Abstract
Neuroendocrine mechanisms underlying complementary behaviors like male-typical mounting and female-typical receptivity are most often studied independently in males and females, respectively. Cnemidophorus uniparens is a unisexual lizard species consisting only of females that alternately express male- and female-like pseudosexual behavior across the ovarian cycle. Intact, postovulatory (PostOv), and ovariectomized (OVX), androgen-implanted animals [OVX plus testosterone (T)] exhibit male-like mounting, but not receptivity, whereas intact, preovulatory (PreOv), and OVX lizards injected with estradiol [OVX plus estrogen (E)] express receptivity, but not mounting. We tested whether the serotonergic system in the preoptic area (POA) and ventromedial nucleus of the hypothalamus (VMN) gates the reciprocal inhibition characterizing this alternating expression of mounting and receptivity. Serotonergic signaling at the POA appears to be key to gating male-like behavior. Postovulatory and OVX plus T animals have lower intracellular serotonin (5-HT) levels, and greater abundance of inhibitory 5-HT1A receptor mRNA in the POA compared with both PreOv and OVX plus E lizards. Moreover, injecting 5-HT into the POA of OVX plus T animals suppresses mounting, whereas injection into VMN of OVX plus E lizards suppresses receptivity. Although 5-HT levels in the VMN do not differ across the ovarian cycle or between hormonally manipulated animals, PreOv and OVX plus E lizards have a lower abundance of 5-HT2A mRNA in the VMN. Stimulating 5-HT1A receptors using systemic drug administration inhibits mounting, whereas activating 5-HT2A receptors facilitates receptivity. This study illuminates how male- and female-typical sexual behaviors share common neural circuits, and that 5-HT regulates these naturally complementary, and mutually exclusive, behaviors.
TYPICALLY, MOUNTING and receptivity are sexually dimorphic behaviors, with males mounting receptive females. However, the common observation of heterotypical behaviors in both sexes in animals in nature as well as in the laboratory indicates that the brain retains its bisexuality in adulthood (1). The fact that these behavioral displays are mutually exclusive suggests that their expression is gated such that in females, stimulation of the receptivity phenotype simultaneously suppresses the mounting phenotype (and vice versa in males). Although most studies investigating sexual behavior test males only with females (and vice versa), this conclusion is supported by studies that have tested females for both receptivity with male, and mounting with female, stimulus animals (cf. Refs. 2,3,4). Because mammals are gonochoristic, with males and females genetically and hormonally distinct, examination of how the brain regulates the expression of the sex-typical and heterotypical behavior would benefit from the study of organisms that engage in both behaviors as part of their natural history, such as parthenogenetic and hermaphroditic species (5,6). In such species each individual not only displays both sex-typical and heterotypical behavior but does so in an appropriate behavioral or physiological context.
The all-female desert-grassland whiptail lizard, Cnemidophorus uniparens, reproduces by obligate parthenogenesis and consists of a single clone throughout its range (7). Although males are lacking, individuals engage in pseudosexual behaviors that are identical to the courtship and copulatory behavior of closely related sexual species (8,9). Individual lizards exhibit a female-like receptive phenotype before ovulation, switching to a male-like pseudocopulatory phenotype after ovulation. These ovarian and behavioral states are correlated with high levels of estrogen (E) before ovulation and high progesterone levels during the postovulatory (PostOv) state. Although androgens are undetectable in circulation throughout the breeding season, C. uniparens is sensitive to exogenous androgen (10). Ovariectomized (OVX) animals will not display pseudosexual behavior, but administration of exogenous testosterone (T) consistently causes individuals to display robust male-like pseudocopulation, whereas exogenous E induces female-like receptivity. This hormonal specificity also extends to the brain with intracranial implantation of T into the preoptic area (POA) inducing mounting behavior, whereas E implantation into the ventromedial nucleus of the hypothalamus (VMN) elicits receptivity (11,12,13,14). Neither T implanted into the VMN nor E into the POA will elicit female- or male-like pseudosexual behaviors, respectively. Therefore, pseudosexual behaviors are both steroid-hormone dependent and brain-nuclei specific.
Neurotransmitters are an important link between sex steroid hormone action and behavior (15,16). The serotonergic system is responsive to the circulating hormonal milieu, and mediates diverse behaviors in a variety of species, including locomotion in fish (17), and aggression in lizards, crustaceans, and mammals (18,19,20,21). Serotonin (5-HT) inhibits sexual behavior in several vertebrate taxa. For example, the estradiol-induced facilitation of male sexual behavior in the quail is mediated by alterations in serotonergic activity (22). 5-HT’s role in rodent sexual behavior has received considerable attention, and, in general, 5-HT is thought to be inhibitory to sexual behavior in both sexes (23,24). It is noteworthy that an increase in amygdalar serotonergic activity is thought to underlie territorial acquisition, whereas a decrease in raphe serotonergic activity accompanies behavioral sex reversal in the saddleback wrasse (25). Previous research indicates that in C. uniparens, pharmacologically increasing 5-HT levels inhibits male-like pseudosexual behavior in androgen-implanted lizards, whereas decreases in 5-HT levels are correlated with an increased incidence of mounting (26).
In this study we investigate a case for the serotonergic gating of both male- and female-like pseudosexual behavior in naturally cycling and OVX, hormonally manipulated C. uniparens using molecular, pharmacological, and behavioral analyses. Our findings indicate that serotonergic neurotransmission provides a mechanism to regulate the expression of one behavior (e.g. mounting) and the simultaneous suppression of the complementary pattern (e.g. receptivity), and vice versa. These data, and findings in other species, including mammals, suggest that common neural substrates govern male- and female-typical behaviors, and neurochemical signaling between the POA and VMN mediates the complementarity observed during sexual encounters.
Materials and Methods
Animals
Adult C. uniparens were collected in the Sonoran desert in Arizona and New Mexico, and transported to the University of Texas at Austin in the summers of 2006 and 2007. In the laboratory, lizards were group housed in sand-filled terraria placed in environmentally controlled chambers as described in Ref. 27. All animal procedures were performed in accordance with National Institutes of Health and Institutional Animal Care and Use Committee guidelines on the care and use of animals (protocol no. 07022602). All the following experiments were performed between June 2006 and August 2007 using animals measuring 62 ± 3 mm (mean ± sd) in snout vent length. Naturally cycling and hormonally manipulated experimental animals were housed in isolation before experimentation to minimize the effect of behavioral interactions on neurochemical parameters.
Behavioral testing
In all experiments, animals were tested for male-like pseudocopulation (10-min test) and female-like receptivity (2-min test). The order, whether the experimental animal was tested first with a receptive stimulus animal for male-like mounting or with a mounting stimulus animal, was counterbalanced within each group and experiment. To test for male-like pseudocopulation, a receptive stimulus animal (OVX and estradiol injected) was introduced into the test animal’s tank, and the time taken by the experimental animal to mount the stimulus animal was recorded (latency to mount). Mounting behavior involves climbing on the back of the stimulus animal and being aligned along the longitudinal axis. The riding animal then assumes a characteristic doughnut posture by wrapping itself around the stimulus animal (pseudocopulation). Female-like receptivity was tested by introducing the experimental animal into the tank of an OVX and T-implanted stimulus animal that had previously shown robust male-like pseudocopulation. The experimental animal was characterized as being receptive (allowed stimulus animal to mount with no resistance) or nonreceptive (showed resistance to being mounted by biting or fleeing from the stimulus animal). All animals were also tested for general motivation and locomotor activity after testing for pseudosexual behavior by recording the number of steps taken in 1 min, movement while chased by the experimenter’s hand, and the latency to seize a live, moving cricket. Nonreproductive behaviors were not affected in any of the reported experiments, with the effects being specific to pseudosexual behavior (see supplemental Video 1 for pseudosexual behavior, which is which is published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). After testing for pseudosexual and noncopulatory behavior, animals were killed as noted in specific experiments below (HPLC, ICC, intracranial surgery, and pharmacological experiments).
Naturally cycling animals and hormonal manipulations
PostOv and preovulatory (PreOv) ovarian states were determined by abdominal palpation. Female-like receptivity was then tested by introducing the experimental animal into the tank of an OVX and T-implanted stimulus animal that had previously shown robust male-like pseudocopulation. In addition, ovarian morphology was noted after killing the experimental animal. All PreOv animals were characterized by the presence of developing follicles and a receptive phenotype, whereas PostOv animals had corpora lutea and were nonreceptive.
