Abstract
Orally delivered salt stimulates renal salt excretion more effectively than does iv delivered salt. Although the mechanisms that underlie this “postprandial natriuresis” are poorly understood, the peptide uroguanylin (UGn) is thought to be a key mediator. However, the lack of selective assays for UGn gene products has hindered rigorous testing of this hypothesis. Using peptide-specific assays, we now report surprisingly little UGn in rat intestine or plasma. In contrast, prouroguanylin (proUGn), the presumed-inactive precursor of UGn, is plentiful (at least 40 times more abundant than UGn) in both intestine and plasma. The intestine is the likely source of the circulating proUGn because: 1) the proUGn portal to systemic ratio is approximately two under normal conditions, and 2) systemic proUGn levels decrease rapidly after intestinal resection. Together, these data suggest that proUGn itself is actively involved in enterorenal signaling. This is strongly supported by our observation that iv infusion of proUGn at a physiological concentration produces a long-lasting renal natriuresis, whereas previously reported natriuretic effects of UGn have required supraphysiological concentrations. Thus, our data point to proUGn as an endocrine (i.e. circulating) mediator of postprandial natriuresis, and suggest that the propeptide is secreted intact from the intestine into the circulation and processed to an active form at an extravascular site.
AN ORALLY DELIVERED salt load is excreted faster than an equivalent iv salt load (1,2,3,4). Although the mechanisms underlying this “postprandial natriuresis” are not fully understood, an intestinal peptide known as uroguanylin (UGn) has been identified as a likely participant (5,6). Perhaps the strongest evidence for this comes from genetic ablation of the UGn gene in mice, which produces chronic hypertension and significantly retards urinary sodium excretion after an oral sodium load (7,8). In addition, both UGn and its propeptide precursor, prouroguanylin (proUGn), circulate in the plasma (9,10,11), and when plasma levels are increased by iv infusion of exogenous UGn, the rate of urinary salt excretion increases (12,13,14). Furthermore, the principal site of UGn expression in the rat intestine is the enterochromaffin (EC) cell (15,16,17), an intestinal endocrine cell long known to secrete amines and peptides into the interstitium, and, therefore, into the general circulation (18,19). UGn gene expression is regulated in these cells by orally administered salt in the intestine of the intact rat (20) and is also regulated by extracellular hypertonicity in an intestinally derived epithelial cell line (21). Together, these observations are consistent with the hypothesis that UGn functions as the endocrine mediator of an enterorenal hormonal axis that regulates urinary sodium excretion in response to dietary sodium intake.
However, to meet the requirements of this hypothesis, the renal natriuretic response must be initiated by UGn that originates from within the intestine. Somewhat surprisingly, this fundamental principle has not yet been established. Although Northern blot studies have shown that the small intestine is by far the major site for pre-proUGn mRNA expression (10,22,23), detectable amounts of pre-proUGn mRNA and/or immunoreactive UGn-like polypeptides have been reported in a variety of other tissues, including the kidney itself, as well as the colon, heart, stomach, lung, pancreas, brain, testis, ovary, oviduct, epididymis, prostate, parotid gland, submandibular gland, nasal mucosa, and gall bladder (10,22,23,24,25,26,27,28,29,30,31,32). One caveat to these studies is that the methods used have either been indirect (RNA based) or nonquantitative (immunocytochemical), and, where antibodies have been used, they have, for the most part, been unable to discriminate between UGn and proUGn. Thus, the relative potential of each of these tissues to contribute to plasma pools of UGn and proUGn remains undefined.
As a further complication, a proUGn-like polypeptide is readily detected in extracts of rat small intestine using standard Western blotting techniques (15,33), but UGn itself appears to be present in these extracts only at very low levels (23). Thus, if the intestine is the source of UGn that is destined for the kidney, it is not clear when and where propeptide processing takes place. Moreover, the UGn concentrations reported in plasma are also very low, relative to the levels that are required to induce physiological responses in the kidney. Thus, published plasma UGn levels are in the femto-molar to picomolar range, depending on the species (34,35,36), whereas physiological thresholds for renal responses have been established in the nanomolar range by prolonged agonist infusions (14), or have been based on a bolus injection procedure that produces very high doses of agonist over a very short (nonphysiological) time course (37,38). The “pharmacological” nature of the concentrations used in these studies has been a source of concern, with respect to the putative endocrine role of UGn.
To address these issues, we developed a new immunoassay that is specific for proUGn and does not cross-react with free UGn. We used this assay to quantify proUGn levels in tissues and plasma, and to determine the effects of gut ablation on the size of the plasma pool. We also measured UGn levels in parallel, using a quantitative bioassay that does not cross-react with proUGn. Our results demonstrate that the dominant form of the peptide stored in the intestine and secreted into the plasma is proUGn rather than UGn. To investigate the biological significance of this circulating pool of proUGn, we infused exogenous propeptide iv at a “physiological” concentration and measured the subsequent effects on renal function. The prominent natriuresis evoked by this infusion suggests that some form of post-secretory processing of proUGn must occur, to convert the biologically inactive propeptide to its mature, bioactive form.
Materials and Methods
Experimental animals
Male Wistar or Sprague Dawley rats (250–300 g) were purchased from Charles River Laboratories (Wilmington, MA) and maintained for several days on a 12-h light, 12-h dark cycle in an Association for Assessment of Laboratory Animal Care-approved facility with continuously available veterinary care and uninterrupted access to water and standard rat chow. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill, and were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Biochemical studies were performed with Wistar rats under urethane anesthesia. Renal clearance studies were performed with Sprague Dawley rats under pentobarbital anesthesia because this combination provided more stable renal function.
Bacterially expressed proUGn
We subcloned the cDNA sequence encoding proUGn into an expression plasmid (pMal-CM; New England Biolabs, Ipswitch MA) downstream from the Escherichia coli maltose binding protein with an intervening thrombin cleavage site. The insert was PCR amplified from a previously cloned pre-proUGn cDNA (23), with an NdeI restriction site added to the forward primer (5′-CATATGGTCTACATCAAGTACCATGG-3′) and a HindIII restriction site added to the reverse primer (5′-AAGCTTTCAGCAGCCCGTAC-3′). The peptide encoded by the insert (Table 1, plasmid pMal-R) lacks the predicted 21-residue N-terminal signal peptide (39,40) but contains four nonnative residues that were added to the N terminus to generate the thrombin cleavage site. A similar construct was created using the same forward primer and a different reverse primer (5′-AAGCTTTCAGGTCCTCAAGGCCTTGA-3′), in which both the signal peptide and the last 17 amino acids at the proUGn C terminus were deleted (Table 1, plasmid pMal-CΔ). The PCR products were ligated into linearized pMal-CM and introduced into E. coli strain DH5α. After single-colony isolation and amplification in bacteria, the sequence and orientation of the inserts were confirmed.
Table 1.
proUGn constructs used in this study
| Plasmid | Encoded propeptide sequence |
|---|---|
| pMal-R | GSHMVYIKYHGFQVQLESVKKLNELEEKQ MSDPQQQKSGLLPDVCYNPALPLDLQPVCASQE AASTFKALRTIATDECELCINVACTGC |
| pMal-CΔ | GSHMVYIKYHGFQVQLESVKKLNELEEKQ MSDPQQQKSGLLPDVCYNPALPLDLQPVCASQE AASTFKALRT |
Bacterially expressed fusion proteins were affinity purified on amylose beads (New England Biolabs) as described by the manufacturer, and cleaved by incubation with thrombin-coated agarose beads (Sigma Chemical Co., St. Louis, MO). After cleavage, R or CΔ polypeptide was dialyzed against distilled water (SpectraPor 6 dialysis membrane, 1-kDa cutoff limit; Spectrum Laboratories, Rancho Domingo, CA), then purified by a two-step chromatographic procedure. Samples were applied to a 16-mm × 60-cm Hi-Prep Sephacryl S-100-HR size exclusion column (GE Healthcare, Life Sciences, Piscataway, NJ) and eluted with 150 mm NaCl plus 10 mm HEPES (pH 7). Active fractions (identified by Western blotting) were pooled and applied to a VYDAC 218TP1010 C-18 reverse-phase column [The Separations Group Inc. (Grace Vydac), Hesperia, CA], and eluted with a 0–60% linear gradient of acetonitrile over 35 min at 1 ml/min. Active fractions were again confirmed by Western blotting.
We used four methods to quantify the final yield of R after HPLC purification, obtaining comparable results in each case. The first method was by direct protein assay, using a commercially supplied kit (Bio-Rad Laboratories, Inc., Hercules, CA). In addition, we also used the silver stain method (SilverSNAP Stain Kit II, Pierce, Rockford, IL) to compare samples of R side by side with standard curves prepared from known amounts of three different commercially purchased peptides, including: 1) 99% pure recombinant human proUGn (BioVendor Laboratory Medicine, Inc., Modrice, Czech Republic); 2) 99% pure cytochrome C (Sigma Chemical); and 3) 99% pure aprotinin (Sigma Chemical).
Collection of plasma and tissues
Animals were anesthetized (1.6 g urethane/kg body weight, ip). The carotid artery was cannulated with PE 50 tubing for blood withdrawal. After tissue and blood removal, animals were euthanized by anesthetic overdose.
Blood samples were typically obtained from a freshly anesthetized animal but, in some cases, were taken both before and 30 min after surgical removal of the small intestine. In this case, extracellular volume was maintained by infusion of isotonic saline through the jugular vein at 30 μl/min/100 g body weight. Blood was collected into heparinized tubes (Sigma Chemical), centrifuged (16,000 × g for 5 min), and stored frozen at −80 C until used for further analysis.
Individual organs were removed intact, subdivided into smaller pieces as necessary, and rapidly frozen. Before freezing, the intestine was cut longitudinally and rinsed thoroughly with saline to eliminate any contents, and each kidney was flushed intravascularly with 15 ml saline to ensure that it was free of plasma and ultrafiltrate. All frozen tissues were homogenized in buffer [25 mm HEPES (pH 7.4)] containing the following protease inhibitor cocktail (Sigma Chemical): 0.5 mm EDTA, 2.5 mm 4-(2-aminoethyl)-benzenesulfonyl fluoride, 38 μm pepstatin A, 35 μm trans-epoxysuccinyl-l-leucylamido(4-guanidino)butane, 100 μm bestatin, 55 μm leupeptin, and 2 μm aprotinin at a ratio of 4 ml buffer/g tissue. Homogenates were centrifuged at 60,000 × g for 30 min at 4 C, and the supernatant fraction was stored at −80 C.
