Abstract
Dendritic mRNA transport and local translation at individual potentiated synapses may represent an elegant way to form synaptic memory. Recently, we characterized Staufen, a double-stranded RNA-binding protein, in rat hippocampal neurons and showed its presence in large RNA-containing granules, which colocalize with microtubules in dendrites. In this paper, we transiently transfect hippocampal neurons with human Staufen-green fluorescent protein (GFP) and find fluorescent granules in the somatodendritic domain of these cells. Human Stau-GFP granules show the same cellular distribution and size and also contain RNA, as already shown for the endogenous Stau particles. In time-lapse videomicroscopy, we show the bidirectional movement of these Staufen-GFP–labeled granules from the cell body into dendrites and vice versa. The average speed of these particles was 6.4 μm/min with a maximum velocity of 24.3 μm/min. Moreover, we demonstrate that the observed assembly into granules and their subsequent dendritic movement is microtubule dependent. Taken together, we have characterized a novel, nonvesicular, microtubule-dependent transport pathway involving RNA-containing granules with Staufen as a core component. This is the first demonstration in living neurons of movement of an essential protein constituent of the mRNA transport machinery.
INTRODUCTION
The concept of localized mRNAs within a mammalian cell to achieve a spatially restricted answer to a local stimulus has attracted significant attention lately (for review, see St. Johnston, 1995; Carson et al., 1998; Kuhl and Skehel, 1998; Tiedge et al., 1999). Prominent examples of asymmetrically distributed mRNAs range from the budding yeast (ASH1 mRNA; Bertrand et al., 1998), Drosophila (bicoid and oskar; Driever et al., 1988; Ephrussi et al., 1991), Xenopus (Vg1; Deshler et al., 1997), fibroblasts (β-actin mRNA; Bassell et al., 1998) to mammalian oligodendrocytes (myelin-basic protein mRNA; Holmes et al., 1988) and neurons (many mRNAs; for review, see Kuhl and Skehel, 1998). The asymmetric localization of mRNAs to the somatodendritic domain has been observed together with the translational machinery in the nervous system. However, the mechanism of mRNA delivery and functional significance has not been analyzed in molecular detail.
In the past year, the first molecular components of the mRNA trafficking pathway have been identified (for review, see Hazelrigg, 1998). Among these were several potential mRNA-binding proteins, which recognize cis-acting sequences in the 3′-untranslated region (UTR) of localized mRNAs (e.g., Mayford et al., 1996). These proteins include hnRNP A2 in oligodendrocytes (Hoek et al., 1998), actin zipcode-binding protein in fibroblasts and Xenopus (Ross et al., 1997; Deshler et al., 1998), and Staufen in Drosophila (St. Johnston et al., 1991). Upon binding to their cognate mRNAs, these mRNA-binding proteins were then recruited to particles as shown in the Drosophila embryo in oligodendrocytes and neurons (Ainger et al., 1993; Ferrandon et al., 1994; Wang and Hazelrigg, 1994; Knowles et al., 1996). These granules may therefore represent the active transport unit to deliver mRNAs to their final destination within the cell (Wilhelm and Vale, 1993). Finally, data have accumulated suggesting this transport requires intact microtubules (Knowles et al., 1996; Carson et al., 1997) or actin in the case of ASH1 mRNA (Bertrand et al., 1998). Besides mRNA and their cognate RNA-binding protein(s), the described granules may also contain microtubule-associated proteins, potentially a kinesin-like or dynein-like molecular motor protein (or an unconvential myosin V motor in the case of ASH1 mRNA), aminoacyl-tRNA-synthetases, elongation factors, components of the translational machinery, and even subunits of ribosomes (for review, see Carson et al., 1998).
Recently, in vivo labeling using the green fluorescent protein (GFP) has been successfully applied to study mRNA transport in Drosophila and yeast. In Drosophila, the movement of the RNA-binding protein exuperantia was visualized (Wang and Hazelrigg, 1994), whereas in yeast, the fate of the mRNA itself could be indirectly followed using two sophisticated reporter plasmids (Bertrand et al., 1998). In neurons, however, these granules have only recently been visualized using the RNA-specific, fluorescent dye SYTO14 (Knowles et al., 1996). Because SYTO14 labels all RNA, including mitochondrial RNA, the characterization and transport of these granules in living neurons have been problematic. Therefore, labeling of a known protein constitutent would be a more specific approach to visualize these RNA-containing granules. We took advantage of the first known protein component of these granules, the RNA-binding protein Staufen (St. Johnston et al., 1991). Human Staufen binds microtubules and colocalizes with polysomes in HeLa cells (Marión et al., 1999; Wickham et al., 1999). In fixed neurons, rat Staufen localized to the somatodendritic domain of hippocampal neurons in large RNA-containing granules (Kiebler et al., 1999) that colocalize with microtubules. To determine whether Staufen-containing granules move in vivo, we transiently transfected hippocampal neurons with human Stau-GFP (hStau-GFP) and studied the formation of fluorescent granules and their transport along distal dendrites by time lapse video microscopy.
