Abstract
Two sibling foxhounds born to a Leishmania seropositive bitch were presented after testing seropositive for Leishmania. Leishmania infantum infection was detected via histopathology, culture, and quantitative polymerase chain reaction (q-PCR). This is the first report of natural infection with Leishmania infantum with the possibility for vertical transmission in North America.
Résumé
Infection disséminée à Leishmania infantum chez deux chiots Fox hound d’une même portée reliée possiblement à une transmission verticale. Deux chiots Fox hound d’une même portée nés d’une mère séropositive à Leishmania ont été présentés après un contrôle sérologique positif. Une infection à Leishmania infantum a été détectée par histopathologie, culture et amplification en chaîne par polymérase quantitative (ACP-q). Il s’agit du premier rapport d’infection naturelle par Leishmania infantum possiblement relié à une transmission verticale en Amérique du Nord.
(Traduit par Docteur André Blouin)
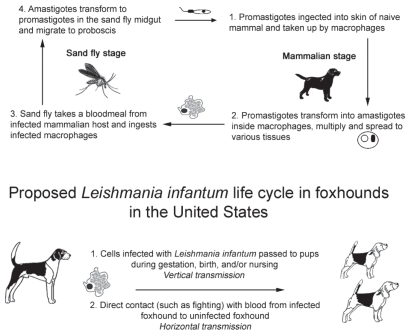
Leishmania infantum is a protozoal parasite that causes visceral leishmaniasis; natural hosts include rodents, small mammals, dogs, and humans (1). Leishmaniasis is classically transmitted to a mammalian host by sand fly bite after which the promastigote form of the parasite is phagocytosed primarily by macrophages (1). Once within host cells, the parasite transforms into the amastigote stage and multiplies, eventually leading to systemic spread of the parasite (Figure 1). Visceral leishmaniasis is clinically characterized by a combination of polyarthritis, skin lesions, epistaxis, fever, weight loss, hepatomegaly, splenomegaly, and renal failure (2). Histologically, there is multifocal histiocytic inflammation with intracellular organisms in affected organs and lymphofollicular hyperplasia within the spleen and lymph nodes, indicating marked immune system stimulation (3).
Figure 1.
The classical Leishmania life cycle requires both a sand fly and a mammalian host for transmission (top). The proposed Leishmania infantum life cycle in the North American foxhound population has a prominent role for vertical transmission (bottom).
Although endemic for centuries in southern central and South America, the Middle East, the Mediterranean basin, and central Asia and Africa, this disease has only recently been described in foxhounds in Canada and the United States (4). Previously, sporadic cases have been reported in Canada and the United States, usually in human and canine travelers returning home from endemic areas (5). There have also been isolated cases of autochthonous visceral leishmaniasis, in which dogs have become infected without exposure to endemic regions or other infected animals (5,6). One of these reports presented a foxhound with visceral leishmaniasis in a research colony in the United States (7). In 1999, a foxhound kennel in New York State reported that 4 foxhounds were infected with Leishmania infantum, a causative agent of canine visceral leishmaniasis. The infected dogs were detected using serology and culture (8,9). By 2005, there were 60 kennels in Nova Scotia, Ontario, and 22 states with seropositive foxhounds (2). This report describes canine visceral leishmaniasis in 2 littermates born to a seropositive bitch. This is the first report of natural disease with the possibility for vertical transmission, or passage of a pathogen from mother directly to pups, of Leishmania infantum in foxhounds in North America.
Case description
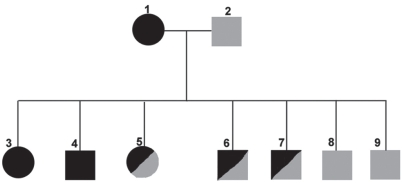
Two 19-month-old American foxhounds, one female (case No. 1) and one male (case No. 2), were donated to Iowa State University (ISU), Department of Veterinary Pathology in February of 2007 following seropositivity (1:512) to Leishmania in January. Both animals were born in a litter of 7 to a bitch that was seronegative at the time of breeding, but became seropositive (1:64) for Leishmania while pregnant in August of 2005. The pups nursed for 6 wk and were then weaned. Two weeks following weaning, the bitch died of renal failure secondary to visceral leishmaniasis. At that time, no other hounds in the pack were seropositive for leishmaniasis and all dogs were allowed to co-mingle in the kennel. Currently, 3 other siblings are Leishmania-infection positive according to quantitative real-time polymerase chain reaction amplification of kinetoplast DNA (q-PCR), but are not yet seropositive (Figure 2).
