Abstract
Rationale
The development of sensitization to amphetamine (AMPH) is dependent on increases in excitatory outflow from the medial prefrontal cortex (mPFC) to subcortical centers. These projections are clearly important for the progressive enhancement of the behavioral response during drug administration that persists through withdrawal.
Objectives
The objective of this study was to identify the mPFC subcortical pathway(s) activated by a sensitizing regimen of AMPH.
Methods
Using retrograde labeling techniques, Fos activation was evaluated in the predominant projection pathways of the mPFC of sensitized rats following a challenge injection of AMPH.
Results
There was a significant increase in Fos-immunoreactive cells in the mPFC, nucleus accumbens (NAc), basolateral amygdala (BLA), and lateral hypothalamus (LH) of rats treated repeatedly with AMPH when compared to vehicle-treated controls. The mPFC pyramidal neurons that project to the LH, but not the NAc or BLA, show a significant induction of Fos after repeated AMPH treatment. In addition, we found a dramatic increase in Fos-activated orexin neurons.
Conclusions
The LH, a region implicated in natural and drug reward processes, may play a role in the development and persistence of sensitization to repeated AMPH through its connections with the mPFC and possibly through its orexin neurons.
Keywords: dopamine, psychostimulant, behavioral sensitization, prelimbic cortex, infralimbic cortex, reward, addiction, Fluoro-Gold, orexin
INTRODUCTION
The neural circuits engaged following repeated amphetamine (AMPH) administration are not fully understood. However, there are data to support neuroadaptations in specific brain regions after such treatment (Robinson and Berridge 2000; Wolf 2002). Persistent structural modifications (Robinson and Kolb 1997; 1999), as well as changes in membrane properties and firing patterns of medial prefrontal cortical (mPFC) projection neurons and some of their target cells, follow repeated psychostimulant exposure (Henry and White 1995; Miller and Marshall 2004; Nasif et al. 2005; Nogueira et al. 2006; Onn and Grace 2000; Peterson et al. 2000; Thomas and Everitt 2001; Trantham et al. 2002; White et al. 1993; White et al. 1995; Zhang et al. 2002; Zhang et al. 1998). In addition, immunohistochemistry or gene expression for immediate early genes are able to map drug-activated neural circuits (de Souza and Meredith 1999; Dragunow and Robertson 1987). Repeated psychostimulant exposure induces c-fos and its protein product, Fos, in mPFC cells and their target neurons, including those in the lateral hypothalamus (LH), basolateral amygdala (BLA), and ventral tegmental area (VTA) (Ciccocioppo et al. 2001; Fadel et al. 2002; Harris et al. 2005; Hedou et al. 2002; Miller and Marshall 2004; Morshedi and Meredith 2007; Nikulina et al. 2004; Rademacher et al. 2006).
The neural circuitry activated by behavioral sensitization to repeated psychostimulant administration is complex with excitatory glutamatergic projections from the prefrontal cortex being of particular importance (Wolf 1998). Several pieces of evidence implicate the circuit between the mPFC and nucleus accumbens (NAc) in sensitized behavior. Lesions of mPFC areas that project to the NAc core block the expression of sensitization and increases in extracellular glutamate in the NAc in response to a drug challenge (Pierce et al. 1998). Further, mPFC-NAc projection neurons show persistent changes in burst firing and membrane oscillations following repeated AMPH treatment (Onn and Grace 2000). The behavioral changes that underlie the transition to compulsive drug seeking may also result from persistent alterations in other neural connections, such as those involving the LH or BLA (Ahmed et al. 2005; Fuchs et al. 2005; but see Miller and Marshall 2005). It is known from neurophysiological and behavioral studies that glutamate signaling from the prefrontal cortex exerts an “executive control” over behavioral output (Schultz et al. 2000; Wolf 2002) and, given the broad implications of multiple targets mediating the response, it is crucial to identify which prefrontal output pathways are involved. Therefore, evaluating Fos expression after drug administration can aid in the identification of activated pathways.
There are several reasons to predict that Fos will be differentially activated in mPFC projection neurons following a behaviorally sensitizing drug regimen. First, the subcortical targets of dorsal and ventral mPFC projections only partially overlap (Vertes 2004) and axonal collateralization of many mPFC projection neurons is restricted, at least in terms of subcortical targets (Gabbott et al. 2005). Second, psychostimulants induce Fos in a heterogeneous manner, depending upon the context of drug exposure (Feldpausch et al. 1998; Hedou et al. 2002; Nikulina et al. 2004; Ostrander et al. 2003; Uslaner et al. 2001) and, finally, lesions or inactivation of only certain mPFC targets (e.g. NAc, BLA, and VTA) disrupt drug-seeking behavior in experimental animals (Brown and Fibiger 1993; Fuchs and See 2002; Ito et al. 2004; McFarland and Kalivas 2001; McLaughlin and See 2003).
The current investigation aimed to establish which mPFC projection pathways are activated by repeated exposure to AMPH. Given the magnitude of mPFC projections to the LH, NAc, and BLA, approximately 27%, 23%, and 8% of layer V infralimbic (IL) and prelimbic (PrL) cortical neurons, respectively, (Gabbott et al. 2005) and their roles in drug-related behaviors, mPFC neurons that project to these regions are likely candidates to become engaged following AMPH exposure.
We selected a repeated, intermittent AMPH injection schedule that has previously been shown to induce robust behavioral sensitization and mPFC neuronal adaptations (Morshedi and Meredith 2007; Onn and Grace 2000; Paulson et al. 1991; Robinson and Camp 1987; Robinson and Kolb 1999). Three weeks after the injection protocol and following a challenge injection of AMPH, we studied Fos induction in mPFC pyramidal neurons in the deep layers of the IL and PrL cortices of rats. We identified the activated pathways and their targets, respectively, by co-localizing Fos immunoreactivity with retrogradely transported Fluoro-Gold (FG) in the mPFC and Fos induction alone in the NAc, BLA, and LH.
Materials and Methods
Animals, drugs, and behavior
All experiments were conducted in accordance with the Declaration of Helsinki and the Guide for the Care and Use of Laboratory Animals and approved by the Rosalind Franklin University Institutional Animal Care and Use Committee. Thirty-six, male Sprague-Dawley rats (Harlan, Indianapolis, IN) were divided into 3 groups. Rats in all groups weighed 125−139 g at the start of experiments. All animals were handled for 2 days prior to the start of experiments and were group-housed on a 12 h light-dark cycle with food and water available ad libitum. The first group of rats received repeated D-amphetamine sulfate (AMPH in 0.9% saline, i.p.) injections: 3 mg/kg for 5 days, except for the first and last day when they received 1.5 mg/kg, followed by 2 drug-free days and repeated for 3 weeks. The second group of rats was administered repeated saline injections (vehicle in the same volume) following the same procedure as the first group. A third group of rats, which served as sham controls for the surgical procedure (n = 3), received AMPH injections as above. Behavioral analysis was conducted using rats treated with AMPH (n = 16) or vehicle (n = 16). Rats were placed in a Plexiglas box equipped with two banks of infrared sensors that recorded movement and animal location (AccuScan Instruments, Inc., Columbus, OH, USA). On the first and last days of treatment, animals were habituated to the box, administered drug, and their horizontal movement recorded for 45 min. Locomotor sensitization was evaluated by comparing movement (total distance traveled) on the last injection day to that on the first day. After 3 weeks of AMPH or vehicle treatment, all rats underwent 3 weeks of withdrawal before euthanasia. One week prior to this, rats were anesthetized and received stereotaxically-placed injections of FG into one of several different targets of the mPFC (see below). Following surgery, all rats were handled daily for 5 days to avoid handling stress and its potential for Fos induction. On the final day, the AMPH-treated rats received a challenge injection of AMPH (1.5 mg/kg, i.p.) and vehicle-treated rats, a saline injection. Ninety minutes later, all animals were euthanized.