MATERIALS AND METHODS
Materials and Reagents
The following antibodies were used in the indicated dilutions: polyclonal antibody against human Staufen (Kiebler et al., 1999) at 1:300; polyclonal anti-GFP antibody (antiserum D2) (Wacker et al., 1997) at 1:300; and monoclonal anti-tubulin antibody (Amersham, Arlington Heights, IL; N356, 1:10,000). Phalloidin (phalloidin-rhodamine; Molecular Probes, Eugene, OR; R-415, 1:500) staining was performed as described (Bradke and Dotti, 1999). As secondary antibodies, an FITC-conjugated donkey anti-rabbit immunoglobulin (Ig) (Amersham; N1034, 1:100), a rhodamine-conjugated goat anti-rabbit IgG (Cappel, West Chester, PA; 55674, 1:100), a lissamine-rhodamine–conjugated goat anti-mouse IgG (Dianova, Hamburg, Germany; 715-085-151, 1:400), and an FITC-conjugated sheep anti-mouse Ig (Amersham; N1031, 1:50) were used. The following drugs were used: nocodazol (Sigma, St. Louis, MO; M-1404; final concentration, 20 μM) and latrunculin B (Calbiochem, La Jolla, CA; 428020-Q; final concentration, 12.6 μM). The cloning of the human Staufen-GFP (S65T mutation) construct was described by Wickham et al. (1999).
Hippocampal Cell Culture and Transient Transfection Protocol
Primary hippocampal neurons derived from rat embryos were cultured following the protocol of Goslin and Banker (1997) and de Hoop et al. (1998). Adult primary hippocampal neurons (stage 5) were transfected using a modified Ca2+-phosphate precipitation protocol (Haubensack et al., 1998). In brief, neurons grown on glass coverslips were transferred into 2 ml of conditioned culture medium in a 3.5 cm culture dish. The Ca2+-phosphate precipitate was prepared by dropwise adding 60 μl of 2× BBS (280 mM NaCl, 1.5 mM Na2HPO4, 50 mM N,N-bis[2-hydroxyethyl]-2-aminoethanesulfonic acid, pH 7.1) to 3.5 μg of plasmid cDNA (1 μg/μl stock in 10 mM Tris-HCl, pH 8.5) dissolved in 60 μl of 250 mM CaCl2 and incubated for 90 s at room temperature. The precipitate was added to the neurons, and the cells were incubated for 2 h at 2.5% CO2 and 37°C. Neurons were washed twice with HEPES-buffered saline, returned to the original medium, and incubated overnight at 5% CO2 and 36.5°C before fixation or performing subsequent experiments. To not saturate the cell with overexpressed or aggregated hStau-GFP, we chose a short time of expression (16–20 h). This allowed the detection of individual particles in dendrites and their subsequent intracellular transport.
SYTO14 labeling of cells was essentially performed as described (Knowles et al., 1996; Kiebler et al., 1999) with the following modification. In brief, cells were incubated in SYTO14-containing (1 μM) conditioned N2 medium for 15 min at 37°C and 5% CO2. Neurons were briefly rinsed twice with N2 medium and then fixed.
Immunocytochemistry of neurons was performed as described by Kiebler et al. (1999). For microtubule staining, neurons were extracted before fixation using 0.1% saponin in microtubule-stabilizing buffer (2 mM MgCl2, 10 mM EGTA, 60 mM 1,4-piperazinediethanesulfonic acid, pH 7.05) for 15 s and shortly rinsed in microtubule-stabilizing buffer.
Blotting
Stau-GFP transfected neurons as well as control neurons were incubated in conditioned medium containing 2 mm sodium butyrate. After 17 h, cells were lysed in 0.1% SDS and methanol-chloroform extracted (Kiebler et al., 1999). Lysates of three neuron-containing dishes (3.5 cm) were pooled, run on a 10% minigel (Bio-Rad, Hercules, CA), and blotted onto nitrocellulose. Nonspecific binding sites were blocked by incubation for 2 h in blocking buffer (5% low-fat milk powder in PBS), and then filters were incubated for 2 h in blocking buffer and anti-GFP antiserum D2 (Wacker et al., 1997). Intermediate wash steps were carried out with 0.2% Tween 20 in PBS. Detection of bound antibodies was performed with HRP-coupled donkey anti-rabbit secondary antibodies (Amersham) for 30 min in blocking buffer followed by ECL detection (Amersham).
Microscopy
Time-lapse video microscopy of living transiently transfected neurons grown on glass coverslips was performed using a metal slide. For recording at physiological temperature, the objective was heated at 36°C by an objective heating ring (Bioptechs, Butler, PA) (Bradke and Dotti, 1998). The following setup was used: a Zeiss (Thornwoodd, NY) Axiovert 135 inverted microscope with a 63× Plan apochromat objective and a 100-W HBO mercury arc bulb (Osram, Berlin, Germany), a shutter driver (Uniblitz D122; Vincent Associates, Rochester, NY), standard FITC and rhodamine filters, and a Cohu (San Diego, CA) charge-coupled device (CCD) camera controlled by a CCD camera control (C2400; Hamamatsu, Hamamatsu City, Japan). Images were taken either every 8 or 10 s using the Scion 1.58 software package (National Institutes of Health). Fluorescent microscopy was performed with a Zeiss Axioskop using a 63× objective, standard FITC and rhodamine filters, a 100-W HBO mercury arc lamp, and a Cohu CCD camera controlled by the NIH Image 1.59 software package.
