Abstract
The mechanisms by which estradiol exerts specific actions on neural function are unclear. In brain the actions of estrogen receptor (ER) α are well documented, whereas the functions of ERβ are not yet fully elucidated. Here, we report that ERβ inhibits the activity of ERα in an anatomically specific manner within the neonatal (postnatal d 7) brain. Using selective agonists we demonstrate that the selective activation of ERα in the relative absence of ERβ activation induces progesterone receptor expression to a greater extent than estradiol alone in the ventromedial nucleus, but not the medial preoptic nucleus, despite high ERα expression. Selective activation of ERβ attenuates the ERα-mediated increase in progesterone receptor expression in the ventromedial nucleus but has no effect in medial preoptic nucleus. These results suggest that ERα/ERβ interactions may regulate the effects of estrogens on neural development and reveal the neonatal brain as a unique model in which to study the specificity of steroid-induced gene expression.
STEROID HORMONE RECEPTORS, as nuclear transcription factors, exert powerful effects on a broad range of neural functions (1,2). Estradiol, although best known for its role in neuroendocrine function and reproductive behaviors in rodents (for review, see Ref. 1), has recently been associated with a number of neurological disorders, including Parkinson’s disease (3), Alzheimer’s disease (3), and stroke (4). Estrogens and selective estrogen receptor modulators (SERMs) are commonly used clinically, despite a poor understanding of how these treatments might influence brain function (5,6). A growing literature clearly demonstrates that the actions of estradiol are tissue specific and developmentally dependent, creating the possibility for unwanted and potentially dangerous side effects of estrogen treatment (7,8). Therefore, it becomes essential to elucidate the mechanisms by which estradiol and estrogen receptors (ERs) exert their specific actions within the brain.
To date, two nuclear ERs, ERα and ERβ, have been identified (9). These two receptors share a highly conserved DNA binding domain but poor to moderate homology in their N-terminal domain (containing AF-1) and ligand binding domain (containing AF-2). Despite the moderate homology at the ligand binding domain, both ERα and ERβ bind to estradiol with a similar affinity (9). However, due to differences in the AF-1 and AF-2 domains, the intracellular actions of ERα and ERβ may differ significantly (10). ERα and ERβ are expressed at high levels within specific brain regions and are often colocalized within cells (11,12,13,14,15). Although a role for ERα in a variety of neural and behavioral functions has been well documented (for review, see Ref. 1), a clear and direct function of ERβ at the cellular level in brain has remained elusive (for review, see Ref. 16). In contrast, in vitro studies consistently reveal an inhibitory action of ERβ on ERα transcriptional activity when coexpressed in the same cells (17,18,19). Such an interaction between ERα and ERβ has not been demonstrated at the cellular level in vivo, within developing brain. Although several reports have demonstrated a role for ERβ activity in behavioral outcomes (16,20,21,22), the cellular mechanisms underlying the actions of ERβ on ERα activity have yet to be demonstrated in vivo.
Within specific regions of the brain, a highly robust bioassay for ERα activity is the induction of progesterone receptor (PR) gene expression (14,23,24,25,26,27). In adult female rats, estradiol dramatically increases PR within both the medial preoptic nucleus (MPN) and the ventromedial nucleus (VMN) of the hypothalamus. However, previous studies from our laboratory demonstrate that the regulation of PR expression by estradiol is anatomically and developmentally specific (25). For example, in neonatal females, estradiol increases PR levels in the MPN at least 50-fold but does not significantly alter PR levels in the VMN of the same animals. PR expression within the VMN becomes increasingly more responsive to estradiol as development ensues. ERα protein is expressed at high levels throughout development in both regions (28,29), suggesting that ERα activity is transiently inhibited in the VMN, but not the MPN, during development.
Interestingly, ERβ expression is high in the neonatal female VMN but is relativity low in the MPN (13,28). In addition, ERβ expression in the VMN gradually decreases as the animal ages. These findings, together with in vitro studies implicating ERβ in the inhibition of ERα, suggest that ERβ function may be responsible for the reduced sensitivity of the VMN to estradiol during development. To test this hypothesis, the actions of the ER subtypes were dissociated using the in vivo administration of ERα and ERβ selective agonists. Results suggest that activation of ERβ inhibits the ERα dependent induction of PR expression within the developing brain in a regionally specific manner. These results implicate ERβ in the specificity of estrogen signaling in vivo and, more specifically, in the proper development of steroid-sensitive brain areas. In addition, these findings introduce the neonatal female VMN as a unique biologically relevant model in which to examine the mechanisms underlying the regulation and specificity of steroid-induced gene expression.
Materials and Methods
Animals
Subjects were offspring of timed pregnant female Sprague Dawley rats (60–80 d of age; Taconic Laboratories, Germantown, NY). Animals were housed on a 14-h light, 10-h dark cycle at a constant temperature of 25 ± 2 C, with food and water available ad libitum. All females were allowed to deliver their pups normally. All animal procedures used in this study were approved by the Institutional Animal Care and Use Committee at the State University of New York at Albany.
All animals were treated on postnatal d 5 and 6 (P5 and P6, respectively), and tissue was collected on postnatal d 7 (P7). All treatment groups were assigned to at least three different litters to control for litter effects. In addition, all animals were housed in a mixed sex litter until tissue was collected. The time period for tissue collection was chosen based on previous studies demonstrating that a single dosage of estradiol benzoate (EB) at this age does not induce PR within 48 h in the VMN but strongly induces PR within the MPN (25).