Quantitative T84 cell assay for GC-C-stimulating activity
Plasma and intestinal extracts were bioassayed for UGn-like activity, based on the method of Currie et al. (41). This assay measures the cyclic GMP (cGMP) responses that are evoked by ligands that activate GC-C; in rats the known ligands are UGn and its structurally related analog guanylin (Gn) (22,23,41). Neither proUGn nor proguanylin (proGn) has significant activity in this assay (23,42,43).
To measure UGn losses from plasma or tissue homogenates during the processing steps that preceded the bioassay, we processed duplicate samples in parallel, one to measure the endogenous peptide and the other spiked with 30 pmol synthetic rat UGn (Peptides Intl., Louisville, KY) for recovery calculations. Samples were mixed with sufficient acetonitrile to bring the final concentration to 50%, placed on ice for 15 min to precipitate high molecular mass protein, then centrifuged for 10 min at 3000 × g. The pellets were discarded. Supernatant fractions were reduced under vacuum to 50% of their original volumes, to evaporate all of the acetonitrile, then reconstituted back to their original volumes with water. Each reconstituted sample was then loaded onto a high-capacity C-18 Sep-Pak (Vac C18 Cartridge, 6 cc/1 g; Waters Corp., Milford, MA). Sep-Paks were washed with 8 ml water, then eluted with 8 ml 10% acetonitrile to recover UGn, leaving proUGn bound to the column matrix. The UGn-containing fraction was dried completely in a centrifugal vacuum concentrator (Savant SpeedVac, Thermo Electron Corp., Waltham, MA), then reconstituted with 250 μl bioassay medium [1 mm 3-isobutyl-1-methylxanthine, 0.03 mm phenol red, 137 mm NaCl, 5.4 mm KCl, 0.25 mm Na2HPO4, 0.44 mm KH2PO4, 1.3 mm CaCl2, 1.0 mm MgSO4, 4.2 mm NaHCO3, and 10 mm HEPES (buffered at pH 7)]. Two 100-μl aliquots were withdrawn from the reconstituted sample and independently analyzed for UGn content by bioassay.
Typically, a standard curve was also constructed, using known amounts of synthetic rat UGn (Peptides Intl.). The responses evoked by the standards were fit using the Michaelis-Menten equation: (pmol cGMP per well) = (maximum response × concentrationUGn)/(EC50 + concentrationUGn). An estimate for the EC50 for rat UGn was obtained from a previously published study (44), and the value of maximum velocity was varied to achieve an optimal fit by eye. Standards and unknowns were assayed in duplicate or triplicate. Results for each unknown are reported as pmol UGn-like activity per well (mean ± sem), as determined by interpolation from the standard curve, followed by a correction for sample loss during processing, based on the activity recovered from the spiked samples (34 ± 8% recovery, n = 10). No difference in recovery was apparent between spiked gut and spiked plasma samples.
Western blotting and quantitative immunoassay for proUGn
We developed a Western blot-based immunoassay to measure tissue and plasma levels of rat proUGn. This assay uses a standard curve constructed with known amounts of our bacterially expressed recombinant rat proUGn (see above), using either antibody 6910 or 6912 (15,23) as the primary detection antibody. To perform the assay, the five left-hand lanes of a gradient gel (4–12% polyacrylamide; Invitrogen Corp., Carlsbad, CA) were loaded with a dilution series of the recombinant standard, typically spanning the range from 10–500 fmol. The remaining lanes were loaded with test samples. After electrophoresis, protein was electroblotted onto a 0.2-μm nitrocellulose membrane (Schleicher and & Schuell/Whatman, Inc., Florham Park, NJ) and blocked overnight with 1.8% teleostean fish gel (Sigma Chemical) in Tris-buffered saline (TBS) [0.05 m Tris plus 0.15 m NaCl (pH 7.4)]. The membrane was then washed with TBS containing 0.1% Tween 20 (TBS-T), incubated for 2 h with primary antibody (diluted 1:500 in TBS-T), washed again with TBS-T, incubated for 1 h in the dark with infrared-emitting secondary antibody (IRDye 800-coupled goat-antirabbit; Rockland Inc., Gilbertsville, PA; diluted 1:2000 in TBS-T), and washed in the dark for 1 h with TBS-T, followed by 20 min with TBS. Immunoreactive proteins were then detected and quantified with an Odyssey infrared blot imaging system (LI-COR Biosciences, Lincoln, NE).
This assay is linear (r2 = 0.98 ± 0.003, n = 19) up to at least 5 pmol, with a lower detection limit of approximately 0.01 pmol. The average coefficient of variation for identical test samples run in the same assay is 11 ± 2% (mean for 11 independent assays, ± sem). The sensitivity of the assay is adequate for extracts of intestinal tissue, which provide a strong proUGn signal when 50 μg total protein is run on a gel. The amount of proUGn present in 75 μl plasma is also well within the range that we can detect with our assay, but analysis of plasma samples required a pre-extraction procedure, using a Superdex 75 10/300 GL size exclusion column (GE Healthcare, Life Sciences) to separate proUGn from high-abundance plasma proteins that would otherwise have interfered with electrophoresis. The Superdex column was eluted with 150 mm NaCl plus 10 mm HEPES (pH 7), and proUGn-containing fractions were pooled and dialyzed against distilled water, using the 1-kDa cutoff dialysis membrane described previously. Dialyzed samples were dried under vacuum, resuspended in boiling sodium dodecyl sulfate sample buffer, and electrophoresed as described previously. Recovery of proUGn from the chromatography and dialysis steps was 30 ± 2% (n = 5). Correction for these sample losses has been included in all of our plasma calculations.
Renal function studies
An experimental group (eight rats, mean body weight 297 ± 6 g) received an iv infusion of recombinant human proUGn (hu-proUGn; BioVendor Laboratory Medicine), and a control group (nine rats, mean body weight 309 ± 9 g) received isotonic saline in place of peptide. Animals were anesthetized with sodium pentobarbital (60 mg/kg body weight ip). Supplemental doses of anesthetic were provided as needed. Animals breathed spontaneously through a tracheotomy tube (PE 240) installed at the start of each experiment. The femoral artery was cannulated with PE 50 tubing for arterial pressure measurement and blood withdrawal. The ureters were exposed through a midline abdominal incision and cannulated with PE 10 tubing about 1 cm from each kidney. Urine was collected over 20-min intervals, and the volume was determined gravimetrically. Blood samples (50 μl) were collected at the midpoints of each urine collection. A continuous iv infusion of 0.9% NaCl was provided into the left jugular vein at a rate of 10 μl/min/100 g body weight. A portion of this infusion (10 μl/min) was provided through a PE 10 cannula connected to a 1-ml syringe. The remainder, provided through a separate cannula, contained 3 mg/ml fluorescein isothiocyanate (FITC)-conjugated inulin (Sigma Chemical). The inulin infusion solution was predialyzed overnight across a 1-kDa cutoff membrane against isotonic saline to remove unconjugated FITC and small inulin polymers, then filtered through a 0.2-μm membrane (Steriflip; Millipore Inc., Billerica, MA).
Glomerular filtration rate (GFR) was determined from the clearance of FITC-labeled inulin using the quantitative fluorescence technique described by Lorenz (7). FITC-inulin concentrations in plasma and urine samples were analyzed in 10-μl capillary tubes (Microcap; Clay Adams, Inc.) with an epifluorescence microscope (Zeiss Axioscope; Carl Zeiss MicroImaging, Inc., Thornwood, NY) fitted with a charge-coupled device camera (Hamamatsu Orca; Hamamatsu Corp., Bridgewater, NJ). Urine samples were diluted with HEPES-buffered saline to normalize the pH and bring the FITC emission intensity into the range of the charge-coupled device detector. Metamorph imaging software (Molecular Devices, Sunnyvale, CA) was used to measure the mean pixel intensity from a central region of each sample over a precisely timed exposure period. Background values were measured in tail vein plasma and bladder urine collected from each rat before inulin infusion. Absolute inulin concentrations in plasma and urine were obtained from a standard curve prepared from the infusion solution used in that experiment. Sodium and potassium concentrations in plasma and urine were determined by flame photometry (IL Model 943; Instrumentation Laboratory Co., Lexington, MA). Blood pressure and heart rate were displayed on an analog monitor (Model 50110 with pressure transducer Model 56360; Stoelting Instruments, Wood Dale, IL) and directed to a universal serial bus-analog/digital module (Model KUSB 3100; Keithley Instruments, Cleveland, OH). Analog/digital output was displayed on a personal computer using acquisition software constructed around the DTx_EZ open source system (Data Translation, Inc., Marlboro, MA). Data were analyzed with the Prism 5 graphing and analysis program (GraphPad Software Inc., San Diego, CA).
Each experiment consisted of seven 20-min clearance periods. After two control periods, rats in the experimental group received recombinant hu-proUGn via the 10-μl/min iv infusion line (described previously) during the third, fourth, and fifth clearance periods at a rate of 1.66 μg/min/kg body weight (equivalent to 172 pmol/min/kg body weight, or ∼50 pmol/min for a 300 g animal). The experiment then continued for two post-infusion clearance periods. Control rats were treated in exactly the same way as the experimental group, except no proUGn was infused. At the end of the last collection period, animals were euthanized by anesthetic overdose.
