Abstract
Background
Chronic alcoholics have increased susceptibility to and severity of infection, which are likely to be a result of impaired immune defense mechanisms. The contribution of dendritic cells (DC) to these immune defense changes is not well understood. Alterations in DC numbers, dendropoiesis, and lifespan have not been specifically studied in vivo in chronic ethanol (EtOH) exposure models. As DC play an essential role in initiating immune responses, alterations in these DC characteristics would help explain changes observed in adaptive immune responses.
Methods
Mice received 20% EtOH (w/v) in the drinking water for up to 28 weeks, with mouse chow ad libitum. In EtOH-fed and water control mice, DC were enumerated by flow cytometry. The effect of EtOH on DC precursor numbers was determined by differentiation in vitro in the presence of granulocyte-macrophage colony-stimulating factor and interleukin-4, and the effect of an EtOH environment on untreated DC differentiation was measured following bone marrow transfer to irradiated hosts. DC turnover rate was also examined by bromodeoxyuridine incorporation and loss.
Results
The percentage and absolute numbers of DC were decreased in spleen and increased in thymus beginning as early as 4 weeks of EtOH feeding. In addition, the overall cellularity of spleen and thymus were altered by this regimen. However, chronic EtOH consumption did not adversely affect DC precursor numbers, differentiation abilities, or turnover rates.
Conclusions
Decreased splenic DC numbers observed following chronic murine EtOH consumption are not because of altered DC precursor numbers or differentiation, nor increased DC turnover rate. Similarly, increased thymic DC numbers are not the result of alterations in DC precursor differentiation or turnover rate. Compartment size plays a role in determining splenic and thymic DC numbers following chronic EtOH feeding. EtOH-induced alterations in total DC numbers provide several mechanisms to partially explain why chronic alcoholics have increased susceptibility to infections.
Keywords: Dendritic Cells, Ethanol, Precursor, Dendropoiesis, Mouse
Alcoholism is A serious disease that currently affects nearly 18 million Americans. Chronic alcoholics have increased susceptibility to and severity of infection (reviewed in Cook, 1998; MacGregor and Louria, 1997; Szabo, 1999), and evidence is strong that this is because of impaired innate and adaptive immunity. Dendritic cells (DC) play a key role in the initiation of adaptive immune responses, and various aspects of DC function are negatively impacted by acute and chronic ethanol (EtOH) exposure in humans (Dolganiuc et al., 2003; Laso et al., 2007; Mandrekar et al., 2004) and mice (Aloman et al., 2007; Heinz and Waltenbaugh, 2007; Lau et al., 2006, 2007; Ness et al., 2008). In addition to dysfunction of individual DC, alterations in DC numbers could contribute to impaired immune responses because of lack of sufficient antigen presentation interactions to stimulate a robust T cell response or because of increased induction of regulatory T cells (Treg).
Dendritic cells are bone marrow (BM)-derived leukocytes responsible for uptake, processing, and presentation of antigen to T cells (reviewed in Banchereau and Steinman, 1998; Bell et al., 1999; Lanzavecchia and Sallusto, 2001). Immature DC continuously sample the environment. When pathogens are encountered in an inflammatory environment, DC migrate to T cell zones of lymph nodes (LN) or spleen, and undergo maturational changes making them effective and unique activators of naive T cells. DC are also found in the thymus, where they play an important role in negative selection of developing T cells (Anderson et al., 1998; Brocker et al., 1997; Gallegos and Bevan, 2004).
Murine DC are identified by the expression of CD11c and major histocompatability complex (MHC) class II. In the spleen, immunostimulatory DC can be divided into conventional DC (cDC) and plasmacytoid DC (pDC) subsets based on differential expression of B220; cDC are B220- and pDC are B220+ (Anjuere et al., 1999; Martin et al., 2002; Vremec and Shortman, 1997; Vremec et al., 1992). Classical DC can be further subdivided based on CD11b, CD4, and CD8 expression (Heath et al., 2004; Kamath et al., 2002). Whereas all of these DC populations present antigen to T cells, some specialized functions have been described for various subsets. For example, CD8-cDC produce high levels of interferon (IFN)γ, whereas CD8+ cDC secrete high levels of interleukin (IL)-12 p40/p70 (Hochrein et al., 2001). pDC are the major source of IFNα following viral stimulation, leading to cytotoxic T lymphocyte activation (Dalod et al., 2003). CD8+ DC are the main DC subset found in the thymus (Wu et al., 1995) where they play a role in negative selection of thymocytes (Gallegos and Bevan, 2004). pDC have also been described in the thymus, but their function in that location is currently unclear (Asselin-Paturel et al., 2003).
While the detailed lineage of DC subsets is not entirely understood, it is clear that DC share a common origin with other hematopoietic cells at early BM precursor stages (Reid, 1997). Turnover rates of these DC subsets differ, although in general, all are quite short-lived compared with some B and T cell populations. Splenic cDC have a very rapid turnover rate of 3 to 4 days (Kamath et al., 2000), whereas thymic CD8+ DC and pDC have slightly longer lifespans with turnover rates of 10 and 14 days, respectively (Kamath et al., 2002; O’Keeffe et al., 2002).
The purpose of this study was to examine the effects of chronic EtOH exposure on DC numbers in various lymphoid tissues, and the mechanisms potentially responsible for observed changes. The Meadows-Cook EtOH in water model was employed (Blank et al., 1992; Meadows et al., 1992; Song et al., 2002), which allows long-term maintenance of mice on EtOH with appropriate weight gain and no evidence of steroid-induced stress (Cook et al., 2007). The results show that EtOH-fed mice had decreased numbers and frequencies of splenic DC but increased thymic DC numbers. Chronic EtOH exposure does not affect BM DC precursor numbers, and an EtOH environment does not influence the differentiation of DC from non-EtOH exposed BM precursors. Furthermore, DC turnover rates were unchanged by chronic EtOH feeding in both spleen and thymus, indicating that altered DC lifespan is not responsible for changes in DC numbers. Total cellularities of spleen and thymus were altered by EtOH feeding, and the size of the splenic or thymic compartment appears to play a substantial role in determining total DC numbers in these organs. Loss of splenic DC provides a potential mechanism for suboptimal T cell activation in response to pathogens, and increased thymic DC numbers could be a contributing factor to increased Treg in chronic EtOH-fed mice. Thus, both of these changes may provide novel means by which alcoholics are more susceptible to severe infections.
MATERIALS AND METHODS
Mice
Six- to seven-week-old female C57Bl/6 (CD45.2), C3H/HeJ, and BALB/c mice were obtained from the National Cancer Institute (Frederick, MD). Pep3bBoy/J (CD45.1 congenic mice on a C57Bl/6 background) mice were obtained from Jackson Laboratories (Bar Harbor, ME). All mice were maintained in a specific pathogen-free facility at the University of Iowa, and all animal procedures were approved by the animal care use committee at the University of Iowa.
EtOH Administration
After a 1-week acclimation period, control and EtOH groups were created by random separation of mice from the same lot. EtOH was provided in the drinking water as the sole water source at 10% (w/v) for 2 days, 15% for 5 days, and 20% for 1 to 28 weeks. All time-points for EtOH consumption are reported as duration on 20% EtOH. The mice were provided laboratory chow ad libitum in the bedding in all cases, and age-matched controls were given the same double-distilled water as that used for mixing the EtOH solutions (Cook et al., 2007).
Determination of DC Precursor Number
C57Bl/6 BM cells were flushed from femurs, washed with balanced salt solution (BSS), centrifuged through FicoLite-LM, and resuspended in sterile staining buffer (consisting of 5% newborn calf serum in BSS). Lineage– (lin–) cells containing DC precursors were purified to >98% using Miltenyi’s lineage cell depletion kit on the AutoMACS (Miltenyi Biotec, Auburn, CA). This kit contains biotinylated-anti-CD5, B220, CD11b, Gr-1, 7-4 (a neutrophil marker), and Ter-119 (lineage markers) followed by antibiotin magnetic microbeads and isolates lineage negative BM cells by negative selection.
Lin– cells were placed at 100 to 5000 cells/well into 96-well plates containing McCoy’s media enriched with 10% fetal calf serum, essential and nonessential amino acids, sodium pyruvate, minimal essential medium, vitamins, penicillin/streptomycin, glutamine, 2-mercapto-ethanol, IL-4 (0.5 ng/ml), and granulocyte-macrophage colony-stimulating factor (GM-CSF, 20 ng/ml) (Peprotech, Rocky Hill, NJ) using a Becton-Dickinson fluorescence-activated cell sorter (FACS) DiVa with a cloner platform. Plates were incubated at 37°C in a 5% CO2 incubator and scored after 7 days for cell growth and colony type.