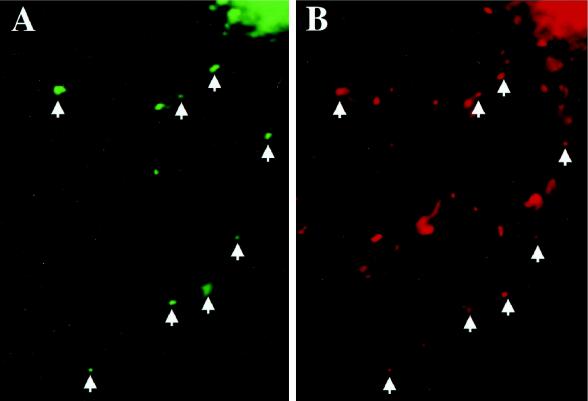
For the Staufen-GFP and SYTO14 colocalization experiment (see Figure 3) the following filter sets were used: 1) enhanced GFP (EGFP) filter (excitation spectra, 470 ± 15 nm; emission spectra, 510 ± 10 nm); and 2) SYTO14 filter (excitation spectra, 546 ± 6 nm; emission spectra, 585 ± 20 nm). Although SYTO14 bound to RNA has its absorbance maximum at 521 nm and the emission maximum at 547 nm, we found a residual SYTO14 signal in the EGFP filter. For that reason, we devised a new method to separate both signals from each other. An image was taken with the SYTO14 filter that detected SYTO14 but not Staufen-GFP, and then this signal was depleted by photobleaching, another image was taken, demonstrating that there was no signal left, and finally a third picture was taken with the EGFP filter that now exclusively represented the Staufen-GFP signal. The first and the third pictures were compared in NIH Image, and individual Staufen-GFP–positive granules were scored for the presence of RNA. In total, 579 granules from 29 cells were examined: of these, 380 were found to contain RNA representing 65.6% of all particles. A representative picture of one of these cells is shown in Figure 3.
Figure 3.
Human Staufen-GFP positive granules colocalize with RNA in transiently transfected rat hippocampal neurons. RNA was visualized using SYTO14 (Knowles et al., 1996; Kiebler et al., 1999), and the degree of colocalization with hStau-GFP was revealed by fluorescent microscopy (see MATERIALS AND METHODS). Individual green fluorescent hStau-GFP granules (A) were analyzed for the presence of RNA detected by SYTO14 labeling of the same cell (B). Arrowheads indicate hStau-GFP granules containing RNA. Overall, 65.6% of analyzed hStau-GFP granules were RNA positive. The additional SYTO14 labeling in B might reflect both RNA-positive granules containing endogenous Staufen as well as mitochondria (Knowles et al., 1996).
Image Analysis and Quantitation
Untransfected cells did not show detectable autofluorescence under identical illumination conditions (our unpublished observations). For quantitatation, transiently transfected cells were randomly chosen by green fluorescence and examined by phase contrast for health, and a fluorescent image was taken and evaluated. To calculate the velocity of individual granules, the distance traveled was measured between two adjacent video frames with the NIH Image 1.59 software package and divided by the time. To quantitate the maximal velocity, 18 different particles from 10 independent transfections were tracked; from these 42 different velocities were measured. The average speed was calculated from videos in which particles were moving at least during three or more consecutive frames (average number of frames, 7.9). In total, 14 different particles were tracked and analyzed.
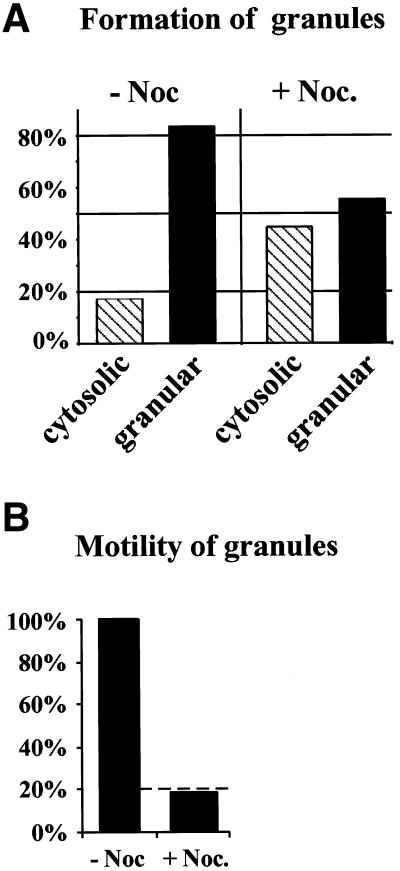
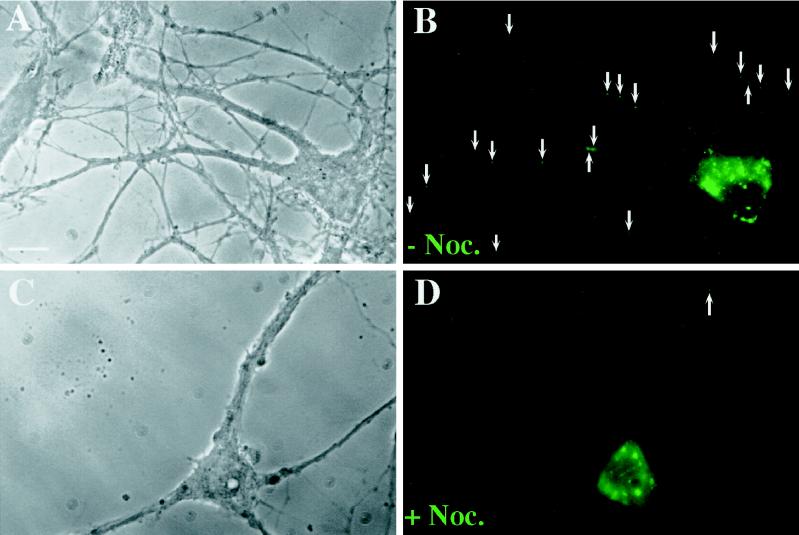
To quantitate the effect of nocodazole on the localization of fluorescent granules, hStau-GFP–expressing neurons were grouped into two different categories (see Figure 7A): 1) neurons with the typical granular expression pattern of hStau-GFP (“localized”), as shown in Figure 2; and 2) neurons with an aberrant expression pattern with no apparent granules (“mislocalized”). In 42 control cells, only 7 cells (17%) showed a cytosolic expression pattern versus 26 of 58 nocodazole-treated cells (45%). This represents a 168% increase of transfected cells with mislocalized hStau-GFP. To analyze the effect of nocodazole on the transport of hStau-GFP–labeled granules, neurons with localized hStau-GFP were examined for the presence of granules in distal (>12 μm apart from the cell body) dendrites (see Figure 7B). Twenty-nine of the 32 mock-treated cells with localized expression had three or more hStau-GFP granules in distal parts of the dendrites versus 5 of 32 nocodazole-treated cells.