Results
Extracts of rat intestine contain intact proUGn
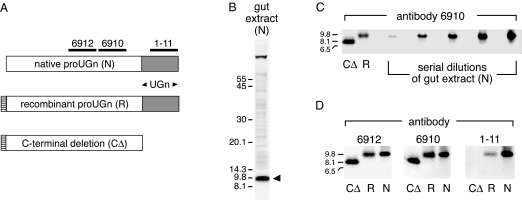
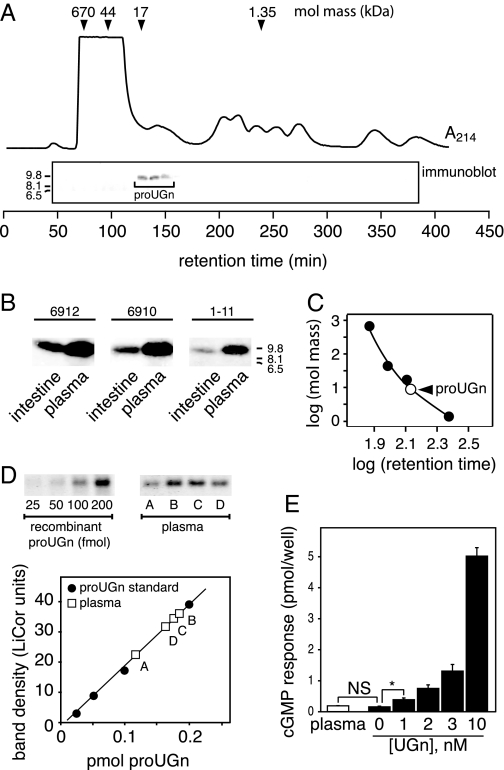
A previous study identified an immunoreactive molecule in rat gut extracts whose intestinal expression pattern closely paralleled that of proUGn mRNA, and whose size on an immunoblot approximated the size predicted for the proUGn polypeptide (33). Positive staining with two different anti-proUGn antibodies confirmed that this native polypeptide was structurally related to proUGn, but it remained unclear whether it was proUGn itself, or a large processing fragment derived from proUGn during the production of UGn. Therefore, in the present study, we have used a bacterial expression system to generate a recombinant rat proUGn standard (R) and a truncated version of rat proUGn from which the C-terminal UGn sequence had been deleted (CΔ); these two standards were then compared with the native proUGn-like molecule on immunoblots, using three different antipeptide antibodies (6912, 6910, and 1–11). The specificities of the antibodies and the structures of the recombinant standards are presented schematically in Fig. 1A.
Figure 1.
Biochemical confirmation that gut extracts contain authentic proUGn. A, Depiction of native rat proUGn (N) showing domains targeted by three independent antipeptide antibodies. Recombinant constructs are aligned beneath the proUGn schematic. R is identical to authentic rat proUGn, except that four extra, nonnative residues were added to its N terminus to generate a thrombin cleavage site. CΔ is identical to R, except that 17 residues were deleted from its C terminus. B, An extract of small intestine immunoblotted with antibody 6912. The arrowhead marks the putative proUGn band. The scale gives molecular masses of standards in kDa. C, Side-by-side comparison of CΔ, R, and N. A dilution series of gut extract containing N was loaded in adjacent lanes of the gel, from left to right, to investigate the possible presence of low-molecular mass proUGn processing fragments. D, A single gel was loaded with samples of CΔ, R, and N, and then cut into three pieces, each of which was probed with a different antibody, as indicated.
Figure 1B shows an immunoblot of an extract of small intestine stained with antibody 6912. As in previous studies, we observed a prominent immunoreactive polypeptide (marked by the black arrowhead) whose apparent molecular mass was consistent with the size predicted for proUGn (∼9.4 kDa). This native molecule (N) was just slightly smaller than R, and much larger than CΔ (Fig. 1C). The slight size discrepancy between N and R, observed on many, but not all, gels, is consistent with the addition of four extra N-terminal amino acids to the recombinant protein, as required to generate a thrombin cleavage site in the fusion protein precursor (see Materials and Methods; Fig. 1A). In addition, like R (and unlike CΔ), N was recognized by all three antibodies (Fig. 1D), confirming that it contains the intact C terminus of proUGn. Thus, based on its size, and its pattern of immunological reactivity, N must correspond to full-length proUGn. Furthermore, as shown in Fig. 1C, we could not detect proUGn processing fragments (molecules whose size was comparable to CΔ, or smaller), even when we deliberately overloaded the gel with a large excess of gut extract: up to 120 μg total protein, the limit that can be electrophoresed without serious distortion of the gel.
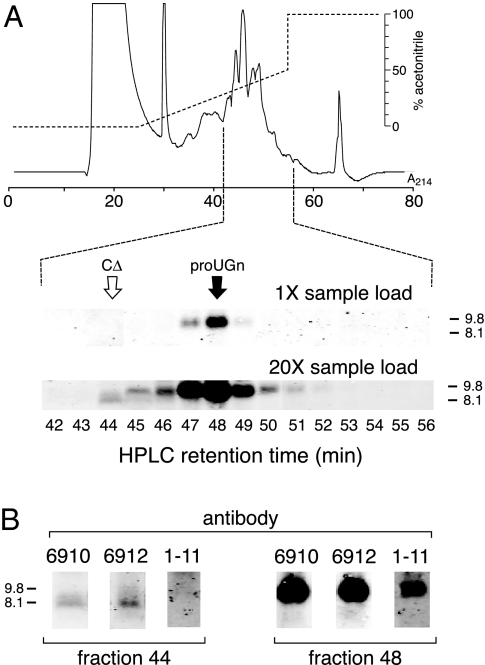
To look more rigorously for proUGn processing fragments, we used HPLC to enrich our samples before applying them to gels. We first passed a crude gut extract over a Sephacryl column and pooled the fractions containing molecules in the 1- to 17-kDa range. This pooled material was concentrated and applied to a C18 reverse-phase column to fractionate the peptides of interest. Immunoblots of individual C-18 column fractions are shown in Fig. 2, A and B. At a moderate sample load, a prominent peak of immunoreactivity was observed in fraction 48 (Fig. 2A, 1× sample load). This molecule must be full-length proUGn because it co-eluted from the C-18 column with our recombinant proUGn standard (the retention time of R is indicated by the black arrow), had the same molecular mass as R on the gel, and was recognized by all three anti-proUGn antibodies (Fig. 2B, fraction 48). When we overloaded a gel with material from the same column fractions (Fig. 2A, 20× sample load), we could still observe the proUGn peak centered in fraction 48, though it was greatly broadened due to overloading. In addition, we could now detect a minor immunoreactive species in fraction 44. We believe that this latter molecule represents a C-terminally truncated processing fragment of proUGn because it co-eluted from the C-18 column with our CΔ standard (the retention time of CΔ is indicated by the white arrow), had the same molecular mass as CΔ on the gel, and was recognized by antibodies 6910 and 6912, but not 1–11 (Fig. 2B, fraction 44). However, the lack of reactivity with antibody 1–11 could also reflect the very small amounts of the candidate molecule in the sample because antibody 1–11 binds less well to full-length rat proUGn than do antibodies 6910 and 6912 (Fig. 2B, fraction 48). If the CΔ-like molecule is, indeed, a proUGn processing fragment, then its abundance is extremely low, relative to the abundance of full-length proUGn (Fig. 2A). Furthermore, no other candidate-processing fragment was observed in any other HPLC fraction, including the early and late fractions, which were tested but are not shown in the figure.
Figure 2.
C-18 reverse-phase HPLC analysis of the native proUGn-like immunoreactivity present in rat jejunum. A, Half of the material from a single jejunum was pre-enriched on a Sephacryl column, then loaded onto a C-18 column. The elution gradient is shown by the upper dashed line, and the UV profile by the solid trace. A Western blot was performed with antibody 6910 on either 1.25% of each fraction (1× sample load) or 25% of each fraction (20× sample load). Only the fractions eluting between 42 and 56 min contained immunoreactive polypeptides of appropriate sizes (<12 kDa). For simplicity, only these fractions are shown in the figure. The retention times of two recombinant standards (R and CΔ), as determined in separate column runs, are indicated by the black and white arrows, respectively. The structures of these standards are given in Fig. 1A. B, Fractions eluting at 44 and 48 min from the C-18 reverse-phase column (in A) were analyzed by Western blotting. Blots were probed with three different antibodies (6912, 6910, and 1–11), as indicated. Each lane was loaded with 25% of the indicated fraction. Only the 3- to 12-kDa regions of the blots are shown.
Quantitation of proUGn expression in the rat gut
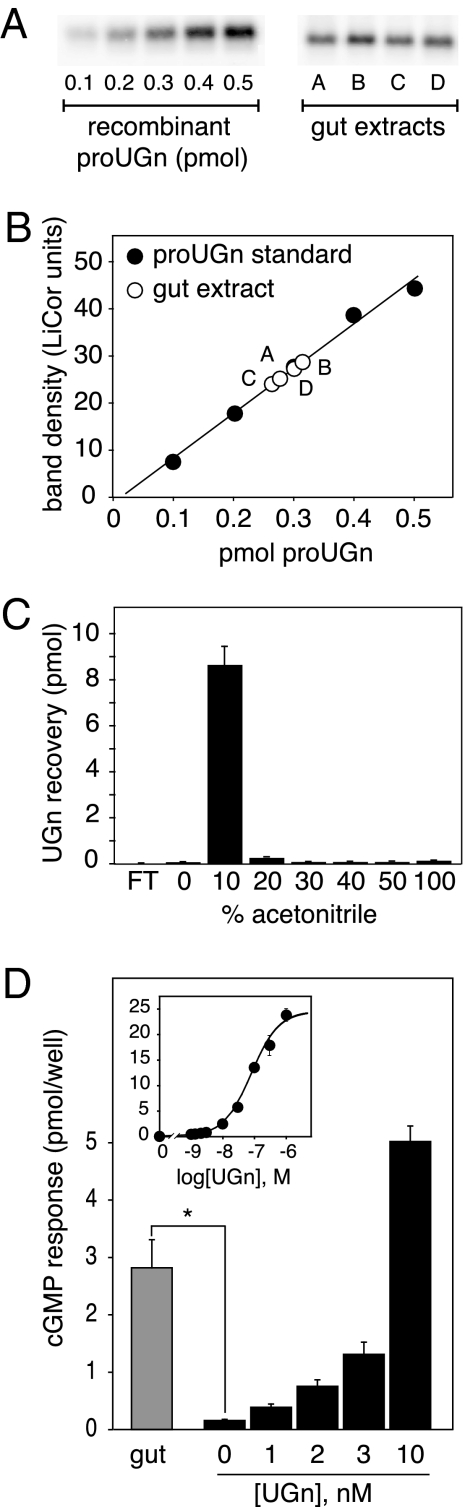
Having established that the small intestine contains authentic, full-length proUGn, we then developed an assay to measure the levels of the propeptide in tissue extracts. To ensure that the assay results would reflect the total tissue pool of proUGn, we removed the entire length of the small intestine, transected it at the midpoint, and prepared separate extracts from the proximal and distal halves without discarding any tissue. Using the assay procedure described in the Materials and Methods (and shown in Fig. 3, A and B), we established a specific activity of 12.6 ± 1.0 fmol proUGn/μg protein (n = 21) in proximal extracts, and 10.0 ± 1.0 fmol proUGn/μg protein (n = 17) in distal extracts. This corresponds to a tissue content of 1.32 ± 0.16 nmol proUGn in the proximal half, and 0.98 ± 0.12 nmol proUGn in the distal half, or a total of approximately 2.3 nmol for the small intestine as a whole (Table 2).