Bone Marrow Reconstitution
Recipient C57Bl/6 (CD45.2) EtOH-fed mice and controls were lethally irradiated (split dose 500 cGy and 600 cGy, 4 h between doses) using a 81–16A JL Shepherd Co. (San Fernando, CA) irradiator with a 137Cs source, and reconstituted intravenously in the retro-orbital plexus with 1 ×106 nucleated BM cells from untreated donor Pep3bBoy/J (CD45.1 congenic) mice. EtOH or water feeding was continued following reconstitution for the respective groups throughout the course of the experiment. Spleen, thymus, LN, and peripheral blood (PB) DC numbers were assessed 2, 4, 8, and 12 weeks after reconstitution.
Bromodeoxyuridine Incorporation
C57Bl/6 EtOH-fed mice and controls were administered 5 mg bromodeoxyuridine (BrdU) (Sigma, St. Louis, MO) in saline intra-peritoneally (i.p.) on day 0, and maintained on 0.8 mg/ml BrdU in drinking water through day 14, at which time BrdU was discontinued. Tissues were harvested on day 1, 7, 14, 15, 18, and 21 after initiation of BrdU feeding, and stained for DC markers and BrdU using a BrdU flow kit (Becton Dickinson, San Diego, CA).
Flow Cytometric Reagents
The following monoclonal antibodies were used for 4 and 5-color flow cytometric analyses: N418, a hamster antimouse CD11c (Metlay et al., 1990); GK1.5, a rat IgG2b antimouse CD4 (Dialynas et al., 1983); 6B2, a rat IgG2a antimouse CD45R (B220) (Morse et al., 1982); M5/114.15.2, a rat IgG2b antimouse I-A and I-E (Bhattacharya et al., 1981); 104, a rat IgG2a antimouse CD45.2 (Shen, 1981); A20, a rat IgG2a antimouse CD45.1 (Shen, 1981); M1/70, a rat IgG2b antimouse CD11b (Mac-1) (Springer et al., 1979); and 536.72, a rat IgG2a antimouse CD8 (Ledbetter and Herzenberg, 1979). These monoclonal antibodies were prepared by ammonium sulfate precipitation from serum-free (HB101) culture supernatants. Polyclonal purified rat IgG (Jackson ImmunoResearch, West Grove, PA) was used for controls. The antibodies were conjugated with fluorescein isothiocyanate (FITC), biotin, phycoerythrin (PE), or cyanine 5.18 using standard protocols. PECy7-avidin was purchased from eBioscience (San Diego, CA).
Flow Cytometric Analysis
Spleen and thymus were injected with 1 ml of a solution containing liberase (0.1 U/ml)/DNAse (40 U/ml) (Roche Diagnostics, Indianapolis, IN) in RPMI 1640 (Invitrogen, Carlsbad, CA) and incubated for 30 minutes at 37°C. LN were minced and incubated in 1 ml of the same solution. Single cell suspensions were washed with BSS and centrifuged through Ficolite LM (Atlanta Biologicals, Lawrenceville, GA). Viable mononuclear cells were collected from the interface, washed in BSS, and resuspended in BSS containing 5% fetal calf serum and 0.1% NaN3. BM was prepared for staining as described under Determination of DC Precursor Number.
Staining was performed with 5 ×105 cells (spleen, thymus, LN, BM) or 50 to 70 μl of whole blood by incubating the cells for 20 minutes at 4°C with fluoresceinated, biotinylated, cyanine 5.18 conjugated, or PE-conjugated antibodies, followed by the appropriate avidin reagent. Equal volumes of 2.4G2, an anti-CD16/32 (FcγRIII/II) (Unkeless, 1979), and rat serum were added in the first incubation to eliminate background staining caused by Ab binding to FcγR. Following staining, erythrocytes were lysed using FACS Lysing Solution (BD Biosciences, San Jose, CA). Cells were fixed with 1% formaldehyde in 1.25×phosphate-buffered saline.
Cells from reconstituted mice were analyzed on a Becton-Dickinson FACS Vantage flow cytometer equipped with a primary argon laser and a rhodamine 6G CR599 dye head laser (Coherent, Palo Alto, CA) pumped by a secondary argon ion laser. All other flow cytometric experiments were analyzed on a Becton-Dickinson Calibur flow cytometer equipped with a primary 488 air-cooled laser and a secondary 635 diode laser. Residual dead cells and cell aggregates were excluded by low angle and orthogonal light scatter. At least 50,000 cells/sample were examined. Where necessary, spectral overlaps between FITC and PE; cyanine and Texas Red; and PE and PE-Cy7 were corrected with electronic compensation. The FACS data were collected in CellQuest (BD Biosciences, San Jose, CA) and analyzed using Flowjo (TreeStar, Ashland, OR). Final graphic output was obtained with Canvas software (Deneba Software, Miami, FL). Two-color contours are represented as 5% probability plots. All fluorescence intensities are represented on a 4-decade log scale.
Statistical Analysis
Two tailed Student’s t-test analyses were performed using instat software (GraphPad Software, San Diego, CA). Statistical significance = p ≤ 0.05.
RESULTS
Alterations in DC Numbers in EtOH-Fed Mice
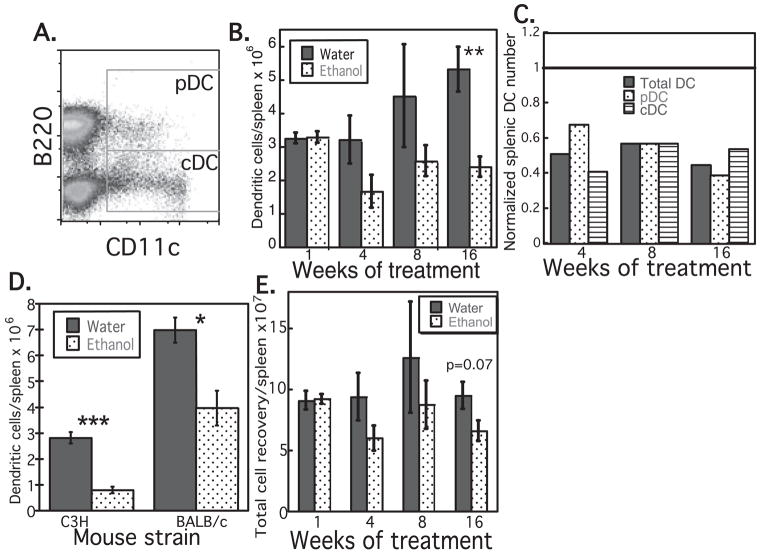
To determine whether quantitative deficiency of DC could contribute to diminished initiation of immune responses and increased risk of infection following chronic EtOH exposure, DC were counted in spleen, thymus, LN, and PB following 1 to 16 weeks of EtOH feeding, and in age-matched controls. As shown in Fig. 1, chronic EtOH exposure resulted in a 40 to 55% decrease in total splenic DC numbers in C57Bl/6 mice. This decrease was observed after as little as 4 weeks of EtOH feeding, and persisted to a similar degree through at least 16 weeks of EtOH exposure. The deficiency impacted both cDC and pDC to similar extents (Fig. 1C). Deficiencies in DC numbers were also identified in C3H and BALB/c mice (Fig. 1D). In all strains studied, loss of splenic DC was attributable partially to decreased DC frequency and partially to decreased total cellularity (and weight) of the spleen (Fig. 1E and data not shown). No EtOH-associated loss of splenic DC was observed at day 7 of EtOH feeding (Fig. 1B), thus this phenomenon appears to be associated specifically with chronic rather than subacute EtOH exposure.
Fig. 1.
Splenic dendritic cell (DC) numbers are decreased in ethanol (EtOH) fed mice, beginning at 4 weeks of treatment. (A) Gating strategy for splenic plasmacytoid DC (pDC; CD11cloB220+) and conventional DC (cDC; CD11c+B220-). Both populations are major histocompatability complex class II+ (data not shown). Total DC were determined as the combination of these 2 gates. (B) Decreased number of splenic DC in C57Bl/6 EtOH-fed mice, n = 4 to 11 mice/group. (C) Decreased number of splenic total DC, cDC, and pDC in C57Bl/6 EtOH-fed mice, normalized to the number found in age-matched controls (represented as 1). (D) Decreased number of total splenic DC in C3H and BALB/c EtOH-fed mice after 13 weeks of feeding. DC numbers in EtOH-fed mice normalized to water controls: C3H = 0.28, BALB/c = 0.57, n = 4 to 6 mice/group. (E) Decreased total splenocyte recovery from C57Bl/6 EtOH mice, n = 7 to 11 mice/group. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 for water vs. EtOH. Error bars = SEM.