Treatments
Experiment 1: selective ERα-mediated transactivation of PR gene in the brain.
On P5 and P6, female pups received either EB (20 μg/kg, n = 8), the ERα-selective agonist, 1,3,5 Tris(4-hydroxyphenyl)-4-propyl-1H-pyrazole (PPT) (1, 2, or 3 mg/kg sc, n = 9, 10, 12), or an equal volume of the vehicle [0.01 cc/g 10% dimethyl sulfoxide (DMSO) in sesame oil, sc, n = 10]. PPT selectively binds to ERα with a 400-fold preference over ERβ and has been a potent activator of ERα transcriptional activity, but not ERβ, in transfected cell lines (30,31). The range of PPT dosages was based on previous studies reporting that PPT in the range of 1–3 mg/kg can induce PR within the rodent brain similarly to 17β-estradiol during neonatal life (24).
Experiment 2: selective activation of ERα and ERβ.
On P5 and P6, female pups received either EB (20 μg/kg, n = 8), PPT (2 mg/kg, n = 8) alone, or PPT in combination with the ERβ selective agonist diarylpropionitrile (DPN) (0.5, 3.5, or 5 mg/kg, n = 10, 9, 7), or an equal volume of the vehicle (0.01 cc/g 10% DMSO in sesame oil, n = 11). DPN demonstrates a 40-fold higher binding preference for ERβ and has been 30-fold more potent in activating transcription using multiple reporter genes in vitro (31,32). The dosages of DPN were chosen from previously published studies in which DPN was used to manipulate ERβ activity in the brains of rodents (24,33,34). A range of dosages has been used previously (32) to study the role of ERβ in many different behavioral paradigms. Due to the variability of dosages, we chose to use a broad range of dosages to find the most effective level of this selective agonist.
Experiment 3: selective activation of ERβ alone.
On P5 and P6, female pups received the most effective dosage of DPN from experiment 2 (3.5 mg/kg) or an equal volume of the vehicle (0.01 cc/g 10% DMSO in sesame oil). A total of 3.5 mg/kg DPN provided the strongest attenuation of PPT when compared with 0.5 and 5 mg/kg, and was therefore used within this experiment. Tissue collection and immunocytochemistry were performed as below.
Experiment 4: localization of ERα, ERβ, and PR via immunocytochemistry.
P7 females were allowed to reach P7 with no drug treatments. Brains were collected for ERα, ERβ, and PR via immunocytochemistry as described below.
Tissue collection
For all experiments animals were anesthetized by hypothermia and killed by rapid decapitation on P7. Brains were removed from the skull and quickly immersion fixed in 5% acrolein in 0.1 m phosphate buffer (PB) (pH 7.6) for 6 h, cryoprotected in 30% sucrose in 0.1 m PB, and cut at 50 μm in the coronal plane. Sections were stored in cryoprotectant (30% sucrose, 0.1% polyvinyl-pyrrolidone-40 in ethylene glycol and 0.1 m PB) at −20 C until processing for immunocytochemistry.
For experiment 4, animals processed for ERβ immunocytochemistry were fixed as described previously using a 3% acrolein in 0.1 m PB solution for 6 h.
Immunocytochemistry
Immunocytochemistry was performed, as previously described (35,36), on free floating sections using a rabbit polyclonal antiserum (Dako Corp. Inc., Glostrup, Denmark) directed against the DNA binding domain of the human PR. This antibody detects both the A and B isoform of PR, and its specificity has been well documented (14,35). All incubations were performed at room temperature unless otherwise stated. Sections were rinsed in Tris-buffered saline (TBS) (pH 7.6) (3 × 5 min), incubated in 1% sodium borohydride in TBS (10 min), rinsed in TBS (4 × 5 min), incubated in TBS containing 20% normal goat serum, 1% H2O2, and 1% BSA for 30 min. PR antiserum was diluted 1:1000 in TBS containing 0.3% Triton X-100, 2% normal goat serum (referred to as TTG) for 72 h at 4 C. Sections were rinsed in TTG (3 × 5 min), incubated in biotinylated goat antirabbit IgG (Vector Laboratories, Burlingame, CA) at a concentration of 5 μg/ml in TTG for 90 min. Sections were rinsed in TTG (2 × 5 min), in TBS (2 × 5 min), then incubated in avidin-biotin complex reagent (Vectastain Elite Kit; Vector Laboratories) for 60 min. Sections were rinsed in TBS (3 × 5 min), then incubated in TBS containing 0.05% diaminobenzidine, 0.75 mm nickel ammonium sulfate, 0.15% β-d-glucose, 0.04% ammonium chloride, and 0.2% glucose oxidase for approximately 15 min. Sections were rinsed in TBS (3 × 5 min), mounted on gelatin-coated slides and coverslipped with Permount (Fisher Scientific, Pittsburgh, PA).
In experiment 4, immunocytochemistry was performed as described previously with the following changes: ERα was detected using polyclonal antisera (C1355; Upstate Signaling, Lake Placid, NY) directed against the last 15 amino acids in the rat ERα for 48 h at 1:2000.