Figure 3.
ProUGn and UGn levels in extracts of rat small intestine. A, Western blot assay for proUGn, including a standard curve constructed with known quantities of recombinant rat proUGn and samples of gut extract (A–D). A number of similar assays were performed to obtain the values presented in Table 2. B, Numerical values derived from the blots in A. C, Sep-Pak elution profile of an authentic UGn sample. After applying the peptide to the Sep-Pak, the flow through (FT) was collected, followed by sequential elution with increasingly hydrophobic solvent. Peptide recovery in each fraction was measured with the T84 cell bioassay (see Materials and Methods). Bioactivity eluted from the Sep-Pak in the 10% acetonitrile fraction. The native bioactivity in gut extracts elutes with an identical profile (data not shown). D, Averaged responses of T84 cells to UGn extracted from 1 ml jejunal extract (gray bar, mean ± sem, n = 5), after Sep-Pak purification as in panel C. Responses to gut samples are compared with responses obtained with known amounts of synthetic UGn (black bars). The inset shows the responses to a full range of synthetic UGn standards, as performed in a typical assay. UGn concentrations in the biological samples are determined by interpolation on this standard curve (see Materials and Methods). The asteriskindicates P< 0.01 by two-tailed ttest.
Table 2.
proUGn and UGn levels of tissues and plasma
| proUGn
|
UGn-like bioactivity
|
|||
|---|---|---|---|---|
| fmol/μg protein (n) | pmol/animal (n) | fmol/μg protein (n) | pmol/animal (n) | |
| Small intestine, proximal half | 12.6 ± 1.0 (21) | 1322 ± 160 (21) | ≤0.54 ± 0.16 (5)a | 56.8 ± 17 (5) |
| Small intestine, distal half | 10.0 ± 1.0 (17) | 978 ± 122 (21) | ||
| Small intestine, total | 11.3 ± 1.0 (21) | 2300 ± 262 (21) | ||
| Kidney | 2.1 ± 0.3 (12) | 247 ± 27 (6) | ||
| Colon | 1.1 ± 0.3 (5) | 27 ± 7 (5) | ||
| pmol/ml (n) | Estimated pmol/animalb (n) | pmol/ml (n) | ||
| Arterial plasma | 10.3 ± 1.7 (21) | Approximately 93 (21) | Not detectable (5) (<0.25)c | |
This represents an upper limit because a portion (∼20%) of the bioactivity is chromatographically distinct from authentic UGn.
Based on an estimated plasma volume of 9 ml for a typical 300-g animal.
This represents an upper limit, calculated on the basis of the detection limit of the assay.
Extracts of rat intestine contain relatively little UGn
To compare the proUGn values reported in Table 2 with levels of UGn in the same regions of intestine, we measured the amount of biologically active peptide in gut extracts, using a well-established T84 cell-based bioassay (see Materials and Methods). This assay is highly selective for UGn and the related intestinal peptide Gn, but does not recognize proUGn.
Initial pilot studies revealed that the amount of UGn-like bioactivity in gut extracts was near the detection limit of the bioassay. Therefore, to concentrate our samples before assay, we passed 1-ml aliquots of each of them over a C-18 Sep-Pak, washed the Sep-Pak with water, and used 10% acetonitrile to elute all of the bound activity (Fig. 3C). The 10% Sep-Pak fraction from each gut extract was then dried, resuspended in a small amount of bioassay medium, and applied to the T84 cells. After Sep-Pak enrichment, all gut-derived samples evoked small, reproducible responses in the cells (Fig. 3D). By interpolating the responses on a standard curve constructed with known amounts of synthetic UGn (Fig. 3D, black bars and inset), we could calculate that each sample contained, on average, 0.52 ± 0.27 pmol UGn-like bioactivity. To correct for any losses that might have occurred due to irreversible binding to the Sep-Pak, or to proteolysis at any step during sample processing, we added 30 pmol UGn to small frozen pieces of gut tissue, then homogenized these spiked samples, and subjected them to the same processing steps outlined previously. On average, 34 ± 8% (n = 10) of the spiked material was recovered. Considering this recovery factor, we could then calculate that extracts of proximal small intestine contained, on average, 0.54 ± 0.16 fmol UGn-like bioactivity/μg protein (mean ± sem, n = 5; Table 2).
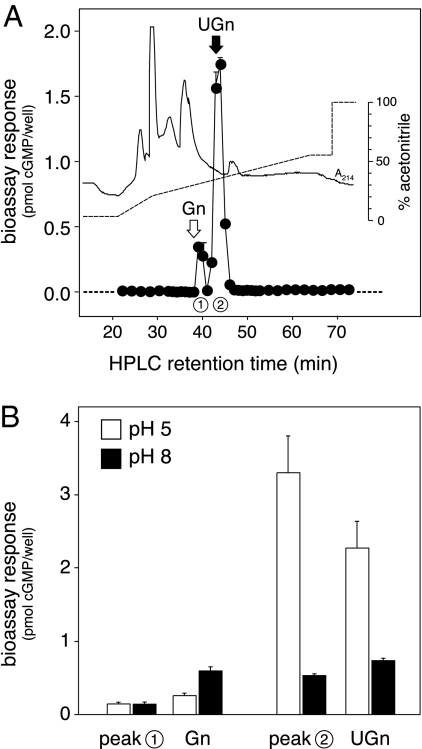
However, not all of the bioactive material in these extracts was necessarily equivalent to UGn because T84 cells respond to both UGn and Gn, and rat jejunum contains both of these peptides (23,33,41). To investigate the identity of the molecule(s) responsible for the bioactivity, we Sep-Pak purified a larger amount of jejunal extract (6 ml) and fractionated this scaled-up preparation by reverse-phase HPLC. Column fractions were dried, reconstituted, and bioassayed to establish the elution profile of the biologically active species (Fig. 4A). One major and one minor peak of activity were evident. The retention time of the major peak (labeled 2 in Fig. 4A) was identical to that of a synthetic rat UGn standard (marked by the black arrow), whereas the retention time of the minor peak (labeled 1 in Fig. 4A) was very similar to that of a synthetic rat Gn standard (marked by the white arrow). As a further test of the identities of the endogenous bioactive molecules, we examined the pH dependencies of the responses that they evoked in T84 cells because it has previously been well documented that responses to UGn are greater at low pH, whereas responses to Gn are greater at high pH (45). As shown in Fig. 4B, the responses to peak 2 and authentic rat UGn were affected identically by pH, whereas the response to peak 1 resembled neither UGn nor Gn in its pH dependency. Thus, by two criteria (retention time and pH dependency), the majority of the biological activity present in crude extracts of proximal small intestine is attributable to authentic UGn. The identity of the minor peak remains unconfirmed, though it most likely represents a Gn-related peptide. Thus, the data presented in Fig. 4 are generally consistent with previous studies showing that rats and mice express higher levels of proUGn than proGn in the small intestine (both at the mRNA and propeptide levels), and higher levels of proGn than proUGn in colon (22,23,33,46,47). However, it should be noted that this differential pattern of peptide distribution is not observed in other species, such as opossum (10,48) or human (26).
Figure 4.
HPLC and bioassay analysis of UGn-like bioactivity from proximal small intestine. A, Extracts were prepared independently from four separate animals, and a 1.5-ml aliquot was withdrawn from each. These aliquots were individually purified on C-18 Sep-Paks, as in Fig. 3C, then combined, applied to a C-18 reverse-phase column, and eluted with a gradient of acetonitrile, as indicated by the dashed line. UV absorbance is given by the solid trace. Fractions were dried, resuspended in bioassay medium, and assayed in duplicate using the T84 cell bioassay. The symbols plot the average of the duplicates, and the error bars give the range; in most cases, the error bars are hidden beneath the symbol. The black and white arrows indicate the retention times of synthetic UGn and Gn standards. This experiment was repeated a second time with very similar results (data not shown). B, Aliquots of material in peak 1 (eluting at 39 min) and peak 2 (eluting at 43 min) were assayed at pH 5 (white bars) and pH 8 (black bars), side by side with authentic Gn and UGn standards.
Furthermore, although Fig. 4 demonstrates that most of the UGn-like bioactivity reported in Table 2 is indistinguishable from authentic UGn, this nevertheless represents only a tiny fraction (∼4%) of the total UGn-related peptide stored in the tissue. The bulk of the peptide is present in the form of intact proUGn (Table 2).
Intact proUGn circulates at much higher levels in rat plasma than does UGn
The approximate 23:1 ratio of proUGn to UGn in the intestine suggests that the intact propeptide might be the preferred secretory product. To verify that proUGn is a constituent of rat plasma, we performed Western blots after fractionating plasma on a size exclusion column. This size fractionation step was required to separate proUGn-sized peptides from very abundant higher molecular mass plasma proteins that interfere with electrophoresis. The blots revealed the presence of an approximate 9.4-kDa proUGn-like immunoreactive polypeptide that eluted between 125 and 150 min (Fig. 5A). To confirm the identity of this peptide, we compared its properties with those of intestinal proUGn. The two molecules migrated identically on a high-resolution gradient gel, and both were recognized by all three of the anti-proUGn antibodies (Fig. 5B). Thus, the plasma molecule was immunologically and electrophoretically identical to the full-length proUGn molecule found in intestinal extracts. Based on its column behavior, proUGn in plasma had a size comparable with that of an approximate 10-kDa globular protein (Fig. 5C). Thus, it was not complexed with any high molecular mass protein, such as a plasma binding protein.