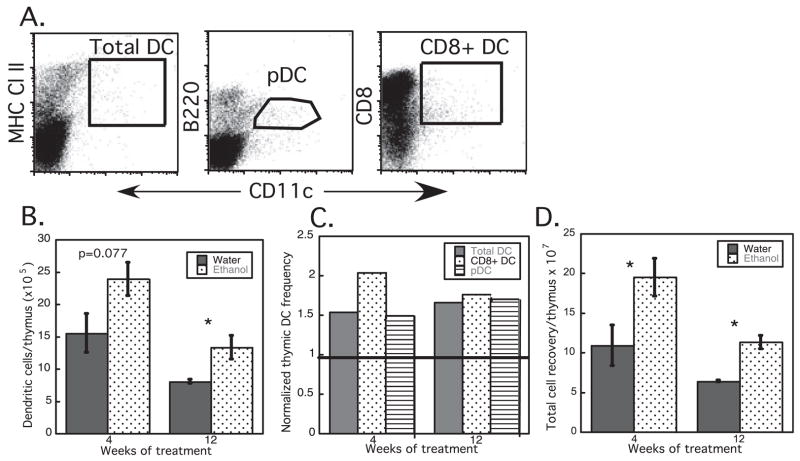
In contrast to splenic DC, thymic DC numbers were increased approximately 1.5×following chronic EtOH feeding, compared with age-matched water fed controls (Fig. 2). As with splenic DC, increases were evident in less than 4 weeks of EtOH feeding, and persisted to a similar degree for as long as 12 weeks. Age-related decreases in total thymic cellularity remained apparent in control and EtOH-fed mice, and contributed to decreased total thymic DC numbers between 4 and 12 weeks of treatment in both EtOH-fed and control groups. Both major DC subsets in the thymus (CD8+ DC and pDC) were increased to similar extents by EtOH exposure (Fig. 2C). Gain in thymic DC numbers after EtOH feeding appeared to be attributable primarily to increased total thymic cellularity (and weight) (Fig. 2D and data not shown).
Fig. 2.
Thymic dendritic cell (DC) numbers are increased in ethanol (EtOH)-fed mice, beginning at 4 weeks of treatment. (A) Gating strategy for total thymic DC (CD11c+ MHC Cl II+), plasmacytoid DC (pDC; CD11c+B220+), and CD8+ DC (CD11c+CD8+) subsets. (B) Increased number of total thymic DC in C57Bl/6 EtOH-fed mice. (C) Increased number of total DC, pDC, and CD8+ DC in thymi of EtOH-fed mice, normalized to the number found in age-matched controls (represented as 1). (D) Increased total thymocyte recovery from EtOH mice, n = 4 mice/group. *p ≤ 0.05 for water vs. EtOH. Error bars = SEM.
Dendritic cell numbers in peripheral (skin-draining) LN and in PB were also quantitated in EtOH-fed mice. No difference was noted in LN DC numbers through 16 weeks of EtOH feeding, and PB DC numbers were unaffected by EtOH exposure through at least 8 weeks of treatment, although at 16 weeks total DC and pDC in PB showed a trend towards decreased numbers in EtOH-fed mice relative to controls (data not shown).
Alterations in splenic and thymic DC numbers resulting from EtOH feeding could be because of changes in DC precursor cell numbers or differentiation ability, or altered DC lifespan. To date, no published information is available regarding the effects of chronic EtOH exposure on dendropoiesis or DC turnover rates. Thus, further studies were undertaken to address these possible mechanisms for altered DC numbers following chronic EtOH feeding.
Effects of EtOH on BM DC Precursor Frequency
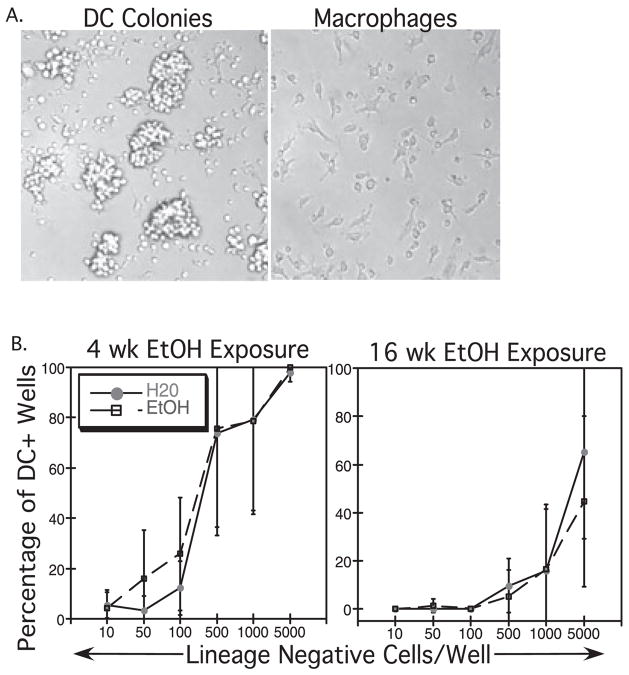
To determine whether the altered DC numbers seen in Et-OH-exposed mice were because of an effect of EtOH on BM DC precursor frequency, purified lin– BM cells from control and EtOH-fed mice [containing early DC precursors, including the common myeloid progenitors (CMP) and common lymphoid progenitors (CLP)] (Manz et al., 2001) were cultured at increasing concentrations in the presence of GM-CSF and IL-4 for 7 days. This combination of cytokines is commonly used to support the in vitro differentiation of cDC. Wells were scored for presence of DC colonies and macrophages which were identified by characteristic morphology, as seen in Fig. 3A. DC were identified as small, round cells seen either in clusters or individually. Macrophages were unclustered, large cells with elongated pseudopodia, adherent to the bottom of the wells. The percentages of wells containing DC corresponding to each precursor cell number initially placed into the well are shown in Fig. 3B. EtOH and water fed mice showed no difference in the frequency of DC precursors at 4, 8, or 16 weeks of EtOH exposure (Fig. 3B and data not shown). The number of total lin– precursor cells recovered from BM, as well as total BM cellularity was not different between EtOH and water fed mice (data not shown). DC colony sizes arising from individual lin– BM cells were generally equivalent between EtOH and control groups (data not shown). Thus, the loss of splenic cDC numbers cannot be attributed to decreased DC precursor frequency in the BM of EtOH-fed mice. While the frequency of pDC precursors was not specifically tested in an assay in which pDC are produced in vitro, pDC and cDC are ultimately thought to arise primarily from the same flt3+ myeloid precursor (D’Amico and Wu, 2003). Thus it is unlikely that alterations in pDC numbers can be explained by altered precursor frequency.
Fig. 3.
Bone marrow (BM) dendritic cell (DC) precursor frequency is not altered following ethanol (EtOH) treatment. Lineage negative (lin–) BM cells were purified and placed at limiting dilution into granulocyte-macrophage colony-stimulating factor/interleukin-4 containing media. Wells were scored for DC colonies after 7 days of culture. (A) DC and macrophages were easily distinguished in vitro by morphology. (B) The percentages of wells with DC colonies were compared with the number of lin– precursor cells initially plated in each well. For example, after 4 weeks of EtOH feeding, 100% of the wells contained DC colonies when 5000 lin– precursors were placed in the well. Lin– BM from mice treated with EtOH for 4 or 16 weeks showed an equivalent frequency of DC precursors compared with control mice. Data shown are representative of at least 3 experiments/time-point. Error bars = SD.