Surgical and Perfusion Procedures
Rats were anesthetized for surgery with equithesin (3 ml/kg, i.p.: 1% sodium pentobarbital, 4.25% chloral hydrate in 10% ethanol) and secured in a Kopf stereotaxic apparatus. Glass micropipettes with tips broken to +/−25μm diameter, filled with 2% FG (Fluorochrome, Denver, CO) in 0.9% saline were positioned using stereotaxic coordinates aimed at one of the following targets: NAc (relative to bregma: anterior-posterior (AP): 1.8 mm, medial-lateral (ML): −1.2 mm; relative to skull: dorsal-ventral (DV): −7.6 mm), BLA (relative to bregma: AP: −2.8 mm, ML: −5.0 mm; relative to skull: DV: −8.5 mm), or LH (relative to bregma: AP: −2.9 mm, ML: −1.3 mm; relative to skull: DV: −8.2 mm) (Paxinos and Watson 2005). The tracer was iontophoresed for 15 min using an alternating (7 s on, 7 s off) current of +8 μA. Pipettes were left in place for 10 min to prevent leakage along the pipette tract during removal. Sham surgeries, using the same coordinates as above, were conducted by reversing the polarity of the current (−8 μA) during iontophoresis, thereby preventing deposit of the tracer.
One week following surgery and 90 min after the challenge injection of saline or drug, rats were deeply anesthetized with sodium pentobarbital (100 mg/kg, i.p.) and transcardially perfused with ice-cold 2.5% sucrose in 0.1 M phosphate buffered saline, followed by 4% buffered paraformaldehyde and 2.5% sucrose. All solutions were made at the same time and the duration of perfusion was kept uniform. The brains were removed, post-fixed for 2 h in the same fixative without sucrose, and transferred to 30% sucrose buffer overnight at 4°C. Coronal sections (70 μm) were cut on a freezing microtome. Sections through the mPFC and the FG injection sites were collected in 0.1 M phosphate buffer, cryoprotected, and stored at −20°C until processing.
Immunohistochemistry
All solutions for the antibody reactions were prepared as a single batch and sections from all groups of animals were processed at the same time.
FG immunohistochemistry
Sections were blocked for 1 h in 5% normal goat serum (NGS) and 0.3% Triton-X 100 (Tx), and then incubated for 48 h at 4°C in rabbit anti-FG (1:5000; AB153; Chemicon, Temecula, CA) with 1% NGS and 0.2% Tx. Sections were incubated for 90 min in biotinylated goat anti-rabbit IgG (1:200; Vector Laboratories, Burlingame, CA) with 1% NGS at room temperature (RT), followed by 1 h in avidin biotin complex (ABC; Elite Kit, Vector Laboratories). Sections were then reacted with 0.05% nickel-enhanced 3,3’-diaminobenzidine (DAB) with 0.01% H2O2 (DAB-Ni), rinsed thoroughly, mounted from gelatin onto slides, dried, and dehydrated before coverslipping.
Fos immunohistochemistry
Sections were blocked for 1 h in 10% NGS and 0.3% Tx, and then incubated for 48 h at 4°C in rabbit anti-(c)-Fos (1:20000; PC38; Calbiochem, San Diego, CA) with 4% NGS and 0.3% Tx. Sections were incubated for 2 h in biotinylated goat anti-rabbit IgG (1:200) with 4% NGS and 0.3% Tx at RT, followed by 1 h in ABC. Sections were then reacted in 0.05% DAB with 0.01% H2O2, rinsed thoroughly, mounted, and coverslipped as described above.
Fos/FG dual immunohistochemistry
Sections were immunolabeled sequentially with antibodies against Fos followed by those against FG, as described above. Briefly, sections were blocked in normal serum, then incubated in rabbit anti-(c)-Fos, diluted 1:30000. Secondary (biotinylated goat anti-rabbit IgG, diluted 1:200) and ABC incubations were followed by a reaction in 0.05% DAB with 0.01% H2O2. Sections were extensively rinsed and then blocked with avidin D (Biotin Blocking Kit, Vector Laboratories), in order to prevent cross-reactivity of the antibodies. This step was followed by incubation in rabbit anti-FG, diluted 1:5000, as above, but with the addition of the biotin solution (Biotin Blocking Kit). Following secondary (biotinylated goat anti-rabbit IgG, diluted 1:200) and ABC incubations, the sections were reacted with the Vector SG substrate kit (blue reaction product, Vector Laboratories). Sections were mounted and topped with coverslips.
Because both primary antibodies were raised in rabbit, cross-reactivity of the secondary antibodies used to label FG with bound Fos primary antisera was tested by omitting the primary antibody to FG (Miller and Marshall 2005). We found equal numbers of Fos-labeled cells in the IL and PrL when compared to sections processed with the single immunohistochemistry for Fos. Thus, even though a rabbit secondary antibody was used in both reactions, no aberrant labeling occurred in either the IL or PrL. Omitting both primary antibodies produced no labeling.
Fos/orexin dual immunohistochemistry
In order determine if Fos was found in LH orexin neurons, dual immunohistochemistry was performed. Sections were immunolabeled sequentially with antibodies against Fos followed by those against orexin, as above with a few differences. Briefly, sections were blocked in normal serum, then incubated in rabbit anti-(c)-Fos, diluted 1:5000, overnight. Secondary, biotinylated donkey anti-rabbit IgG (1:400; Jackson Immunoresearch, West Grove, PA), and ABC incubations were followed by a reaction in 0.05% DAB with 0.01% H2O2. Sections were extensively rinsed and then incubated in goat anti-orexin A (1:1000; sc-8070; Santa Cruz Biotechnology, Santa Cruz, CA). Following secondary, biotinylated horse anti-goat IgG (1:200; Vector Laboratories), and ABC incubations, the sections were reacted with the Vector SG substrate kit. Sections were mounted and topped with coverslips. No cross-reactivity with Fos labeling was seen when the primary antibody for orexin was omitted. Omitting both primary antibodies produced no labeling.
Image Analysis and Quantification
Slides were coded so that the investigator was blind to the treatment groups during analysis. For the density measurements of Fos-immunoreactive cells, images were captured at 2 different rostrocaudal levels (mPFC: from bregma: 2.7 and 3.2 mm; NAc: from bregma: 2.1 and 2.5 mm; BLA and LH: from bregma: −2.7 and −3.2 mm) (Paxinos and Watson 2005) at 10X magnification (Nikon E600 microscope) using a digital camera (Optronics, Goleta, CA). The densities (cells/mm2) of Fos-immunoreactive cells were quantified in the deep layers of the IL and PrL cortices of the mPFC, the shell and core of the NAc, the BLA, and the LH using ImageJ software (U.S. National Institutes of Health, Bethesda, MD).