Figure 5.
Authentic, full-length proUGn circulates in plasma. A, Chromatography of rat plasma on a Sephacryl S-100 HR column. Retention times and molecular masses of standards used to calibrate the column are indicated at the top of the panel. The solid trace shows the UV absorption profile. An immunoblot was performed with antibody 6912 on each fraction eluting between 45 and 380 min. B, Samples of partially purified plasma proUGn are compared with samples of partially purified intestinal proUGn run on the same gel and probed with multiple antibodies. C, Log-log plot of molecular mass vs. retention time for proUGn compared with standards run on the same column (taken from A). D, Quantitative assay for plasma proUGn, performed as in Fig. 3. Plasma was partially purified on a size exclusion column before the assay. E, Quantitative assay for plasma UGn, performed on 3-ml samples of plasma (white bar, mean ± sem, n = 5), as in Fig. 3. The asteriskindicates P <0.05 by two-tailed ttest. mol mass, Molecular mass; NS, not significant.
To quantify the levels of proUGn in plasma, we analyzed 100 μl samples on a smaller, higher-resolution Superdex size exclusion column, which can process multiple samples more efficiently. Arterial plasma was obtained from 21 independent animals. Each sample was individually applied to the column, and the proUGn-containing fractions from each sample were pooled and subjected to the proUGn-specific immunoassay (Fig. 5D). On the basis of these measurements, including a correction for recovery from the column, we determined that proUGn circulates in plasma at 10.3 ± 1.7 pmol/ml (mean ± sem, n = 21). Therefore, assuming a plasma volume of 9 ml for a 300-g rat (49), the estimated circulating pool of proUGn amounts to approximately 93 pmol, corresponding to approximately 4% of the total intestinal pool (Table 2).
For comparison we then used the T84 cell bioassay procedure described previously to measure UGn-like bioactivity in plasma. Again, we pre-fractionated each plasma sample on a Sep-Pak before applying it to the reporter cells. However, even when we loaded up to 3 ml plasma onto the Sep-Pak, we did not recover any signal that could correspond to endogenous UGn (Fig. 5E, white bar). By contrast, plasma samples spiked with 30 pmol UGn provoked robust responses, with the same 34% recovery that was observed with gut samples (data not shown). By taking the response to 10−9 m UGn as the limit of detection for the bioassay (Fig. 5E, black bars), and factoring in the appropriate volumes and the recovery factor, we calculate that the upper limit for the plasma concentration of UGn is 0.25 pmol/ml. Thus, the plasma concentration of UGn is less than 2.5% of the plasma concentration of proUGn (Table 2). In fact, the percentage is probably much lower because sensitive RIA measurements have established a circulating level of 0.003 pmol/ml for UGn in human plasma (50). Note that the published assays of UGn levels in rat plasma report only “total UGn-like” immunoreactivity, without separating UGn from proUGn (35,36); see the Discussion for further elaboration of this point.
The intestine is the likely source of circulating proUGn
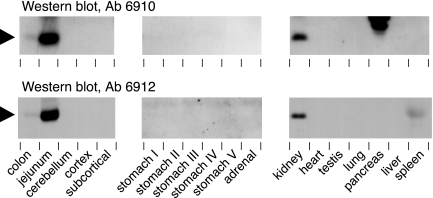
The small intestine is known to be the most abundant site of proUGn mRNA expression in the rat (22,23). However, as summarized in the introductory section, a number of other organs also express proUGn mRNA in Northern blot or PCR-based assays and/or UGn-like immunoreactivity in immunocytochemical studies. To assess which if any of these organs might contain enough proUGn to serve as the source of the circulating propeptide pool, we performed Western blots on a variety of tissue extracts, using antibodies 6910 and 6912 (Fig. 6). Although nonspecific labeling was evident in some tissues, particularly in the molecular mass range above 30 kDa (data not shown), only a few tissues contained a band of the appropriate size that could be detected by both antibodies. This polypeptide (marked by the black arrowhead) was abundant in jejunum and also present, though at very much lower levels, in colon, in good agreement with the previously defined pattern of intestinal proUGn expression in rats (33,51). In addition, we observed moderate levels of a comparable peptide in the kidney. No credible proUGn signal was observed in any other tissue. A faint band of the right size was recognized by antibody 6912, but not antibody 6910, in the spleen. This immunoreactive material may represent rat pro-lymphoguanylin, a UGn-related peptide that was first identified in opossum spleen (52), and that might, in theory, cross-react with antibody 6912 but not 6910. In addition, the pancreas contains several immunoreactive polypeptides that are recognized only by antibody 6910. We believe that these most likely represent nonspecific interactions because the pancreatic polypeptides are significantly larger than any of the known propeptides that belong to the UGn family (proUGn, proGn, and pro-lymphoguanylin), and are recognized by only one of the two anti-proUGn antibodies.
Figure 6.
Western blot analysis of proUGn expression in a variety of tissues. Brain was divided into three regions: cerebellum, cortex, and subcortex (all of the structures within the forebrain that lie beneath the cortex). Stomach was cut into five approximately equal portions to provide rostral, intermediate, and caudal samples (labeled I–V). Other tissues were removed intact, and extracts were made from the whole organ. Comparable aliquots (60 μg protein) from each tissue were tested with two different antibodies (6910 and 6912). The position of the proUGn band is marked by the black arrowheads. Polypeptides in the pancreas and spleen that are detected by only one of the two antibodies cannot represent authentic proUGn, and most likely represent molecules that contain cross-reacting epitopes.
We then used our gel-based proUGn assay to quantify propeptide levels in the colon and kidney for comparison to the levels in small intestine. The results are shown in Table 2. The total proUGn content of the small intestine is approximately 10 times greater than that of the kidney, and approximately 85 times greater than that of the colon. Thus, although our results do not exclude the possibility that some of the other tissues that we tested may contain levels of proUGn that fall below the detection limit of our gel-based assay, it is, nevertheless, reasonable to consider only the intestine and kidney as plausible tissue sources of the high levels of proUGn observed in plasma, with small intestine being the best candidate.
Direct evidence for intestinal secretion of proUGn
To investigate more directly the hypothesis that circulating proUGn is derived from the intestine, we used our proUGn assay to measure the level of propeptide in the venous outflow of the intestine (portal vein plasma) relative to that in the systemic circulation (carotid artery plasma). In paired measurements, portal plasma contained almost twice as much proUGn as did systemic plasma obtained at the same time from the same animal (portal levels were 177 ± 16% of arterial levels; n = 20; P = 0.002, paired t test), providing strong evidence that the intestine is a source of circulating proUGn. In contrast, the kidney does not appear to play a significant role in maintaining the plasma pool. Paired measurements of proUGn levels in the renal vein and renal artery revealed that there was significantly less proUGn in the venous outflow from the kidney than was present in the arterial input to the kidney: renal vein levels were 63 ± 5% of renal artery levels (n = 8; P = 0.02, paired t test). Thus, the net contribution of the kidney is to remove proUGn from the plasma, rather than to supply the propeptide to the plasma.
As a final test of the origin of circulating proUGn, we measured levels in systemic plasma before and after complete resection of the small intestine. At 30 min after resection, proUGn levels were reduced to 27 ± 6% of the pre-resection level (n = 3; P = 0.01, paired t test), whereas in control animals maintained for 30 min under identical conditions, but with no intestinal resection, plasma proUGn levels remained constant: levels at 30 min were 104 ± 2% of the “zero time” control (n = 3). These effects of resection, coupled with the regional plasma measurements described previously, indicate that most, and very likely all, of the proUGn in the circulation is derived from the gut.
Infused proUGn stimulates renal sodium excretion
The data presented to this point demonstrate that, relative to UGn, proUGn is by far the dominant molecular species stored in the intestine and circulating in the plasma. This indicates that secretion of proUGn, rather than UGn, is the likely endocrine response of the intestine to salt intake. Might the presumably inactive propeptide somehow be capable of triggering physiological responses in the kidney? To investigate this question, we infused a commercially available recombinant human form of the propeptide (hu-proUGn) into anesthetized rats and evaluated its effects on renal function; note that our recombinant rat propeptide was not available in sufficient quantities for these studies.
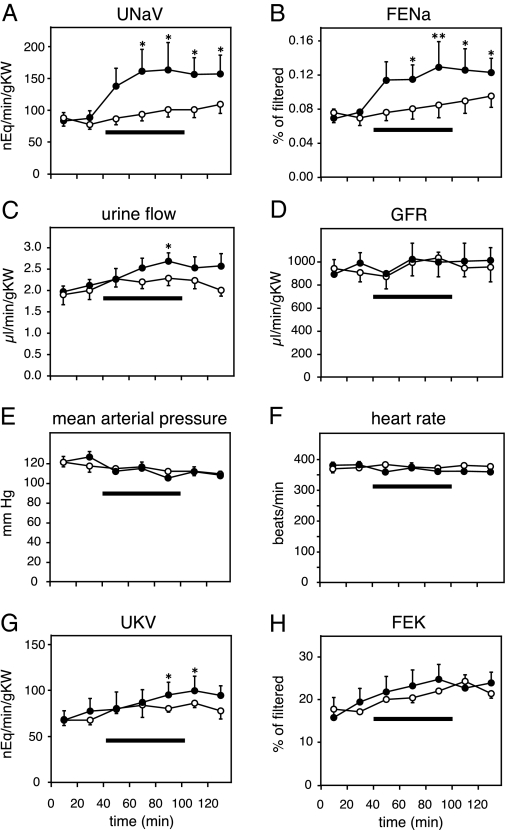
In control animals, sodium excretion, urine flow, GFR, blood pressure, heart rate, and potassium excretion did not change significantly over the course of the experiment (Fig. 7, A–H, white symbols). In contrast, iv infusion of 100 μg/kg body weight hu-proUGn over 60 min increased total urinary sodium excretion by approximately 70% (Fig. 7A, black symbols), fractional sodium excretion by approximately 50% (Fig. 7B, black symbols), and urine flow by approximately 20% (Fig. 7C, black symbols). These natriuretic and diuretic responses had a relatively slow onset and long duration, outlasting the application of the peptide by more than 40 min, and were not accompanied by changes in GFR, mean arterial pressure, or heart rate (Fig. 7, D–F, black symbols). Potassium excretion was also stimulated slightly by propeptide infusion, but the kaliuresis developed very slowly, and the statistical significance of the response was marginal (Fig. 7, G and H, black symbols).