Effects of an EtOH Environment on DC Precursor Differentiation
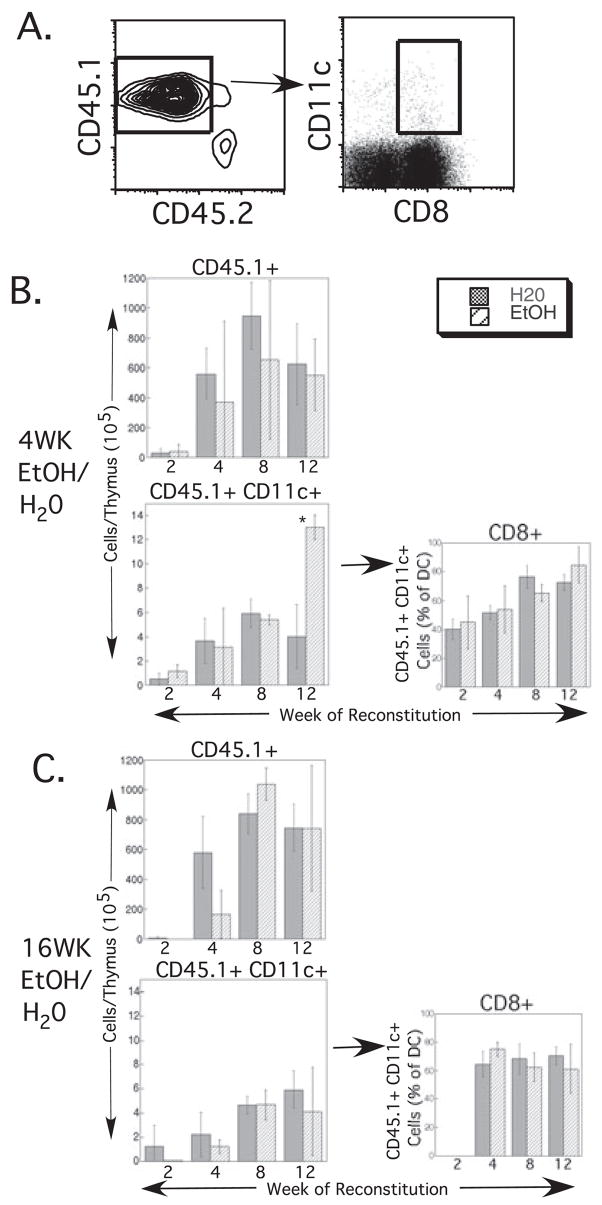
An alternate explanation for decreased splenic DC numbers in EtOH mice (Fig. 1) that does not require an adverse effect of EtOH directly on DC precursors is that chronic EtOH feeding alters factors in the BM or splenic environment and thereby indirectly decreases splenic DC production from precursor cells. Similarly, alterations in the BM or thymic environment because of EtOH feeding could result in the observed increase in thymic DC numbers (Fig. 2). As thymic and splenic DC with similar phenotypes do not follow identical differentiation pathways (Ardavin et al., 1993; D’Amico and Wu, 2003; Wu et al., 1995), it is formally possible that alterations in the BM environment could simultaneously result in decreases in splenic DC and increases in thymic DC. To examine the effect of an EtOH environment on the differentiation of normal BM cells (including DC precursors), BM cells from untreated congenic donors were transferred into EtOH-fed or control recipient mice after lethal irradiation. Recipient mice continued their respective treatment (EtOH or water feeding) following reconstitution. Donor reconstitution was examined at 2, 4, 8, and 12 weeks after irradiation. Dendritic cell content of spleen and thymus, as well as LN and PB were examined at each time-point. Donor-derived cells were identified as CD45.1+ (Figs 4A and 5A).
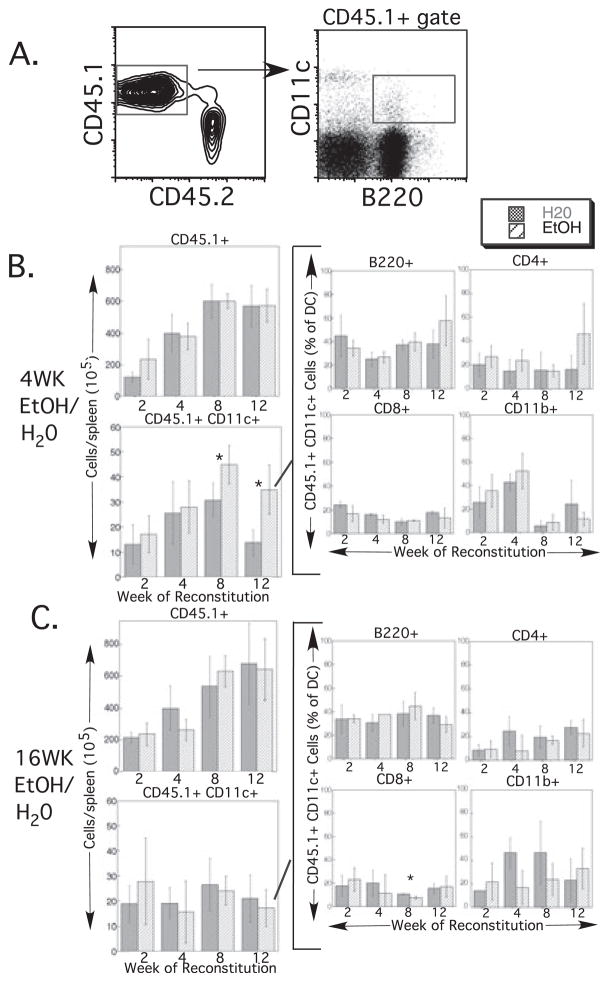
Fig. 4.
Splenic dendritic cell (DC) reconstitution by untreated bone marrow (BM) precursors is unchanged by ethanol (EtOH) feeding. (A) Representative gating strategy used for identifying donor-derived DC subsets following BM reconstitution. CD45.1+ (donor-derived) cells were gated, and within that population CD11c+ cells (DC) that were also positive for each of the subset markers (B220, shown; CD8, CD4, or CD11b) were gated. (B) Reconstitution of 4-week- and (C) 16-week-EtOH-treated recipients and controls. Weeks on EtOH represent length of feeding at the time of BM transfer; weeks of reconstitution represent additional time on the same (EtOH or water) regimen after BM transfer. For each exposure period, the upper left graph shows the total number of donor-derived splenocytes at each time-point examined. The lower left graph represents total donor-derived splenic DC numbers, which were then subdivided into 4 DC subsets (center and right columns of graphs). Time-points with error bars had ≥3 mice/group. The 2 time-points without error bars represent 2 mice/group. Error bars = SD. *p ≤ 0.05 for water vs. EtOH.
Fig. 5.
Thymic dendritic cell (DC) reconstitution by untreated bone marrow (BM) precursors is unchanged by ethanol (EtOH) feeding. (A) Representative gating strategy used for identifying donor-derived DC subsets following BM reconstitution. CD45.1+ (donor-derived) cells were gated, and within that population CD11c+ cells that were also CD8+ were gated. (B). Reconstitution of 4-week- (C) and 16-week-EtOH-treated recipients and controls. Weeks on EtOH represent length of feeding at the time of BM transfer; weeks of reconstitution represent additional time on the same (EtOH or water) regimen since BM transfer. For each exposure period, the upper left graph shows the total number of donor-derived thymocytes at each time-point examined. The lower left graph represents total donor-derived thymic DC numbers, which were then assessed for the CD8+ DC subset (graphs in the right column). n ≥ 3 mice/group. CD8+ DC percentages were not determined at 2 weeks after BM transfer in 16-week-EtOH-fed- or control mice because of low total cell counts. Error bars = SD. *p ≤ 0.05 for water vs. EtOH.
In the spleen, 4 or 16 weeks of EtOH feeding prior to BM transfer did not affect total donor-derived splenic cell numbers, as seen in the upper left plots in Fig. 4B and 4C. Reconstitution proceeded as expected with increasing donor cell numbers from 2 to 8 weeks, and then counts leveled off from 8 to 12 weeks. Total DC numbers were also not adversely affected by EtOH treatment (lower left plots, Fig. 4B and 4C). Surprisingly, DC actually were present in higher numbers at late time-points following BM transfer to 4-week EtOH-fed mice compared with controls. All analyzed subsets of DC (B220+, CD4+, CD8+, and CD11b+) were present at all time-points after reconstitution in both EtOH-fed and control mice (Fig. 4B and 4C, center and right plots). A statistically significant decrease in the fraction of DC from 16-week-EtOH-fed mice that were CD8+ was seen at 8 weeks after reconstitution (Fig. 4C), but the biologic significance of the small difference is likely to be minimal, and the difference disappeared by 12 weeks after reconstitution. BM of reconstituted mice was studied at the same time-points for donor-derived populations containing early hematopoietic precursors, B cells, T cells, myeloid cells, and DC. Reconstitution proceeded as expected with increasing cell numbers from 2 to 8 weeks and a plateau at 12 weeks, and no differences between control and EtOH-fed recipients were identified (data not shown). In summary, a chronic EtOH environment had no adverse effect on the ability of untreated BM cells to reconstitute the BM compartment or produce splenic DC, indicating that the observed decrease in splenic DC numbers in EtOH-fed mice (Fig. 1) is unlikely to be the result of adverse environmental changes on DC differentiation in the BM or spleen.