ERβ immunoreactivity (ir) was detected using mouse monoclonal human ERβ (hERβ) antibody (hERβNT-221.3; Ligand Pharmaceuticals, Inc., San Diego, CA) raised against a synthetic peptide corresponding to the 14-amino acid N-terminal sequence of hERβ (1–485 form) at a dilution of 1 μg/ml. The specificity of this antibody has been previously published (14). After primary incubation for 72 h, tissue was incubated in a biotinylated goat antimouse IgG (Vector Laboratories) at a concentration of 4 μg/ml in TTG.
Analysis
For both experiments a representative, anatomically matched section through the rostral MPN and caudal VMN of each animal was selected for image analysis by an experimenter blind to the treatment group. Animals from which an anatomical match could not be found due to damaged sections were excluded from analysis. Microscope images of the PR-ir in the MPN and ventrolateral VMN (VMNvl) were captured with a Nikon Eclipse E600 microscope (Nikon Corp., Tokyo, Japan) fitted with a SPOT Insight camera (Diagnostic Instruments, Sterling Heights, MI) connected to a Dell Inspiron 8600 laptop (Dell Computer Corp., Round Rock, TX). National Institutes of Health Image software (W. Rasband, National Institutes of Health, Bethesda, MD) was used to analyze captured images. Briefly, the relative total amount of PR-ir above background levels was determined according to previously published methods (37,38,39). Only cells that had a gray level darker than a defined criteria above background were counted. The relative amount of PR-ir in the MPN and VMNvl was determined by measuring the area (μm2) covered by “thresholded” pixels [i.e. those pixels with a gray level higher than a defined “threshold” density (specific immunoreactive staining)]. “Threshold” was determined as a constant function over and above background OD (i.e. gray level), and was defined as the OD three times the sd higher than the mean background density. The mean background density was measured in a region devoid of PR-ir, immediately lateral to the analyzed region containing PR-ir. For experiments 1 and 2, statistical analyses were performed using a one-way ANOVA (P < 0.05), followed by preplanned, pairwise comparisons using Student-Newman-Keuls post hoc analysis (P < 0.05). For experiment 3, statistical analysis was performed using a Student’s t test (P < 0.05).
Results
Experiment 1: selective activation of ERα in the relative absence of ERβ activation
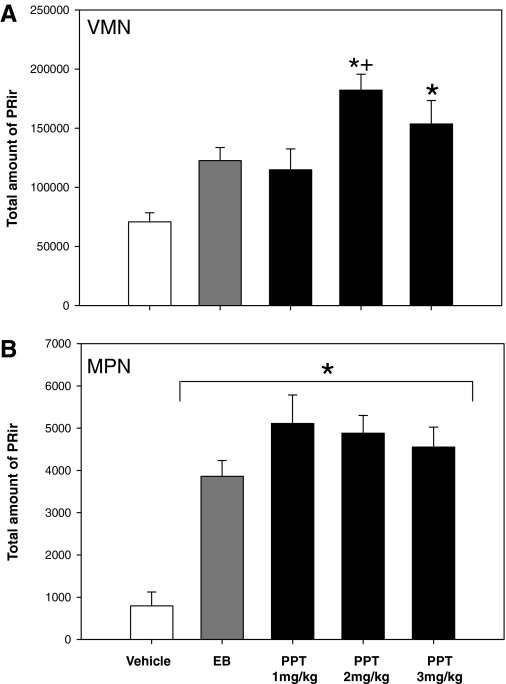
In the VMN the selective activation of ERα alone induced PR over and above EB treatment. In addition, PPT (2 mg/kg) induced PR above that of vehicle controls. In contrast, in the MPN, both EB and all doses of PPT dramatically increased PR levels above that of vehicle controls.
In the neonatal VMN, one-way ANOVA revealed a main effect of treatment [F(4,48) = 7.674; P < 0.001; Fig. 1A]. Post hoc analysis revealed that PR-ir levels were significantly higher in the PPT group (2 mg/kg) compared with EB treatment (P < 0.05). In addition, PPT at 2 and 3 mg/kg increased PR levels compared with oil-treated controls (P < 0.001), whereas EB treatment did not significantly alter PR levels compared with controls (P = 0.072).
Figure 1.
Selective activation of ERα, in the relative absence of ERβ activation, induces PR expression over and above estradiol in the VMN, but not the MPN. The mean (sem) total amount of PR-ir within the VMNvl or the MPN of female rats on P7 treated with vehicle (n = 10), EB (20 μg/kg, n = 8), or the ERα selective agonist, PPT, at 1, 2, or 3 mg/kg (n = 9, 10, 12) on P5 and P6. A, VMNvl. PR-ir levels were significantly higher after PPT treatment (2 mg/kg) compared with EB treatment. PPT (2 and 3 mg/kg) significantly increased PR-ir levels compared with vehicle treatment. B, MPN. PPT treatment (all doses) and EB treatment significantly increased levels of PR-ir compared with vehicle treatment. PR-ir levels did not differ between EB and PPT treatments at any dose. *, Significantly different from vehicle (P < 0.001); +, significantly different from EB (P < 0.05).
In the MPN, one-way ANOVA revealed a significant main effect of treatment [F(4,51) = 13.271; P < 0.001; Fig. 1B]. Post hoc analysis revealed that EB and PPT at all doses significantly increased PR-ir levels compared with vehicle-treated controls (P < 0.001). EB and PPT treatments were not different from one another.