Figure 7.
The transport of hStau-GFP particles into distal dendrites is microtubule dependent. Transfected neurons were treated as described in Figure 6. Pictures of 42 mock-treated and 58 nocodazole-treated cells were taken and analyzed for granular (localized) versus cytosolic (mislocalized) fluorescence (A) and subsequently for localization of hStau-GFP granules in distal (>12 μm apart from the cell body) dendrites (B).
Figure 2.
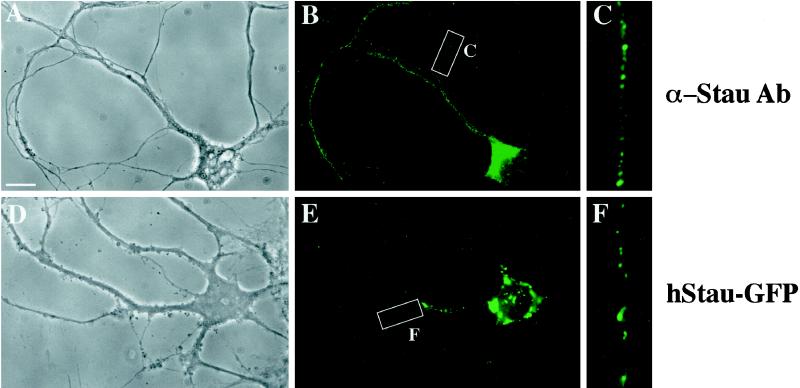
Expressed human Staufen-GFP and endogenous rat Staufen protein show a comparable punctate, dendritic expression pattern in hippocampal neurons. Adult rat hippocampal neurons were either fixed and labeled with anti-hStau antibodies (A–C) or transiently transfected with hStau-GFP and fixed, and green fluorescence was recorded (D–F). (A–C) Phase-contrast and hStau immunofluorescence of a representative neuron. C is an enlarged section of the white box in B. (D–F) Phase-contrast and hStau-GFP fluorescence of a representative neuron. F is an enlarged section of the white box in E. The small precipitate seen in D is due to CaPi transfection. Note that two different types of fluorescent granules were routinely observed (E): large granules around the nuclear membrane and smaller granules in the periphery of the neurons. Bar, 10 μm.
RESULTS AND DISCUSSION
Hippocampal neurons have been previously shown to be an appropriate model system to study the trafficking of heterologously expressed proteins (De Strooper et al., 1995; Simons et al., 1996; Haubensack et al., 1998). Hence we used this cell system to analyze the trafficking of Staufen by transiently transfecting adult neurons with a plasmid coding for a fusion protein of human Staufen and GFP (hStau-GFP) (Wickham et al., 1999). Our goal was twofold: first, to determine whether hStau-GFP is indeed incorporated into large RNA-containing granules as does the endogenous rat Staufen (rStau) (Kiebler et al., 1999), and second, to study assembly of these particles and their subsequent dendritic transport in adult living hippocampal neurons.
Transfected Staufen-GFP is Present in Large RNA-containing Granules
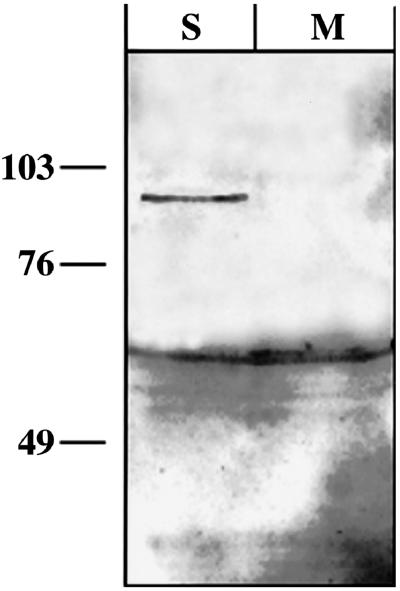
We first tested whether hStau-GFP is expressed as a full-length fusion protein in rat hippocampal neurons. Therefore, we transiently transfected rat hippocampal neurons with hStau-GFP and performed Western blotting on extracts both from transfected and untreated neurons. As shown in Figure 1, we found that a specific 92-kDa band is detected with a GFP antibody, demonstrating that neurons exclusively express the correct fusion protein consisting of hStau (65 kDa) and GFP (27 kDa) and that there is no proteolytic cleavage.