Figure 7.
Infused hu-proUGn is natriuretic and diuretic in rat renal clearance studies. Horizontal bars indicate the duration of hu-proUGn infusion (100 μg/kg body weight over 60 min) in the experimental group (filled circles, n = 8). Control rats (open circles, n = 9) received isotonic saline in place of proUGn. Asterisks indicate a significant difference between the associated data point and the first clearance period, as determined by one-way ANOVA with repeated measures, followed by the Bonferroni method for post hoc testing (*, P < 0.05; **, P < 0.01). nEq, Nanoequivalents; gKW, grams of kidney weight; UNaV, urine sodium concentration times urine flow; FENa, fractional sodium excretion; GFR, glomerular filtration rate; UKV, urine potassium concentration times urine flow; FEK, fractional potassium excretion.
Preliminary quantitative studies revealed that our infusion protocol produced a 3.2 ± 0.36-fold increase in plasma proUGn levels (mean ± range, n = 2). Thus, the renal responses observed in Fig. 7 were evoked by peptide infusions that fell within a “physiological” range. The absence of hemodynamic effects of proUGn suggests a direct tubular effect of the peptide on sodium excretion, and this is confirmed by the significant increase in fractional sodium excretion during and after peptide infusion. The prolonged time course of the natriuretic response to hu-proUGn in our studies is comparable with the prolonged responses evoked by UGn in the isolated perfused kidney (12,14,53) or the intact mouse (13,38). However, the kaliuretic responses to propeptide infusion are much less pronounced than those observed in previous studies with UGn.
Discussion
A number of observations, summarized in the introductory section, have led to the hypothesis that UGn serves as the endocrine mediator of an enterorenal signaling pathway that enhances renal salt excretion after oral salt intake (5,6). Most studies investigating this hypothesis have focused on UGn itself. In this and a companion publication (54), we provide evidence that the UGn precursor, proUGn, plays a previously underappreciated role in the enterorenal natriuretic axis. The underpinnings of this idea are provided by two key observations, both presented previously. First, nearly all of the UGn that is stored within the small intestine is in the form of intact proUGn (Figs. 1–3 and Table 2). This observation is consistent with the fact that none of the potential monobasic and dibasic cleavage sites in rat proUGn matches the consensus sequence for cleavage by conventional prohormone convertases (55). Second, plasma levels of proUGn are also very high (Fig. 5D and Table 2), whereas circulating UGn levels are so low that they fall below the limit of detection of our assay (Fig. 5E and Table 2).
The UGn and proUGn measurements given in Table 2 can be compared with previously published RIA values for both tissue and plasma pools of “UGn-like immunoreactivity” (11,16,34,35,36,56,57). In particular, the proUGn levels determined with our newly developed assay are substantially higher than those published previously. However, the RIA procedures used in all prior studies have used antibodies directed exclusively against the C-terminal amino acid sequence that defines UGn. This introduces several complications. First, any such antibody will recognize both UGn and proUGn (Fig. 1A), and, thus, unless the two target molecules are separated before performing the measurement, the assay will reflect the contributions of both. Second, even after separation of proUGn from UGn, the concentration of proUGn cannot be measured accurately because the standard curve is established with UGn, and, in general, anti-UGn antibodies cross-react poorly with intact proUGn. Recognizing this problem some studies have proteolytically converted proUGn to free UGn before performing the assay (16), but, even so, the results provide only a lower limit for proUGn levels because it is not possible to assess the stoichiometry of the conversion.
Our new gel-based proUGn assay eliminates these problems because it detects proUGn directly, does not cross-react with UGn, requires no proteolysis, and is based on an authentic rat proUGn standard. We have used this assay to quantify proUGn levels in the plasma, small intestine, colon, and kidney of the rat (Table 2).
The tissue proUGn values obtained in our current studies are consistent with tissue levels that have been established for other mammalian gastrointestinal peptides, such as secretin and cholecystokinin, which range from 50–500 pmol/g wet weight, calculated as the sum of all the known forms of these peptides, both processed and unprocessed, that reside in the small intestine (58). In addition, a sampling of published values for the circulating levels of a variety of peptide hormones (59,60,61,62,63) reveals that, whereas our estimate of the plasma proUGn concentration is significantly higher than the levels observed for peptides that are processed either before or coincident with secretion (e.g. gastrin, cholecystokinin, atrial natriuretic peptide, and B-type natriuretic peptide), it is nevertheless well below the levels established for two circulating propeptides that are only converted to mature peptides subsequent to secretion: angiotensinogen, the precursor for angiotensin II, and T kininogen, a rat-specific propeptide that is the precursor for ile-ser-bradykinin. Thus, there is biological precedent for the idea that plasma levels of unprocessed propeptides can be very high. Our data also suggest that the intestine must maintain a very high rate of proUGn synthesis and secretion to generate such a large, steady-state plasma pool of propeptide in the face of the rapid plasma clearance processes that are described in our companion publication (54).
In addition to high levels of proUGn, our measurements also demonstrate comparatively low levels of UGn, both in gut extracts and in plasma. The bioassay technique that we used to measure UGn is not as sensitive as an RIA or a Western blot. Nevertheless, it was adequate to detect a small pool of biologically active UGn in extracts of proximal small intestine (Fig. 3C), which, upon quantification (Table 2), revealed a proUGn to UGn ratio on the order of 23:1. However, the assay was not sufficiently sensitive to detect bioactive UGn in plasma, even when the plasma sample was scaled up to 3 ml, representing nearly one third of the plasma in an individual animal. Thus, we can only set an upper limit on the level of UGn in plasma (Table 2) and establish a minimal proUGn to UGn ratio of 40:1.
One strikingly consistent feature of these measurements is that proUGn levels are very much higher than UGn levels, both in gut extracts and in plasma; indeed, the slightly different proUGn to UGn ratios in the two compartments may only reflect the potential sources of error in our measurements. Therefore, in terms of an endocrine (tissue-to-tissue) signaling role, this suggests that circulating proUGn might be functionally more important than circulating UGn. However, this concept can only be valid if circulating proUGn is capable of eliciting appropriate responses from the target tissue. To investigate this latter possibility, we infused recombinant hu-proUGn into anesthetized rats and measured their renal function. As shown in Fig. 7, both sodium and fluid excretion were significantly elevated in response to the propeptide.
Direct plasma measurements demonstrated that our infusion protocol produced an approximate 3-fold increase in plasma proUGn levels. This can be contrasted with previously published studies of UGn, in which the peptide had to be infused at supraphysiological levels to evoke renal responses (14,38). Thus, in contrast to UGn, proUGn appears to act at levels that are appropriate for an enterorenal-signaling molecule.
This not only provides further support for proUGn as a plausible candidate for the endocrine mediator of the postulated enterorenal signaling axis but also raises the question of how and where such a presumably inactive propeptide might be converted to active form. Experiments presented in our companion publication (54) address this question directly. By analyzing the fate of infused, radiolabeled proUGn, these biochemical studies demonstrate that the propeptide: 1) remains intact throughout its short dwell time in the plasma; 2) is rapidly cleared from the plasma by the kidney; and 3) is processed intrarenally to a spectrum of metabolites, including one that has the chromatographic characteristics of UGn. Furthermore, other than small amounts of free cysteine and methionine, none of these metabolites is returned to the circulation. Thus, any physiological responses that such metabolites may trigger, such as the natriuresis and diuresis demonstrated here after infusion of recombinant hu-proUGn, must be initiated exclusively within the nephron. In good agreement with this, the hu-proUGn-evoked increase in fractional sodium excretion that we report here, along with the lack of observable changes in blood pressure or GFR, is fully consistent with a direct intratubular natriuretic effect.
This “target organ-mediated” model for propeptide processing is unusual, and it is worth considering why it might be of particular utility in the case of UGn and proUGn:
UGn, like all GC-C agonists, is highly dependent on disulfide-mediated folding for activity, and, if unfolded, will preferentially refold in an inactive conformation (64). However, there is an “intramolecular chaperone” domain at the N terminus of the prosequence that specifies the correct disulfide pairing during synthesis, and permits correct refolding of the C terminus after oxidation (65). Thus, the increased stability and oxidation resistance of full-length proUGn over UGn provides one rationale for favoring the propeptide as the circulating form.
When UGn is retained within the prosequence, it has little or no biological activity on GC-C-expressing cells. Consequently, minimizing the level of free UGn in the plasma will limit potential extrarenal systemic actions [e.g. activation of GC-C receptors in the pancreas (27,29), reproductive organs (30), and liver (66)].
Embedding UGn within the prosequence may protect it from plasma, endothelial, and tissue ecto-proteases.
The EC cells that synthesize proUGn have long been known to release their previously identified secretory products (serotonin and substance P) both apically, into the intestinal lumen, and basolaterally into the circulation (67,68,69,70). This raises the interesting possibility that the cleavage products of proUGn that are produced in the lumen of the gut may be distinct from those produced in the lumen of the proximal tubule.
It is appropriate to acknowledge that the biochemical experiments described in our companion publication (54) do not exclude the possibility that proUGn may be converted to UGn in tissues that were not studied, although, if this occurs, any UGn-mediated effects must be strictly local because no measurable amount of UGn was returned to the plasma, even during our longest infusion studies. In addition, it is well documented that small amounts of UGn do, indeed, circulate in plasma (9,10,11), and this might become quantitatively more important at certain times of day or in response to specific environmental stimuli. Thus, we must leave open the possibility that a more conventional UGn-based endocrine pathway may operate in parallel with the propeptide-based mechanism that we have described here.
In summary, the biochemical and physiological studies presented previously, in combination with the pulse-chase studies of infused proUGn that are described in our companion publication (54), suggest a unique model for proUGn processing. Not only does processing appear to be primarily a post-secretory event, but, to our knowledge, it is unique among endocrine mechanisms identified to date because it also occurs only after the circulating propeptide has reached (and been sequestered within) one of its principal target organs, the renal tubules. Therefore, we propose that the N-terminal prosequence may serve as a specialized delivery vehicle that shields UGn, either preventing its destruction or minimizing its physiological actions, during its passage from the gut to the kidney.