Experiment 2: selective activation of ERα and ERβ
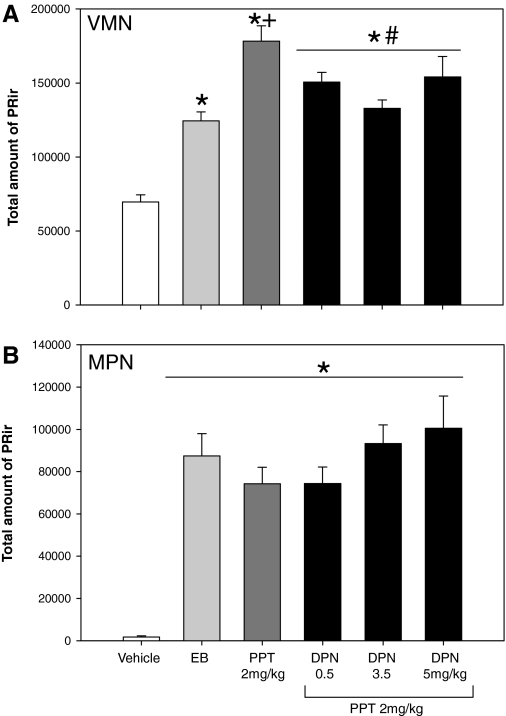
Consistent with experiment 1, PPT induced PR levels in the VMN significantly greater than EB treatment. Furthermore, the effect of PPT was attenuated with the addition of the ERβ agonist, DPN. The combination of PPT and DPN significantly decreased PR levels compared with PPT alone but were similar to those of EB treatment.
In the VMN, one-way ANOVA revealed a main effect of treatment [F(5,52) = 25.483; P < 0.001; Fig. 2A]. Post hoc analysis revealed that levels of PR-ir were higher in the EB treated group compared with the oil-treated group (P < 0.05). PPT significantly increased PR levels over and above that of EB (P < 0.001). Furthermore, the effect of PPT was significantly attenuated by the addition of DPN. All three doses of DPN in combination with PPT decreased PR levels compared with PPT alone (P < 0.001 for 3.5 mg/kg, and P < 0.05 for 0.5 and 5 mg/kg DPN). In contrast, in the MPN, all treatments significantly increased PR levels compared with vehicle controls, but there were no differences between the treatment groups. One-way ANOVA revealed a significant main effect of treatment [F(5,56) = 22.277; P < 0.001; Fig. 2B]. Post hoc analysis revealed that all treatments (EB, PPT, and PPT plus DPN) significantly increased PR levels compared with vehicle treatment (P < 0.001). PR levels did not differ between the PPT plus DPN treated groups and the EB treated group.
Figure 2.
Selective activation of ERβ attenuates the induction of PR expression by ERα in the VMN, but not the MPN. The mean (sem) total amount of PR-ir within the VMNvl or the MPN of female rats on P7 treated with vehicle (n = 11), EB (20 μg/kg, n = 8), or the ERα selective agonist, PPT (2 mg/kg, n = 8) or PPT in combination with the ERβ selective agonist, DPN, at 0.5, 3.5, or 5 mg/kg (n = 10, 9, 7) on P5 and P6. A, VMNvl. PR-ir levels were significantly higher after PPT treatment (2 mg/kg) compared with EB treatment. The effect of PPT was significantly attenuated by the addition of DPN at all doses. PR-ir was significantly higher in all treatment groups compared with vehicle. B, MPN. PPT treatment alone, or PPT in combination with any dose of DPN, did not significantly alter PR-ir levels compared with EB treatment. PR-ir was significantly higher in all treatment groups compared with vehicle. *, Significantly different from vehicle (P < 0.001); +, significantly different from EB (P < 0.01); #, significantly different from PPT (3.5 mg/kg DPN P < 0.001; 0.5 and 5 mg/kg DPN, P < 0.05).
Experiment 3: selective activation of ERβ alone
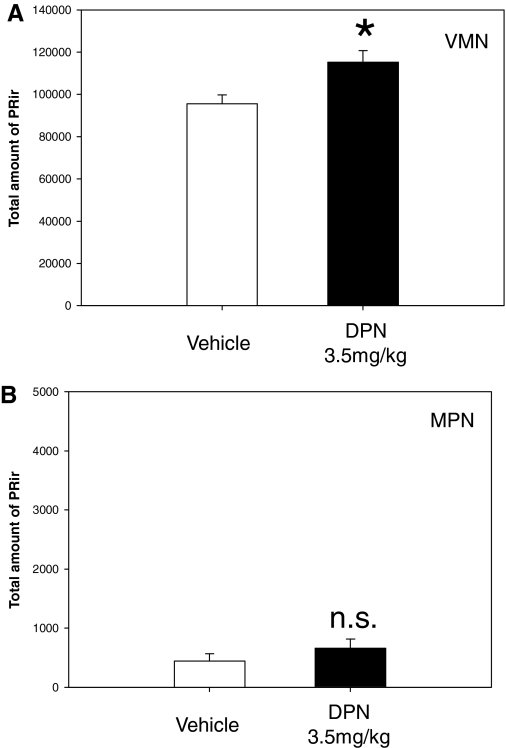
Selective activation of ERβ alone had no effect on PR-ir levels in the MPN, suggesting that DPN, at the dose used, had little effect on ERα activity. DPN alone increased PR-ir levels slightly in the VMN (P < 0.05; Fig. 3A). PR-ir levels did not significantly differ between the DPN treated group and vehicle-treated group in the MPN (Fig. 3B).