Figure 2.
Pedigree of American foxhounds with Leishmania infantum infection as presented in this report. Females are denoted by circles, males by squares. Black indicates CDC confirmed seropositive for L. infantum. Half black indicates L. infantum q-PCR positive but to date seronegative. Grey indicates status unknown. Dogs 3 and 4 are presented in this report. Dog 8 was euthanized due to poor body condition prior to q-PCR testing. Dog 9 was lost to follow-up.
Case No. 1 was a 19-kg female, in fair body condition with a dull hair coat and a nonpainful mass in the cranial abdomen that was attributed to splenomegaly. Case No. 2 was a 36-kg male that presented with a serosanguineous nasal discharge and dull hair coat but was in good body condition. Both dogs were active initially, but progressively lost body condition over time. Case No. 2 rapidly lost condition the last 2 wk before euthanasia, losing a total of 3.2 kg in the 2 mo he was housed at ISU.
Initial clinical pathologic work included a complete blood (cell) count (CBC), chemistry panel and urinalysis for both cases. Case No. 1 had a nonregenerative anemia [hematocrit 22.8%; reference interval (RI): 37.0% to 55.0%; mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and red cell distribution width (RDW) were all within the RI]. There was evidence of renal compromise, including azotemia (blood urea nitrogen 31.8 mmol/L; RI: 3.6 to 10.7 mmol/L, creatinine 309.4 μmol/L; RI: 8.8 to 106.1 μmol/L), hyperphosphatemia (3.5 mmol/L; RI: 1.0 to 2.0 mmol/L), hypermagnesemia (2.2 mmol/L; RI: 0.9 to 1.2 mmol/L), and 4+ proteinuria (urine dipstick).
Complete blood count results from case No. 2 included a nonregenerative anemia (hematocrit 24.3%; RI: 37.0% to 55.0%; MCV, MCH, and RDW were all within the RI). There was mild thrombocytopenia (189 × 109/L; reference range 200–500 × 109/L). Clinical chemistry findings included mild hyponatremia (139 mmol/L; RI: 141 to 154 mmol/L) and signs of hepatocellular injury, including elevated alkaline phosphatase (ALP) (249 U/L; RI: 20 to 115 U/L), elevated alanine transferase (ALT) (211 U/L; RI: 24 to 105 U/L), and mild hypercholesterolemia (7.2 mmol/L; RI: 3.6 to 7.0 mmol/L). Other clinical chemistry abnormalities included hyperproteinemia (91 g/L; RI: 52 to 71 g/L), attributed to hypergammaglobulinemia and hypoalbuminemia (20 g/L; RI: 32 to 43 g/L). Urinalysis findings included 4+ proteinuria (urine dipstick). Other parameters were within normal limits. Whole blood was collected from both animals for q-PCR amplification of kinetoplast DNA using Leishmania infantum-specific primers, and was positive for both dogs.
The female dog (case No. 1) was euthanized 2 wk following presentation due to marked weight loss and renal compromise. At necropsy, she was emaciated and all of the lymph nodes, including peripheral, mesenteric, and mediastinal, were moderately to markedly enlarged and the liver and spleen were diffusely enlarged. The male dog (case No. 2) was euthanized following 2 mo of monitoring via CBC, chemistry panels, and q-PCR, during which time he had a marked increase in alanine transferase (336 U/L; RI: 24–105 U/L). At necropsy, this dog was thin to emaciated and the liver and spleen were diffusely and markedly enlarged, pale, and firm. All lymph nodes, including peripheral, mesenteric, and mediastinal nodes, were markedly enlarged and both kidneys were moderately enlarged and diffusely pale.