In the hormonally manipulated conditions, the adult C. uniparens was OVX and implanted with an empty 12-mm SILASTIC brand capsule (Dow Corning, Corp., Midland, MI; 1.47-mm inner diameter, 1.96-mm outer diameter) or with a capsule packed with T (OVX plus T). Independent cohorts of animals were OVX and not implanted but administered ip injections of 0.5 μg estradiol benzoate on 3 d consecutively, 6 d after ovariectomy (OVX plus E). In all instances OVX individuals failed to show either male-like mounting or female-like receptivity in the behavioral tests. In addition, each steroid hormone-treated animal served as its own control in that it was tested for the behavior opposite of that induced by the steroid hormone. The route of hormone administration was dictated by the animal’s ability to tolerate the hormone. For example, estradiol-filled SILASTIC brand capsules result in the animal’s death within 1-wk implantation, and, therefore, estradiol needs to be injected rather than implanted (9).
HPLC analysis
Tissue collection for HPLC analysis.
Naturally cycling and hormonally manipulated lizards were decapitated 30 min after behavioral testing, brains rapidly dissected, frozen on dry ice, and stored at −80 C until the time of sectioning. Using a cryostat (Microm HM 500 OM; Microm, Walldorf, Germany), 200-μm coronal sections were thaw mounted onto Superfrost Plus slides (Erie Scientific Co., Portsmouth, NH). Sections were then rapidly frozen using a cooling block set at −20 C (Physitemp Instruments, Inc., Clifton, NJ), and the POA, VMN, and dorsal cortex (CxD) were dissected using a 300-μm diameter micropunch, as per Refs. 28 and 29. Tissue samples were assayed independently of each other and not pooled. The punched tissue was ejected into ice-cold 70 μl homogenization solution: a mixture of 60 μl homogenization buffer (0.1 m Perchloric acid; Sigma-Aldrich, St. Louis, MO) containing 347 μm sodium bisulfate (Sigma-Aldrich) and 134 μm EDTA disodium salt (Fluka Chemical Corp., Milwaukee, WI), and 10 μl 100 nm Epinine-internal standard (Sigma-Aldrich). Tissue samples in homogenization solution were then stored at −80 C overnight and freeze thawed after 24 h. The thawed samples were centrifuged at 14,000 rpm at 4 C for 20 min, after which the supernate was collected and used for HPLC analysis. Protein content in the resulting pellet was determined by resuspending and agitating the pellet in 45 μl ice-cold 0.3 N NaOH for 24 h at 4 C, and performing a modified Bradford assay thereafter (Pierce, Rockford, IL).
HPLC.
Levels of 5-HT and dopamine (DA) in the POA and VMN were determined by HPLC-electrochemical detection using modifications of Ref. 30 with the assistance of Dr. Herng-Hsiang Lo in the Center for Research on Environmental Disease Analytical Instrumentation Facility Core (University of Texas-Austin). In brief, 50 μl sample was injected into an HPLC system that comprised of a Shimadzu SCL-10A system controller, LC-10AD pump, an SIL-10A auto-sampler (Shimadzu, Columbia, MD), and coupled with a four-channel CoulArray electrochemical detector (ESA, Chelmsford, MA). The isocratic mobile phase contains 4 mm citric acid, 8 mm ammonium acetate, 120 μm 1-octanesulfonic acid sodium salt, 60 μm EDTA disodium in water, and 5% MeOH (pH 3.5). The flow rate of the mobile phase remained at 1 ml/min. Separation was achieved by a 4.6 × 80-mm reverse-phase HR-80, 3 μm particle size column (ESA). The potential of channels 1–4 of CoulArray was set at −50, 0, 300, and 400 mV, respectively. Peak area (nC) of neurochemicals at the corresponding retention time on the chromatogram resulted from 300 mV and was used to quantify the amount based on the standard curve of each neurotransmitter. Recovery of internal standard was consistently high across all experimental runs (95–100%), making it unnecessary to correct for recovery of the internal standard. Neurochemical levels were expressed as pg/μg of protein in the microdissected tissue extract.
Tryptophan hydroxylase (TrpH) immunocytochemistry (ICC)
TrpH ICC was conducted on brain tissue obtained from naturally cycling and hormonally manipulated animals that had been fixed in 4% paraformaldehyde after decapitation. Methods for TrpH ICC and quantification were similar to tyrosine hydroxylase ICC described previously in Ref. 31, with the exception that sections were incubated for 2 d with a sheep polyclonal antibody that detects TrpH (AB1541, 1:250; CHEMICON International, Inc., Temecula, CA) and cells visualized using a biotinylated goat antisheep secondary antibody (1:200; Vector Laboratories, Burlingame, CA), avidin-biotin-complex, 3′3-diaminobenzidine HCl system. Sections incubated in the absence of a primary antibody served as controls.
Cloning of C. uniparens 5-HT1A and 5-HT2A receptors, and in situ hybridization
Total RNA extracted from the C. uniparens brain using TRIzol reagent (Invitrogen Corp., Carlsbad, CA) was reverse transcribed using the first-strand cDNA synthesis kit (Invitrogen) and oligo (deoxythymidine) primers. A 77-bp fragment of the 5-HT1A receptor (with 96% identity to rat, mouse, and human 5-HT1A receptor fragments, GenBank accession no. EF628370) and a 155-bp fragment of C. uniparens 5-HT2A receptor (with 95, 93, and 85% identity to rat, mouse, and human 5-HT2A receptor fragments, respectively, GenBank accession no. EF628369) were cloned from this cDNA using a nested primer design and two rounds of PCR. The following primers obtained from Integrated DNA Technologies (Coralville, IA) were used in the PCRs: 5-HT1A, outer primer pair 5′-CTGCAGAACGTGGCCAAYTAYYTNAT-3′, 5′-ACAGGATGAAGGTGCCCATDATDAT-3′, and inner primer pair 5′-CTGGACCGGTACTGGGCNATHAC-3′, 5′-ACAGGATGAAGGTGCCCATDATDAT-3′; and 5-HT2A, outer primer pair 5′-GACATGCTGCTGGGCTTCYTNGTNATGCC-3′, 5′-CACCATGATGGTCAGGGGNAYRAARAA-3′, and inner primer pair 5′-TGGGCATCTCCATGCCNATHCCNGT-3′, 5′-CACCATGATGGTCAGGGGNAYRAARAA-3′.
In situ hybridization was conducted as per Ref. 32. Briefly, 20-μm fresh frozen coronal sections were sectioned on a cryostat and thaw mounted on Superfrost Plus slides. Slides were then fixed in 4% paraformaldehyde, acetylated, and dehydrated before storage at −80 C. Riboprobes specific to the lizard 5-HT1A and 5-HT2A receptors were transcribed from inserts ligated into the TOPO PCRII vector (Invitrogen) using T7 and SP6 RNA polymerases. All cRNA probes were transcribed using 35S-labeled uridine 5′-triphosphate (PerkinElmer, Inc., Wellesley, MA). Slides were incubated for 16–18 h at 55 C with hybridization buffer [50% formamide, 0.6 m NaCl, 10 mm Tris (pH 7.4), 1× Denhardt’s solution, 10 mm dithiothreitol, 250 μg/ml yeast transfer RNA, 10% dextran sulfate, and 50 μg/ml herring sperm DNA] and 35S-uridine 5′-triphosphate labeled receptor-specific riboprobes at a concentration of 1 × 106 cpm/150 μl. After hybridization the tissue was washed in 2× saline sodium citrate (SSC) at RT, treated with RNase A (10 μg/ml) at 37 C for 20 min, followed by stringent washes in decreasing concentrations of SSC, with a final wash in 0.25× SSC at RT. Slides were air-dried and exposed to BioMAX-MR (Eastman Kodak Co., Rochester, NY) for 2 wk. Sense riboprobes did not yield significant hybridization (supplemental Fig. 1, B and C), confirming the specificity of the signal observed with the antisense riboprobes. Based on our controls (sense hybridization, RNase pretreatment), as well as our Dig-labeled in situ hybridization, the receptors do indeed seem to be widely expressed much like in other systems studied [in lizards (33), rats (34), and mice (35)]. 5-HT1A and 5-HT2A receptor mRNA levels were analyzed using Scion Image (Scion Corp., Frederick, MD) after performing in situ hybridization. To correct for nonlinearity, 14C standards were used for calibration purposes. The use of C-14 standards when using 35-S labeled probes is routine practice because of a similar emission spectrum of 35-S and C-14 [in primates (36) and rats (37,38)]. OD measurements using grid sizes of 5 × 5 pixels were obtained from both sides of three to four individual sections from each animal after the specific regions were outlined.