In the thymus, 4 or 16 weeks of EtOH exposure did not significantly alter total donor-derived cell numbers after reconstitution, as seen in Fig. 5B and 5C (upper left plots). As in the spleen, reconstitution proceeded as expected with increasing donor cell numbers from 2 to 8 weeks, followed by stable numbers from 8 to 12 weeks. Four-week EtOH-fed mice had significantly higher donor-derived DC numbers at 12 weeks after reconstitution compared with control mice (Fig. 5B, lower left plot), a finding that did not persist in 16 week EtOH-fed mice (Fig. 5C, lower left plot). The frequency of CD8+ DC, the major thymic DC subset, as a fraction of total thymic DC remained unaffected by EtOH exposure (Fig. 5B and 5C, right plots). Thus, chronic EtOH feeding did not promote the ability of normal BM cells to produce thymic DC, indicating that the observed increase in thymic DC numbers in EtOH-fed mice (Fig. 2) is unlikely to be the result of adverse environmental changes on DC differentiation in the BM or thymus.
Although no significant differences were seen in LN or PB DC numbers following 4, 8, or 16 weeks of EtOH feeding, DC numbers were evaluated in these tissues in the EtOH-fed and control mice that received normal BM transfer as part of their thorough evaluation. No significant differences or persistent trends in donor DC numbers and/or percentages were identified in these locations (data not shown).
Effects of EtOH and Radiation on Total Splenic and Thymic Cellularity
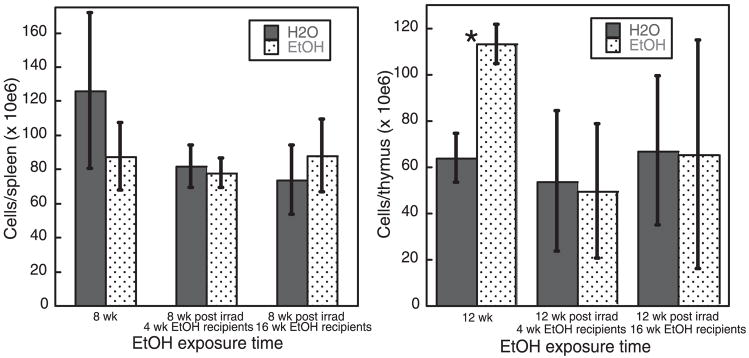
On the basis of the data in Figs 1 and 2 indicating EtOH-induced alterations in splenic and thymic DC numbers as early as 4 weeks following initiation of EtOH feeding, we anticipated that by late time-points following BM transfer, the number of splenic DC found in EtOH-fed recipients would decrease compared with controls, and the number of thymic DC would increase as the transferred BM cells and their progeny became chronically EtOH-exposed. However, with the exception of thymic DC found at 12 weeks after BM transfer in mice that were EtOH-fed for 4 weeks prior to transfer (Fig. 5B), this did not occur. Instead, the numbers of donor-derived DC were generally equivalent between EtOH-fed and control mice, following BM transfer. To determine if this effect was specific to DC or more generally applicable to total organ cellularity, total splenic and thymic cellularity of EtOH-fed and control mice that received irradiation and BM transfer were compared with these cell numbers in otherwise untreated EtOH-fed and control mice (Fig. 6). The BM and its progeny in each pair of bars received equivalent EtOH (or water) exposure (although the total EtOH exposure of the mice varied depending on when the BM transfer occurred). As shown in Fig. 6 (first pair of bars in each plot), total thymic and splenic cellularity of EtOH-fed mice that received no additional treatment paralleled DC numbers found in these locations (Figs 1 and 2). Total splenic cellularity was decreased in EtOH-fed mice, and total thymic cellularity was increased. However, following irradiation and BM transfer, continued EtOH feeding had no effect on the total cellularity of the organs (Fig. 6, second and third pair of bars in each plot). The lack of difference in total organ cellularity parallels the lack of difference in donor-derived DC numbers following BM transfer into EtOH-fed or control mice (Figs 4 and 5). Thus, we hypothesize that the size or total cellularity of the organ is a major determinant in the number of DC present within the organ. EtOH feeding clearly alters organ cellularity, contributing to the observed alterations in splenic and thymic DC numbers in otherwise unmanipulated mice. However, following irradiation, EtOH appears to have no additional impact on organ cellularity, leading to no difference in DC numbers in this environment.
Fig. 6.
Total splenic and thymic cellularity in nonirradiated ethanol (EtOH)-fed and control mice, and in similar mice following reconstitution. Cell counts were performed at the indicated time-points. Plotted time-points represent equivalent amounts of EtOH exposure of hematopoietic precursors, either in the absence of or following lethal irradiation. Error bars = SEM. *p ≤ 0.05 for water vs. EtOH, n = 4 mice/time-point.
Effects of EtOH on DC Turnover
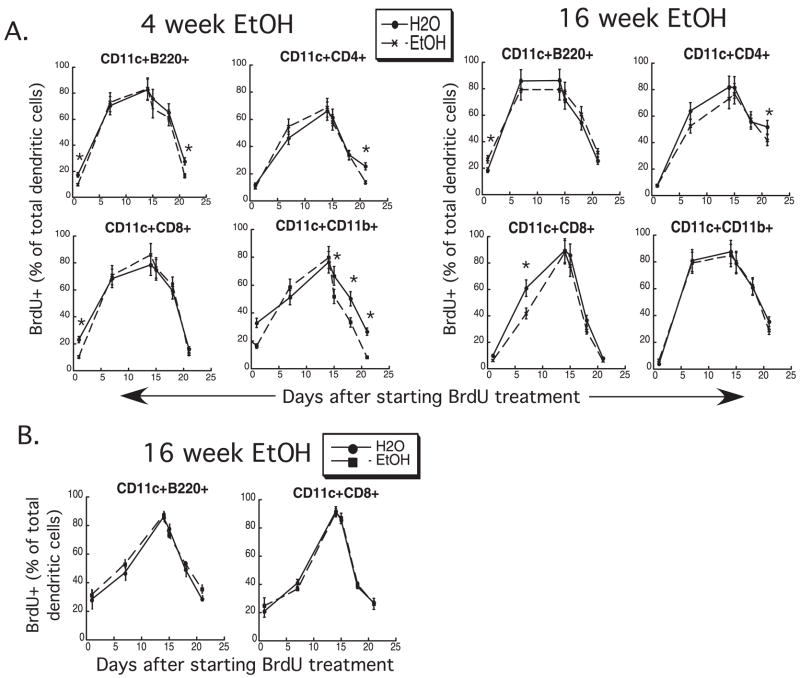
The data discussed so far provide evidence that altered DC numbers are not the result of altered dendropoiesis. Quantitative changes in DC numbers could also be affected by alterations in DC turnover rates in spleen and/or thymus. To examine whether EtOH affects DC turnover rates, 4- and 16-week-EtOH-fed mice were given an i.p. bolus of BrdU and then maintained on BrdU for up to 14 days. BrdU administration was then discontinued, and some mice were followed for up to a week later. Spleens and thymi were harvested on various days after BrdU administration was initiated. BrdU incorporation rate measures DC production from BM precursors that incorporate BrdU prior to entry into the spleen, as well as DC production from local proliferation (Liu et al., 2007), (Diao et al., 2007), and rate of BrdU loss measures primarily death rate for DC, as these cells are largely terminally located in spleen and thymus and only a tiny fraction migrate from these tissues (Kamath et al., 2000).
As seen in Fig. 7A, BrdU incorporation in EtOH-exposed splenic DC showed no consistent decrement compared with controls. B220+ DC (pDC) showed significantly less incorporation of BrdU on the first day of administration in both 4-and 16-week EtOH-fed mice, but the difference did not persist until day 7 of BrdU administration. BrdU loss from CD11b+ DC, a subset of cDC, was accelerated in 4-week EtOH-fed mice, indicating a potential contribution of increased death rate to the decreased splenic DC numbers seen at this time-point (Fig. 1). However, this effect did not persist at 16 weeks of EtOH feeding, thus its overall contribution to persistently decreased splenic DC numbers is probably minimal.
Fig. 7.
Dendritic cell (DC) turnover is unaffected by ethanol (EtOH) feeding. DC were labeled with bromodeoxyuridine (BrdU) by a single bolus injection followed by 14 days of BrdU in the drinking water. On day 15, BrdU was removed from the water source, and DC was assessed for loss of labeling over the subsequent week. (A) Splenic DC turnover. (B) Thymic DC turnover. *p ≤ 0.05 for water vs. EtOH. Error bars = SD, n = 3 mice/time-point.
Bromodeoxyuridine incorporation and loss from thymic DC was studied only at 16 weeks of EtOH feeding, and no effect from EtOH on BrdU incorporation or loss was noted on either thymic DC subset (Fig. 7B). Thus increased production and/or decreased rate of DC death do not appear to contribute to the increased thymic DC numbers in chronic EtOH-fed mice (Fig. 2).