Co-localized Fos and FG cells were analyzed at 20X magnification. For each animal, the number of dually labeled neurons and the total number of neurons labeled with FG were counted in the deep layers of the PrL and IL at the rostrocaudal levels described above and ipsilateral to the FG injection site. The total surface area analyzed for the PrL was approximately 5.0 mm2 (average of 2.5 mm2 per section) and for the IL was approximately 1.9 mm2 (average of 0.9 mm2 per section). Dually labeled neurons have an amber nucleus (Fos-immunoreactive cell) surrounded by blue cytoplasm (FG-immunoreactive neuron). Ratios of Fos/FG dually labeled neurons to total number of FG-immunoreactive neurons were calculated. The total numbers of neurons labeled with FG are expressed as a mean number per group (Table 1). To address the issue of possible FG suppression of Fos (Franklin and Druhan 2000), we also quantified the density of Fos-immunoreactive cells after sham surgeries (n = 3).
Table 1.
Mean numbers of FG-immunoreactive cells in the mPFC retrogradely labeled from injection targets for each group
| |
IL |
PrL |
||
|---|---|---|---|---|
| Target | Saline | AMPH | AMPH | Saline |
| Nucleus accumbens | 203 ± 22 | 254 ± 39 | 286 ± 40 | 315 ± 31 |
| Basolateral amygdala | 95 ± 10 | 104 ± 8 | 36 ± 6 | 53 ± 10 |
| Lateral hypothalamus | 394 ± 21 | 298 ± 51 | 315 ± 39 | 249 ± 57 |
Co-localized Fos and orexin cells were analyzed at 20X magnification. For each animal, the number of dually labeled neurons within the LH (lateral to 0.4 mm medial to the fornix) was counted at 3 different rostrocaudal levels (from bregma: −2.7, −3.2, and −3.7 mm) (Paxinos and Watson 2005) and contralateral to the FG injection site. Dually labeled neurons have an amber nucleus (Fos-immunoreactive cell) surrounded by blue cytoplasm (orexin-immunoreactive neuron). This analysis was assisted by marking counted cells with different markers depending upon whether they were singly or dually labeled (Neurolucida, Microbrightfield, Williston, VT). The ratio of Fos/orexin dually labeled neurons to the total number of orexin-immunoreactive neurons was calculated.
Statistical Analysis
Paired Student's t-tests were used to compare within groups’ locomotor activity on the first and last day of treatments and Student's t-tests to compare between groups’ locomotor activity. Differences in the densities of Fos-immunoreactive nuclei between each region analyzed and each treatment group (repeated AMPH or saline) were compared using a two-way multifactorial analysis of variance (ANOVA). Differences among individual means were subsequently verified using Holm-Sidak post hoc tests. Student's t-tests were used to compare the percent of dually-labeled (Fos/FG) neurons to FG-labeled neurons within the IL and PrL between AMPH and vehicle groups for each target region (Miller and Marshall 2005) as well as the percent of dually-labeled (Fos/orexin) neurons to total orexin-labeled neurons in the LH between treatment groups. A Student's t-test was also employed to determine if FG suppressed Fos immunoreactivity by comparing the density of Fos-immunoreactive cells of FG-iontophoresed and sham surgery groups within the IL and PrL.
RESULTS
Locomotor sensitization
We found that AMPH increases locomotor activity and sensitization becomes evident with repeated administration. There was a significant increase in the total distance traveled between the first and the last test session (difference: +1018 ± 182 cm, t = −5.594, df = 30, p < 0.001) for animals administered AMPH repeatedly, whereas locomotor activity in the vehicle treated group was not different between the first and the last sessions (difference: +85 ± 52 cm, t = −1.641, df = 30, p = 0.116).
Verification of FG injection sites
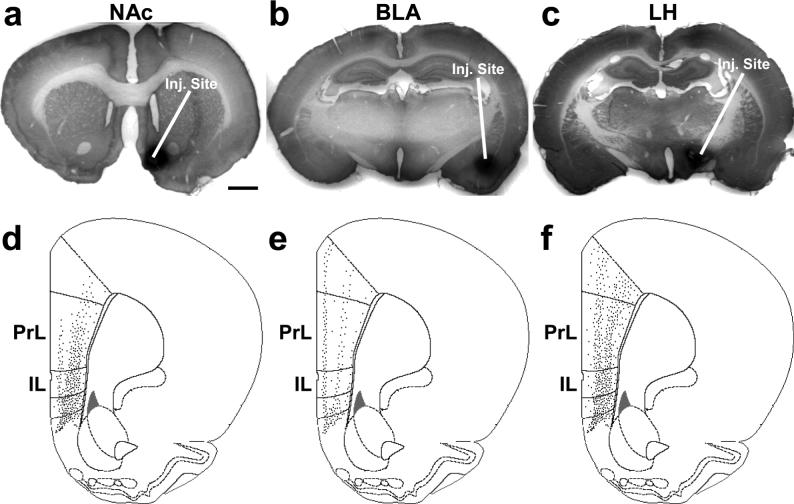
We placed the retrograde tracer, FG, into target regions of mPFC output neurons. Only animals with minimal or no spread of FG deposit beyond the intended region (NAc, BLA, LH) were included in the final analyses. Figures 1a-c illustrate typical FG injection sites within the NAc, BLA, and LH. The injection sites had minimal tissue damage. Injections of FG into the NAc were centered in the medial portion of the nucleus, including both core and shell areas (Fig. 1a), and targeted the intermediate to caudal NAc. In the BLA, FG deposition encompassed the entire complex (Fig. 1b) and was centered in the anterior BLA, previously shown to receive the densest projections from the mPFC (Sesack et al. 1989; Vertes 2004). In the LH, injection sites were typically centered lateral to the fornix and included the majority of the LH (Fig. 1c). The injections were central to the rostrocaudal extent of the LH.
Figure 1.
Representative micrographs showing typical FG injection sites for the (a) NAc, (b) BLA, and (c) LH. Diagrams representing the corresponding ipsilateral areal distributions of neurons (black dots) in the mPFC retrogradely labeled from the (d) NAc, (e) BLA, and (f) LH (each drawing represents a coronal section at approximately +2.7 mm from bregma) (Paxinos and Watson 2005). FG injections centered in the (d) NAc and (f) LH produced similar dense distributions of retrogradely labeled neurons primarily in the deep layers of the IL and PrL, while injections centered in the (e) BLA labeled neurons in layer II and the deeper layers of the IL and PrL. Scale bars: a (also valid for b,c), 2 mm.
