Abstract
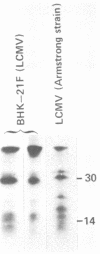
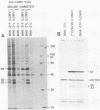
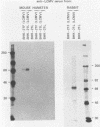
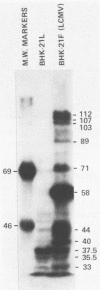
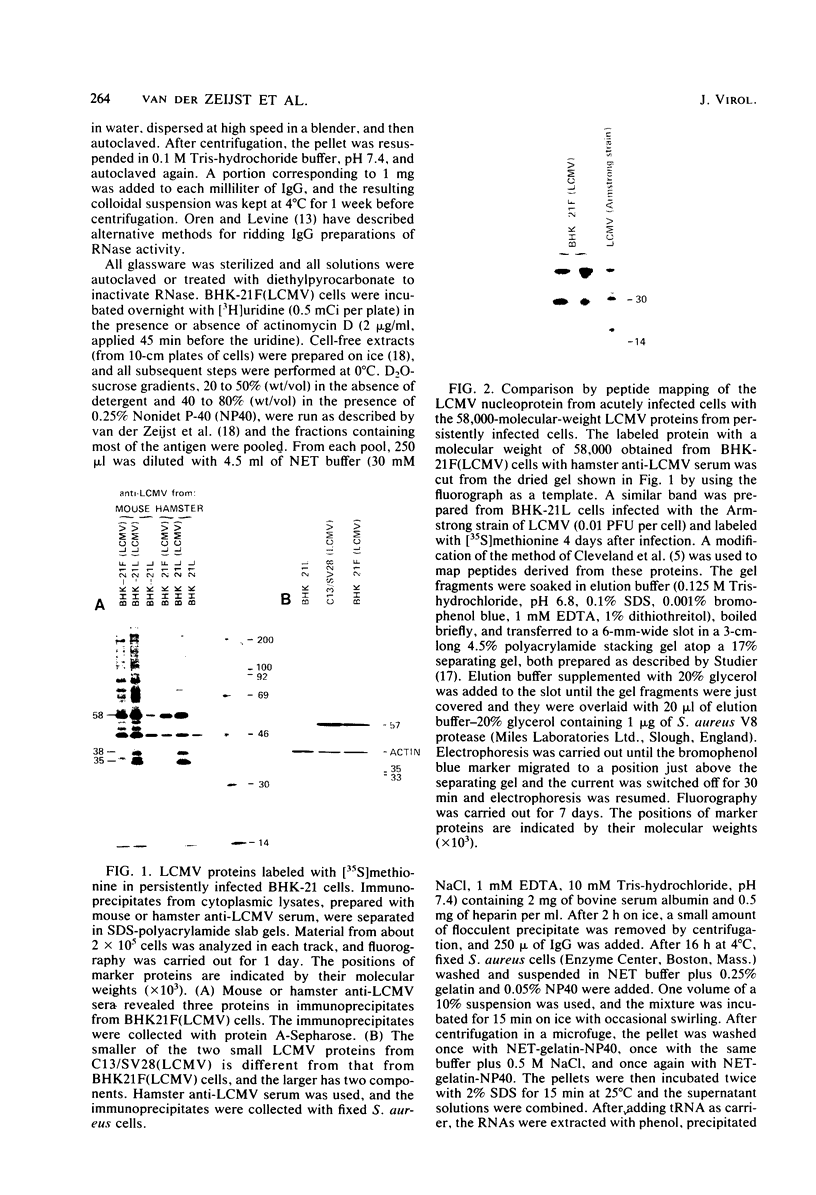
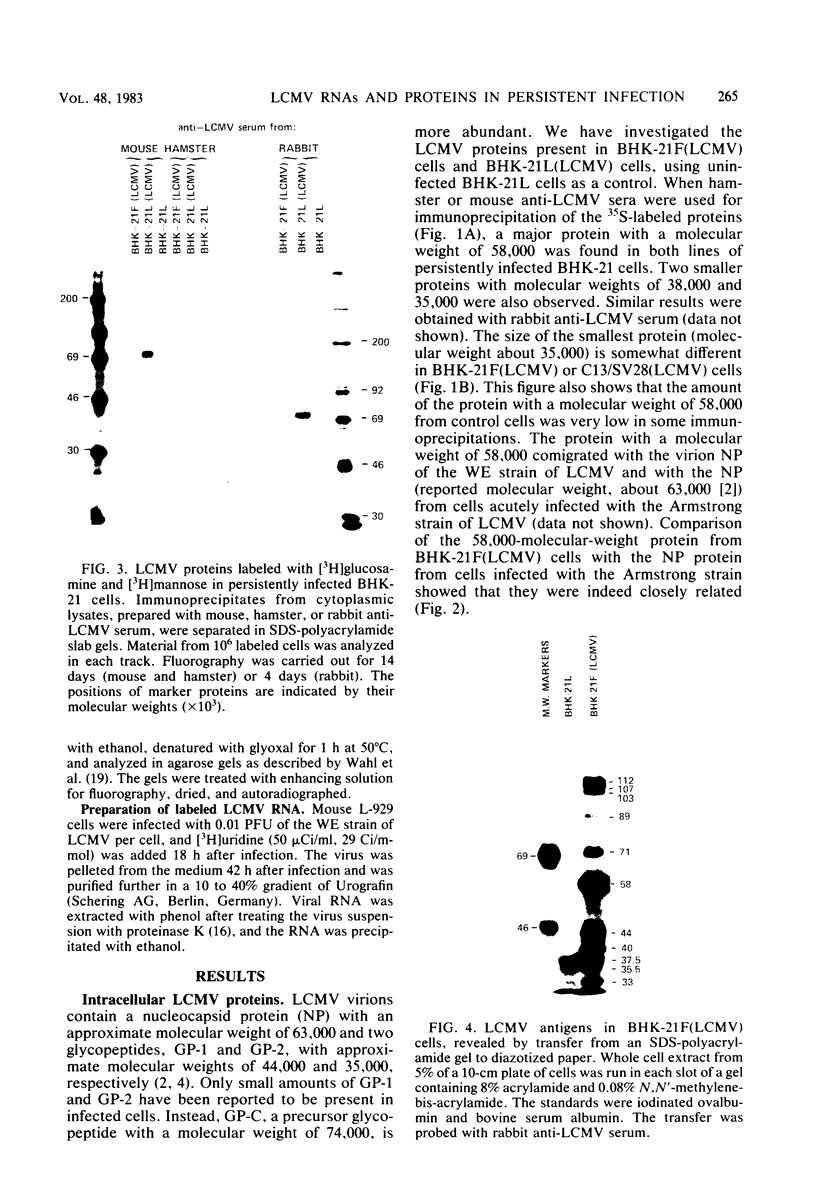
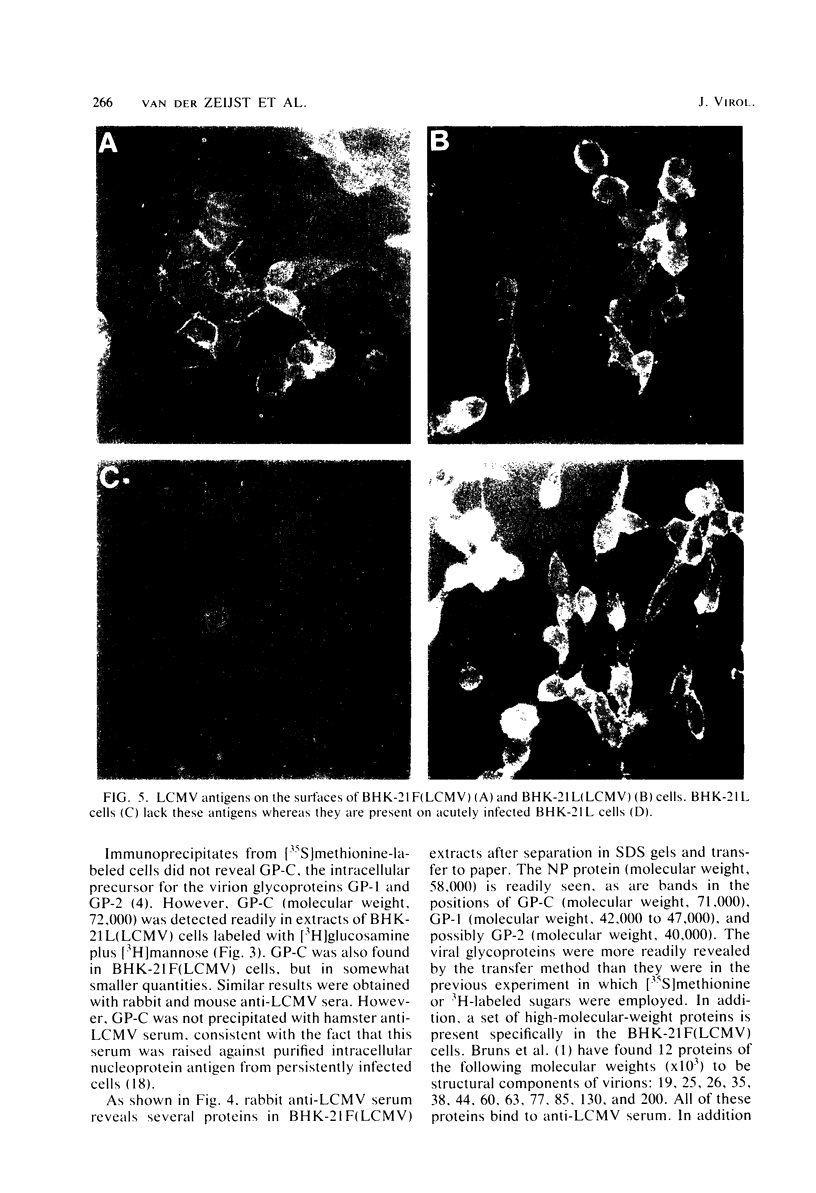
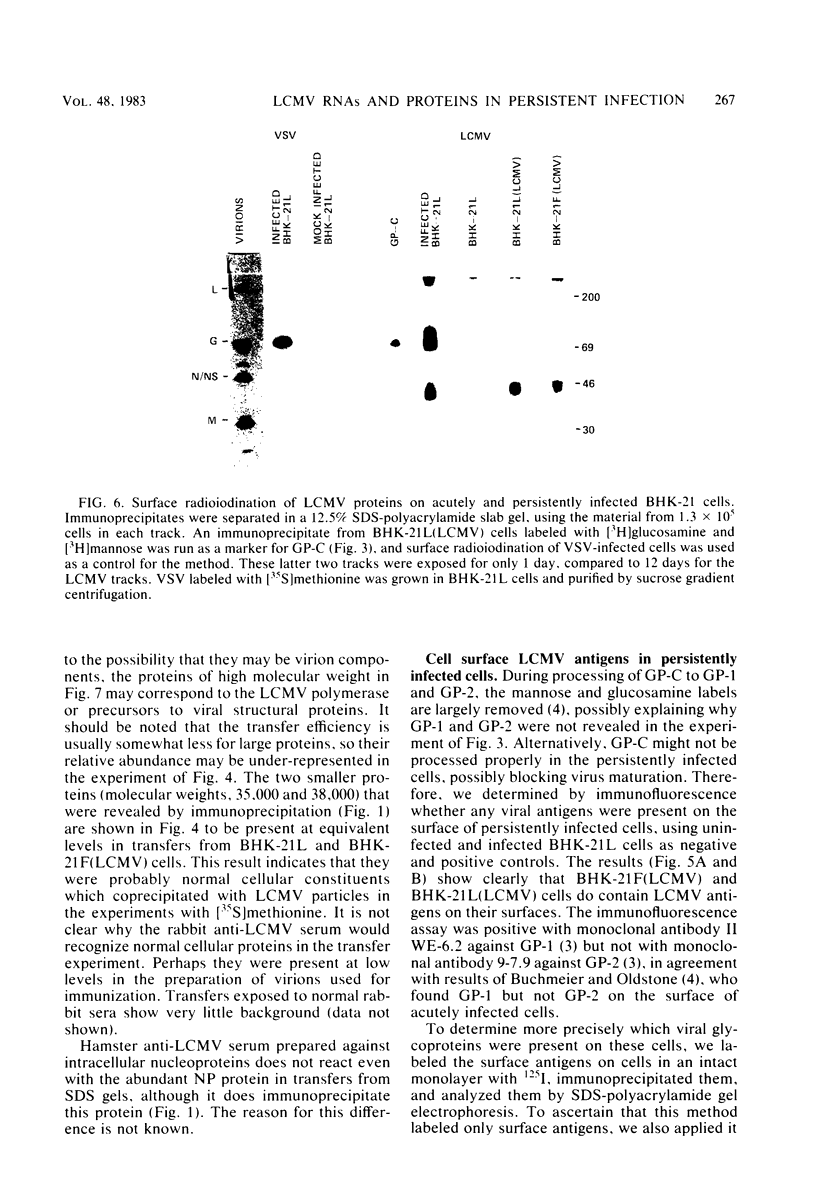
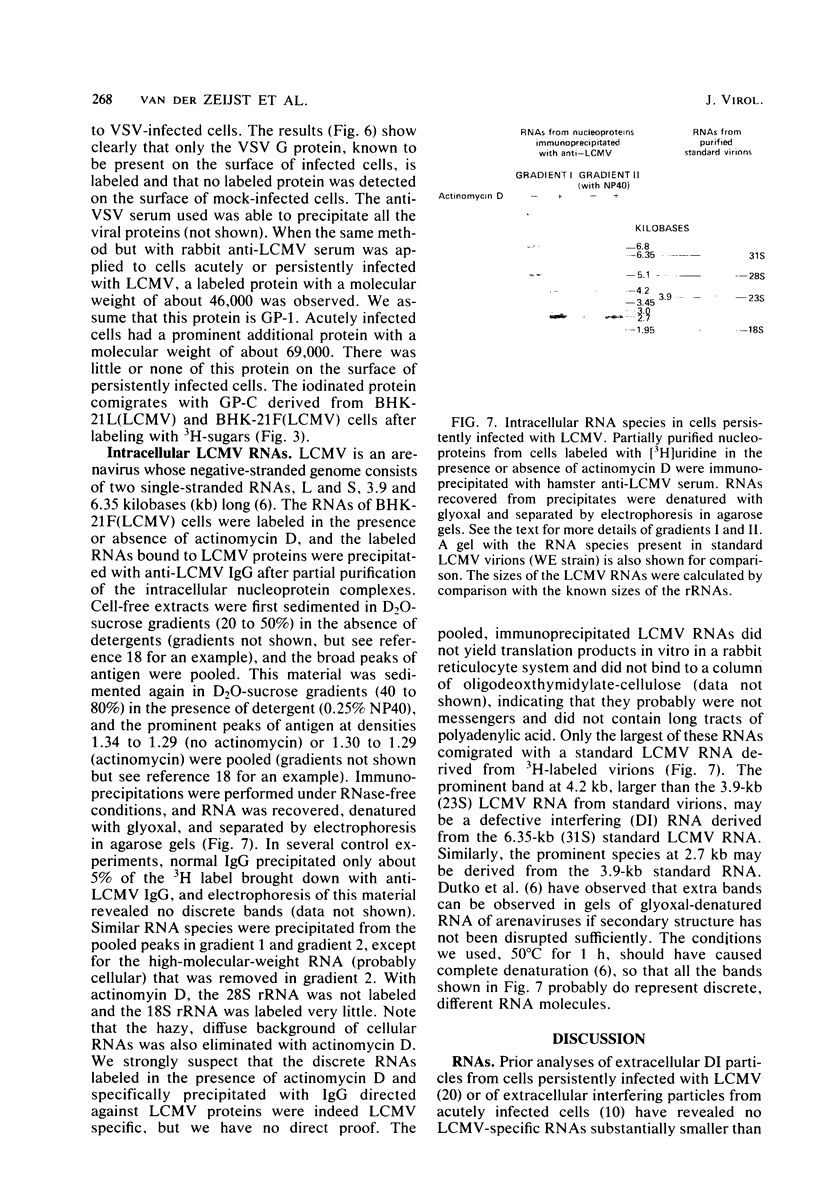
Some Syrian hamster cell lines persistently infected with lymphocytic choriomeningitis virus (LCMV) do not produce extracellular virus particles but do contain intracytoplasmic infectious material. The proteins of these cells were labeled with [35S]methionine or with [3H]glucosamine and [3H]mannose, and immunoprecipitates were prepared with anti-LCMV sera. A substantial amount of the LCMV nucleocapsid protein (molecular weight about 58,000) was detected, along with GP-C, the precursor of the virion glycoproteins GP-1 and GP-2. GP-1 and GP-2 themselves were not detected. A new method of transferring proteins electrophoretically from sodium dodecyl sulfate-polyacrylamide gels to diazotized paper in high yield revealed