Figure 3.
DPN alone does not activate ERα in the MPN. The mean (sem) total amount of PR-ir within the VMNvl or the MPN of female rats on P7 treated with vehicle (n = 11), or DPN at 3.5 mg/kg (n = 10) on P5 and P6. A, VMNvl. PR-ir levels were significantly higher after DPN treatment compared with vehicle. B, MPN. DPN treatment had no significant (n.s.) effect on PR-ir. *, Significantly different from vehicle (P < 0.05).
Experiment 4: ERβ, ERα, and PR expression in the neonatal female VMN and MPN
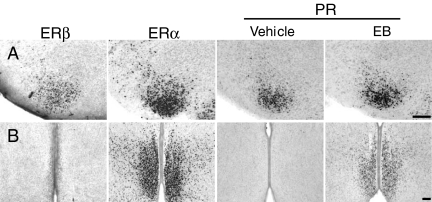
ERβ, ERα, and PR are all expressed at high levels within the VMNvl of P7 females (Fig. 4A). Furthermore, the anatomical distribution of immunoreactive nuclei fall within the same region of the VMNvl, suggesting that these three receptors overlap in their expression within the VMN.
Figure 4.
Differential ERβ expression coincides with the ability of estradiol to induce PR in the VMN and MPN. ERβ, ERα, and PR immunoreactive nuclei within (A) the VMNvl or (B) the MPN of P7 female rats. PR-ir is shown in females treated with vehicle or with EB (20 μg/kg) on P5 and P6. Magnification bars, 100 μm.
In the MPN, although ERα is expressed at high levels, ERβ and PR expression is virtually absent (Fig. 4B). EB treatment dramatically alters PR-ir in the MPN but does little to PR-ir in the VMNvl. The ability of EB treatment to induce PR-ir in these regions coincides with the differential expression of ERβ in MPN and VMN.
Discussion
The present results implicate ERβ as an inhibitor of ERα transcriptional activity in the brain, representing a cellular level function for ERβ in vivo. Selective activation of ERα by PPT, in the relative absence of ERβ activation, induced PR expression in the neonatal VMN to a greater extent than estradiol, which similarly activates both ERα and ERβ. The PPT-induced increase was attenuated when ERβ was activated with the selective agonist, DPN. These results suggest that activation of ERβ by estradiol in the VMN inhibits ERα transcriptional activity, thereby suppressing ERα-mediated transactivation of the PR gene in this brain region. The inhibition of ERα transcriptional activity by ERβ was specific to the VMN, which expresses both ERα and ERβ. In the MPN, an area that expresses high levels of ERα but very low levels of ERβ (28), PR was equally induced by EB and PPT, and the ERβ agonist DPN had no effect, indicating an absence of an ERα/ERβ interaction in this region. These data implicate ERβ in the anatomically and developmentally specific effects of estradiol in the brain.
Estradiol can dramatically influence brain development as clearly demonstrated in the sexual differentiation of the rodent brain (22,40). However, the actions of estradiol are often anatomically specific, such that estradiol can exert strong effects in one area of the brain, whereas having little to no influence in other areas despite ample ERα expression (41,42). For example, in the neonatal female rat brain, PR expression is increased 50-fold within the MPN after a single injection of estradiol 48 h before tissue collection (25). In stark contrast, PR expression is not altered in the VMN of the same animals and only becomes more responsive to estradiol later in postnatal development. Here, we demonstrate a potential mechanism underlying the regional specificity of estradiol action in developing brain.
Treatment with DPN alone (at the most effective dose from experiment 2) had no effect on PR-ir in the MPN in which ERβ expression is very low, demonstrating that this dose of DPN was selective for ERβ and did not significantly activate ERα. Interestingly, DPN alone increased PR levels slightly within the ERβ-rich VMN of the same animals. This may be explained by findings from in vitro studies demonstrating that ERβ, in the absence of ERα activity, can form homodimers capable of weakly inducing the transcription of ERα-driven reporter genes (7,43,44,45). The present findings also document doses of PPT and DPN that when combined, mimic the effect of estradiol in our paradigm. It is noteworthy that DPN at the highest dose had a diminished inhibitory effect on PR-ir levels compared with lower doses in experiment 2. This is most likely due to the activation of ERα by high levels of DPN. Furthermore, we noticed a similar effect with the highest dose of PPT in experiment 1, suggesting that at high doses, the selectivity of these agonists may be diminished in vivo.
The differential regulation of estradiol signaling in the MPN and VMN can be attributed to the differential expression of ERβ, and may be essential for the proper development of these two reproductively important brain regions as previously reported (36,37,39). The VMN is important for successful mating behavior in females (46,47), whereas the MPN is essential for the complex repertoire of maternal behaviors after parturition (48). The present results are consistent with the idea that interactions between ERα and ERβ within the developing VMN may be critical to protect the VMN from defeminization by estradiol during important periods of development, thus ensuring proper feminization of the VMN and subsequent adult female sexual behavior.
