doi: 10.1093/nar/10.24.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusky M., Botchan M. Inhibition of SV40 replication in simian cells by specific pBR322 DNA sequences. Nature. 1981 Sep 3;293(5827):79–81. doi: 10.1038/293079a0. [DOI] [PubMed] [Google Scholar]
- Milman G., Herzberg M. Efficient DNA transfection and rapid assay for thymidine kinase activity and viral antigenic determinants. Somatic Cell Genet. 1981 Mar;7(2):161–170. doi: 10.1007/BF01567655. [DOI] [PubMed] [Google Scholar]
- Perucho M., Hanahan D., Wigler M. Genetic and physical linkage of exogenous sequences in transformed cells. Cell. 1980 Nov;22(1 Pt 1):309–317. doi: 10.1016/0092-8674(80)90178-6. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sambrook J. The isolation of defective variants of simian virus 40 whose genomes contain sequences derived from adenovirus 2 DNA. J Gen Virol. 1978 Feb;38(2):313–327. doi: 10.1099/0022-1317-38-2-313. [DOI] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Smith G. R., Kunes S. M., Schultz D. W., Taylor A., Triman K. L. Structure of chi hotspots of generalized recombination. Cell. 1981 May;24(2):429–436. doi: 10.1016/0092-8674(81)90333-0. [DOI] [PubMed] [Google Scholar]
- Soeda E., Arrand J. R., Smolar N., Walsh J. E., Griffin B. E. Coding potential and regulatory signals of the polyoma virus genome. Nature. 1980 Jan 31;283(5746):445–453. doi: 10.1038/283445a0. [DOI] [PubMed] [Google Scholar]
- Stahl F. W. Special sites in generalized recombination. Annu Rev Genet. 1979;13:7–24. doi: 10.1146/annurev.ge.13.120179.000255. [DOI] [PubMed] [Google Scholar]
- Stringer J. R. DNA sequence homology and chromosomal deletion at a site of SV40 DNA integration. Nature. 1982 Mar 25;296(5855):363–366. doi: 10.1038/296363a0. [DOI] [PubMed] [Google Scholar]
- Villarreal L. P., Berg P. Hybridization in situ of SV40 plaques: detection of recombinant SV40 virus carrying specific sequences of nonviral DNA. Science. 1977 Apr 8;196(4286):183–185. doi: 10.1126/science.191907. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal H. Genomic arrangement of an adenovirus-simian virus 40 hybrid virus, Ad2+ND4del. J Virol. 1981 Nov;40(2):526–532. doi: 10.1128/jvi.40.2.526-532.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. H., Berget P. B., Pipas J. M. Somatic cells efficiently join unrelated DNA segments end-to-end. Mol Cell Biol. 1982 Oct;2(10):1258–1269. doi: 10.1128/mcb.2.10.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocour E., Keshet I. Indiscriminate recombination in simian virus 40-infected monkey cells. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4861–4865. doi: 10.1073/pnas.77.8.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocour E., Lavie V., Keshet I. Structure of simian virus 40-phiX174 recombinant genomes isolated from single cells. J Virol. 1983 Oct;48(1):229–238. doi: 10.1128/jvi.48.1.229-238.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zain B. S., Roberts R. J. Characterization and sequence analysis of a recombination site in the hybrid virus Ad2+ND. J Mol Biol. 1978 Mar 25;120(1):13–31. doi: 10.1016/0022-2836(78)90293-0. [DOI] [PubMed] [Google Scholar]