Acknowledgments
We thank Jennifer Holmes for help with bacterial expression, Todd DeVries, Randall Rhyne, Tailun Zhao, Christopher Cazzolla, and Dorothy Riguera for technical assistance, James Anderson and Bill Arendshorst for invaluable collegial encouragement, Richard Cheney for generous access to his fluorescence microscope, and Kathleen Dunlap and Christopher Cazzolla for helpful comments on the manuscript.
Footnotes
This work was supported by awards from the National Institutes of Health (RO1-HL078980), the American Heart Association (Grant-in-Aid NC97GS34 and 0755397U), and the Maren Foundation (to M.F.G.), and a Pilot/Feasability Award from the University of North Carolina Center for Gastrointestinal Biology and Disease (DK34987) (to N.G.M.).
Disclosure Summary: R.C.F., S.J.Y., Z.L., and M.N. have nothing to declare. N.G.M., X.Q., and M.F.G. are inventors on United States Patent Application No. 11/596,493.
First Published Online May 22, 2008
Abbreviations: cGMP, Cyclic GMP; EC, enterochromaffin; FITC, fluorescein isothiocyanate; GFR, glomerular filtration rate; Gn, guanylin; proGn, proguanylin; proUGn, prouroguanylin; TBS, Tris-buffered saline; TBS-T, Tris-buffered saline containing 0.1% Tween 20; UGn, uroguanylin.
References
- Lennane RJ, Carey RM, Goodwin TJ, Peart WS 1975 A comparison of natriuresis after oral and intravenous sodium loading in sodium-depleted man: evidence for a gastrointestinal or portal monitor of sodium intake. Clin Sci Mol Med 49:437–440 [DOI] [PubMed] [Google Scholar]
- Carey RM, Smith JR, Ortt EM 1976 Gastrointestinal control of sodium excretion in sodium-depleted conscious rabbits. Am J Physiol 230:1504–1508 [DOI] [PubMed] [Google Scholar]
- Carey RM 1978 Evidence for a splanchnic sodium input monitor regulating renal sodium excretion in man. Lack of dependence upon aldosterone. Circ Res 43:19–23 [DOI] [PubMed] [Google Scholar]
- Singer DR, Markandu ND, Buckley MG, Miller MA, Sagnella GA, MacGregor GA 1998 Contrasting endocrine responses to acute oral compared with intravenous sodium loading in normal humans. Am J Physiol 274(1 Pt 2):F111–F119 [DOI] [PubMed] [Google Scholar]
- Forte Jr LR 2004 Uroguanylin and guanylin peptides: pharmacology and experimental therapeutics. Pharmacol Ther 104:137–162 [DOI] [PubMed] [Google Scholar]
- Forte LR 2003 A novel role for uroguanylin in the regulation of sodium balance. J Clin Invest 112:1138–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz JN, Nieman M, Sabo J, Sanford LP, Hawkins JA, Elitsur N, Gawenis LR, Clarke LL, Cohen MB 2003 Uroguanylin knockout mice have increased blood pressure and impaired natriuretic response to enteral NaCl load. J Clin Invest 112:1244–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elitsur N, Lorenz JN, Hawkins JA, Rudolph JA, Witte D, Yang LE, McDonough AA, Cohen MB 2006 The proximal convoluted tubule is a target for the uroguanylin-regulated natriuretic response. J Pediatr Gastroenterol Nutr 43(Suppl 1):S74–S81 [DOI] [PubMed] [Google Scholar]
- Hess R, Kuhn M, Schulz-Knappe P, Raida M, Fuchs M, Klodt J, Adermann K, Kaever V, Cetin Y, Forssmann WG 1995 GCAP-II: isolation and characterization of the circulating form of human uroguanylin. FEBS Lett 374:34–38 [DOI] [PubMed] [Google Scholar]
- Fan X, Hamra FK, Freeman RH, Eber SL, Krause WJ, Lim RW, Pace VM, Currie MG, Forte LR 1996 Uroguanylin: cloning of preprouroguanylin cDNA, mRNA expression in the intestine and heart and isolation of uroguanylin and prouroguanylin from plasma. Biochem Biophys Res Commun 219:457–462 [DOI] [PubMed] [Google Scholar]
- Kinoshita H, Fujimoto S, Nakazato M, Yokota N, Date Y, Yamaguchi H, Hisanaga S, Eto T 1997 Urine and plasma levels of uroguanylin and its molecular forms in renal diseases. Kidney Int 52:1028–1034 [DOI] [PubMed] [Google Scholar]
- Forte LR, Fan X, Hamra FK 1996 Salt and water homeostasis: uroguanylin is a circulating peptide hormone with natriuretic activity. Am J Kidney Dis 28:296–304 [DOI] [PubMed] [Google Scholar]
- Carrithers SL, Hill MJ, Johnson BR, O'Hara SM, Jackson BA, Ott CE, Lorenz J, Mann EA, Giannella RA, Forte LR, Greenberg RN 1999 Renal effects of uroguanylin and guanylin in vivo. Braz J Med Biol Res 32:1337–1344 [DOI] [PubMed] [Google Scholar]
- Fonteles MC, Greenberg RN, Monteiro HS, Currie MG, Forte LR 1998 Natriuretic and kaliuretic activities of guanylin and uroguanylin in the isolated perfused rat kidney. Am J Physiol 275(2 Pt 2):F191–F197 [DOI] [PubMed] [Google Scholar]
- Perkins A, Goy MF, Li Z 1997 Uroguanylin is expressed by enterochromaffin cells in the rat gastrointestinal tract. Gastroenterology 113:1007–1014 [DOI] [PubMed] [Google Scholar]
- Nakazato M, Yamaguchi H, Date Y, Miyazato M, Kangawa K, Goy MF, Chino N, Matsukura S 1998 Tissue distribution, cellular source, and structural analysis of rat immunoreactive uroguanylin. Endocrinology 139:5247–5254 [DOI] [PubMed] [Google Scholar]
- Cui L, Blanchard RK, Coy LM, Cousins RJ 2000 Prouroguanylin overproduction and localization in the intestine of zinc-deficient rats. J Nutr 130:2726–2732 [DOI] [PubMed] [Google Scholar]
- Forsberg EJ, Miller RJ 1983 Regulation of serotonin release from rabbit intestinal enterochromaffin cells. J Pharmacol Exp Ther 227:755–766 [PubMed] [Google Scholar]
- Nilsson O, Ahlman H, Geffard M, Dahlstrom A, Ericson LE 1987 Bipolarity of duodenal enterochromaffin cells in the rat. Cell Tissue Res 248:49–54 [DOI] [PubMed] [Google Scholar]
- Carrithers SL, Jackson BA, Cai WY, Greenberg RN, Ott CE 2002 Site-specific effects of dietary salt intake on guanylin and uroguanylin mRNA expression in rat intestine. Regul Pept 107:87–95 [DOI] [PubMed] [Google Scholar]
- Steinbrecher KA, Rudolph JA, Luo G, Cohen MB 2002 Coordinate upregulation of guanylin and uroguanylin expression by hypertonicity in HT29–18-N2 cells. Am J Physiol Cell Physiol 283:C1729–C1737 [DOI] [PubMed] [Google Scholar]
- Miyazato M, Nakazato M, Matsukura S, Kangawa K, Matsuo H 1996 Uroguanylin gene expression in the alimentary tract and extra-gastrointestinal tissues. FEBS Lett 398:170–174 [DOI] [PubMed] [Google Scholar]
- Li Z, Perkins AG, Peters MF, Campa MJ, Goy MF 1997 Purification, cDNA sequence, and tissue distribution of rat uroguanylin. Regul Pept 68:45–56 [DOI] [PubMed] [Google Scholar]
- Hill O, Cetin Y, Cieslak A, Magert HJ, Forssmann WG 1995 A new human guanylate cyclase-activating peptide (GCAP-II, uroguanylin): precursor cDNA and colonic expression. Biochim Biophys Acta 1253:146–149 [DOI] [PubMed] [Google Scholar]
- Fan X, Wang Y, London RM, Eber SL, Krause WJ, Freeman RH, Forte LR 1997 Signaling pathways for guanylin and uroguanylin in the digestive, renal, central nervous, reproductive, and lymphoid systems. Endocrinology 138:4636–4648 [DOI] [PubMed] [Google Scholar]
- Magert HJ, Reinecke M, David I, Raab HR, Adermann K, Zucht HD, Hill O, Hess R, Forssmann WG 1998 Uroguanylin: gene structure, expression, processing as a peptide hormone, and co-storage with somatostatin in gastrointestinal D-cells. Regul Pept 73:165–176 [DOI] [PubMed] [Google Scholar]
- Kulaksiz H, Cetin Y 2001 Uroguanylin and guanylate cyclase C in the human pancreas: expression and mutuality of ligand/receptor localization as indicators of intercellular paracrine signaling pathways. J Endocrinol 170:267–275 [DOI] [PubMed] [Google Scholar]
- Kulaksiz H, Rausch U, Vaccaro R, Renda TG, Cetin Y 2001 Guanylin and uroguanylin in the parotid and submandibular glands: potential intrinsic regulators of electrolyte secretion in salivary glands. Histochem Cell Biol 115:527–533 [DOI] [PubMed] [Google Scholar]
- Kulaksiz H, Cetin Y 2002 The electrolyte/fluid secretion stimulatory peptides guanylin and uroguanylin and their common functional coupling proteins in the rat pancreas: a correlative study of expression and cell-specific localization. Pancreas 25:170–175 [DOI] [PubMed] [Google Scholar]
- Jaleel M, London RM, Eber SL, Forte LR, Visweswariah SS 2002 Expression of the receptor guanylyl cyclase C and its ligands in reproductive tissues of the rat: a potential role for a novel signaling pathway in the epididymis. Biol Reprod 67:1975–1980 [DOI] [PubMed] [Google Scholar]
- Maake C, Auf der Maur F, Jovanovic K, Reinecke M, Hauri D, John H 2003 Occurrence and localization of uroguanylin in the aging human prostate. Histochem Cell Biol 119:69–76 [DOI] [PubMed] [Google Scholar]