Intracranial injection of 5-HT
Surgeries were performed as per Ref. 39. Two weeks after implantation, OVX plus T animals were tested for pseudosexual behavior for 3 d consecutively. All animals used in subsequent experimentation mounted a receptive stimulus animal on all 3 d and were nonreceptive. On the day of surgery, animals were tested for pseudosexual and nonreproductive behavior. Animals were then anesthetized using hypothermia (Institutional Animal Care and Use Committee protocol no. 07022602). After verification of the absence of flexor reflex in response to limb extension or toe pinch, the animals were mounted into a modified stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). As part of the modification, a small plastic support stick was taped securely to the bite bar. The anesthetized animals were positioned in the stereotaxic apparatus with their upper jaws above the bite bar and its body resting horizontally on the plastic support stick. The animals were covered with ice to maintain hypothermic conditions. A dental drill with a 1-mm round dental burr was used to drill a hole into the animals’ skull based on stereotaxic coordinates (see below). A 30-gauge steel cannula attached to polyethylene tubing was inserted to the desired depth, and the compound was injected in a controlled manner using a syringe pump (Razel Scientific, St. Albans, VT). After the injection, the cannula was slowly withdrawn and the skull hole covered using Gelfoam (Pharmacia Corp., Kalamazoo, MI). Animals were then placed back into home tanks immediately and typically recovered within 10 min. Pseudosexual and nonreproductive behavior was tested 20 min after injection. Animals were killed 20 min after behavioral testing, with the brain being fresh frozen on dry ice. Forty micrometer coronal sections were obtained using a cryostat and examined to verify the site of injection.
A separate group of animals was OVX and injected with 0.5 μg estradiol benzoate (OVX plus E) once daily for 3 d consecutively, 2 wk after ovariectomy. These animals that expressed receptive phenotypes were then injected and treated as described previously.
Animals in this experiment received 1 μl unilateral injections of either 0.9% saline or 10 μg 5-HT creatinine sulfate complex over a period of 5 min. Both compounds were mixed with a 0.25% solution of toluidine blue to visualize the site of injection. Using the point of intersection of the two frontal parietal scales and the interparietal scale as the reference point, injections were targeted at the POA (0.1 mm posterior and 0.3 mm ventral to reference point) in the OVX plus T animals, and at the VMN (0.16 mm posterior and 0.38 mm ventral to reference) in the OVX plus E animals. To ensure that the suppression of mounting in OVX plus T animals was related to signaling at serotonergic receptors, we also injected OVX plus T animals with 1.5 mg/kg methysergide maleate ip (a broad 5-HT receptor antagonist) and then injected saline or 5-HT into the POA of these animals 1 h later.
8-Hydroxy-2-(dipropylamino) tetralin (8-OH-DPAT) and 1-(2,5-dimethoxy-4-iodophenyl)-2- aminopropane (DOI) experiments
Three weeks after surgery, OVX plus T animals were tested for pseudosexual behavior on 3 d consecutively. All animals used in the experiment mounted a receptive stimulus animal on all 3 d and were nonreceptive. On the injection day, experimental animals were first tested for pseudosexual behavior (before drug) and nonreproductive behavior. They were then injected ip either with 0.9% saline or 1 mg/kg 8-OH-DPAT. Animals were then tested for pseudosexual behavior and nonreproductive behavior 20 and 45 min after the injection (20 and 45 min after drug, respectively).
A separate group of animals was OVX and allowed to recover for 2 wk. Baseline behavioral testing resulted in no pseudosexual behavior being exhibited by any of these animals. Animals were then subjected to the same injection paradigm as OVX plus T animals. A week after this injection day, OVX animals were injected with 0.5 μg estradiol benzoate (OVX plus E) once daily for 3 d consecutively. One day later, OVX plus E animals showed receptive behavior but did not mount stimulus animals. OVX plus E animals were then injected with either saline or 8-OH-DPAT and tested as described previously. Drug treatments were the same in animals across OVX and OVX plus E states, i.e. animals injected with 8-OH-DPAT in the OVX state were once again injected with 8-OH-DPAT in the OVX plus E state.
A similar experiment was conducted on OVX, OVX plus T, and OVX plus E animals with 1 mg/kg DOI injected ip. In an independent experiment, animals were pretreated with dimethylsulfoxide (vehicle) or 3 mg/kg ketanserin (a 5-HT2A antagonist) ip 20 min before DOI administration to investigate specifically the role of the 5-HT2A receptor in female-typical receptivity.
Statistical analyses
All statistical analyses were conducted using SPSS v12.0 for Windows (SPSS, Inc., Chicago, IL) with significance set at P < 0.05.
HPLC analysis.
Analysis for each region was achieved by conducting multivariate ANOVAs with 5-HT and DA as the dependent variables and hormone or ovarian state as the independent variable. Tukey honestly significant difference (HSD) values were computed for post hoc comparisons and reported.
In situ hybridization.
For each gene, a univariate ANOVA was conducted using hormonal state as the independent variable, and mRNA level as dependent variables.
5-HT administration into the POA.
A univariate ANOVA was conducted using preinjection mount latency as a covariate, treatment (saline or 5-HT) and placement (in or out of the POA) as independent variables, and the postinjection mount latency as the dependent variable. Tukey HSD was used for the purpose of post hoc comparisons.
5-HT administration into the VMN.
Data were analyzed using a binary logistic regression with treatment, placement, and treatment × placement interaction as predictors of receptivity.
8-OH-DPAT administration.
A split plot ANOVA was used to analyze these data using time (0, 20, and 45 min after injection) and treatment (saline or DPAT) as independent variables, with the latency to mount being the dependent variable. Post hoc analysis was accomplished using Tukey HSD.
DOI administration.
Treatment (saline or DOI) and time (0, 20, and 50 min after injection) were used to predict receptivity by conducting a binary logistic regression.
TrpH ICC.
A univariate ANOVA was used to analyze differences in TrpH-immunoreactive cell numbers in the POA and VMN of OVX plus T and OVX plus E lizards with hormonal manipulation and region as the independent variables, and cell number as the dependent variable.
Results
Neurochemical levels in naturally cycling and hormonally manipulated C. uniparens
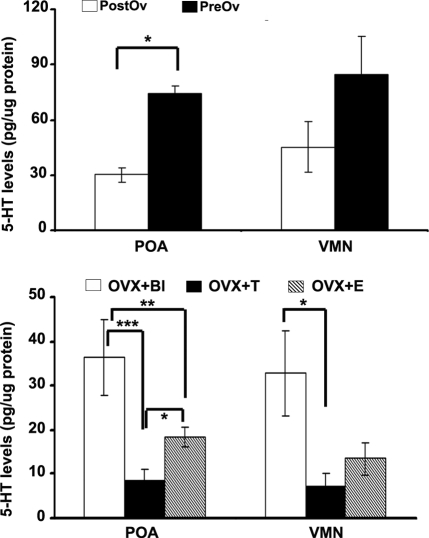
PostOv animals had significantly lower 5-HT levels than PreOv animals in the POA (5-HT: F1,8 = 7.538; P < 0.05) (Fig. 1, top panel). No significant differences in 5-HT levels were observed in the VMN across ovarian states. DA levels were not significantly different between PreOv and PostOv animals in any of the regions examined (DA: P > 0.05).
Figure 1.
5-HT levels vary in the POA of the unisexual lizard (C. uniparens) during the natural cycle (top panel), as well as after hormonal manipulation (bottom panel). Naturally cycling PostOv lizards (n = 5) have lower levels of 5-HT relative to PreOv animals (n = 4) in the POA; a similar pattern is observed in OVX and androgen-implanted lizards (OVX plus T; n = 5) relative to OVX and estradiol-injected animals (OVX plus E; n = 4). OVX lizards (n = 5) have higher 5-HT levels in the POA compared with OVX plus T and OVX plus E animals. No significant differences were detected in the VMN of naturally cycling animals. OVX lizards (n = 5) had significantly higher 5-HT levels than OVX plus T animals in the VMN. Data are expressed as mean ± sem. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
There was a significant effect of hormonal manipulation on 5-HT but not on DA levels in the POA (5-HT: F2,11 = 40.089, P < 0.001; DA: F2,11 = 2.554, P > 0.05). In the POA, OVX plus T animals had significantly lower 5-HT levels than both OVX plus E animals (P < 0.05) and OVX animals (P < 0.001), whereas OVX plus E animals had significantly lower 5-HT levels than OVX controls (P < 0.01) (Fig. 1, bottom panel). In the VMN, hormonal manipulation significantly affected 5-HT but not DA levels (5-HT: F2,11 = 4.440, P < 0.05; DA: F2,11 = 0.616, P > 0.05). OVX plus T animals had significantly lower 5-HT levels than OVX animals (P < 0.05).