Retrograde transport of FG
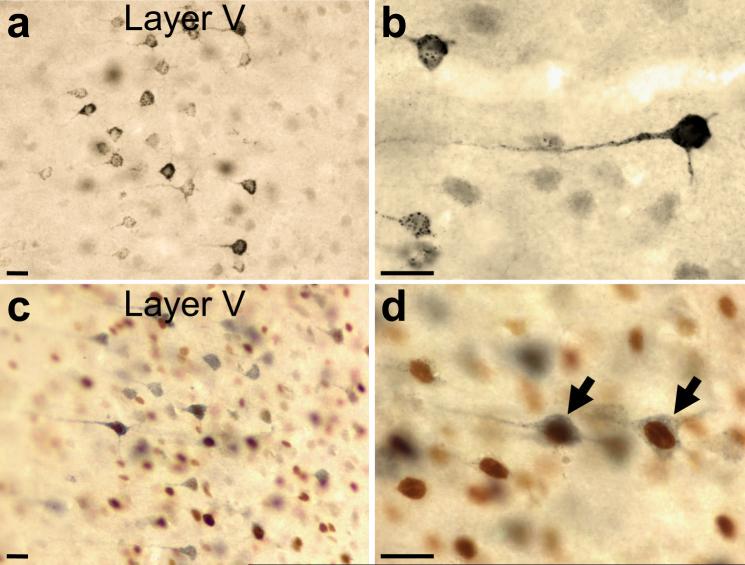
Pyramidal neurons, retrogradely labeled with FG, typically showed a granular, blue-black cytoplasm with no label over the nucleus (Figs. 2a-b). Figures 1d-f are diagrammatic representations of the distribution of FG immunolabeled neurons from a representative caudal section of the mPFC (from bregma: +2.7) for each target region. Because axons of mPFC projection neurons rarely collateralize (Gabbott et al. 2005; Pinto and Sesack 2000), the retrogradely labeled cells predominantly represent single output pathways. Injections of FG into the NAc and LH retrogradely labeled more neurons in the deep layers of the IL and PrL cortices than those placed in the BLA (Table 1; Figs. 1d-f). Injections targeting the NAc retrogradely labeled pyramidal neurons primarily in the deep layers (III-VI) of the IL and PrL cortices (Fig. 1d). These FG injections also labeled a small population in the anterior cingulate (AC) cortex, especially if the injection site was situated more dorsally (one case). In addition, labeled cells were seen in the claustrum and the anterior insular, lateral and ventral orbital, dorsal penduncular, and piriform cortices. Injections in the BLA retrogradely labeled cells in layers II and V of the IL and PrL cortices, with a few neurons in the AC (Fig. 1e). The anterior insular, dorsal peduncular, and ventral orbital cortices also contained a few labeled cells. Tracer injection in the LH retrogradely labeled cells in the deep layers, especially layer V of the IL and PrL (Fig. 1f). A few labeled cells were also found in the AC, anterior insular, and dorsal peduncular cortices and even fewer were located in the ventral and lateral orbital cortices.
Figure 2.
Representative micrographs of deep layers of the mPFC showing FG-filled neurons (DAB-Ni) at (a) 20X and (b) 60X, and neurons dually immunolabeled for Fos (DAB) and FG (Vector SG) at (c) 20X and (d) 60X. Dually immunolabeled neurons (arrows) have an amber nucleus (Fos-immunoreactive cell) surrounded by blue cytoplasm (FG-immunoreactive neuron). Scale bars: a-d, 25 μm.
Fos immunoreactivity
We analyzed the density of Fos immunoreactivity in the IL and PrL areas of the mPFC and their major projection targets, i.e. the NAc shell and core, BLA, and LH (Fig. 3). The statistical analysis demonstrated significant differences between treatment groups (F1,42 = 169.4, p < 0.001) and between regions (F5,42 = 10.0, p < 0.001). A significant interaction was also found between regions and treatment groups (F5,42 = 4.1, p = 0.004), indicating that the density of Fosimmunoreactive cells for each region was dependent on treatment. Post-hoc analyses revealed that all regions except the NAc shell (p = 0.052, nonsignificant trend), showed a significant increase in the density of Fos-immunoreactive cells after repeated AMPH treatment compared to vehicle (P < 0.001; Fig. 3). Between regions, the Fos-immunoreactive cell density in the IL, PrL, NAc core, and LH was significantly greater than the density in the NAc shell (p < 0.001), the density of Fos in the PrL, NAc core, and LH was also significantly greater than the density in the BLA (p < 0.001), and the density in the NAc core was greater than in the IL (p < 0.001) following repeated AMPH treatment. Comparisons between regions also revealed that within the repeated AMPH group, the IL, PrL, NAc core, and BLA had the greatest increases in Fosimmunoreactive cell densities, all of which were significant. Finally, we found no significant differences in Fos-immunoreactive cell density between the vehicle groups for all regions.
Figure 3.
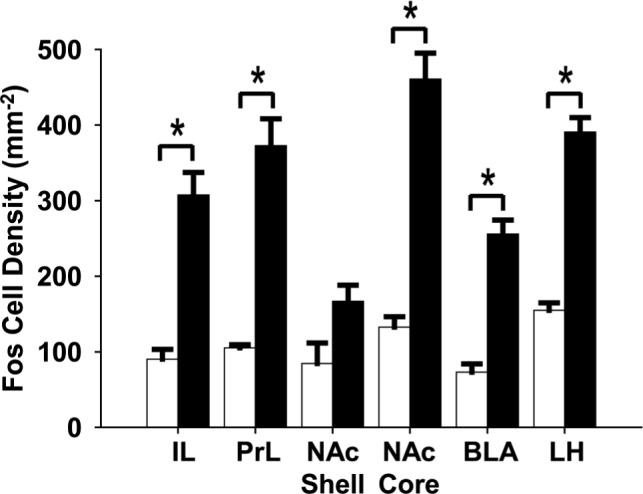
Bar graphs showing the density of Fos immunolabeled cells after repeated AMPH or vehicle administration in the IL and PrL areas and their major target regions (NAc shell and core, BLA, and LH); filled bars, repeated AMPH administration (n = 5) and open bars, vehicle (n = 5). *p < 0.001, repeated AMPH group has a significantly higher density of Fos-immunoreactive cells in the IL, PrL, NAc core, BLA, and LH when compared to vehicle.