Figure 1.
Transiently transfected hippocampal neurons express the full-length human Staufen-GFP fusion protein. Western blots of extracts from either transiently transfected (S) or mock-treated (M) rat hippocampal neurons were probed with a polyclonal GFP antibody. The antibody detects a 92-kDa band in the transfected but not in the untransfected neurons; this represents the expected size for the hStau-GFP fusion protein (65 + 27 kDa). Additionally, the antibody detects a 61-kDa band in all neuronal extracts, most likely representing an unspecific cross-reaction. The sizes of the molecular mass markers are indicated on the left in kilodaltons.
We then analyzed the pattern of expression and localization of expressed fluorescent hStau-GFP (Figure 2, D–F) and compared that with the endogenous rStau, as visualized by immunofluorescence with an anti-hStau antibody (Figure 2, A–C). Sixteen to 20 h after transfection, hStau-GFP started to appear in small and large granules in the cell body and dendrites of these neurons (Figure 2, D–F). In contrast, cells transfected with GFP alone show an evenly distributed fluorescence throughout the whole cell. The observed hStau-GFP granules (Figure 2, D–F) were of the same size and showed the same cellular distribution as their endogenous rStau counterparts (Figure 2, A–C). However, it must be noted that the expression of hStau-GFP yielded fewer granules in dendrites (Figure 2F) compared with the endogenous Staufen (Figure 2C) and a reduced cytosolic background. There are several explanations for that phenomenon. First, we only allowed a moderate expression rate after transfection to not saturate the cell (see MATERIALS AND METHODS). Second, we only overexpressed Staufen and not any RNA; there is also increasing experimental evidence in other cells that Stau-containing granules can only move if newly synthesized mRNA is provided by the cells. In hippocampal neurons, we observed two different types of Staufen granules: 1) larger granules restricted to the cell body and 2) smaller granules in the periphery of the cell body and in dendrites (see Figures 2E and 6B).
Figure 6.
Nocodazole treatment of hippocampal neurons results in a diminished number of Stau-GFP particles in distal dendrites. Hippocampal neurons were transiently transfected as described in Figure 2 and the following day were treated with nocodazole (20 μM) for 3.5 h or mock treated. Cells were fixed, and fluorescence was recorded. (A and B) A representative cell is shown containing 19 Stau-GFP granules (arrows) in distal dendrites (>12 μm apart from the cell body). (C and D) A typical nocodazole-treated cell with a granular expression pattern is shown containing one granule (arrow) in distal dendrites. Bar, 10 μm.
To test whether those hStau-GFP granules indeed contain RNA, we transiently transfected rat hippocampal neurons with hStau-GFP and subsequently labeled RNA using the RNA-specific dye SYTO14 (Knowles et al., 1996; Kiebler et al., 1999). Based on corresponding pairs of fluorescent images, we examined whether individual hStau-GFP granules colocalize with ribonucleoprotein particles. Figure 3 shows a representative example of a hippocampal neuron in which the majority of hStau-GFP granules clearly contain RNA. In total, we analyzed 579 hStau-GFP granules from 29 different cells and found that 65.6% of these granules were also SYTO14 positive. Interestingly, larger granules found in the cell body were often SYTO14 negative, whereas the majority of the smaller granules in the periphery of the cell body and in dendrites were SYTO14 positive (Figure 3). Additionally, some of the observed SYTO14-negative hStau-GFP granules might represent recycling granules migrating in the retrograde direction toward the cell body (see below). Finally, taken into account that an immunolabeling of GFP-labeled secretory granules in neuroendocrine cells yielded a 75% colocalization of all green fluorescent structures (Kaether et al., 1997), the calculated value (65.6%) of colocalization comes close to the expected theoretical upper limit of this quantitation method. In conclusion, these results demonstrate that hStau-GFP, like the endogenous Staufen, is dendritically targeted in RNA-containing granules.