DISCUSSION
The negative effects of chronic EtOH exposure on dendritic cell function, and the contribution that these alterations may have on the ability to combat infection are beginning to be appreciated (Aloman et al., 2007; Laso et al., 2007; Lau et al., 2006, 2007; Ness et al., 2008). Alcoholic humans with liver disease have decreased numbers of DC in PB (Laso et al., 2007). Mice maintained on modified Lieber-deCarli diets for 11 days show no loss in the percentage of splenic DC (Heinz and Waltenbaugh, 2007), but to date, only 1 report exists regarding the effect of chronic EtOH feeding on DC numbers in murine models of chronic alcoholism. When mice are maintained on complete liquid (Lieber-DeCarli) diets for 8 weeks and simultaneously infected with a Flt3L-producing plasmid, EtOH-fed mice had decreased splenic DC numbers (Aloman et al., 2007). Numerous reports indicate that corticosterone levels were elevated in such feeding regimens (Jerrells et al., 1990; Kruger and Jerrells, 1994; Padgett et al., 2000; Tabakoff et al., 1978), and this alteration may potentially have contributed to the observed decrease in DC numbers in the Aloman study. Lack of sufficient DC to process and present antigen to naive T cells would be expected to decrease the likelihood of efficient T cell responses to pathogens. The purpose of this study was to determine if DC numbers in primary and secondary lymphoid tissues are altered following chronic EtOH feeding in the absence of steroid-induced stress, and to investigate the mechanisms by which any observed quantitative DC alterations might occur.
Chronic EtOH feeding using an EtOH in water model that allows treatment of mice for many months without evidence of steroid-related stress effect (Cook et al., 2007) resulted in decreased splenic DC numbers in multiple murine strains in as little as 4 weeks (Fig. 1). These results corroborate and extend the previous report of decreased splenic DC in chronic EtOH-fed mice (Aloman et al., 2007) in an environment where steroid-related stress is not a potential contributing factor. Decreased DC numbers in the Meadows-Cook model do not correlate with alterations in alanine transaminase (ALT) as seen in humans (Laso et al., 2007), as mice are in general more resistant to EtOH-induced liver pathology than humans, and ALT levels are unaffected at up to 21 weeks of EtOH feeding (Song et al., 2002 and RC, unpublished observations). The relative loss of splenic DC was greatest in C3H mice, whereas C57Bl/6 and BALB/c mice showed similar degrees of DC loss (see Fig. 1C and legend to Fig. 1D). The observed strain differences may be because of differential EtOH consumption, metabolism, and/or sensitivity of the lymphoid environment (including DC) to the toxic effects of EtOH or its metabolites. Loss of splenic DC numbers is anticipated to result in decreased naïve antigen-specific T cell activation and a poorer adaptive immune response to pathogens, providing a mechanism to explain increased incidence and severity of infections in chronic alcoholics. In fact, the effect of DC loss may be amplified at the T cell activation stage of the immune response, as a 2-fold loss of DC has been reported to result up to 4-fold decrease in activated T cells (Martín-Fontecha et al., 2003). Furthermore, multiple DC encounters promote increased T cell activation and IFNγ production, thus decreased DC numbers might be expected to result in a qualitatively deficient T cell response (Celli et al., 2005).
Several DC intrinsic mechanisms could be responsible for the observed EtOH-induced decrease in splenic DC, including decreased DC precursor frequency or differentiation, altered migration through the blood from BM to spleen, and/or increased DC death. Examination of splenic DC precursor frequency and turnover (Figs 3 and 7) revealed no differences between EtOH-fed and control DC. The ability of EtOH-exposed DC to migrate into spleen was not specifically examined in this study, and remains a potential mechanism that may contribute to decreased splenic DC numbers. However, following subcutaneous transfer, EtOH exposure for up to 8 weeks in the Meadows-Cook model had no effect on the ability of splenic DC to traffic to the draining lymph node (Lau et al., 2007). Furthermore, if a DC-intrinsic migration defect contributed significantly to decreased homeostatic splenic DC numbers in EtOH-fed mice, this should have been reflected in some degree of decreased BrdU incorporation, as most of the BrdU+ DC acquired this label in the BM rather than after entry to the spleen (Liu et al., 2007).
Additionally, environmental differences might influence DC number, including factors that influence DC differentiation from precursors, and factors that influence the size of the compartment that is being filled with DC. The first possibility was excluded by the observation of no difference in DC numbers produced from untreated BM transferred into EtOH and control mice (Fig. 4). However, this study supports the possibility that decreased compartment size is a large contributor to decreased splenic DC numbers in EtOH-fed mice (Fig. 6). Homeostatic factors that maintain splenic volume following organogenesis are not entirely understood. Lymphotoxin α1β2 (LTα1β2)-LTβ receptor (LTβR) interactions clearly contribute to the maintenance of normal spleen size, as disruption of this interaction leads to lower weight spleens (Ettinger et al., 1996). Furthermore, disruption of this axis results in decreased splenic DC numbers (Abe et al., 2003; Kabashima et al., 2005; Wu et al., 1999). Additional contributors to the maintenance of normal DC numbers in the spleen include CXCL13, CCL19, and CCL21 produced by B cells and splenic stromal cells. Absence of these chemokines leads to loss of splenic DC (Ngo et al., 1999; Yu et al., 2002), and decreased levels might contribute specifically to the decreased percentage of DC found following chronic EtOH feeding (independent of overall spleen size). Complete disruption of LT or homeostatic chemokine interaction with their receptors results in variable degrees of disrupted splenic architecture (Muller et al., 2003), which we have not observed prior to 32 weeks of EtOH feeding in our model (T. Waldschmidt, AJS and KJN, unpublished observations). However, partial disruption of these axes by EtOH might result in decreased splenic size and DC numbers while maintaining grossly normal architecture.
In contrast to the spleen, thymic DC were increased in number following chronic EtOH exposure (Fig. 2). Thymic DC play an important role in negative selection of both CD4+ and CD8+ thymocytes (Gallegos and Bevan, 2004). They originate from 2 sources: differentiation from an intra-thymic precursor that is likely to give rise to thymocytes, and circulating immature DC that carry extrathymic antigen from peripheral tissues to the thymic medulla (Bonasio et al., 2006; Donskoy and Goldschneider, 2003). The presence of the latter DC increases the frequency of Treg in the thymus. Although we did not specifically attempt to distinguish these 2 thymic DC subsets, total DC, CD8+ DC, and pDC were equivalently increased in thymi of EtOH-fed mice, suggesting that both lineages of thymic DC are affected by EtOH exposure. Increased thymic DC numbers as a result of chronic EtOH feeding provides a potential mechanism that could contribute to the increase in natural Treg observed in this EtOH feeding model (RC and J. Colgan, unpublished observations).
Investigation into the mechanism(s) by which thymic DC are increased in the presence of chronic EtOH exposure revealed no evidence for an environmental effect on the ability of BM DC precursors (the ultimate source of both thymic DC lineages) to produce DC (Fig. 5) or altered thymic DC turnover rates (Fig. 7). Rather, as for the spleen, the size of the thymic compartment appears to be a major contributor in the increase in thymic DC number (Fig. 6). We have previously noted an increase in thymic cellularity at early time-points in the Meadows-Cook model (Cook et al., 2007). Factors controlling thymic size in adulthood include sex steroids which are well-known to contribute to thymic atrophy. The effect of chronic EtOH feeding on sex steroid levels remains unknown. Clearly, they are not entirely absent, as mice maintained on the Meadows-Cook EtOH regimen are fertile (R.C. and B. Young, unpublished observation). Furthermore, thymic DC numbers in EtOH-fed mice do decline between 4 and 12 weeks of EtOH feeding, in parallel to the decline seen in control mice. These changes are likely because of aging effects on the thymus. However, diminished sex steroid levels could contribute to increased thymic size in chronic EtOH-fed mice.
As in the spleen, it is possible that altered migration of DC into the thymus contributes to increased DC numbers. The specific chemokines involved in normal DC migration from peripheral tissues into thymus remain unknown, but they are Gαi-mediated and pertussis toxin-sensitive, similar to chemokines mediating lymphocyte homeostasis in lymphoid tissue (von Andrian and Mackay, 2000; Bonasio et al., 2006). As the chemokines that mediate normal thymic DC migration are identified, levels present in thymi of EtOH-fed mice will be of interest.
The data presented here show for the first time that DC numbers are quantitatively increased in thymus, and confirm that DC numbers are decreased in spleen by EtOH feeding in a murine model of chronic alcoholism. Additionally, we have previously shown that Langerhans cell numbers are significantly decreased in this same model (Ness et al., 2008). Together, these changes would be expected to favor a decreased activation of adaptive immunity following pathogen encounter, and potentially increased production of Treg, leading to active suppression of immunity. Furthermore, we provide evidence indicating that chronic EtOH exposure does not affect DC precursor numbers or differentiation, or turnover rates within spleen or thymus. Rather, total splenic or thymic compartment size appears to be a major contributor to DC numbers in these organs, with a smaller contribution (leading to decreased frequency of splenic DC) potentially the result of altered homeostatic DC migration. The role of LT and homeostatic chemokines in this process remains to be investigated.