several additional LCMV proteins present specifically in the persistently infected cells, at apparent molecular weights (X10(3] of 112, 107, 103, 89, 71 (probably GP-C), 58 (nucleocapsid protein), 42 to 47 (probably GP-1), and 40 (possibly GP-2). By iodinating intact cells with I3, GP-1 but not GP-2 or GP-C was revealed on the surfaces of the persistently infected cells, whereas both GP-1 and GP-C were found on the surfaces of acutely infected cells. The absence of GP-C from the plasma membrane of the persistently infected cells might be related to defective maturation of the virus in these cells. Cytoplasmic viral nucleoprotein complexes were labeled with [3H]uridine in the presence or absence of actinomycin D, purified partially by sedimentation in D2O-sucrose gradients, and adsorbed to fixed Staphylococus aureus cells in the presence of anti-LCMV immunoglobulin G. Several discrete species of viral RNA were released from the immune complexes with sodium dodecyl sulfate. Some were appreciably smaller than the 31S and 23S species of standard LCMV virions, indicating that defective interfering viral RNAs are probably present in the persistently infected cells. Ribosomal 28S and 18S RNAs, labeled only in the absence of actinomycin D, were coprecipitated with anti-LCMV serum but not with control serum, indicating their association with LCMV nucleoproteins in the cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruns M., Martínez Peralta L., Lehmann-Grube F. Lymphocytic choriomeningitis virus. III. Structural proteins of the virion. J Gen Virol. 1983 Mar;64(Pt 3):599–611. doi: 10.1099/0022-1317-64-3-599. [DOI] [PubMed] [Google Scholar]
- Buchmeier M. J., Elder J. H., Oldstone M. B. Protein structure of lymphocytic choriomeningitis virus: identification of the virus structural and cell associated polypeptides. Virology. 1978 Aug;89(1):133–145. doi: 10.1016/0042-6822(78)90047-8. [DOI] [PubMed] [Google Scholar]
- Buchmeier M. J., Lewicki H. A., Tomori O., Oldstone M. B. Monoclonal antibodies to lymphocytic choriomeningitis and pichinde viruses: generation, characterization, and cross-reactivity with other arenaviruses. Virology. 1981 Aug;113(1):73–85. doi: 10.1016/0042-6822(81)90137-9. [DOI] [PubMed] [Google Scholar]