Time-Lapse Fluorescent Microscopy Visualizes the Saltatory, Bidirectional Movement of hStau-GFP Granules into Dendrites of Living Neurons
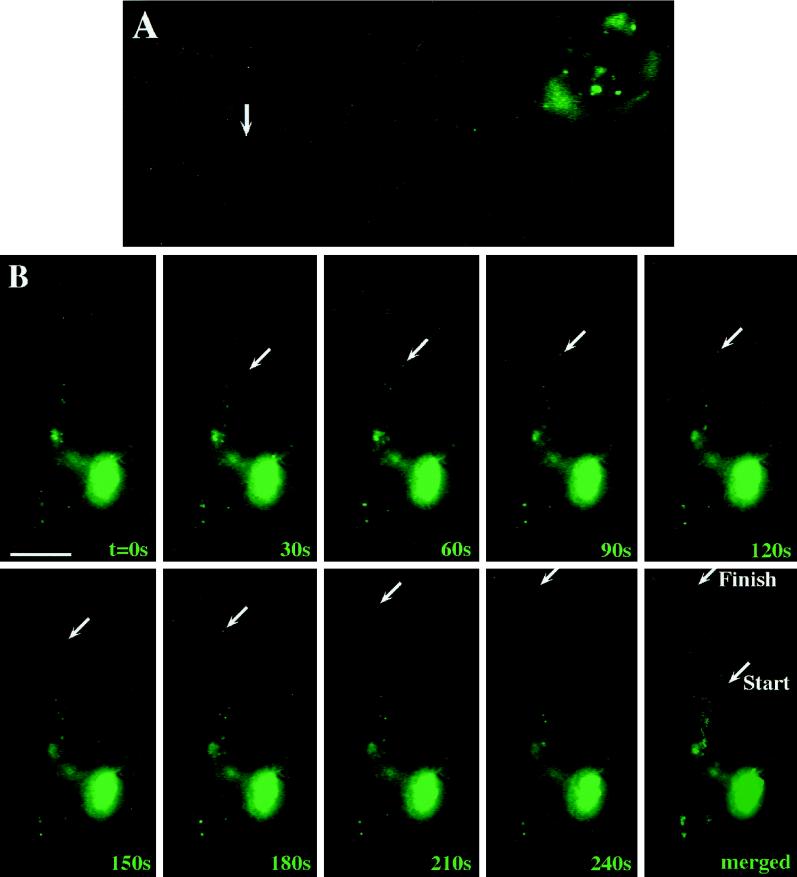
We next sought to perform time-lapse video microscopy to follow the intracellular movement of these fluorescent granules in neurons. For this reason, transiently transfected adult hippocampal neurons were transferred to a video chamber, and the movement of individual green fluorescent granules was recorded using a heated objective (Figure 4). Figure 4A is the corresponding figure to Video 1; Figure 4B is the corresponding figure to Video 2; moving particles are labeled by an arrow (online versions of both figures in QuickTime format are available at www.molbiolcell.org). In contrast to previous experiments shown in Figure 2, individual video frames were more highly integrated to detect the smaller granules in processes.
Figure 4.
Individual hStau-GFP particles are transported into dendrites of living hippocampal neurons. Hippocampal neurons on glass coverslips were transfected with hStau-GFP as described in Figure 2, and individual motilities of green fluorescent granules were observed by time-lapse video microscopy. (A) Corresponding figure to Video 1; (B) corresponding figure to Video 2. In both videos as well as in B, moving particles are labeled by an arrow and were followed during its anterograde transport into the dendrite. The individual video frames were more highly integrated compared with Fig. 2 to detect individual granules in processes. Elapsed time is indicated in seconds in the lower right of each video frame in B. The last panel in B shows a merged picture of all frames depicting both the Brownian movement of most particles as well as the discontinuous, directed motility of the chosen granule. Bar, 10 μm.
As shown in Figure 4 and the two videos, we frequently observed one or more fluorescent granules moving from the cell body into distal dendrites (anterograde transport). However, retrograde transport was also frequently observed in adult neurons (Video 2). The anterograde transport of these particles into the distal part of the dendrite was then followed. Interestingly, almost all particles in motion were moving in a saltatory and not a linear, uninterrupted manner. In most cases, particles were moving for some 30–60 s and then suddenly stopped and remain stationary for some time. These pauses can be of significant durance until these particles continue their movement. In general, three different types of movements occurred: 1) particles in a stationary phase or showing Brownian movement, 2) particles moving in one direction only, and 3) particles moving bidirectionally.
During video microscopy, we observed two types of granules in living neurons: larger granules around the nuclear membrane, which seemed to be stationary, and smaller granules moving toward the periphery of the neurons. The large granules could correspond to newly synthesized hStau-GFP protein, which still has not bound to their cognate mRNA. This is further supported by the finding that those larger granules were often SYTO14-negative (Figure 3). In this scenario, one would then hypothesize that upon contact with newly synthesized mRNA, transport-competent granules form and begin to move toward the periphery, as seen in the videos representing active transport units. Indeed, we observed in some cases smaller granules attached to larger particles, which might be in the process of pinching off (Köhrmann and Kiebler, unpublished observation). Taken together, the videos and Figure 4B faithfully represent Staufen dynamics. The fact that only a small percentage of granules actually move in a neuron has also been found in SYTO14-labeled cortical neurons (Knowles and Kosik, 1997).
Measurement of the Velocity and Average Speed of Staufen-GFP Granules in Living Neurons
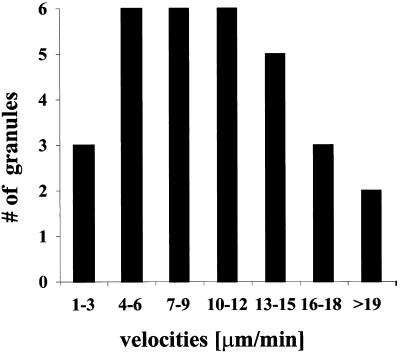
We then went on to characterize the observed transport of granules by determining the velocities and average speed of individual particles. We therefore analyzed 18 different particles to calculate their velocities and measured 42 distances that the particles traveled between two adjacent frames. Figure 5 shows a Gaussian distribution of the measured velocities arranged in increasing intervals.
Figure 5.