Pathway-specific Fos expression
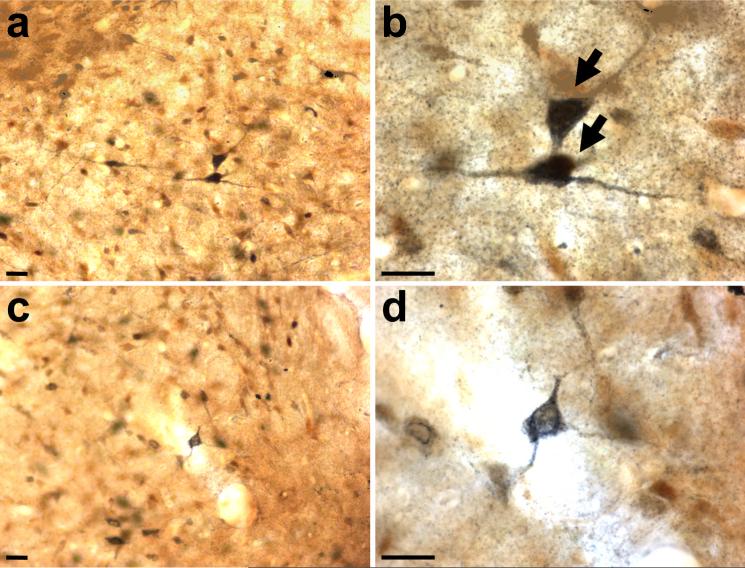
Overall, Fos immunoreactivity in the mPFC is elevated following repeated AMPH treatment, and Fos is differentially activated in mPFC efferent pathways. In order to evaluate pathway-specific Fos expression, we counted cells that co-localized FG and Fos in the deep layers of the IL and PrL and found that the dually labeled cells appeared in both cortical areas for all the projection targets studied (Figs. 2c-d, 4). There was no difference in the relative density of FG-immunolabeled neurons in the single and dual-labeled preparations (compare Figs. 2a and 2c). Quantitative analysis revealed that neither the mPFC-NAc nor the mPFC-BLA efferent neurons differed in their Fos expression between repeated AMPH- and vehicle-treated groups (Figs. 4a-b). Specifically, there was no difference between groups for the mPFC-NAc projection neurons in the IL (p = 0.249) or the PrL (p = 0.983). There was also no difference between groups for the mPFC-BLA pyramidal cells in the IL (p = 0.413) or the PrL (p = 0.256). There was, however, a significant increase in Fos expression in neurons of the mPFC-LH pathway in both the IL and PrL areas after repeated AMPH treatment when compared to vehicle-treated controls (Fig. 4c). Finally, there was no difference in Fos induction in both the PrL and IL areas of the mPFC for animals that had sham surgeries as compared to those with Fluoro-Gold injections in one of the three targets described above (Student's t-test, not significant).
Figure 4.
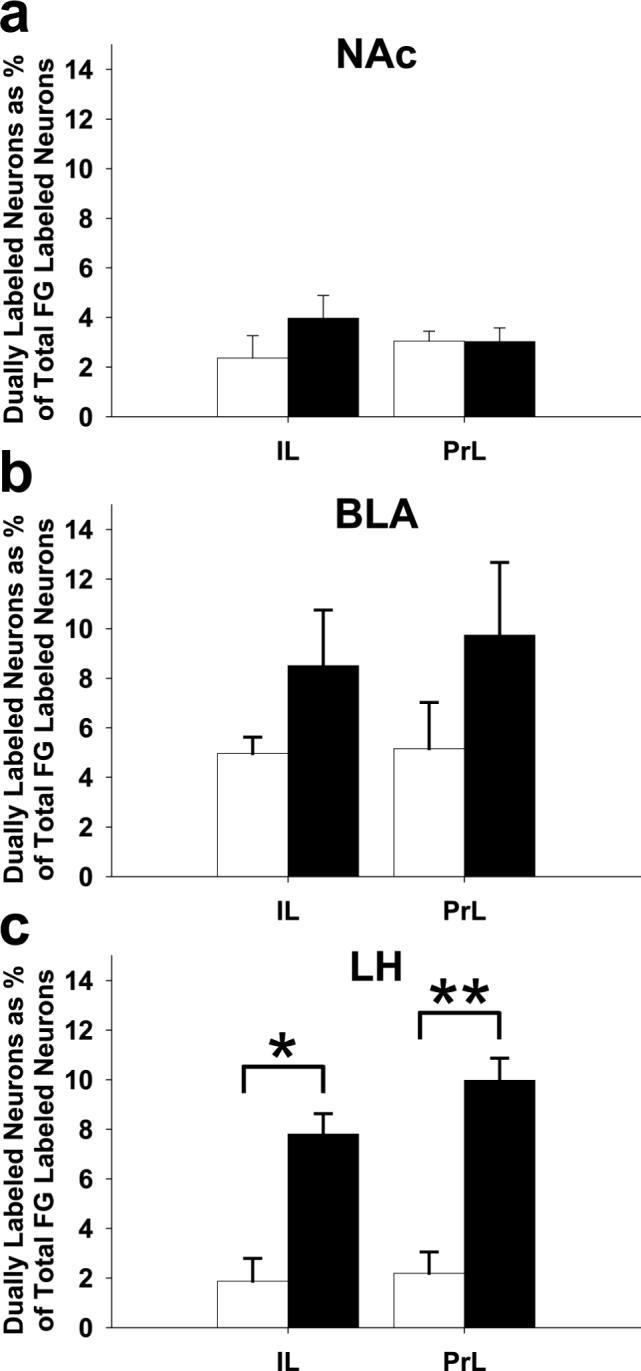
Bar graphs showing pathway-specific Fos induction after repeated AMPH or vehicle administration. The proportion of colocalized FG- and Fos- immunolabeled neurons in the IL or PrL areas of the mPFC is shown following FG deposition in the (a) NAc (n = 5 per group), (b) BLA (AMPH, n = 5/group), or (c) LH (AMPH, n = 6 and vehicle, n = 4). Open bars, vehicle; filled bars, repeated AMPH administration. *p < 0.005, **p < 0.001, repeated AMPH group has a significantly higher density of co-localized cells in mPFC-LH efferent neurons compared to vehicle.
Given the increase in Fos in the mPFC-LH pathway and the induction of Fos in the LH, we dually labeled LH neurons for Fos and orexin immunohistochemistry. Comparing AMPH (n = 4) to vehicle (n = 3) treatments, we found that the orexin-immunoreactive neurons co-localized with Fos (Fig. 5). Indeed, the proportion of orexin neurons that expressed Fos significantly increased following repeated AMPH treatment when compared to vehicle (61.0 ± 4.9% and 17.4 ± 2.6% respectively; p < 0.001).
Figure 5.
Representative micrographs of the LH. (a) Dually immunolabeled neurons (arrows) with an amber nucleus (Fos-immunoreactive cell) surrounded by blue cytoplasm (orexin-immunoreactive neuron) after AMPH treatment. (b) Orexin-immunoreactive neuron (singly labeled) after vehicle treatment. Scale bar: a (also valid for b), 25 μm.
DISCUSSION
Our principle finding is that repeated AMPH exposure differentially activates mPFC projection pathways. While neurons projecting to the LH increase their Fos expression, those that project to the NAc or the BLA do not. Although the relationship between Fos induction and neuronal firing is complex (Labiner et al. 1993), our results indicate a preferential activation of the mPFC-LH pathway following a sensitizing regimen of repeated AMPH administration. We also found a dramatic increase in Fos-activated orexin neurons, suggesting a role for LH orexin neurons in the behavioral sensitization to this drug.
Technical considerations
The medial forebrain bundle, containing dopaminergic fibers from the VTA, runs through the LH, which could potentially confound our tracing results. Nevertheless, there are several reasons that we can be confident that our results reflect the projection to LH and not to sites found further caudally. Although FG is not readily taken up by fibers of passage (Pieribone and Aston-Jones 1988; Schmued and Fallon 1986), it can be picked up by axons passing through certain regions (Dado et al. 1990). Nevertheless, FG is very sensitive, especially if iontophoretically applied. Furthermore, mPFC projections to the VTA and targets further caudally are very sparse (<9% of projections and only from layer V; Gabbott et al. 2005). Moreover, others have demonstrated that the mPFC-VTA pathway is not activated by AMPH treatment (Berod and Colussi-Mas 2005). Therefore, the fibers of passage that could have picked up the FG infusion in the LH would have labeled few neurons in the mPFC.