- Lee SH, Paeng JP, Jung HH, Lee SH, Lee HM, Kwon SY, Lim KJ, Jung KY 2004 Expression of guanylin and uroguanylin mRNA in human nasal mucosa and nasal polyps. Acta Otolaryngol 124:179–185 [DOI] [PubMed] [Google Scholar]
- Qian X, Prabhakar S, Nandi A, Visweswariah SS, Goy MF 2000 Expression of GC-C, a receptor-guanylate cyclase, and its endogenous ligands uroguanylin and guanylin along the rostrocaudal axis of the intestine. Endocrinology 141:3210–3224 [DOI] [PubMed] [Google Scholar]
- Nakazato M, Yamaguchi H, Kinoshita H, Kangawa K, Matsuo H, Chino N, Matsukura S 1996 Identification of biologically active and inactive human uroguanylins in plasma and urine and their increases in renal insufficiency. Biochem Biophys Res Commun 220:586–593 [DOI] [PubMed] [Google Scholar]
- Fukae H, Kinoshita H, Fujimoto S, Kita T, Nakazato M, Eto T 2002 Changes in urinary levels and renal expression of uroguanylin on low or high salt diets in rats. Nephron 92:373–378 [DOI] [PubMed] [Google Scholar]
- Kikuchi M, Fujimoto S, Fukae H, Kinoshita H, Kita T, Nakazato M, Eto T 2005 Role of uroguanylin, a Peptide with natriuretic activity, in rats with experimental nephrotic syndrome. J Am Soc Nephrol 16:392–397 [DOI] [PubMed] [Google Scholar]
- Greenberg RN, Hill M, Crytzer J, Krause WJ, Eber SL, Hamra FK, Forte LR 1997 Comparison of effects of uroguanylin, guanylin, and Escherichia coli heat-stable enterotoxin STa in mouse intestine and kidney: evidence that uroguanylin is an intestinal natriuretic hormone. J Investig Med 45:276–282 [PubMed] [Google Scholar]
- Carrithers SL, Ott CE, Hill MJ, Johnson BR, Cai W, Chang JJ, Shah RG, Sun C, Mann EA, Fonteles MC, Forte LR, Jackson BA, Giannella RA, Greenberg RN 2004 Guanylin and uroguanylin induce natriuresis in mice lacking guanylyl cyclase-C receptor. Kidney Int 65:40–53 [DOI] [PubMed] [Google Scholar]
- Miyazato M, Nakazato M, Yamaguchi H, Date Y, Kojima M, Kangawa K, Matsuo H, Matsukura S 1996 Cloning and characterization of a cDNA encoding a precursor for human uroguanylin. Biochem Biophys Res Commun 219:644–648 [DOI] [PubMed] [Google Scholar]
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S 2004 Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 340:783–795 [DOI] [PubMed] [Google Scholar]
- Currie MG, Fok KF, Kato J, Moore RJ, Hamra FK, Duffin KL, Smith CE 1992 Guanylin: an endogenous activator of intestinal guanylate cyclase. Proc Natl Acad Sci USA 89:947–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz S, Chrisman TD, Garbers DL 1992 Cloning and expression of guanylin. Its existence in various mammalian tissues. J Biol Chem 267:16019–16021 [PubMed] [Google Scholar]
- Hamra FK, Fan X, Krause WJ, Freeman RH, Chin DT, Smith CE, Currie MG, Forte LR 1996 Prouroguanylin and proguanylin: purification from colon, structure, and modulation of bioactivity by proteases. Endocrinology 137:257–265 [DOI] [PubMed] [Google Scholar]
- Fan X, Hamra FK, London RM, Eber SL, Krause WJ, Freeman RH, Smith CE, Currie MG, Forte LR 1997 Structure and activity of uroguanylin and guanylin from the intestine and urine of rats. Am J Physiol 273(5 Pt 1):E957–E964 [DOI] [PubMed] [Google Scholar]
- Hamra FK, Eber SL, Chin DT, Currie MG, Forte LR 1997 Regulation of intestinal uroguanylin/guanylin receptor-mediated responses by mucosal acidity. Proc Natl Acad Sci USA 94:2705–2710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RK, Cousins RJ 1997 Upregulation of rat intestinal uroguanylin mRNA by dietary zinc restriction. Am J Physiol 272(5 Pt 1):G972–G978 [DOI] [PubMed] [Google Scholar]
- Whitaker TL, Witte DP, Scott MC, Cohen MB 1997 Uroguanylin and guanylin: distinct but overlapping patterns of messenger RNA expression in mouse intestine. Gastroenterology 113:1000–1006 [DOI] [PubMed] [Google Scholar]
- London RM, Krause WJ, Fan X, Eber SL, Forte LR 1997 Signal transduction pathways via guanylin and uroguanylin in stomach and intestine. Am J Physiol 273(1 Pt 1):G93–G105 [DOI] [PubMed] [Google Scholar]
- Pass G, Freeth G 1993 The rat. ANZCCART News 6:1–4 [Google Scholar]
- Kinoshita H, Nakazato M, Yamaguchi H, Matsukura S, Fujimoto S, Eto T 1997 Increased plasma guanylin levels in patients with impaired renal function. Clin Nephrol 47:28–32 [PubMed] [Google Scholar]
- Scheving LA, Jin WH 1999 Circadian regulation of uroguanylin and guanylin in the rat intestine. Am J Physiol 277(6 Pt 1):C1177–C1183 [DOI] [PubMed] [Google Scholar]
- Forte LR, Eber SL, Fan X, London RM, Wang Y, Rowland LM, Chin DT, Freeman RH, Krause WJ 1999 Lymphoguanylin: cloning and characterization of a unique member of the guanylin peptide family. Endocrinology 140:1800–1806 [DOI] [PubMed] [Google Scholar]
- Santos-Neto MS, Carvalho AF, Monteiro HS, Forte LR, Fonteles MC 2006 Interaction of atrial natriuretic peptide, urodilatin, guanylin and uroguanylin in the isolated perfused rat kidney. Regul Pept 136:14–22 [DOI] [PubMed] [Google Scholar]
- Qian X, Moss NG, Fellner RC, Goy MF 2008 Circulating prouroguanylin is processed to its active natriuretic form exclusively within the renal tubules. Endocrinology 149:4510–4518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckert P, Brunak S, Blom N 2004 Prediction of proprotein convertase cleavage sites. Protein Eng Des Sel 17:107–112 [DOI] [PubMed] [Google Scholar]
- Fukae H, Kinoshita H, Fujimoto S, Nakazato M, Eto T 2000 Plasma concentration of uroguanylin in patients on maintenance dialysis therapy. Nephron 84:206–210 [DOI] [PubMed] [Google Scholar]
- Kinoshita H, Fujimoto S, Fukae H, Yokota N, Hisanaga S, Nakazato M, Eto T 1999 Plasma and urine levels of uroguanylin, a new natriuretic peptide, in nephrotic syndrome. Nephron 81:160–164 [DOI] [PubMed] [Google Scholar]
- Rehfeld JF 1998 The new biology of gastrointestinal hormones. Physiol Rev 78:1087–1108 [DOI] [PubMed] [Google Scholar]
- Varro A, Yegen B, Dockray GJ 1993 Control of tissue progastrin concentrations in the rat. Exp Physiol 78:327–336 [DOI] [PubMed] [Google Scholar]
- Liddle RA, Goldfine ID, Williams JA 1984 Bioassay of plasma cholecystokinin in rats: effects of food, trypsin inhibitor, and alcohol. Gastroenterology 87:542–549 [PubMed] [Google Scholar]
- Clerico A, Del Ry S, Giannessi D 2000 Measurement of cardiac natriuretic hormones (atrial natriuretic peptide, brain natriuretic peptide, and related peptides) in clinical practice: the need for a new generation of immunoassay methods. Clin Chem 46:1529–1534 [PubMed] [Google Scholar]
- Clauser E, Bouhnik J, Gonzalez MF, Corvol P, Menard J 1984 Influence of converting-enzyme inhibition on rat des-angiotensin I-angiotensinogen. Am J Physiol 246(2 Pt 1):E129–E133 [DOI] [PubMed] [Google Scholar]
- Bouhnik J, Savoie F, Baussant T, Michaud A, Alhenc-Gelas F, Corvol P 1988 Effect of thyroidectomy on rat T-kininogen. Am J Physiol 255(4 Pt 1):E411–E415 [DOI] [PubMed] [Google Scholar]
- Hidaka Y, Ohno M, Hemmasi B, Hill O, Forssmann WG, Shimonishi Y 1998 In vitro disulfide-coupled folding of guanylyl cyclase-activating peptide and its precursor protein. Biochemistry 37:8498–8507 [DOI] [PubMed] [Google Scholar]
- Hidaka Y, Shimono C, Ohno M, Okumura N, Adermann K, Forssmann WG, Shimonishi Y 2000 Dual function of the propeptide of prouroguanylin in the folding of the mature peptide: disulfide-coupled folding and dimerization. J Biol Chem 275:25155–25162 [DOI] [PubMed] [Google Scholar]
- Laney Jr DW, Bezerra JA, Kosiba JL, Degen SJ, Cohen MB 1994 Upregulation of Escherichia coli heat-stable enterotoxin receptor in regenerating rat liver. Am J Physiol 266(5 Pt 1):G899–G906 [DOI] [PubMed] [Google Scholar]
- Bulbring E, Lin RC 1958 The effect of intraluminal application of 5-hydroxytryptamine and 5-hydroxytryptophan on peristalsis; the local production of 5-HT and its release in relation to intraluminal pressure and propulsive activity. J Physiol 140:381–407 [PMC free article] [PubMed] [Google Scholar]
- Burks TF, Long JP 1966 5-Hydroxytryptamine release into dog intestinal vasculature. Am J Physiol 211:619–625 [DOI] [PubMed] [Google Scholar]
- Gamse R, Mroz E, Leeman S, Lembeck F 1978 The intestine as source of immunoreactive substance P in plasma of the cat. Naunyn Schmiedebergs Arch Pharmacol 305:17–21 [DOI] [PubMed] [Google Scholar]
- Ahlman H, DeMagistris L, Zinner M, Jaffe BM 1981 Release of immunoreactive serotonin into the lumen of the feline gut in response to vagal nerve stimulation. Science 213:1254–1255 [DOI] [PubMed] [Google Scholar]