Acknowledgments
This work is part of collaborative projects in alcohol immunology funded by NIH Interactive Research Program Grants AA-014405 (RTC), AA-014400 (T. J. Waldschmidt), AA-014406 (AJS), AA-014418 (Z. K. Ballas), University of Iowa Carver College of Medicine; and AA-012450 (T.R. Jerrells), University of Nebraska Medical Center. We thank Ruth Coleman for excellent mouse colony maintenance, and Teresa Duling for expertise with flow cytometry.
This work was supported by NIH RO1 AA014405 and AA014406.
References
- Abe K, Yarovinsky FO, Murakami T, Shakhov AN, Tumanov AV, Ito D, Drutskaya LN, Pfeffer K, Kuprash DV, Komschlies KL, Nedospasov SA. Distinct contributions of TNF and LT cytokines to the development of dendritic cells in vitro and their recruitment in vivo. Blood. 2003;101:1477–1483. doi: 10.1182/blood.V101.4.1477. [DOI] [PubMed] [Google Scholar]
- Aloman C, Gehring S, Wintermeyer P, Kuzushita N, Wands JR. Chronic ethanol consumption impairs cellular immune responses against HCV HS5 protein due to dendritic cell dysfunction. Gastroenterol. 2007;132:698–708. doi: 10.1053/j.gastro.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Anderson G, Partington KM, Jenkinson EJ. Differential effects of peptide diversity and stromal cell type in positive and negative selection in the thymus. J Immunol. 1998;161:6599–6603. [PubMed] [Google Scholar]
- von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. New Engl J Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- Anjuere F, Martin P, Ferrero I, Fraga ML, del Hoyo GM, Wright N, Ardavin C. Definition of dendritic cell subpopulations present in the spleen, Peyer’s patches, lymph nodes, and skin of the mouse. Blood. 1999;93:590–598. [PubMed] [Google Scholar]
- Ardavin C, Wu L, Li C-L, Shortman K. Thymic dendritic cells and T cells develop simultaneously in the thymus from a common precursor population. Nature. 1993;362:761–763. doi: 10.1038/362761a0. [DOI] [PubMed] [Google Scholar]
- Asselin-Paturel C, Brizard G, Pin J-J, Briere F, Trinchieri G. Mouse strain differences in plasmacytoid dendritic cell freequency and function revealed by a novel monoclonal antibody. J Immunol. 2003;171:6466–6477. doi: 10.4049/jimmunol.171.12.6466. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Bell D, Young JW, Banchereau J. Dendritic cells. Adv Immunol. 1999;72:255–324. doi: 10.1016/s0065-2776(08)60023-1. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Dorf ME, Springer TA. A shared alloantigenic determinant on Ia antigens encoded by the I-A and I-E subregions: evidence for I region gene duplication. J Immunol. 1981;127:2488–2495. [PubMed] [Google Scholar]
- Blank SE, Johannson J-O, Origines MM, Meadows GG. Modulation of NK cell activity by moderate intensity endurance training and chronic ethanol consumption. Am Physiol Soc. 1992;72:8–14. doi: 10.1152/jappl.1992.72.1.8. [DOI] [PubMed] [Google Scholar]
- Bonasio R, Scimone ML, Schaerli P, Grabie N, Lichtman AH, von Andrian UH. Clonal deletion of thymocytes by circulating dendritic cells homing to thymus. Nat Immunol. 2006;7:1092–1100. doi: 10.1038/ni1385. [DOI] [PubMed] [Google Scholar]
- Brocker T, Riedinger M, Karjalainen K. Targeted expression of major histocompatibility complex (MHC) class II molecules demonstrates that dendritic cells can induce negative but not positive selection of thymocytes in vivo. J Exp Med. 1997;185:541–550. doi: 10.1084/jem.185.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celli S, Garcia Z, Bousso P. CD4 T cells integrate signals delivered during successive DC encounters in vivo. J Exp Med. 2005;202:1271–1278. doi: 10.1084/jem.20051018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RT. Alcohol abuse, alcoholism, and damage to the immune system – a review. Alcohol Clin Exp Res. 1998;22:1927–1942. [PubMed] [Google Scholar]
- Cook RT, Schlueter AJ, Coleman RA, Tygrett L, Ballas ZK, Jerrells TR, Nashelsky MB, Ray NB, Haugen RH, Waldschmidt TJ. Thymocytes, pre-B cells and organ changes in a mouse model of chronic ethanol ingestion. Absence of subset-specific glucocorticoid-induced immune cell loss. Alcohol Clin Exp Res. 2007;31:1746–1758. doi: 10.1111/j.1530-0277.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amico A, Wu L. The early progenitors of mouse dendritic cells and plasmacytoid predendritic cells are within the bone marrow hematopoietic precursors expressing flt3. J Exp Med. 2003;198:293–303. doi: 10.1084/jem.20030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalod M, Hamilton T, Salomon R, Salazar-Mather TP, Henry SC, Hamilton JD, Biron CA. Dendritic cell responses to early murine cytomegalovirus infection: subset functional specialization and differential regulation by interferon α/β. J Exp Med. 2003;197:885–898. doi: 10.1084/jem.20021522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dialynas DP, Quan ZS, Wall KA, Pierres A, Quintans J, Loken MR, Pierres M, Fitch FW. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983;131:2445–2451. [PubMed] [Google Scholar]
- Diao J, Winter E, Chen W, Xu F, Cattral MS. Antigen transmission by replicating antigen-bearing dendritic cells. J Immunol. 2007;179:2713–2721. doi: 10.4049/jimmunol.179.5.2713. [DOI] [PubMed] [Google Scholar]
- Dolganiuc A, Kodys K, Kopasz A, Marshall C, Mandrekar P, Szabo G. Additive inhibition of dendritic cell allostimulatory capacity by alcohol and hepatitis C is not restored by DC maturation and involves abnormal IL-10 and IL-2 induction. Alcohol Clin Exp Res. 2003;27:1023–1031. doi: 10.1097/01.ALC.0000071745.63433.32. [DOI] [PubMed] [Google Scholar]
- Donskoy E, Goldschneider I. Two developmentally distinct populations of dendritic cells inhabit adult mouse thymus: demonstration by differential importation of hematogenous precursors under steady-state conditions. J Immunol. 2003;170:3514–3521. doi: 10.4049/jimmunol.170.7.3514. [DOI] [PubMed] [Google Scholar]
- Ettinger R, Browning JL, Michie SA, van Ewijk W, McDevitt HO. Disrupted splenic architecture, but normal lymph node development in mice expressing a soluble lymphotoxin-β receptor-IgG1 fusion protein. Proc Natl Acad Sci USA. 1996;93:13102–13107. doi: 10.1073/pnas.93.23.13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos AM, Bevan MJ. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J Exp Med. 2004;200:1039–1049. doi: 10.1084/jem.20041457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath WR, Belz GT, Behrens GMN, Smith CM, Forehan IA, Parish IA, Davey GM, Wilson NS, Carbone FR. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- Heinz R, Waltenbaugh C. Ethanol-consumption modifies dendritic cell antigen presentation in mice. Alcohol Clin Exp Res. 2007;31:1759–1771. doi: 10.1111/j.1530-0277.2007.00479.x. [DOI] [PubMed] [Google Scholar]
- Hochrein H, Shortman K, Vremec D, Scott B, Hertzog P, O’Keeffe M. Differential production of IL-12, IFN-α, and IFN-γ by mouse dendritic cell subsets. J Immunol. 2001;166:5448–5455. doi: 10.4049/jimmunol.166.9.5448. [DOI] [PubMed] [Google Scholar]
- Jerrells TR, Smith W, Eckardt MJ. Murine model of ethanol-induced immunosuppression. Alcohol Clin Exp Res. 1990;14:546–550. doi: 10.1111/j.1530-0277.1990.tb01197.x. [DOI] [PubMed] [Google Scholar]
- Kabashima K, Banks TA, Ansel KM, Lu TT, Ware CF, Cyster JG. Intrinsic lymphotoxin-β receptor requirement for homeostasis of lymphoid tissue dendritic cells. Immunity. 2005;22:439–450. doi: 10.1016/j.immuni.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Kamath AT, Henri S, Battye F, Tough DF, Shortman K. Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood. 2002;100:1734–1741. [PubMed] [Google Scholar]