Gaussian distribution of measured velocities of individual hStau-GFP particles. Cells were transiently transfected, and fluorescence recording was performed as described in Figure 4. The velocities of moving hStau-GFP particles were calculated from the distance traveled between two adjacent video frames. Forty-two velocities from 18 particles were analyzed by time-lapse video microscopy, the resulting velocities arranged in increasing intervals and plotted. The mean velocity was 11.5 μm/min.
We next determined the average speed as described in MATERIALS AND METHODS and calculated a value of 6.4 ± 3.2 (SEM) μm/min by analyzing 14 different hStau-GFP granules moving over an extended distance in adult hippocampal neurons. Videos 1 (Figure 4A) and 2 (Figure 4B) show two exceptional examples of such a movement. In Video 1 (Figure 4A), the particle observed travels 27.3 μm during the recorded 2.13 min, resulting in an average speed of 12.8 μm/min; in Video 2 (Figure 4B), the observed particle travels 22.5 μm during 4 min, which results in an average speed of 5.6 μm/min. The observed overall average speed of 6.4 μm/min is more than one magnitude slower than vesicular, fast axonal transport (up to 278 μm/min) (Brady et al., 1982) or vesicular, dendritic transport (120 μm/min) (Kaether and Dotti, unpublished results).
The measured speed, however, is in very good agreement with studies on RNA transport performed on oligodendrocytes, rat brain sections, and sympathetic and cortical neurons (Ainger et al., 1993; Knowles et al., 1996; Muslimov et al., 1997; Wallace et al., 1998). In oligodendrocytes, microinjected myelin-basic protein mRNA moved with a transport rate of ∼6–12 μm/min (Ainger et al., 1993). Wallace et al. (1998) studied the transport of the Arc/arg3.1 mRNA to dendrites by in situ hybridization and determined a transport rate of 5.0 μm/min. Muslimov et al. (1997) microinjected BC1, a small noncoding RNA transcript, into sympathetic neurons and found a delivery rate of 4.0 μm/min. Finally, Knowles et al. (1996) labeled RNA-containing granules in young cortical neurons with SYTO14 and measured an average rate of 6.0 μm/min. In conclusion, these transport rates strongly argue that the RNA-binding protein Staufen is the first known protein in neurons being recruited to discrete RNA-containing particles, which are then transported with the same kinetics than described for granular RNA transport.
Recruitment of hStau-GFP into Granules and Their Subsequent Transport into Dendrites Are Microtubule Dependent in Living Neurons
We next sought to examine whether these granules move along microtubules into dendrites of hippocampal neurons. To study the effect of microtubule depolymerization on the localization and transport of hStau-GFP, we transiently transfected adult hippocampal neurons and incubated the neurons in the presence or absence of nocodazole. Green fluorescence analysis showed that microtubule depolymerization resulted in a significant change in the overall expression pattern of hStau-GFP in all cells observed (Figure 6). In control neurons, only 17% of the cells had an evenly distributed, “nonlocalized” expression of hStau-GFP, whereas all other cells showed the granular expression pattern as described in Figure 2. In nocodazole-treated cells, however, 45% of all transfected cells had a nonclustered, evenly distributed pattern with no apparent granules both in the cell body and in dendrites. This represents a 168% increase of cells with the described aberrant expression pattern of mislocalized hStau-GFP (Figure 7A). This finding clearly indicates that the recruitment and formation of hStau-GFP into granules requires an intact cytoskeleton.
We next analyzed the effect of nocodazole on the transport of hStau-GFP particles into the distal part of dendrites. For this we focused our attention on those cells that had a granular expression pattern (Figures 6 and 7B). Whereas 83% of the control cells (29 of 35) with a granular expression pattern had more than two granules in distal parts of the dendrites, only 16% (5 of 32) of the nocodazole-treated cells showed the same phenotype. When normalized, this reflects an 81% suppression of the number of neurons with fluorescent, dendritically transported hStau-GFP granules (Figure 7B). To study the role of the actin on hStau-GFP expression pattern, we used latrunculin B (Spector et al., 1989), a G-actin–sequestering drug. Latrunculin B had no effect on both the localization and transport of hStau-GFP granules.
A similar microtubule dependence for mRNA transport has been observed in Drosophila embryos (Ferrandon et al., 1994) and cortical neurons (Knowles et al., 1996). In the embryo, the microtubule-depolymerizing drug colcemid but not the actin-depolymerizing drug cytochalasin B prevented both the formation of Staufen-bcd-3′-UTR particles after injection of bcd-3′-UTR mRNA into embryos as well as their subsequent movement. Ferrandon et al. (1994) introduced a model in which the binding of Staufen to its target RNA induces a conformational change in the Staufen protein. In our opinion it is conceivable that this would allow the Staufen-RNA complex to assemble either directly or indirectly to microtubules thereby leading to the assembly of the granules at the site of transport. In mammalian neurons this is further corroborated by the fact that human Staufen contains a (low-affinity) tubulin binding domain (Wickham et al., 1999) allowing the Staufen–RNA complex to bind to the cytoskeleton directly. In cortical neurons, colchicine, another microtubule-depolymerizing drug, but not cytochalasin D prevented the transport of RNA-labeled granules into neurites of 4-d-old neurons. Taken together, we show for the first time that hStau-GFP is recruited to RNA-containing granules in adult hippocampal neurons. The existence of fluorescent granules now enabled us to examine their motility in living neurons. Video microscopy revealed that this transport occurs in a saltatory manner with many particles remaining in a stationary phase during recording and allowed us to calculate an average speed of 6.4 μm/min. Finally, we demonstrate that both the assembly and the dendritic transport of these granules require intact microtubules.