Results from Kudwa et al. (22) support this hypothesis by demonstrating that selective activation of ERα or ERβ during development alters female sexual behavior in adulthood.
Additional work demonstrates that a role for ERβ is not exclusive to sexual behavior but, rather, may mediate numerous neurological functions. ERβ has been implicated in modulating behaviors such as spatial ability (21), anxiety (49,50), and aggression [(51), and see Ref. 52 for an extensive review]. Furthermore, Bodo et al. (34) have suggested that ERβ may be mediating the effects of estradiol through interactions with ERα. For example, Imwalle et al. (50) have demonstrated that ERβ knockout (KO) mice have higher anxiety levels compared with ERα KO females and wild-type mice, suggesting that ERβ may modulate the effects of estradiol through antagonistic effects on ERα activity. In addition, the ability of estradiol to induce PR expression in the medial preoptic area of adult ERα KO and ERβ KO mice and ERαβKO mice suggests that ERα and ERβ interact in complex ways to regulate the brain and behavior (27). Our results are consistent with many of these observations and provide a possible model to study the cellular mechanisms underlying the modulation of ERα by ERβ within the brain.
The present results elucidating a novel role for ERβ in vivo are consistent with in vitro work reporting that ERβ inhibits the transcriptional activity of ERα by direct physical interactions between the two receptors, (i.e. heterodimerization and/or cofactor recruitment) (16,17,19). Present findings strongly suggest that ERα, ERβ, and PR are expressed within overlapping cell populations within the VMN, but not the MPN, creating the possibility that ERα and ERβ could have direct interactions with one another in PR expressing cells. However, the intriguing possibility also exists that indirect, transsynaptic regulation of ERα activity by ERβ occurs in the developing brain. In vitro studies (17) as well as in vivo studies in adult brain (53,54) demonstrate that estradiol increases the transcription of the PR gene. Therefore, changes in PR-ir levels in the present study are likely attributable to changes in transcription of the PR gene, but the possibility also exits that alterations in translation or turnover rate of PR protein may also occur within the developing brain.
The present studies elucidate mechanisms underlying estrogen signaling in brain, which becomes an increasingly important issue as the clinical use of estrogens and SERMs increases. The present results implicate ERβ in the inhibition of ERα transcriptional activity in the anatomically and developmentally specific actions of estradiol within the developing brain. Furthermore, we introduce the neonatal female VMN as a powerful in vivo model in which to study the mechanisms by which specificity of steroid-induced gene expression is achieved.
Footnotes
This work was supported by National Science Foundation Grant IOB0447492 (to C.K.W.), March of Dimes National Research Grant (to C.K.W.), and National Institutes of Health Grant DK61935 (to M.J.T.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online May 29, 2008
Abbreviations: DMSO, Dimethyl sulfoxide; DPN, diarylpropionitrile; EB, estradiol benzoate; ER, estrogen receptor; ir, immunoreactivity; KO, knockout; MPN, medial preoptic nucleus; PB, phosphate buffer; P5, postnatal d 5; P6, postnatal d 6; P7, postnatal d 7; PPT, 1,3,5 Tris (4-hydroxyphenyl)-4-propyl-1H-pyrazole; PR, progesterone receptor; SERM, selective estrogen receptor modulator; TBS, Tris-buffered saline; VMN, ventromedial nucleus; VMNvl, ventrolateral ventromedial nucleus.
References
- Mcewen B 2002 Estrogen actions throughout the brain. Recent Prog Horm Res 57:357–384 [DOI] [PubMed] [Google Scholar]
- Hall JM, Couse JF, Korach KS 2001 The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem 276:36869–36872 [DOI] [PubMed] [Google Scholar]
- Sawada H, Shimohama S 2000 Neuroprotective effects of estradiol in mesencephalic dopaminergic neurons. Neurosci Biobehav Rev 24:143–147 [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Dellovade TL, Shughrue PJ 2003 Neuroprotection by estrogen in animal models of global and focal ischemia. Ann NY Acad Sci 1007:89–100 [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen BS, Katzenellenbogen JA 2002 Biomedicine. Defining the “S” in SERMs. Science 295:2380–2381 [DOI] [PubMed] [Google Scholar]
- McDonnell DP 1999 The molecular pharmacology of SERMS. Trends Endocrinol Metab 10:301–311 [DOI] [PubMed] [Google Scholar]
- Matthews J, Gustafsson JA 2003 Estrogen signaling: a subtle balance between ERα and ERβ. Mol Interv 3:281–292 [DOI] [PubMed] [Google Scholar]
- Schultz JR, Petz LN, Nardulli AM 2005 Cell-and ligand-specific regulation of promoters containing activator protein-1 and sp1 sites by estrogen receptors α and β. J Biol Chem 280:347 [DOI] [PubMed] [Google Scholar]