5-HT receptor subtype mRNA expression in brain nuclei of naturally cycling and hormonally manipulated C. uniparens
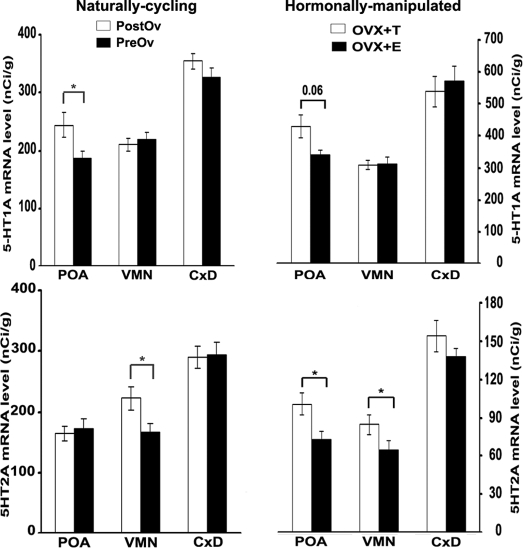
PreOv animals have less 5-HT1A mRNA in the POA compared with PostOv lizards (F1,14 = 5.370, P < 0.05) (supplemental Fig. 1, B and C, and Fig. 2). Similar levels were measured in the CxD and VMN across the ovarian cycle. 5-HT2A mRNA levels in the VMN were significantly lower in the PreOv group compared with PostOv animals (F1,14 = 5.372; P < 0.05) (Fig. 2). Levels in the POA and CxD were unchanged across both groups.
Figure 2.
5-HT receptor subtype mRNA expression in the POA, VMN, and dorsolateral cortex (CxD) vary in the unisexual lizard (C. uniparens) during the natural cycle (left panel), as well as after hormonal manipulation (right panel) as measured by radioactive in situ hybridization using riboprobes specific to lizard 5-HT1A and 5-HT2A receptors. PostOv lizards have significantly more 5-HT1A mRNA in the POA than PreOv animals; OVX and androgen-implanted (OVX plus T) showed the same trend compared with OVX and estradiol-injected lizards (OVX plus E). There are no significant differences in 5-HT1A mRNA levels in the CxD and VMN. PostOv and OVX plus T-implanted animals have more 5-HT2A mRNA in the POA compared with PreOv and OVX plus E-injected lizards, respectively, whereas 5-HT2A mRNA levels in the VMN were significantly higher in the OVX plus T-implanted group compared with OVX plus E-injected animals. No significant differences in 5-HT2A mRNA levels were detected in the CxD. Data are expressed as mean ± sem (n = 7 per group). *, P < 0.05.
5-HT1A mRNA in the POA of OVX plus T-implanted lizards tended to be greater than that found in OVX plus E-injected animals (F1,11 = 4.594; P = 0.06) (Fig. 2). Similar levels were measured in the CxD and VMN across both hormonal groups. 5-HT2A mRNA levels in the POA were greater in OVX plus T-implanted lizards compared with OVX plus E-injected animals (F1,11 = 4.760; P = 0.05) (Fig. 2). In the VMN, 5-HT2A mRNA levels were significantly lower in the OVX plus E-injected group compared with OVX plus T-implanted animals (F1,11 = 5.815; P < 0.05). Levels in the CxD were unchanged across both groups.
Effect of intracranial injection of 5-HT in the POA and VMN
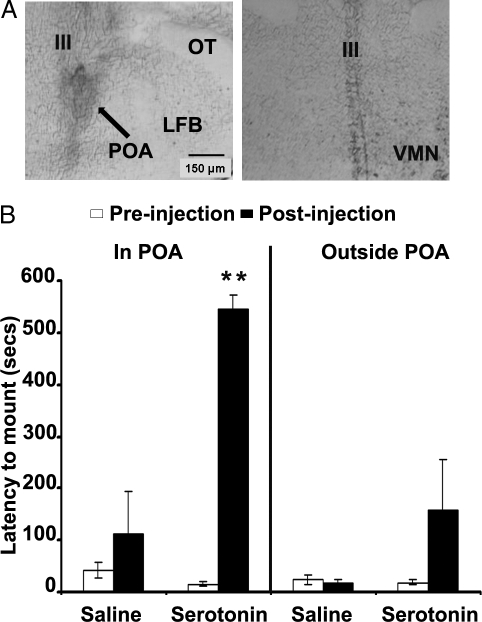
There was a significant interaction between treatment (saline or 5-HT) and placement (within or outside the POA) on the latency to mount a stimulus receptive animal by OVX plus T-implanted lizards (F1,24 = 9.967; P < 0.01) (Fig. 3B). Tukey post hoc analysis indicated that animals injected with 5-HT into the POA took significantly longer to mount the stimulus animals compared with all other groups (P < 0.01). No other significant differences between groups were noted. None of the test animals expressed female-like receptivity. Furthermore, ip injection of methysergide (a broad 5-HT receptor antagonist) 1 h before intracranial injection of 5-HT prevented the suppression of male-like pseudocopulation as observed previously (latency to mount: 545 ± 27, mean seconds ± sem) in four of four OVX plus T animals (latency to mount: 91 ± 31).
Figure 3.
A, Intracranial injection of 5-HT into the POA inhibits mounting behavior of OVX androgen-implanted unisexual C. uniparens lizards. Lizards that were robustly and reliably mounting receptive stimulus animals during baseline tests (n = 29) were injected with either saline or 5-HT (in POA-saline, n = 7 and 5-HT, n = 9; outside POA-saline, n = 7 and 5-HT, n = 6). Top panels are representative photomicrographs of the site of injection (arrow) into the POA (left) but not into the VMN of the same animal (right). B, Only when 5-HT was injected into the POA was the latency to mount the stimulus receptive significantly increased. Data are expressed as mean ± sem. **, P < 0.01. LFB, Lateral forebrain bundle; OT, optic tract; III, third ventricle.
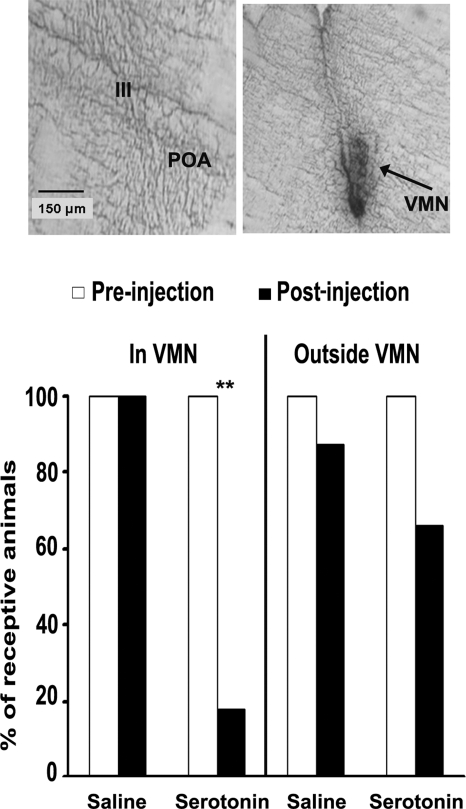
Intracranial injection (saline or 5-HT) was a significant predictor of receptivity in OVX plus E animals after the injection with receptivity being suppressed in a greater percentage of 5-HT injected animals compared with saline-injected controls (X2 = 11.255; P < 0.01; binary logistic regression with treatment, placement, and treatment × placement interaction as predictors of receptivity) (Fig. 4, bottom graph). Examination of the data indicates that receptivity is suppressed by 5-HT injection into the VMN, and not outside this brain nucleus. Male-like pseudocopulation was not exhibited by any of the animals.
Figure 4.
Intracranial injection of 5-HT into the VMN inhibits receptive behavior of OVX estradiol-injected unisexual C. uniparens lizards. Lizards that were robustly and reliably receptive to the mounting behavior of stimulus animals during baseline tests (n = 29) were injected with either saline or 5-HT (in VMN-saline, n = 7 and 5-HT, n = 9; outside VMN-saline, n = 7 and 5-HT, n = 6). Top panels are representative photomicrographs of the site of injection into the VMN (arrow) (right) but not into the POA of the same animal (left). Bottom graph indicates that only when 5-HT was injected into the VMN was the percentage of animals showing receptivity decreased (**, P < 0.01). Data represented as the percentage of animals showing a receptive phenotype. III, Third ventricle.
Effect of a 5-HT1A receptor agonist (8-OH-DPAT) on pseudosexual behavior
Intraperitoneal administration of 8-OH-DPAT to OVX plus T lizards significantly increased the latency to mount stimulus receptive females (F2,32 = 649.68; P < 0.001) (Table 1). Tukey post hoc analysis indicated that OVX plus T animals injected with DPAT took significantly longer to mount the stimulus animals 20 min after drug administration compared with all other groups (P < 0.001). No other significant differences between groups were noted. 8-OH-DPAT did not affect the receptivity exhibited by OVX plus E lizards (data not shown).
Table 1.