In order to address the potential suppression of Fos expression by FG uptake (Franklin and Druhan 2000), we conducted sham surgeries where no FG was iontophoresed. We found no significant difference in the density of Fos immunoreactivity in the mPFC between the sham surgical and FG groups.
Activation of mPFC target regions
Interestingly, all target regions demonstrated an increase in Fos induction, but the increase is only borderline in the shell of NAc. This is in agreement with Simpson and colleagues (1995), who showed that Fos is significantly induced in core but not shell neurons of AMPH-sensitized rats. Moreover, sensitization could be associated with alterations in glutamate and dopamine release in the NAc core, but not the shell (Cadoni et al. 2000; Giorgi et al. 2005; Pierce et al. 1996; but see Hedou et al. 1999; Pierce and Kalivas 1995). Nevertheless, there is a strong trend towards significance in the shell with our results, which agrees with earlier work by Hedou and colleagues (2002). The shell has long been thought to mediate the hedonic or reinforcing responses to psychostimulants, whereas the core is key for the expression of motivation (reviewed in Di Chiara 2002). The core may serve as an interface between motivation and action and, in the case of behavioral sensitization, link repeated drug exposure to increased locomotor activity through its robust connections with motor output nuclei (Heimer et al. 1991).
Implications of differential mPFC efferent activation
A key question behind these investigations is why drug craving persists long after drug use. We know that long lasting changes in behavior are associated with chronic exposure to drugs of abuse (Robinson and Berridge 2000). These behavioral changes also persist despite adverse consequences to the individual and can lead to drug relapse after years of abstinence (Self and Nestler 1998). In animal models, repeated exposure to AMPH progressively enhances locomotor activity (behavioral sensitization), an effect that persists after treatment and can be measured by a drug challenge (Wolf 1998). The change in the behavioral response has been associated with reinstatement of drug-seeking behavior (De Vries et al. 1998) and may involve enhanced activation/plasticity of particular brain circuits, particularly the mPFC (Pierce and Kalivas 1997). While repeated AMPH treatment is known to increase mPFC Fos expression (Hedou et al. 2002; present results), no studies have directly implicated the mPFC-LH pathway in psychostimulant exposure.
Surprisingly, the present results show that the mPFC-NAc pathway does not significantly induce Fos following repeated AMPH administration. Others have suggested that increases in glutamate release in the NAc of sensitized animals is due to augmented activation of the mPFC (reviewed in Everitt and Wolf 2002; Pierce and Kalivas 1997). Onn and Grace (2000) have demonstrated an increase in corticoaccumbal neuron firing after withdrawal from repeated AMPH treatment, however their data did not reach significance and the numbers of neurons examined were a very small percentage of the total number in that pathway. Moreover, other electrophysiological data indicate that dopaminergic pathway stimulation reduces mPFC excitation of NAc neurons through dopamine receptor activation (Brady and O'Donnell 2004).
The mPFC-BLA pathway was not activated with the present paradigm. Inter-animal variability could have interfered with these results due to the relatively small number of rats used in the present study. However, we believe that this is unlikely. Miller and Marshall (2005) reported enhanced Fos activation of BLA-mPFC and BLA-NAc pathways with a conditioned drug response but found no difference in the mPFC projections to BLA or NAc using group sizes of similar magnitude. The variability is most likely due to the widespread terminal fields in the BLA and the relatively few projections from the mPFC (Gabbott et al. 2005). Moreover, Rosenkranz and Grace (2001) have shown that increases in dopamine, as occurs with AMPH administration, can remove the mPFC-induced suppression of BLA output, data consistent with a net zero change in the activation of the mPFC-BLA pathway.
The LH plays an important role in homeostasis and motivated behaviors. LH neurons contain melanin-concentrating hormone (MCH) and orexin. These neuroactive peptides are found predominantly in the LH (Peyron et al. 1998) and have been strongly implicated in the sensitizing effects of psychostimulants (Borgland et al. 2006; Smith et al. 2005; present results). In addition, MCH and orexin receptors are expressed throughout regions implicated in reward, including the VTA, NAc, BLA, and mPFC (Marcus et al. 2001; Saito et al. 2001). Axons of orexin neurons form dense projections to the VTA and mPFC and also innervate the BLA and NAc, though to a lesser extent (Fadel and Deutch 2002). In fact, orexin neurons that project to the VTA are almost exclusively found in the LH (Fadel and Deutch 2002). Furthermore, orexin and dopamine fibers co-distribute in the mPFC (Fadel and Deutch 2002) indicating that mPFC neurons innervated by dopaminergic neurons from the VTA may also be regulated by the LH.
Medial PFC projections directly innervate orexin neurons throughout the hypothalamus, but most densely in the LH (Yoshida et al. 2006). The LH neurons that are contacted by deep layer mPFC neurons presumably play an important role in integrating signals for arousal (de Lecea et al. 2006; Sutcliffe and de Lecea 2002). Others have shown that orexin administration elicits increases in locomotor activity, resembling AMPH administration, through noradrenergic and dopaminergic systems (Hagan et al. 1999; Nakamura et al. 2000). Moreover, Fos seems to be exclusively activated in subsets of orexin-containing neurons by acute AMPH treatment (Fadel et al. 2002). Our results demonstrate, for the first time, a role for the orexin neurons in behavioral sensitization, perhaps due to orexin activation of VTA neurons (Borgland et al. 2006; Korotkova et al. 2003). Therefore, selective activation of mPFC efferents to the LH after repeated AMPH treatment argues strongly for a link between LH orexin neurons and sensitizing behaviors.
Conclusion
Our study employed a repeated AMPH treatment procedure known to induce persistent behavioral, structural, and physiological plasticity associated with the mPFC (Morshedi and Meredith 2007; Onn and Grace 2000; Robinson and Kolb 1997; 1999). We have demonstrated that repeated AMPH treatment selectively engages mPFC efferents to the LH, while those to the NAc and BLA remain unchanged. The LH, a region known to be involved in natural reward, has more recently been implicated in drug reward. Therefore, repeated AMPH treatment, by specifically activating mPFC excitatory projections to the LH, could play a role in usurping natural reward circuitry for activation of drug-focused behaviors.
Acknowledgements
The work was supported by a USPHS grant from the NIH, DA16662. We thank Drs. Anthony West and David Rademacher for their critical reading of the manuscript and Mrs. Jennifer Jackolin for her technical assistance. All experiments were conducted in accordance with the current laws of the United States of America.