- Kuiper G, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA 1996 Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 93:5925–5930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kian Tee M, Rogatsky I, Tzagarakis-Foster C, Cvoro A, An J, Christy RJ, Yamamoto KR, Leitman DC 2004 Estradiol and selective estrogen receptor modulators differentially regulate target genes with estrogen receptors α and β. Mol Biol Cell 15:1262–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K, Ochiai I, Nishi M, Kawata M 2002 Colocalization and ligand-dependent discrete distribution of the estrogen receptor (ER) α and ERβ. Mol Endocrinol 16:2215–2230 [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I 1997 Comparative distribution of estrogen receptor-α; and β; mRNA in the rat central nervous system. J Comp Neurol 388:507–525 [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Nagai A, Ikeda MA, Hayashi S 2003 Sexually dimorphic and estrogen-dependent expression of estrogen receptor β in the ventromedial hypothalamus during rat postnatal development. Endocrinology 144:5098–5104 [DOI] [PubMed] [Google Scholar]
- Greco B, Allegretto EA, Tetel MJ, Blaustein JD 2001 Coexpression of ERβ with ERα and progestin receptor proteins in the female rat forebrain: effects of estradiol treatment. Endocrinology 142:5172–5181 [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Scrimo PJ, Merchenthaler I 1998 Evidence of the colocalization of estrogen receptor-β mRNA and estrogen receptor-α immunoreactivity in neurons of the rat forebrain. Endocrinology 139:5267–5270 [DOI] [PubMed] [Google Scholar]
- Pettersson K, Gustafsson JA 2001 Role of estrogen receptor β in estrogen action. Annu Rev Physiol 63:165–192 [DOI] [PubMed] [Google Scholar]
- Matthews J, Wihlen B, Tujague M, Wan J, Strom A, Gustafsson JA 2006 Estrogen receptor (ER) β modulates ERα-mediated transcriptional activation by altering the recruitment of c-Fos and c-Jun to estrogen-responsive promoters. Mol Endocrinol 20:534–543 [DOI] [PubMed] [Google Scholar]
- Pettersson K, Delaunay F, Gustafsson JA 2000 Estrogen receptor β acts as a dominant regulator of estrogen signaling. Oncogene 19:4970–4978 [DOI] [PubMed] [Google Scholar]
- Hall JM, Mcdonnell DP 1999 The estrogen receptor β-isoform (ERβ) of the human estrogen receptor modulates ERα transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology 140:5566–5578 [DOI] [PubMed] [Google Scholar]
- Fugger HN, Foster TC, Gustafsson J, Rissman EF 2000 Novel effects of estradiol and estrogen receptor α and β on cognitive function. Brain Res 883:258–264 [DOI] [PubMed] [Google Scholar]
- Rissman EF, Heck AL, Leonard JE, Shupnik MA, Gustafsson J 2002 Disruption of estrogen receptor β gene impairs spatial learning in female mice. Proc Natl Acad Sci USA 99:3996–4001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudwa AE, Michopoulos V, Gatewood JD, Rissman EE 2006 Roles of estrogen receptors α and β in differentiation of mouse sexual behavior. Neuroscience 138:921–928 [DOI] [PubMed] [Google Scholar]
- Wagner CK, Pfau JL, De Vries GJ, Merchenthaler IJ 2001 Sex differences in progesterone receptor immunoreactivity in neonatal mouse brain depend on estrogen receptor α expression. J Neurobiol 47:176–182 [DOI] [PubMed] [Google Scholar]
- Chung WC, Pak TR, Weiser MJ, Hinds LR, Andersen ME, Handa RJ 2006 Progestin receptor expression in the developing rat brain depends upon activation of estrogen receptor α and not estrogen receptor β. Brain Res 1082:50–60 [DOI] [PubMed] [Google Scholar]
- Quadros PS, Wagner CK 2008 Regulation of progesterone receptor expression by estradiol is dependent on age, sex and region in the rat brain. Endocrinology 149:3054–3061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt CA, Rissman EF, Shupnik MA, Blaustein JD 1998 Induction of progestin receptors by estradiol in the forebrain of estrogen receptor-α gene-disrupted mice. J Neurosci 18:9556–9563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudwa AE, Gustafsson JA, Rissman EF 2004 Estrogen receptor β modulates estradiol induction of progestin receptor immunoreactivity in male, but not in female, mouse medial preoptic area. Endocrinology 145:4500–4506 [DOI] [PubMed] [Google Scholar]
- Perez SE, Chen EY, Mufson EJ 2003 Distribution of estrogen receptor α and β immunoreactive profiles in the postnatal rat brain. Brain Res Dev Brain Res 145:117–139 [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Nagai A 2006 Differential expression of the estrogen receptors α and β during postnatal development of the rat cerebellum. Brain Res 1083:39–49 [DOI] [PubMed] [Google Scholar]
- Shiau AK, Barstad D, Radek JT, Meyers MJ, Nettles KW, Katzenellenbogen BS, Katzenellenbogen JA, Agard DA, Greene GL 2002 Structural characterization of a subtype-selective ligand reveals a novel mode of estrogen receptor antagonism. Nat Struct Biol 9:359–364 [DOI] [PubMed] [Google Scholar]
- Harrington WR, Sheng S, Barnett DH, Petz LN, Katzenellenbogen JA, Katzenellenbogen BS 2003 Activities of estrogen receptor α- and β-selective ligands at diverse estrogen responsive gene sites mediating transactivation or transrepression. Mol Cell Endocrinol 206:13–22 [DOI] [PubMed] [Google Scholar]