Stimulation of 5-HT2A receptors by DOI stimulates receptive behavior, in OVX, androgen-treated (OVX plus T) C. uniparens: 8-OH-DPAT, dependent variable, latency to mount (sec)
| Saline
|
DPAT
|
||||
|---|---|---|---|---|---|
| Before drug | 20 min after drug | 45 min after drug | Before drug | 20 min after drug | 45 min after drug |
| 10.11 ± 1.78 | 10.89 ± 1.81 | 9.22 ± 1.27 | 8.11 ± 1.35 | 578.33 ± 21.6a | 14.11 ± 3.38 |
Hormonally manipulated lizards (OVX plus T) were injected ip either with 0.9% saline or 1 mg/kg 8-OH-DPAT (n = 9 per group), and their latency to mount was noted before (before drug), 20 min after injection (20 min after drug), or 45 min after injection (45 min after drug). Data are represented as mean ± sem. Lizards took significantly longer to mount a receptive stimulus animal 20 min after 8-OH-DPAT injection and returned to baseline mount latencies 45 min after injection.
P < 0.001.
Effect of a 5-HT2A/2C receptor agonist (DOI) on pseudosexual behavior
The administration of DOI elicited a receptive phenotype in OVX plus T animals 20 min after drug administration, with animals returning to a nonreceptive state 50 min after injection (X2 = 12.713; P < 0.001) (Table 2). Pretreatment with dimethylsulfoxide and DOI 10 min later elicited receptivity in 75% of OVX plus T animals 20 min after drug administration, whereas pretreatment with ketanserin (3 mg/kg, ip) followed by DOI resulted in none of the OVX plus T animals being receptive. DOI administration had no effect on male-like pseudocopulation in OVX plus T-implanted animals. The receptivity already observed in OVX plus E-injected animals was unaffected by DOI administration (data not shown).
Table 2.
Stimulation of 5-HT2A receptors by DOI stimulates receptive behavior, in OVX, androgen-treated (OVX plus T) C. uniparens: DOI, dependent variable, percentage (%) of receptive animals
| Saline
|
DOI
|
||||
|---|---|---|---|---|---|
| Before drug | 20 min after drug | 50 min after drug | Before drug | 20 min after drug | 50 min after drug |
| 0 | 0 | 0 | 0 | 75 | 0 |
Androgen-treated lizards (OVX plus T) were injected ip either with 0.9% saline or 1 mg/kg DOI (n = 8 per group), and receptivity was noted before (before drug), 20 min after injection (20 min after drug), or 50 min after injection (50 min after drug). Previously nonreceptive OVX plus T lizards demonstrated receptivity 20 min after DOI injection and returned to a nonreceptive state 50 min after injection (P < 0.001). X2 = 12.713; P < 0.001.
Discussion
By definition, in all vertebrates, mating behaviors are complementary, with males mounting receptive females. Although these behaviors emphasize the sexual differentiation of the brain and behavior, male receptivity and female mounting occur normally in many species (1,40,41). Such observations illustrate that even the adult brain retains its bisexual nature in each individual. The observation that during a reproductive encounter, one behavior is facilitated in an individual, whereas its complement is simultaneously suppressed, suggests a reciprocal inhibition that necessitates gating of behavioral repertoires. Our work demonstrates that serotonergic neurotransmission gates the reciprocal inhibition that accompanies mounting and receptive behaviors. We propose that this gating mechanism involves the alteration of 5-HT and 5-HT receptor subtype mRNA levels by the hormonal milieu at the POA and VMN, and signaling via distinct receptor subtypes at these brain nuclei. Low 5-HT levels in the POA of PostOv and OVX plus T animals create a neurochemical environment that is permissive for mounting to be expressed, resulting in male-like pseudosexual behavior being disinhibited, whereas high 5-HT levels in the POA of PreOv and OVX plus E animals result in the inhibition of male-like mounting. It is important to note that the VMN does not appear to be as crucial a node in gating the behavioral state, but in contrast, when serotonergic dynamics at the POA facilitate a male-like pseudosexual phenotype, the female-like receptive phenotype is suppressed by an as yet unknown mechanism. Central to this model are the fluctuations in 5-HT level at the POA, and the subsequent signaling via specific 5-HT receptors.
Neurotransmitter activity in the POA and VMN is critical to the mediation of sexual behavior in other species. In rats, dopaminergic activity in the POA gates the expression of mounting behavior in males, with low extracellular DA levels in the POA characteristic of noncopulating male rats compared with copulating males (42). 5-HT inhibits sexual behavior in both males and females across a variety of mammalian species (43,44,45). Inhibition of 5-HT synthesis by para-chlorophenylalanine increases mounting behavior in both OVX rats, as well as those OVX and implanted with T and E (3,46,47,48). In OVX, E-primed female rats, para-chlorophenylaline increases the lordosis quotient (49). In addition, the destruction of 5-HT neurons by administration of the 5-HT-selective neurotoxin 5,7-DHT into the VMN of OVX and E-implanted female rats induces greater receptivity compared with controls (50). The injection of 5-HT into the POA of male rats suppresses mounting (51), whereas 5-HT injection into the VMN of hormonally primed female rats inhibits lordosis (52). Thus, the literature not only suggests that 5-HT is involved in sex-typical sexual behavior (receptivity) but also in heterotypical sexual behavior (mounting) in the female rat. Related to the idea of gating sexually dimorphic states is the observation that serotonergic neurotransmission at the amygdala, raphe, and POA underlies behavioral sex reversal in the saddleback wrasse (25). Our data are consistent with these findings, and suggest that serotonergic modulation of behavior at the POA and VMN is evolutionarily conserved. The inhibitory effect of 5-HT and signaling via serotonergic receptors on male-like mounting is validated by the short latency of OVX plus T lizards pretreated with methysergide (a broad 5-HT receptor antagonist) and then injected with 5-HT into the POA to mount a stimulus receptive.
Behavioral transitions are usually associated with hormonal transitions, which in turn alter neurotransmitter levels. Hypothalamic 5-HT levels are lower in proestrus female rats than diestrus females as well as intact male rats (53,54,55), with this reduction coinciding with the period of maximal receptivity. In contrast, reduced 5-HT levels in the anterior hypothalamus of male compared with female rats in response to prenatal androgen administration are thought to be correlated with the propensity to display male-typical sexual behavior by the males (56). The administration of estradiol and progesterone also alter 5-HT content in hypothalamic nuclei in female rats (57). Correlations between preoptic aromatase activity, fluctuations in monoamine concentrations, and sexual behavior have also been documented in the male Japanese quail (58). OVX lizards do not show either form of pseudosexual behavior, an observation probably related to the high 5-HT levels in both the POA and VMN of OVX lizards. The observed decreases in 5-HT levels in the POA and VMN by hormonal manipulation (OVX plus T and OVX plus E) present one avenue by which hormones alter the threshold for behaviors to be expressed. Our intracranial surgery data and HPLC analysis indicate that fluctuations in 5-HT levels at the POA and VMN as brought about by the circulating hormonal milieu serve to regulate the expression or suppression of male- and female-like pseudosexual behavior, respectively.
One potential explanation for the fluctuation in 5-HT levels across the ovarian and hormonal states might be differences in the number of 5-HT synthesizing neurons in the POA and VMN. TrpH ICC (supplemental Fig. 1A) did not reveal any differences in the number of 5-HT-synthesizing TrpH immunoreactive cell numbers in the POA or VMN of either the naturally cycling (PostOv, n = 8; PreOv, n = 7) or hormonally manipulated animals (OVX plus T, n = 7; OVX plus E, n = 5) (supplemental Fig. 2). Alternatively, the differing 5-HT levels might result from the differential amount of activation of serotonergic neurons in specific brain nuclei, a possibility that warrants further analysis.
5-HT acts via signaling through a diverse family of receptors, including the 5-HT1A and 5-HT2A receptors. Signaling via the 5-HT1A receptor typically facilitates male-typical sexual behavior, whereas 5-HT2A receptor stimulation is associated with an increase in female-typical receptivity (24,59,60,61). Receptor autoradiography reveals both receptor subtypes in the brain of the green anole lizard, Anolis carolinensis (33); 8-OH-DPAT and DOI bind specifically to 5-HT1A and 5-HT2A/2C receptors and have behavioral effects in a reptile (62). It is important to note that the 5-HT1A receptor-mediated facilitation of male sexual behavior in rats and primates occurs at the level of intromission and ejaculation, whereas the number of mounts is actually decreased (63). The absence of hemipenes in C. uniparens obviates any effect on intromission and ejaculation, and the effect of 8-OH-DPAT on increasing mount latency is consistent with the rat and primate literature. Similarly, the facilitation of receptivity through signaling via the 5-HT2A receptor is in agreement with the mammalian literature, and corroborated by the inhibition of DOI-induced receptivity in C. uniparens by pretreatment with the 5-HT2A receptor antagonist ketanserin.