REFERENCES
- Ahmed SH, Lutjens R, van der Stap LD, Lekic D, Romano-Spica V, Morales M, Koob GF, Repunte-Canonigo V, Sanna PP. Gene expression evidence for remodeling of lateral hypothalamic circuitry in cocaine addiction. Proc Natl Acad Sci U S A. 2005;102:11533–8. doi: 10.1073/pnas.0504438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berod AM, Colussi-Mas J. Afferent projections to the rat ventral tegmental area activated by a single exposure to amphetamine Society for Neuroscience. 2005 Abstract Viewer/Itinerary Planner; Washington, DC: 2005. 2005. [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Brady AM, O'Donnell P. Dopaminergic modulation of prefrontal cortical input to nucleus accumbens neurons in vivo. J Neurosci. 2004;24:1040–9. doi: 10.1523/JNEUROSCI.4178-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EE, Fibiger HC. Differential effects of excitotoxic lesions of the amygdala on cocaine-induced conditioned locomotion and conditioned place preference. Psychopharmacology (Berl) 1993;113:123–30. doi: 10.1007/BF02244344. [DOI] [PubMed] [Google Scholar]
- Cadoni C, Solinas M, Di Chiara G. Psychostimulant sensitization: differential changes in accumbal shell and core dopamine. Eur J Pharmacol. 2000;388:69–76. doi: 10.1016/s0014-2999(99)00824-9. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci U S A. 2001;98:1976–81. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dado RJ, Burstein R, Cliffer KD, Giesler GJ., Jr. Evidence that Fluoro-Gold can be transported avidly through fibers of passage. Brain Res. 1990;533:329–33. doi: 10.1016/0006-8993(90)91358-n. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Jones BE, Boutrel B, Borgland SL, Nishino S, Bubser M, DiLeone R. Addiction and arousal: alternative roles of hypothalamic peptides. J Neurosci. 2006;26:10372–5. doi: 10.1523/JNEUROSCI.3118-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza IE, Meredith GE. NMDA receptor blockade attenuates the haloperidol induction of Fos protein in the dorsal but not the ventral striatum. Synapse. 1999;32:243–53. doi: 10.1002/(SICI)1098-2396(19990615)32:4<243::AID-SYN1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN, Binnekade R, Mulder AH, Vanderschuren LJ. Drug-induced reinstatement of heroin- and cocaine-seeking behaviour following long-term extinction is associated with expression of behavioural sensitization. Eur J Neurosci. 1998;10:3565–71. doi: 10.1046/j.1460-9568.1998.00368.x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Dragunow M, Robertson HA. Kindling stimulation induces c-fos protein(s) in granule cells of the rat dentate gyrus. Nature. 1987;329:441–2. doi: 10.1038/329441a0. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J Neurosci. 2002;22:3312–20. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Bubser M, Deutch AY. Differential activation of orexin neurons by antipsychotic drugs associated with weight gain. J Neurosci. 2002;22:6742–6. doi: 10.1523/JNEUROSCI.22-15-06742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–87. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Feldpausch DL, Needham LM, Stone MP, Althaus JS, Yamamoto BK, Svensson KA, Merchant KM. The role of dopamine D4 receptor in the induction of behavioral sensitization to amphetamine and accompanying biochemical and molecular adaptations. J Pharmacol Exp Ther. 1998;286:497–508. [PubMed] [Google Scholar]
- Franklin TR, Druhan JP. The retrograde tracer fluoro-gold interferes with the expression of fos-related antigens. J Neurosci Methods. 2000;98:1–8. doi: 10.1016/s0165-0270(00)00168-0. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, See RE. Basolateral amygdala inactivation abolishes conditioned stimulus- and heroin-induced reinstatement of extinguished heroin-seeking behavior in rats. Psychopharmacology (Berl) 2002;160:425–33. doi: 10.1007/s00213-001-0997-7. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–77. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Giorgi O, Piras G, Lecca D, Corda MG. Differential activation of dopamine release in the nucleus accumbens core and shell after acute or repeated amphetamine injections: a comparative study in the Roman high- and low-avoidance rat lines. Neuroscience. 2005;135:987–98. doi: 10.1016/j.neuroscience.2005.06.075. [DOI] [PubMed] [Google Scholar]
- Gorelova N, Seamans JK, Yang CR. Mechanisms of dopamine activation of fast-spiking interneurons that exert inhibition in rat prefrontal cortex. J Neurophysiol. 2002;88:3150–66. doi: 10.1152/jn.00335.2002. [DOI] [PubMed] [Google Scholar]
- Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hatcher JP, Hatcher PD, Jones DN, Smith MI, Piper DC, Hunter AJ, Porter RA, Upton N. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A. 1999;96:10911–6. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–9. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Hedou G, Feldon J, Heidbreder CA. Effects of cocaine on dopamine in subregions of the rat prefrontal cortex and their efferents to subterritories of the nucleus accumbens. Eur J Pharmacol. 1999;372:143–55. doi: 10.1016/s0014-2999(99)00218-6. [DOI] [PubMed] [Google Scholar]
- Hedou G, Jongen-Relo AL, Murphy CA, Heidbreder CA, Feldon J. Sensitized Fos expression in subterritories of the rat medial prefrontal cortex and nucleus accumbens following amphetamine sensitization as revealed by stereology. Brain Res. 2002;950:165–79. doi: 10.1016/s0006-8993(02)03034-2. [DOI] [PubMed] [Google Scholar]
- Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- Henry DJ, White FJ. The persistence of behavioral sensitization to cocaine parallels enhanced inhibition of nucleus accumbens neurons. J Neurosci. 1995;15:6287–99. doi: 10.1523/JNEUROSCI.15-09-06287.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7:389–97. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labiner DM, Butler LS, Cao Z, Hosford DA, Shin C, McNamara JO. Induction of c-fos mRNA by kindled seizures: complex relationship with neuronal burst firing. J Neurosci. 1993;13:744–51. doi: 10.1523/JNEUROSCI.13-02-00744.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–63. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Altered prelimbic cortex output during cue-elicited drug seeking. J Neurosci. 2004;24:6889–97. doi: 10.1523/JNEUROSCI.1685-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Altered Fos expression in neural pathways underlying cue-elicited drug seeking in the rat. Eur J Neurosci. 2005;21:1385–93. doi: 10.1111/j.1460-9568.2005.03974.x. [DOI] [PubMed] [Google Scholar]
- Morshedi MM, Meredith GE. Differential laminar effects of amphetamine on prefrontal parvalbumin interneurons. Neuroscience. 2007 doi: 10.1016/j.neuroscience.2007.07.047. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Uramura K, Nambu T, Yada T, Goto K, Yanagisawa M, Sakurai T. Orexin-induced hyperlocomotion and stereotypy are mediated by the dopaminergic system. Brain Res. 2000;873:181–7. doi: 10.1016/s0006-8993(00)02555-5. [DOI] [PubMed] [Google Scholar]