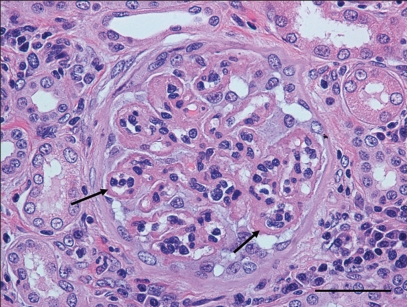
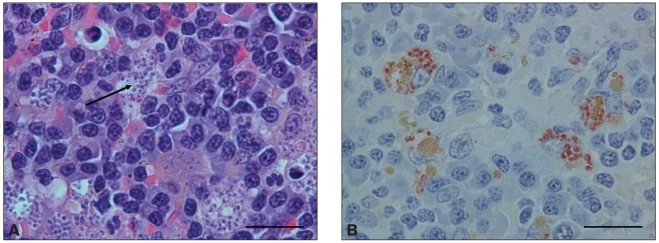
At the time of necropsy, a complete set of tissues from both cases was collected and fixed in 10% neutral buffered formalin. Tissues were routinely processed and stained with hematoxylin and eosin (H&E) for histopathologic evaluation. In both cases the kidneys had severe changes, including multifocal glomeruli with thickened Bowman’s capsules. Approximately 60% to 70% of the glomeruli had markedly thickened and prominent capillary loops with multiple synechia, consistent with membranous glomerulonephritis (Figure 3); this was confirmed by periodic acid silver methenamine (PASM) staining. Within the renal interstitium in both animals there were multifocal to coalescing accumulations of inflammatory cells, primarily lymphocytes, plasma cells, and macrophages, with moderate numbers of macrophages containing intracellular organisms. Diffusely, the spleens of both dogs were markedly hypercellular with numerous well-developed lymphoid follicles and prominent germinal centers. Splenic sinusoids contained numerous macrophages, many of which contained one or more 1- to 3-μm round to oval basophilic organisms consistent with Leishmania (Figure 4a). Within the livers of both animals, the portal areas contained a low to moderate number of lymphocytes, plasma cells, and fewer macrophages, some of which contained one or more Leishmania organisms. All lymph nodes in both dogs were diffusely hypercellular with numerous well-developed follicles with prominent germinal center formation. Sub-capsular and medullary sinuses were filled with numerous macrophages, many of which contained small intracytoplasmic organisms as described previously.
Figure 3.
Kidney (glomerulus) — case No. 1. Note the marked thickening of glomerular capillary loops (arrows) indicative of membranous glomerulonephritis. H&E stain. Bar = 50 μm.
Figure 4.
Spleen — case No. 1. A — Note the multiple amastigotes (arrow) within macrophages. H&E stain; bar = 20 μm. B — Strong anti-Leishmania infantum immunoreactivity is present in the cytoplasm of macrophages. Bar = 20 μm.
Multifocally within the stomach of case No. 1, the vascular walls of medium-sized vessels were multifocally disrupted and the tunica media contained cellular and nuclear debris, fibrin and mineral, consistent with uremia secondary to renal failure. This animal also had intra-histiocytic parasites within the colon, urinary bladder, and bone marrow. In case No. 2, intra-histiocytic parasites were present within the adrenal gland, pancreas, and bone marrow.
Confirmation of Leishmania infantum infection was necessary as the Centers for Disease Control and Prevention (CDC) serologic test is cross-reactive with Trypanosma cruzi antibodies. Immunohistochemistry was used to confirm Leishmania infantum infection in tissues, as PCR and serology only indicate hematogenous infection. This test was performed on fixed tissues from case No. 1 using PCR and canine serum from culture positive case No. 2, and was positive for Leishmania infantum (Figure 4b) (7). This technique can be used as a lower-cost alternative for diagnosis when molecular techniques are not available. Tissue samples, including spleen, popliteal lymph node, and bone marrow were sent to the CDC for culture and q-PCR speciation. Leishmania parasites were cultured from the spleen and bone marrow from case No. 2. The CDC q-PCR analysis was positive, thus confirming disseminated Leishmania donovani infantum (MON1) infection in both cases 1 and 2.
Discussion
A final diagnosis of visceral leishmaniasis was made in both cases. Severe membranous glomerulonephritis was present in both dogs, though clinical signs of renal failure were only present in case No. 1. Glomerulonephritis is a common finding in both canine and human patients with visceral leishmaniasis (10). Case No. 1 had a urine specific gravity of 1.020, and although not indicative of isosthenuria, the actual urine concentration is likely to be lower than reported, as proteinuria will falsely elevate the refractometer urine specific gravity reading. Both dogs exhibited disseminated disease with intra-histiocytic parasites present in multiple tissues.