Another mechanism by which hormones affect neurotransmitter dynamics is by altering mRNA and protein levels of specific receptor subtypes. Differences in 5-HT receptor subtype levels and their activity in OVX, hormonally manipulated female rats are thought to underlie the expression of receptivity or the lack thereof (64). We find that steroid hormones alter serotonergic receptor subtype mRNA levels in the POA and VMN of C. uniparens, and this might be responsible for the form of behavior expressed. These observations are similar to the hypothesis that dopaminergic signaling via D1 or D2 receptors mediates distinct aspects of male rat sexual behavior (65), as well as 5-HT1A and 5-HT2A receptor activities, respectively, mediating an inhibitory and facilitatory role of 5-HT on receptivity in female rats (66).
Having determined the involvement of 5-HT in facilitating and suppressing the complementary behaviors in C. uniparens, we are now in a position to investigate further the interaction between the ovarian hormonal milieu and 5-HT. For example, are steroid receptors present in 5-HT synthesizing neurons as observed in nonhuman primates and rodents (67,68,69), and could they affect processes within these neurons to mediate behavior? Given that interactions between nitric oxide and 5-HT are known to contribute to aggression in mice (70), and 5-HT and DA interact to affect sexual behavior in male rats (71), it would be interesting to examine the same interaction in the whiptail lizard, in light of the demonstrated roles of nitric oxide and DA in mediating sexual behavior in Cnemidophorus lizards (72,73).
The gating of sex-typical behavior has been attributed to both peripheral and central mechanisms. For example, Trpc2 channels in the vomeronasal organ appear to be involved in the suppression of mounting behavior in female mice (74), and in rats light acting via the suprachiasmatic nucleus (the neural generator of circadian rhythms) may be responsible for the appropriate display of sex-typical behaviors (75,76). However, usually when mention is made of the complementarity of sexual behavior, the gating of behavioral expression is attributed to independently acting mechanisms at the POA (mounting) and VMN (receptivity). Our study suggests that serotonergic neurotransmission allows for male-typical mounting and female-typical receptivity to be gated by the appropriate hormonal state, and social context within and between interacting individuals. Social behaviors exhibited by males and females share common neural networks (77), and our data suggest that it is the neurochemistry at the POA and VMN, in concert with the stimulus partner’s qualities, that dictates behavioral output.
The present study not only sheds light on the mechanisms underlying male-typical mounting and female-typical receptivity but also extends our understanding of how brain mechanisms controlling sexually dimorphic behavioral traits may have evolved. By focusing on the neural substrates underlying sex-typical behaviors in a parthenogen, we avoid the confounding factors of genotype and endocrine history inherent in studies with gonochoristic species with genotypic sex determination (9). Sexual behavior in genetic male Cnemidophorus inornatus (the sexual ancestor) is dependent upon testicular androgens, although a polymorphism exists in sensitivity to progesterone, with exogenous progesterone inducing the full suite of sexual behavior in about a third of castrated male C. inornatus. This is presumed to be the basis of the novel neuroendocrine mechanism controlling male-like pseudosexual behavior in the descendant C. uniparens, which is normally initiated by the PostOv progesterone. Although no detectable levels of androgen have ever been measured in the circulatory system of unisexual lizards, they have androgen receptor in the same brain areas as the ancestral species and are extremely sensitive to androgen, showing a robust mounting response when implanted with T. By taking advantage of the conserved nature of male-typical mounting as triggered robustly by T, we are able to focus on how the neurochemistry at specific brain nuclei interact in a circuit to elicit normally sex-typical behaviors independent of the nature of the hormonal trigger. The similarities in neurochemical level and receptor mRNA profiles in PostOv animals and OVX plus T lizards allow us to postulate overarching commonalities in the mechanisms gating the expression of mounting behavior.
The findings reported in this study also speak to how sexually differentiated traits might occur. The canonical concept that the female sex is the default or neutral sex, and males are the organized sex has proved overly simplistic (2). Research on sex determination in humans, transgenic mice, and species lacking sex chromosomes reveals that both sexes are organized, the difference lying in the patterns of expression of gonad-differentiating genes (5,78,79,80). That is, the differentiation of the primordial gonads into a testicular or an ovarian phenotype depends upon a common conserved gene network, with sex specificity lying in the trigger (genotypic such as Sry in mammals or environmental such as temperature in turtles) that modulates the patterns of expression of genes of this gonad-differentiating network.
Given that the first “sex” was female, and E receptor is the most ancestral sex steroid hormone receptor (81,82), it is more appropriate to consider the female and E as ancestral, and the male and androgen as derived, states. Although the concept of ovarian development as the ancestral pathway, with certain triggers sculpting testicular development, has been the subject of extensive investigation, this idea has seldom been discussed within the framework of sexual behavior. This alternate concept of the female as the ancestral, and the male as the derived, sex (5) maintains the element of the “male phenotype” being imposed on what otherwise would be a “female phenotype” but extends research in new theoretical directions. If in fact males are the derived sex, it follows that males may be more like females than females are like males (83). Previously, several lines of evidence were offered to support this idea, such as the relative ease of masculinizing animals compared with the difficulty of defeminizing animals and the resurrection of males in parthenogenetic whiptails (84,85), indicating that the genes of male traits are present in this all-female species. It is widely accepted that in gonochoristic species, both males and females possess the neuronal circuitry and molecular pathways to mediate both mounting and receptivity, with one behavioral trajectory being suppressed as a consequence of sexual differentiation. A novel perspective from the present data lies in the implication that being receptive might be the ancestral reproductive behavior of vertebrates, and the evolution of males and their attendant male-typical behavior required the masking of this receptive phenotype. To our knowledge, this is the first demonstration that a single molecule regulates the simultaneous complementarity of sex-typical behaviors within the same sex, and sheds light on the mechanisms and potential evolution by which homotypical and heterotypical sex behaviors might be balanced within a sex.
Supplementary Material
Acknowledgments
We thank three anonymous reviewers, and Dr. Andrea Gore, Dr. Hans Hofmann, and Sunayana Banerjee for helpful comments regarding the manuscript. Animals caught in the Sonoran desert were temporarily housed at the Southwestern Research Station (Portal, AZ) before being transported to the University of Texas at Austin.
Footnotes
This work was supported by a grant from the National Institute of Mental Health (MH41770) (to D.C.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online May 15, 2008
Abbreviations: CxD, Dorsal cortex; DA, dopamine; DOI, 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane; E, estrogen; HSD, honestly significant difference; 8-OH-DPAT, 8-hydroxy-2-(dipropylamino) tetralin; ICC, immunocytochemistry; OVX, ovariectomized; POA, preoptic area; PostOv, postovulatory; PreOv, preovulatory; 5-HT, serotonin; SSC, standard saline solution; T, testosterone; TrpH, tryptophan hydroxylase; VMN, ventromedial nucleus of the hypothalamus.