- Nasif FJ, Hu XT, White FJ. Repeated cocaine administration increases voltage-sensitive calcium currents in response to membrane depolarization in medial prefrontal cortex pyramidal neurons. J Neurosci. 2005;25:3674–9. doi: 10.1523/JNEUROSCI.0010-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikulina EM, Covington HE, 3rd, Ganschow L, Hammer RP, Jr., Miczek KA. Long-term behavioral and neuronal cross-sensitization to amphetamine induced by repeated brief social defeat stress: Fos in the ventral tegmental area and amygdala. Neuroscience. 2004;123:857–65. doi: 10.1016/j.neuroscience.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Nogueira L, Kalivas PW, Lavin A. Long-term neuroadaptations produced by withdrawal from repeated cocaine treatment: role of dopaminergic receptors in modulating cortical excitability. J Neurosci. 2006;26:12308–13. doi: 10.1523/JNEUROSCI.3206-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onn SP, Grace AA. Amphetamine withdrawal alters bistable states and cellular coupling in rat prefrontal cortex and nucleus accumbens neurons recorded in vivo. J Neurosci. 2000;20:2332–45. doi: 10.1523/JNEUROSCI.20-06-02332.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrander MM, Richtand NM, Herman JP. Stress and amphetamine induce Fos expression in medial prefrontal cortex neurons containing glucocorticoid receptors. Brain Res. 2003;990:209–14. doi: 10.1016/j.brainres.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Camp DM, Robinson TE. Time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology (Berl) 1991;103:480–92. doi: 10.1007/BF02244248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th edn. Elsevier Academic Press, Elsevier Academic Press; 2005. [Google Scholar]
- Peterson JD, Wolf ME, White FJ. Altered responsiveness of medial prefrontal cortex neurons to glutamate and dopamine after withdrawal from repeated amphetamine treatment. Synapse. 2000;36:342–4. doi: 10.1002/(SICI)1098-2396(20000615)36:4<342::AID-SYN11>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–60. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. Amphetamine produces sensitized increases in locomotion and extracellular dopamine preferentially in the nucleus accumbens shell of rats administered repeated cocaine. J Pharmacol Exp Ther. 1995;275:1019–29. [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Reeder DC, Hicks J, Morgan ZR, Kalivas PW. Ibotenic acid lesions of the dorsal prefrontal cortex disrupt the expression of behavioral sensitization to cocaine. Neuroscience. 1998;82:1103–14. doi: 10.1016/s0306-4522(97)00366-7. [DOI] [PubMed] [Google Scholar]
- Pieribone VA, Aston-Jones G. The iontophoretic application of Fluoro-Gold for the study of afferents to deep brain nuclei. Brain Res. 1988;475:259–71. doi: 10.1016/0006-8993(88)90614-2. [DOI] [PubMed] [Google Scholar]
- Pinto A, Sesack SR. Limited collateralization of neurons in the rat prefrontal cortex that project to the nucleus accumbens. Neuroscience. 2000;97:635–42. doi: 10.1016/s0306-4522(00)00042-7. [DOI] [PubMed] [Google Scholar]
- Rademacher DJ, Kovacs B, Shen F, Napier TC, Meredith GE. The neural substrates of amphetamine conditioned place preference: implications for the formation of conditioned stimulus-reward associations. Eur J Neurosci. 2006;24:2089–97. doi: 10.1111/j.1460-9568.2006.05066.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Camp DM. Long-lasting effects of escalating doses of d-amphetamine on brain monoamines, amphetamine-induced stereotyped behavior and spontaneous nocturnal locomotion. Pharmacol Biochem Behav. 1987;26:821–7. doi: 10.1016/0091-3057(87)90616-2. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci. 1997;17:8491–7. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999;11:1598–604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Dopamine attenuates prefrontal cortical suppression of sensory inputs to the basolateral amygdala of rats. J Neurosci. 2001;21:4090–103. doi: 10.1523/JNEUROSCI.21-11-04090.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Cheng M, Leslie FM, Civelli O. Expression of the melanin-concentrating hormone (MCH) receptor mRNA in the rat brain. J Comp Neurol. 2001;435:26–40. doi: 10.1002/cne.1191. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Fallon JH. Fluoro-Gold: a new fluorescent retrograde axonal tracer with numerous unique properties. Brain Res. 1986;377:147–54. doi: 10.1016/0006-8993(86)91199-6. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb Cortex. 2000;10:272–84. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Self DW, Nestler EJ. Relapse to drug-seeking: neural and molecular mechanisms. Drug Alcohol Depend. 1998;51:49–60. doi: 10.1016/s0376-8716(98)00065-9. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–42. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Simpson JN, Wang JQ, McGinty JF. Repeated amphetamine administration induces a prolonged augmentation of phosphorylated cyclase response element-binding protein and Fos-related antigen immunoreactivity in rat striatum. Neuroscience. 1995;69:441–57. doi: 10.1016/0306-4522(95)00274-m. [DOI] [PubMed] [Google Scholar]
- Smith DG, Tzavara ET, Shaw J, Luecke S, Wade M, Davis R, Salhoff C, Nomikos GG, Gehlert DR. Mesolimbic dopamine super-sensitivity in melanin-concentrating hormone-1 receptor-deficient mice. J Neurosci. 2005;25:914–22. doi: 10.1523/JNEUROSCI.4079-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JG, de Lecea L. The hypocretins: setting the arousal threshold. Nat Rev Neurosci. 2002;3:339–49. doi: 10.1038/nrn808. [DOI] [PubMed] [Google Scholar]
- Thomas KL, Everitt BJ. Limbic-cortical-ventral striatal activation during retrieval of a discrete cocaine-associated stimulus: a cellular imaging study with gamma protein kinase C expression. J Neurosci. 2001;21:2526–35. doi: 10.1523/JNEUROSCI.21-07-02526.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trantham H, Szumlinski KK, McFarland K, Kalivas PW, Lavin A. Repeated cocaine administration alters the electrophysiological properties of prefrontal cortical neurons. Neuroscience. 2002;113:749–53. doi: 10.1016/s0306-4522(02)00246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uslaner J, Badiani A, Day HE, Watson SJ, Akil H, Robinson TE. Environmental context modulates the ability of cocaine and amphetamine to induce c-fos mRNA expression in the neocortex, caudate nucleus, and nucleus accumbens. Brain Res. 2001;920:106–16. doi: 10.1016/s0006-8993(01)03040-2. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- White FJ, Hu XT, Henry DJ. Electrophysiological effects of cocaine in the rat nucleus accumbens: microiontophoretic studies. J Pharmacol Exp Ther. 1993;266:1075–84. [PubMed] [Google Scholar]
- White FJ, Hu XT, Zhang XF, Wolf ME. Repeated administration of cocaine or amphetamine alters neuronal responses to glutamate in the mesoaccumbens dopamine system. J Pharmacol Exp Ther. 1995;273:445–54. [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- Wolf ME. Addiction: making the connection between behavioral changes and neuronal plasticity in specific pathways. Mol Interv. 2002;2:146–57. doi: 10.1124/mi.2.3.146. [DOI] [PubMed] [Google Scholar]
- Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494:845–61. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XF, Cooper DC, White FJ. Repeated cocaine treatment decreases whole-cell calcium current in rat nucleus accumbens neurons. J Pharmacol Exp Ther. 2002;301:1119–25. doi: 10.1124/jpet.301.3.1119. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Hu XT, White FJ. Whole-cell plasticity in cocaine withdrawal: reduced sodium currents in nucleus accumbens neurons. J Neurosci. 1998;18:488–98. doi: 10.1523/JNEUROSCI.18-01-00488.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]