At present, diagnosis and control of Leishmaniasis is difficult as dogs can be infected but seronegative for years (11). As serology, an indirect fluorescent antibody assay is the primary diagnostic test used in the United States for surveillance of canine leishmaniasis; it is thought that many infections go undetected in dogs. Reports have shown that q-PCR is a more sensitive test for L. infantum infection in both dogs and humans, and can detect asymptomatic dogs and/or dogs that have yet to seroconvert (2,12).
Dogs are the primary reservoir for Leishmania infection in endemic regions, and are the most significant risk factor predisposing humans to infection (13). This suggests a possible human health threat as the disease has become endemic within the North American foxhound population, particularly since it has not been proven that the sand fly in North America cannot transmit disease (4). In endemic areas, the primary means of transmission is vector borne via the sand fly. Lutzomyia shannoni, a potential sand fly vector, is present in the southern and southeastern United States, but is not present in the Midwest where these pups were born. Many foxhounds from Canada and the northern United States travel to the southern United States for field trials, or dogs are drafted from these areas into Canadian kennels. To date, despite many efforts to find Leishmania-infected sand flies near foxhound kennels, these efforts have been unsuccessful (4). Other mechanisms have been postulated for transmission of canine visceral leishmaniasis in North America and include vector-independent modes such as vertical transmission (transplacental or transmammary) and horizontal transmission by direct contact with infected cells in blood (Figure 1) (4). Transmission has been documented via packed red blood cell transfusion from infected foxhounds (14). The existence and frequency of vertical transmission in endemic areas are unknown due to the overwhelming likelihood of vector contact (15,16).
There is some controversy as to whether canine visceral leishmaniasis can be transmitted vertically. One report of Leishmania chagasi in various breeds of dogs in Brazil indicated that vertical transmission does not occur (17). Other groups, however, have described congenital transmission of visceral leishmaniasis in humans and during experimental Leishmania infection of beagles (18). Placentitis has been associated with Leishmania infantum infection in a coonhound, but fetal infection did not occur (19). Natural vertical transmission has yet to be documented in foxhounds.
Despite evidence indicating that this disease in foxhounds in North America is not vector borne, the disease manifestations are similar to those observed in endemic regions, indicating that the pathology of clinical visceral leishmaniasis is not likely due to the means of transmission. In this case report, we describe 2 sibling foxhounds that were born to a seropostive bitch and were euthanized due to disseminated visceral leishmaniasis. At the time of birth, these dogs and all other foxhounds in the pack were seronegative. This does not rule out horizontal transmission, as q-PCR was not performed on the other foxhounds in the kennel at the time of birth. It does indicate, however, that there was potential for transmission of this disease from the mother to these pups. To our knowledge, this study is the first report of natural disease with the possibility for vertical transmission of Leishmania infantum in foxhounds in North America.
Leishmania infantum infection is endemic in the North American foxhound population. This report indicates that vertical transmission may be a potential route of transmission of the parasite in this population. Serology should be supplemented with q-PCR for diagnosis, as serology is not sufficiently sensitive to detect Leishmania infantum early during infection, and in some cases, there is cross-reaction with T. cruzi.