References
- Beach FA 1938 Sex reversals in the mating pattern of the rat. J Genet Psychol 53:329–334 [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC 1959 Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65:369–382 [DOI] [PubMed] [Google Scholar]
- Sodersten P, Larsson K, Ahlenius S, Engel J 1976 Stimulation of mounting behavior but not lordosis behavior in ovariectomized female rats by p-chlorophenylalanine. Pharmacol Biochem Behav 5:329–333 [DOI] [PubMed] [Google Scholar]
- Lumia AR, Meisel RL, Sachs BD 1981 Induction of female and male mating patterns in female rats by gonadal steroids: effects of neonatal or adult olfactory bulbectomy. J Comp Physiol Psychol 95:497–509 [DOI] [PubMed] [Google Scholar]
- Crews D 1993 The organizational concept and vertebrates without sex chromosomes. Brain Behav Evol 42:202–214 [DOI] [PubMed] [Google Scholar]
- Bass AH, Grober MS 2001 Social and neural modulation of sexual plasticity in teleost fish. Brain Behav Evol 57:293–300 [DOI] [PubMed] [Google Scholar]
- Conant R, Collins JT 2003 Reptiles and amphibians: Eastern and Central North America. New York: Houghton Mifflin Co. [Google Scholar]
- Crews D, Fitzgerald KT 1980 “Sexual” behavior in parthenogenetic lizards (Cnemidophorus). Proc Natl Acad Sci USA 77:499–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D 2005 Evolution of neuroendocrine mechanisms that regulate sexual behavior. Trends Endocrinol Metab 16:354–361 [DOI] [PubMed] [Google Scholar]
- Crews D, Grassman M, Lindzey J 1986 Behavioral facilitation of reproduction in sexual and unisexual whiptail lizards. Proc Natl Acad Sci USA 83:9547–9550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade J, Crews D 1991 The relationship between reproductive state and “sexually” dimorphic brain areas in sexually reproducing and parthenogenetic whiptail lizards. J Comp Neurol 309:507–514 [DOI] [PubMed] [Google Scholar]
- Kingston PA, Crews D 1994 Effects of hypothalamic lesions on courtship and copulatory behavior in sexual and unisexual whiptail lizards. Brain Res 643:349–351 [DOI] [PubMed] [Google Scholar]
- Rand MS, Crews D 1994 The bisexual brain: sex behavior differences and sex differences in parthenogenetic and sexual lizards. Brain Res 663:163–167 [DOI] [PubMed] [Google Scholar]
- Wennstrom KL, Blesius F, Crews D 1999 Volumetric analysis of sexually dimorphic limbic nuclei in normal and sex-reversed whiptail lizards. Brain Res 838:104–109 [DOI] [PubMed] [Google Scholar]
- Hull EM, Lorrain DS, Du J, Matuszewich L, Lumley LA, Putnam SK, Moses J 1999 Hormone-neurotransmitter interactions in the control of sexual behavior. Behav Brain Res 105:105–116 [DOI] [PubMed] [Google Scholar]
- Summers CH 2001 Mechanisms for quick and variable responses. Brain Behav Evol 57:283–292 [DOI] [PubMed] [Google Scholar]
- Lowry CA, Moore FL 2006 Regulation of behavioral responses by corticotropin-releasing factor. Gen Comp Endocrinol 146:19–27 [DOI] [PubMed] [Google Scholar]
- Huber R, Orzeszyna M, Pokorny N, Kravitz EA 1997 Biogenic amines and aggression: experimental approaches in crustaceans. Brain Behav Evol 50:60–68 [DOI] [PubMed] [Google Scholar]
- Woodley SK, Matt KS, Moore MC 2000 Estradiol modulation of central monoamine activity in female mountain spiny lizards. Brain Behav Evol 56:175–183 [DOI] [PubMed] [Google Scholar]
- Summers CH, Summers TR, Moore MC, Korzan WJ, Woodley SK, Ronan PJ, Höglund E, Watt MJ, Greenberg N 2003 Temporal patterns of limbic monoamine and plasma corticosterone response during social stress. Neuroscience 116:553–563 [DOI] [PubMed] [Google Scholar]
- Raleigh MJ, McGuire MT, Brammer GL, Pollack DB, Yuwiler A 1991 Serotonergic mechanisms promote dominance acquisition in adult male vervet monkeys. Brain Res 559:181–190 [DOI] [PubMed] [Google Scholar]
- Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Balthazart J 2006 Estradiol rapidly activates male sexual behavior and affects brain monoamine levels in the quail brain. Behav Brain Res 166:110–123 [DOI] [PubMed] [Google Scholar]
- Hull EM, Muschamp JW, Sato S 2004 Dopamine and serotonin: influences on male sexual behavior. Physiol Behav 83:291–307 [DOI] [PubMed] [Google Scholar]
- Uphouse L 2000 Female gonadal hormones, serotonin, and sexual receptivity. Brain Res Brain Res Rev 33:242–257 [DOI] [PubMed] [Google Scholar]
- Larson ET, Norris DO, Summers CH 2003 Monoaminergic changes associated with socially induced sex reversal in the saddleback wrasse. Neuroscience 119:251–263 [DOI] [PubMed] [Google Scholar]
- Dias BG, Crews D 2006 Serotonergic modulation of male-like pseudocopulatory behavior in the parthenogenetic whiptail lizard, Cnemidophorus uniparens. Horm Behav 50:401–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley SC, Crews D 2004 Species differences in the regulation of tyrosine hydroxylase in Cnemidophorus whiptail lizards. J Neurobiol 60:360–368 [DOI] [PubMed] [Google Scholar]
- Smeets WJ, Steinbusch HW 1988 Distribution of serotonin immunoreactivity in the forebrain and midbrain of the lizard Gekko gecko. J Comp Neurol 271:419–434 [DOI] [PubMed] [Google Scholar]
- Young LJ, Lopreato GF, Horan, K, Crews D 1994 Cloning and in situ hybridization analysis of estrogen receptor, progesterone receptor, and androgen receptor expression in the brain of whiptail lizards (Cnemidophorus uniparens and C. inornatus). J Comp Neurol 347:288–300 [DOI] [PubMed] [Google Scholar]
- Bai F, Lau SS, Monks TJ 1999 Glutathione and N-acetylcysteine conjugates of α-methyldopamine produce serotonergic neurotoxicity: possible role in methylenedioxyamphetamine-mediated neurotoxicity. Chem Res Toxicol 12:1150–1157 [DOI] [PubMed] [Google Scholar]
- Dias BG, Ataya RS, Rushworth D, Zhao J, Crews D 2007 Effect of incubation temperature and androgens on dopaminergic activity in the leopard gecko, Eublepharis macularius. Dev Neurobiol 67:630–636 [DOI] [PubMed] [Google Scholar]
- Dias BG, Banerjee BS, Duman RS, Vaidya VA 2003 Differential regulation of brain derived neurotrophic factor transcripts by antidepressant treatments in the adult rat brain. Neuropharmacology 45:553–563 [DOI] [PubMed] [Google Scholar]
- Clark EC, Baxter Jr LR 2000 Mammal-like striatal functions in Anolis. I. Distribution of serotonin receptor subtypes, and absence of striosome and matrix organization. Brain Behav Evol 56:235–248 [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Mondal AC, Payappagoudar GV, Rizavi HS 2005 Differential regulation of serotonin (5HT)2A receptor mRNA and protein levels after single and repeated stress in rat brain: role in learned helplessness behavior. Neuropharmacology 48:204–214 [DOI] [PubMed] [Google Scholar]
- Schiller L, Donix M, Jähkel M, Oehler J 2006 Serotonin 1A and 2A receptor densities, neurochemical and behavioural characteristics in two closely related mice strains after long-term isolation. Prog Neuropsychopharmacol Biol Psychiatry 30:492–503 [DOI] [PubMed] [Google Scholar]
- Patel PD, Katz M, Karssen AM, Lyons DM 2008 Stress-induced changes in corticosteroid receptor expression in primate hippocampus and prefrontal cortex. Psychoneuroendocrinology 33:360–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Broide RS, Chen Y, Leslie FM 1999 Highly sensitive radioactive in situ hybridization using full length hydrolyzed riboprobes to detect α2 adrenoceptor subtype mRNAs in adult and developing rat brain. Brain Res Brain Res Protoc 3:229–241 [DOI] [PubMed] [Google Scholar]
- Vaidya VA, Marek GJ, Aghajanian GK, Duman RS 1997 5-HT2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. J Neurosci 17:2785–2795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo ML, Crews D 1987 Neural control of male-like pseudocopulatory behavior in the all-female lizard, Cnemidophorus uniparens: effects of intracranial implantation of dihydrotestosterone. Horm Behav 21:181–192 [DOI] [PubMed] [Google Scholar]
- Beach FA, Rasquin P 1942 Masculine copulatory behavior in intact and castrated female rats. Endocrinology 31:393–409 [Google Scholar]
- Bagemihl B 1999 Biological exuberance. New York: St. Martin’s Press [Google Scholar]
- Hull EM, Du J, Lorrain DS, Matuszewich L 1995 Extracellular dopamine in the medial preoptic area: implications for sexual motivation and hormonal control of copulation. J Neurosci 15:7465–7471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlenius S, Larsson K, Svensson L 1980 Further evidence for an inhibitory role of central 5-HT in male rat sexual behavior. Psychopharmacology 68:217–220 [DOI] [PubMed] [Google Scholar]
- Bitran D, Hull EM 1987 Pharmacological analysis of male rat sexual behavior. Neurosci Biobehav Rev 11:365–389 [DOI] [PubMed] [Google Scholar]