- Kamath AT, Pooley J, O’Keeffe MA, Vremec D, Zhan Y, Lew AM, D’Amico A, Wu L, Tough DF, Shortman K. The development, maturation, and turnover rate of mouse spleen dendritic cell populations. J Immunol. 2000;165:6762–6770. doi: 10.4049/jimmunol.165.12.6762. [DOI] [PubMed] [Google Scholar]
- Kruger TE, Jerrells TR. Effects of ethanol consumption and withdrawal on B cell subpopulation in murine bone marrow. Clin Exp Immunol. 1994;96:521–527. doi: 10.1111/j.1365-2249.1994.tb06060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106:263–266. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- Laso FJ, Vaquero JM, Almeida J, Marcos M, Orfao A. Chronic alcohol consumption is associated with changes in the distribution, immunopheno-type, and the inflammatory cytokine secretion profile of circulating dendritic cells. Alcohol Clin Exp Res. 2007;31:846–854. doi: 10.1111/j.1530-0277.2007.00377.x. [DOI] [PubMed] [Google Scholar]
- Lau AH, Abe M, Thomson AW. Ethanol affects the generation, cosignaling molecule expression, and function of plasmacytoid and myeloid dendritic cell subsets in vitro and in vivo. J Leukoc Biol. 2006;79:941–953. doi: 10.1189/jlb.0905517. [DOI] [PubMed] [Google Scholar]
- Lau AH, Thomson AW, Colvin BL. Chronic ethanol exposure affects in vivo migration of hepatic dendritic cells to secondary lymphoid tissue. Hum Immunol. 2007;68:577–585. doi: 10.1016/j.humimm.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Ledbetter JA, Herzenberg LA. Xenogeneic monoclonal antibodies to mouse differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Liu K, Waskow C, Liu X, Yao K, Hoh J, Nussenzweig M. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat Immunol. 2007;8:578–583. doi: 10.1038/ni1462. [DOI] [PubMed] [Google Scholar]
- MacGregor RR, Louria DB. Alcohol and infection. Curr Clin Top Infect Dis. 1997;17:291–315. [PubMed] [Google Scholar]
- Mandrekar P, Catalano D, Dolganiuc A, Kodys K, Szabo G. Inhibition of myeloid dendritic cell accessory cell function and induction of T cell anergy by alcohol correlates with decreased IL-12 production. J Immunol. 2004;173:3398–3407. doi: 10.4049/jimmunol.173.5.3398. [DOI] [PubMed] [Google Scholar]
- Manz MG, Traver D, Miyamoto T, Weissman IL, Akashi K. Dendritic cell potentials of early lymphoid and myeloid precursors. Blood. 2001;97:3333–3341. doi: 10.1182/blood.v97.11.3333. [DOI] [PubMed] [Google Scholar]
- Martin P, Martinez del Hoyo G, Anjuere F, Arias CF, Vargas HH, Fernandez-L A, Parrillas V, Ardavin C. Characterization of a new subpopulation of mouse CD8alpha+ B220+ dendritic cells endowed with type 1 interferon production capacity and tolerogenic potential. Blood. 2002;100:383–390. doi: 10.1182/blood.v100.2.383. [DOI] [PubMed] [Google Scholar]
- Martín-Fontecha A, Sebastiani S, Höpken UE, Uguccioni M, Lipp M, Lanzavecchia A, Sallusto F. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med. 2003;198:615–621. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows GG, Wallendal M, Kosugi A, Wunderlich J, Singer DS. Ethanol induces marked changes in lymphocyte populations and natural killer cell activity in mice. Alcohol Clin Exp Res. 1992;16:474–479. doi: 10.1111/j.1530-0277.1992.tb01403.x. [DOI] [PubMed] [Google Scholar]
- Metlay JP, Witmer-Pack MD, Agger R, Crowley MT, Lawless D, Steinman RM. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J Exp Med. 1990;171:1753–1771. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse HC, Davidson WF, Yetter RA, Coffman RL. A cell-surface antigen shared by B cells and Ly2+ peripheral T cells. Cell Immunol. 1982;70:311–320. doi: 10.1016/0008-8749(82)90332-x. [DOI] [PubMed] [Google Scholar]
- Muller G, Hopken UE, Lipp M. The impact of CCR7 and CXCR5 on lymphoid organ development and systemic immunity. Immunol Rev. 2003;195:117–135. doi: 10.1034/j.1600-065x.2003.00073.x. [DOI] [PubMed] [Google Scholar]
- Ness KJ, Fan J, Wilke WW, Coleman RA, Cook RT, Schlueter AJ. Chronic ethanol consumption decreases murine Langerhans cell numbers and delays migration of Langerhans cells as well as dermal dendritic cells. Alcohol Clin Exp Res. 2008;32:657–668. doi: 10.1111/j.1530-0277.2007.00614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo VN, Korner H, Gunn MD, Schmidt KN, Riminton DS, Cooper MD, Browning JL, Sedgwick JD, Cyster JG. Lymphotoxin α/β and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J Exp Med. 1999;189:403–412. doi: 10.1084/jem.189.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keeffe M, Hochrein H, Vremec D, Caminschi I, Miller JL, Anders EM, Wu L, Lahoud MH, Henri S, Scott B, Hertzog P, Tatarczuch L, Shortman K. Mouse plasmacytoid cells: long-lived cells, heterogeneous in surface phenotype and function that differentiate into CD8+ dendritic cells only after microbial stimulus. J Exp Med. 2002;196:1307–1319. doi: 10.1084/jem.20021031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett EL, Sibley DA, Jerrells TR. Effect of adrenalectomy on ethanol-associated changes in lymphocyte cell numbers and subpopulations in thymus, spleen, and gut-associated lymphoid tissues. Int J Immunopharmacol. 2000;22:285–298. doi: 10.1016/s0192-0561(99)00083-1. [DOI] [PubMed] [Google Scholar]
- Reid CD. The dendritic cell lineage in haemopoiesis. Br J Haematol. 1997;96:217–223. doi: 10.1046/j.1365-2141.1997.d01-2030.x. [DOI] [PubMed] [Google Scholar]
- Shen F-W. Monoclonal antibodies to mouse lymphocyte differentiation alloantigens, in Monoclonal Antibodies and T-Cell Hybridomas. In: Hammerling GJ, Hammerling U, Kearney JF, editors. Perspectives and Technical Advances. Elsevier/North-Holland Biomedical Press; Amsterdam: 1981. pp. 25–31. [Google Scholar]
- Song K, Coleman RA, Zhu X, Alber C, Ballas ZK, Waldschmidt TJ, Cook RT. Chronic ethanol consumption by mice results in activated splenic T cells. J Leukoc Biol. 2002;72:1109–1116. [PubMed] [Google Scholar]
- Springer T, Galfre G, Sacher DS, Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979;9:301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Szabo G. Consequences of alcohol consumption on host defence. Alcohol Alcohol. 1999;34:830–841. doi: 10.1093/alcalc/34.6.830. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Jafee RC, Ritzmann RF. Corticosterone concentrations in mice during ethanol drinking and withdrawal. J Pharm Pharmacol. 1978;30:371–374. doi: 10.1111/j.2042-7158.1978.tb13259.x. [DOI] [PubMed] [Google Scholar]
- Unkeless JC. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979;150:580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vremec D, Shortman K. Dendritic cell subtypes in mouse lymphoid organs. Cross-correlation of surface markers, changes with incubation, and differences among thymus, spleen, and lymph nodes. J Immunol. 1997;159:565–573. [PubMed] [Google Scholar]
- Vremec D, Zorbas M, Scollay R, Saunders DJ, Ardavin CF, Wu L, Shortman K. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J Exp Med. 1992;176:47–58. doi: 10.1084/jem.176.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Vremec D, Ardavin C, Winkel K, Suss G, Georgiou H, Maraskovsky E, Cook W, Shortman K. Mouse thymus dendritic cells: kinetics of development and changes in surface markers during maturation. Eur J Immunol. 1995;25:418–425. doi: 10.1002/eji.1830250217. [DOI] [PubMed] [Google Scholar]
- Wu Q, Wang Y, Wang J, Hedgeman EO, Browning JL, Fu Y-X. The requirement of membrane lymphotoxin for the presence of dendritic cells in lymphoid tissues. J Exp Med. 1999;190:629–638. doi: 10.1084/jem.190.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, Wang Y, Chin RK, Martinez-Pomares L, Gordon S, Kosco-Vibois MH, Cyster JG, Fu Y-X. B cells control the migration of a subset of dendritic cells into B cell follicles via CXC chemokine ligand 13 in a lymphotoxin-dependent fashion. J Immunol. 2002;166:5117–5123. doi: 10.4049/jimmunol.168.10.5117. [DOI] [PubMed] [Google Scholar]