Acknowledgments
We thank Dr. Frank Steuer for providing serology and culture data; our collaborating foxhound huntsmen, huntswomen, and Masters of foxhounds for providing us with these wonderful dogs and other samples for this study; and Dr. Claire Andreasen and Dr. Amanda Fales-Williams for their helpful review of the manuscript. This study was supported by an ACORN grant from the AKC Canine Health Foundation (#799-A). CVJ
References
- 1.Roberts LJ, Handman E, Foote SJ. Science, medicine, and the future: Leishmaniasis. BMJ. 2000;321:801–804. doi: 10.1136/bmj.321.7264.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosypal AC, Troy GC, Duncan RB, Zajac AM, Lindsay DS. Utility of diagnostic tests used in diagnosis of infection in dogs experimentally inoculated with a North American isolate of Leishmania infantum infantum. J Vet Intern Med. 2005;19:802–809. doi: 10.1892/0891-6640(2005)19[802:uodtui]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 3.Jubb KVF, Kennedy PC, Palmer N. Pathology of domestic animals. 4. San Diego: Acad Pr; 1993. [Google Scholar]
- 4.Duprey ZH, Steurer FJ, Rooney JA, et al. Canine visceral leishmaniasis, United States and Canada, 2000–2003. Emerg Infect Dis. 2006;12:440–446. doi: 10.3201/eid1203.050811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schantz PM, Steurer FJ, Duprey ZH, et al. Autochthonous visceral leishmaniasis in dogs in North America. J Am Vet Med Assoc. 2005;226:1316–1322. doi: 10.2460/javma.2005.226.1316. [DOI] [PubMed] [Google Scholar]
- 6.Anderson DC, Buckner RG, Glenn BL, et al. Endemic canine leischmaniasis. Vet Pathol. 1980;17:94–96. doi: 10.1177/030098588001700110. [DOI] [PubMed] [Google Scholar]
- 7.Swenson CL, Silverman J, Stromberg PC, et al. Visceral leischmaniasis in an English foxhound from an Ohio research colony. J Am Vet Med Assoc. 1988;193:1089–1092. [PubMed] [Google Scholar]
- 8.Gaskin AA, Schantz P, Jackson J, et al. Visceral leishmaniasis in a New York foxhound kennel. J Vet Intern Med. 2002;16:34–44. doi: 10.1892/0891-6640(2002)016<0034:vliany>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 9.Tafuri WL, Santos RL, Arantes RM, et al. An alternative immunohistochemical method for detecting Leishmania amastigotes in paraffin-embedded canine tissues. J Immunol Methods. 2004;292:17–23. doi: 10.1016/j.jim.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Costa FA, Goto H, Saldanha LC, et al. Histopathologic patterns of nephropathy in naturally acquired canine visceral leishmaniasis. Vet Pathol. 2003;40:677–684. doi: 10.1354/vp.40-6-677. [DOI] [PubMed] [Google Scholar]
- 11.Rodríguez-Cortés A, Ojeda A, Lopez-Fuertes L, et al. A long term experimental study of canine visceral leishmaniasis. Int J Parasitol. 2007;37:683–693. doi: 10.1016/j.ijpara.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Wortmann G, Hochberg L, Houng HH, et al. Rapid identification of Leishmania complexes by a real-time PCR assay. Am J Trop Med Hyg. 2005;73:999–1004. [PubMed] [Google Scholar]
- 13.Gavgani AS, Mohite H, Edrissian GH, Mohebali M, Davies CR. Domestic dog ownership in Iran is a risk factor for human infection with Leishmania infantum. Am J Trop Med Hyg. 2002;67:511–515. doi: 10.4269/ajtmh.2002.67.511. [DOI] [PubMed] [Google Scholar]
- 14.Owens SD, Oakley DA, Marryott K, et al. Transmission of visceral leishmaniasis through blood transfusions from infected English foxhounds to anemic dogs. J Am Vet Med Assoc. 2001;219:1076–1083. doi: 10.2460/javma.2001.219.1076. [DOI] [PubMed] [Google Scholar]
- 15.Mancianti F, Sozzi S. Isolation of Leishmania from a newborn puppy. Trans R Soc Trop Med Hyg. 1995;89:402. doi: 10.1016/0035-9203(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 16.Tarantino C, Rossi G, Kramer LH, Perrucci S, Cringoli G, Macchioni G. Leishmania infantum and Neospora caninum simultaneous skin infection in a young dog in Italy. Vet Parasitol. 2001;102:77–83. doi: 10.1016/s0304-4017(01)00518-0. [DOI] [PubMed] [Google Scholar]
- 17.Andrade HM, de Toledo Vde P, Marques MJ, et al. Leishmania (Leishmania) chagasi is not vertically transmitted in dogs. Vet Parasitol. 2002;103:71–81. doi: 10.1016/s0304-4017(01)00552-0. [DOI] [PubMed] [Google Scholar]
- 18.Rosypal AC, Troy GC, Zajac AM, Frank G, Lindsay DS. Transplacental transmission of a North American isolate of Leishmania infantum in an experimentally infected beagle. J Parasitol. 2005;91:970–972. doi: 10.1645/GE-483R.1. [DOI] [PubMed] [Google Scholar]
- 19.Dubey JP, Rosypal AC, Pierce V, Scheinberg SN, Lindsay DS. Placentitis associated with leishmaniasis in a dog. J Am Vet Med Assoc. 2005;227:1266–1269. 1250. doi: 10.2460/javma.2005.227.1266. [DOI] [PubMed] [Google Scholar]