- Frasor J, Barnett DH, Danes JM, Hess R, Parlow AF, Katzenellenbogen BS 2003 Response-specific and ligand dose-dependent modulation of estrogen receptor (ER) α activity by ERβ in the uterus. Endocrinology 144:3159–3166 [DOI] [PubMed] [Google Scholar]
- Lund TD, Rovis T, Chung WCJ, Handa RJ 2005 Novel actions of estrogen receptor-β on anxiety-related behaviors. Endocrinology 146:797–807 [DOI] [PubMed] [Google Scholar]
- Bodo C, Kudwa AE, Rissman EF 2006 Both estrogen receptor-α and -β are required for sexual differentiation of the anteroventral periventricular area in mice. Endocrinology 147:415–420 [DOI] [PubMed] [Google Scholar]
- Quadros PS, Pfau JL, Wagner CK 2007 Distribution of progesterone receptor immunoreactivity in the fetal and neonatal rat forebrain. J Comp Neurol 504:42–56 [DOI] [PubMed] [Google Scholar]
- Quadros PS, Goldstein AYN, De Vries GJ, Wagner CK 2002 Regulation of sex differences in progesterone receptor expression in the medial preoptic nucleus of postnatal rats. J Neuroendocrinol 14:761–767 [DOI] [PubMed] [Google Scholar]
- Wagner CK, Nakayama AY, De Vries GJ 1998 Potential role of maternal progesterone in the sexual differentiation of the brain. Endocrinology 139:3658–3661 [DOI] [PubMed] [Google Scholar]
- Wagner CK, Xu J, Pfau JL, Quadros PS, De Vries GJ, Arnold AP 2004 Neonatal mice possessing an Sry transgene show a masculinized pattern of progesterone receptor expression in the brain independent of sex chromosome status. Endocrinology 145:1046–1049 [DOI] [PubMed] [Google Scholar]
- Quadros PS, Pfau JL, Goldstein AYN, De Vries GJ, Wagner CK 2002 Sex differences in progesterone receptor expression: a potential mechanism for estradiol-mediated sexual differentiation. Endocrinology 143:3727–3739 [DOI] [PubMed] [Google Scholar]
- McCarthy MM 2008 Estradiol and the developing brain. Physiol Rev 88:91–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mong JA, Glaser E, McCarthy MM 1999 Gonadal steroids promote glial differentiation and alter neuronal morphology in the developing hypothalamus in a regionally specific manner. J Neurosci 19:1464–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Kaufman LC, Brooks PJ, Pfaff DW, Schwartz-Giblin S 1995 Estrogen modulation of mRNA levels for the two forms of glutamic acid decarboxylase(gad) in female rat brain. J Comp Neurol 360:685–697 [DOI] [PubMed] [Google Scholar]
- Cowley SM, Hoare S, Mosselman S, Parker MG 1997 Estrogen receptors α and β form heterodimers on DNA. J Biol Chem 272:19858–19862 [DOI] [PubMed] [Google Scholar]
- Pettersson K, Grandien K, Kuiper GG, Gustafsson JA 1997 Mouse estrogen receptor β forms estrogen response element-binding heterodimers with estrogen receptor α. Mol Endocrinol 11:1486–1496 [DOI] [PubMed] [Google Scholar]
- Li X, Huang J, Yi P, Bambara RA, Hilf R, Muyan M 2004 Single-chain estrogen receptors (ERs) reveal that the ERα/β heterodimer emulates functions of the ERα dimer in genomic estrogen signaling pathways. Mol Cell Biol 24:7681–7694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis PG, Krieger MS, Barfield RJ, Mcewen BS, Pfaff DW 1982 The site of action of intrahypothalamic estrogen implants in feminine sexual behavior: an autoradiographic analysis. Endocrinology 111:1581–1586 [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Sakuma Y 1979 Deficit in the lordosis reflex of female rats caused by lesions in the ventromedial nucleus of the hypothalamus. J Physiol 288:203–210 [PMC free article] [PubMed] [Google Scholar]
- Numan M 1986 The role of the medial preoptic area in the regulation of maternal behavior in the rat. Ann NY Acad Sci 474:226–233 [DOI] [PubMed] [Google Scholar]
- Krezel W, Dupont S, Krust A, Chambon P, Chapman PF 2001 Increased anxiety and synaptic plasticity in estrogen receptor β-deficient mice. Proc Natl Acad Sci USA 98:12278–12282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imwalle DB, Gustafsson J, Rissman EF 2005 Lack of functional estrogen receptor β influences anxiety behavior and serotonin content in female mice. Physiol Behav 84:157–163 [DOI] [PubMed] [Google Scholar]
- Choleris E, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW, Ogawa S 2003 An estrogen-dependent four-gene micronet regulating social recognition: A study with oxytocin and estrogen receptor-α and -β knockout mice. Proc Natl Acad Sci USA 100:6192–6197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodo C, Rissman EF 2006 New roles for estrogen receptor β in behavior and neuroendocrinology. Front Neuroendocrinol 27:217–232 [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Turcotte JC 1989 Estradiol-induced progestin receptor immunoreactivity is found only in estrogen receptor-immunoreactive cells in guinea pig brain. Neuroendocrinology 49:454–461 [DOI] [PubMed] [Google Scholar]
- Warembourg M, Jolivet A, Milgrom E 1989 Immunohistochemical evidence of the presence of estrogen and progesterone receptors in the same neurons of the guinea pig hypothalamus and preoptic area. Brain Res 480:1–15 [DOI] [PubMed] [Google Scholar]