- Gorzalka BB, Mendelson SD, Watson NV 1990 Serotonin receptor subtypes and sexual behavior. Ann NY Acad Sci 600:435–444 [DOI] [PubMed] [Google Scholar]
- Emery DE, Sachs BD 1976 Hormonal and monoaminergic influences on masculine copulatory behavior in the female rat. Horm Behav 7:341–352 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sierra JF, Naggar AN, Komisaruk BR 1976 Monoaminergic mediation of masculine and feminine copulatory behavior in female rats. Pharmacol Biochem Behav 5:457–463 [DOI] [PubMed] [Google Scholar]
- van de Poll NE, van Dis H, Bermond B 1977 The induction of mounting behavior in female rats by p-chlorophenylalanine. Eur J Pharmacol 41:225–229 [DOI] [PubMed] [Google Scholar]
- Zemlan FP, Ward IL, Crowley WR, Margules DL 1973 Activation of lordotic responding in female rats by suppression of serotonergic activity. Science 179:1010–1011 [DOI] [PubMed] [Google Scholar]
- Frankfurt M, Renner K, Azmitia E, Luine V 1985 Intrahypothalamic 5,7-dihydroxytryptamine: temporal analysis of effects on 5-hydroxytryptamine content in brain nuclei and on facilitated lordosis behavior. Brain Res 340:127–133 [DOI] [PubMed] [Google Scholar]
- Verma S, Chhina GS, Mohan Kumar V, Singh B 1989 Inhibition of male sexual behavior by serotonin application in the medial preoptic area. Physiol Behav 46:327–330 [DOI] [PubMed] [Google Scholar]
- Foreman MM, Moss RL 1978 Role of hypothalamic serotonergic receptors in the control of lordosis behavior in the female rat. Horm Behav 10:97–106 [DOI] [PubMed] [Google Scholar]
- James MD, Hole DR, Wilson CA 1989 Differential involvement of 5-hydroxytryptamine (5HT) in specific hypothalamic areas in the mediation of steroid-induced changes in gonadotrophin release and sexual behaviour in female rats. Neuroendocrinology 49:561–569 [DOI] [PubMed] [Google Scholar]
- Gundlah C, Simon LD, Auerbach SB 1998 Differences in hypothalamic serotonin between estrous phases and gender: an in vivo microdialysis study. Brain Res 785:91–96 [DOI] [PubMed] [Google Scholar]
- Maswood S, Truitt W, Hotema M, Caldarola-Pastuszka M, Uphouse L 1999 Estrous cycle modulation of extracellular serotonin in mediobasal hypothalamus: role of the serotonin transporter and terminal autoreceptors. Brain Res 831:146–154 [DOI] [PubMed] [Google Scholar]
- Borisova NA, Proshlyakova EV, Sapronova AY, Ugrumov MV 1996 Androgen-dependent sex differences in the hypothalamic serotoninergic system. Eur J Endocrinol 134:232–235 [DOI] [PubMed] [Google Scholar]
- Lu H, Yuri K, Ito T, Yoshimoto K, Kawata M 1998 The effects of oestrogen and progesterone on serotonin and its metabolite in the lateral septum, medial preoptic area and ventromedial hypothalamic nucleus of female rats. J Neuroendocrinol 10:919–926 [DOI] [PubMed] [Google Scholar]
- Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Dejace C, Ball GF, Balthazart J 2005 Rapid decreases in preoptic aromatase activity and brain monoamine concentrations after engaging in male sexual behavior. Endocrinology 146:3809–3820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez MI, Farabollini F, Albonetti E, Wilson CA 1994 Interactions between 5-hydroxytryptamine (5-HT) and testosterone in the control of sexual and nonsexual behaviour in male and female rats. Pharmacol Biochem Behav 47:591–601 [DOI] [PubMed] [Google Scholar]
- Morali G, Larsson K 1984 Differential effects of a new serotoninomimetic drug, 8-OH-DPAT, on copulatory behavior and pelvic thrusting pattern in the male rat. Pharmacol Biochem Behav 20:185–187 [DOI] [PubMed] [Google Scholar]
- Bethea CL, Lu NZ, Gundlah C, Streicher JM 2002 Diverse actions of ovarian steroids in the serotonin neural system. Front Neuroendocrinol 23:41–100 [DOI] [PubMed] [Google Scholar]
- Baxter Jr LR, Clark EC, Ackermann RF, Lacan G, Melega WP 2001 Brain mediation of Anolis social dominance displays. II. Differential forebrain serotonin turnover, and effects of specific 5-HT receptor agonists. Brain Behav Evol 57:184–201 [DOI] [PubMed] [Google Scholar]
- Fernandez-Guasti A, Escalante AL, Ahlenius S, Hillegaart V, Larsson K 1992 Stimulation of 5-HT1A and 5-HT1B receptors in brain regions and its effects on male rat sexual behaviour. Eur J Pharmacol 210:121–129 [DOI] [PubMed] [Google Scholar]
- Gonzalez MI, Greengrass P, Russell M, Wilson CA 1997 Comparison of serotonin receptor numbers and activity in specific hypothalamic areas of sexually active and inactive female rats. Neuroendocrinology 66:384–392 [DOI] [PubMed] [Google Scholar]
- Dominguez JM, Hull EM 2005 Dopamine, the medial preoptic area, and male sexual behavior. Physiol Behav 86:356–368 [DOI] [PubMed] [Google Scholar]
- Mendelson SD, Gorzalka BB 1985 A facilitatory role for serotonin in the sexual behavior of the female rat. Pharmacol Biochem Behav 22:1025–1033 [DOI] [PubMed] [Google Scholar]
- Bethea CL 1993 Colocalization of progestin receptors with serotonin in raphe neurons of macaque. Neuroendocrinology 57:1–6 [DOI] [PubMed] [Google Scholar]
- Gundlah C, Lu NZ, Mirkes SJ, Bethea CL 2001 Estrogen receptor β (ERβ) mRNA and protein in serotonin neurons of macaques. Brain Res Mol Brain Res 91:14–22 [DOI] [PubMed] [Google Scholar]
- Alves SE, Weiland NG, Hayashi S, McEwen BS 1998 Immunocytochemical localization of nuclear estrogen receptors and progestin receptors within the rat dorsal raphe nucleus. J Comp Neurol 391:322–334 [PubMed] [Google Scholar]
- Chiavegatto S, Dawson VL, Mamounas LA, Koliatsos VE, Dawson TM, Nelson RJ 2001 Brain serotonin dysfunction accounts for aggression in male mice lacking neuronal nitric oxide synthase. Proc Natl Acad Sci USA 98:1277–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain DS, Riolo JV, Matuszewich L, Hull EM 1999 Lateral hypothalamic serotonin inhibits nucleus accumbens dopamine: implications for sexual satiety. J Neurosci 19:7648–7652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson NS, Weissler E, Crews D 2005 The nitric oxide synthase inhibitor L-NAME suppresses androgen-induced male-like pseudocopulatory behavior in whiptail lizards. Brain Res 1052:236–239 [DOI] [PubMed] [Google Scholar]
- Woolley SC, Sakata JT, Crews D 2004 Tyrosine hydroxylase expression is affected by sexual vigor and social environment in male Cnemidophorus inornatus. J Comp Neurol 476:429–439 [DOI] [PubMed] [Google Scholar]
- Kimchi T, Xu J, Dulac C 2007 A functional circuit underlying male sexual behaviour in the female mouse brain. Nature 448:1009–10014 [DOI] [PubMed] [Google Scholar]
- Sodersten P, Hansen S, Srebro B 1981 Suprachiasmatic lesions disrupt the daily rhythmicity in the sexual behaviour of normal male rats and of male rats treated neonatally with antioestrogen. J Endocrinol 88:125–130 [DOI] [PubMed] [Google Scholar]
- Sodersten P 1984 Sexual differentiation: do males differ from females in behavioral sensitivity to gonadal hormones? Prog Brain Res 61:257–270 [DOI] [PubMed] [Google Scholar]
- Newman SW 1999 The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann NY Acad Sci 877:242–257 [DOI] [PubMed] [Google Scholar]
- Crews D 1996 Temperature-dependent sex determination: the interplay of steroid hormones and temperature. Zoolog Sci 13:1–13 [DOI] [PubMed] [Google Scholar]
- Shoemaker CM, Queen J, Crews D 2007 Response of candidate sex-determining genes to changes in temperature reveals their involvement in the molecular network underlying temperature-dependent sex determination. Mol Endocrinol 21:2750–2763 [DOI] [PubMed] [Google Scholar]
- Brennan J, Capel B 2004 One tissue, two fates: molecular genetic events that underlie testis versus ovary development. Nat Rev Genet 5:509–521 [DOI] [PubMed] [Google Scholar]
- Thornton JW 2001 Evolution of vertebrate steroid receptors from an ancestral estrogen receptor by ligand exploitation and serial genome expansions. Proc Natl Acad Sci USA 98:5671–5676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton JW, Need E, Crews D 2003 Ancient origin of steroid receptors and reconstitution of the ancestral receptor. Science 301:1714–1717 [DOI] [PubMed] [Google Scholar]
- Crews D 1998 The evolutionary antecedents of love. Psychoneuroendocrinology 23:751–764 [DOI] [PubMed] [Google Scholar]
- Wibbels T, Crews D 1994 Putative aromatase inhibitor induces male sex determination in a female unisexual lizard and in a turtle with temperature-dependent sex determination. J Endocrinol 141:295–299 [DOI] [PubMed] [Google Scholar]
- Wennstrom KL, Crews D 1995 Making males from females: The effects of aromatase inhibitors on a parthenogenetic species of whiptail lizard. Gen Comp Endocrinol 99:316–322 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.