- Buchmeier M. J., Oldstone M. B. Protein structure of lymphocytic choriomeningitis virus: evidence for a cell-associated precursor of the virion glycopeptides. Virology. 1979 Nov;99(1):111–120. doi: 10.1016/0042-6822(79)90042-4. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- Meredith R. D., Erlanger B. F. Isolation and characterization of rabbit anti-m7G-5'-P antibodies of high apparent affinity. Nucleic Acids Res. 1979;6(6):2179–2191. doi: 10.1093/nar/6.6.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Oren M., Levine A. J. Immunoselection of simian virus 40 large T antigen messenger rnas from transformed cells. Virology. 1981 Sep;113(2):790–793. doi: 10.1016/0042-6822(81)90210-5. [DOI] [PubMed] [Google Scholar]
- Pedersen I. R. Structural components and replication of arenaviruses. Adv Virus Res. 1979;24:277–330. doi: 10.1016/s0065-3527(08)60396-6. [DOI] [PubMed] [Google Scholar]
- Peralta L. M., Bruns M., Lehmann-Grube F. Biochemical composition of lymphocytic choriomeningitis virus interfering particles. J Gen Virol. 1981 Aug;55(Pt 2):475–479. doi: 10.1099/0022-1317-55-2-475. [DOI] [PubMed] [Google Scholar]
- Seed B. Diazotizable arylamine cellulose papers for the coupling and hybridization of nucleic acids. Nucleic Acids Res. 1982 Mar 11;10(5):1799–1810. doi: 10.1093/nar/10.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaan W. J., Rottier P. J., Horzinek M. C., van der Zeijst B. A. Isolation and identification of virus-specific mRNAs in cells infected with mouse hepatitis virus (MHV-A59). Virology. 1981 Jan 30;108(2):424–434. doi: 10.1016/0042-6822(81)90449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Padgett R. A., Stark G. R. Gene amplification causes overproduction of the first three enzymes of UMP synthesis in N-(phosphonacetyl)-L-aspartate-resistant hamster cells. J Biol Chem. 1979 Sep 10;254(17):8679–8689. [PubMed] [Google Scholar]
- Welsh R. M., Jr, Buchmeier M. J. Protein analysis of defective interfering lymphocytic choriomeningitis virus and persistently infected cells. Virology. 1979 Jul 30;96(2):503–515. doi: 10.1016/0042-6822(79)90107-7. [DOI] [PubMed] [Google Scholar]