Perspectives
The RNA-binding protein Staufen is the first known protein being recruited to discrete particles, which then move into dendrites. Their visualization in living neurons (see attached videos) therefore faithfully represents for the first time the dynamics of this process. Interestingly, both the velocity and the microtubule dependence correlate well with studies in other organisms, suggesting a strong conservation of the mRNA transport mechanism through phylogeny. Moreover, the assay presented here will allow to experimentally address a central hypothesis in neurobiology: is dendritic mRNA transport (and subsequent local translation) to the synapse involved in forming synaptic memories? Finally, the biochemical isolation of these particles will lead to the identification of both transported mRNAs as well as other components of this transport machinery.
Supplementary Material
ACKNOWLEDGMENTS
This paper is dedicated to Walter Neupert at the occasion of his 60th birthday. Special thanks to Lola Ledesma, Bianca Hellias, Eugenia Piddini, Francesca Ruberti, Barbara Grunewald, Jürgen Löschinger, R. Carazo Salas, P. Fortes, H. McBride, P. Scheiffele, and F. Bonhoeffer for discussions and/or critically reading the manuscript. M.A.K. was supported by research fellowships from Deutsche Forschungsgemeinschaft and Human Frontier Science Program; C.K. was supported by a fellowship from the Fritz-Thyssen-Stiftung; C.G.D. was supported by Sonderforschungsbereich grant SFB 317; and L.D.G. was supported by a Natural Sciences and Engineering Research Council of Canada grant.
Footnotes
Online version of this article contains video material for Figure 4. Online version available at www.molbiolcell.org.
REFERENCES
- Ainger K, Avossa D, Morgan F, Hill SJ, Barry C, Barbarese E, Carson JH. Transport and localization of exogenous myelin basic protein mRNA microinjected into oligodendrocytes. J Cell Biol. 1993;123:431–441. doi: 10.1083/jcb.123.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassell G, Zhang H, Byrd AL, Femino AM, Singer RH, Taneja KL, Lifshitz LM, Herman IM, Kosik KS. Sorting of β-actin mRNA and protein to neurites and growth cones in culture. J Neurosci. 1998;18:251–265. doi: 10.1523/JNEUROSCI.18-01-00251.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand E, Chartrand P, Schaefer M, Shenoy SM, Singer RH, Long RM. Localization of ASH1 mRNA particles in living yeast. Mol Cell. 1998;2:437–445. doi: 10.1016/s1097-2765(00)80143-4. [DOI] [PubMed] [Google Scholar]
- Bradke F, Dotti CG. Microinjection and Transgenesis: Strategies and Protocols, ed A. Cid-Arregui and A. Garcia-Carrancá. Heidelberg: Springer-Verlag; 1998. Videomicroscopy of living microinjected hippocampal neurons in culture; pp. 81–94. [Google Scholar]
- Bradke F, Dotti CG. The role of local actin instability in axon formation. Science. 1999;283:1931–1934. doi: 10.1126/science.283.5409.1931. [DOI] [PubMed] [Google Scholar]
- Brady ST, Lasek RJ, Allen RD. Fast axonal transport in extruded cytoplasm from squid giant axon. Science. 1982;218:1129–1131. doi: 10.1126/science.6183745. [DOI] [PubMed] [Google Scholar]
- Carson JH, Kwon S, Barbarese E. RNA trafficking in myelinating cells. Curr Opin Neurobiol. 1998;8:607–612. doi: 10.1016/s0959-4388(98)80088-3. [DOI] [PubMed] [Google Scholar]
- Carson JH, Worboys K, Ainger K, Barbarese E. Translocation of myelin basic protein mRNA in oligodendrocytes requires microtubules and kinesin. Cell Motil Cytoskeleton. 1997;38:318–328. doi: 10.1002/(SICI)1097-0169(1997)38:4<318::AID-CM2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- de Hoop MJ, Meyn L, Dotti CG. Culturing hippocampal neurons and astrocytes from fetal rodent brain. In: Celis JE, editor. Cell Biology: A Laboratory Handbook. 2nd ed. San Diego: Academic Press; 1998. pp. 154–163. [Google Scholar]
- De Strooper B, Simons M, Multhaup G, Van Leuven F, Beyreuther K, Dotti CG. Production of intracellular amyloid-containing fragments in hippocampal neurons expressing human amyloid precursor protein and protection against amyloidogenesis by subtle amino acid substitutions in the rhodent sequence. EMBO J. 1995;14:4932–4938. doi: 10.1002/j.1460-2075.1995.tb00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshler JO, Highett MI, Abramson T, Schnapp BJ. A highly conserved RNA-binding protein for cytoplasmic mRNA localization in vertebrates. Curr Biol. 1998;8:489–496. doi: 10.1016/s0960-9822(98)70200-3. [DOI] [PubMed] [Google Scholar]
- Deshler JO, Highett MI, Schnapp BJ. Localization of Xenopus Vg1 mRNA by VERA protein and the endoplasmic reticulum. Science. 1997;276:1128–1131. doi: 10.1126/science.276.5315.1128. [DOI] [PubMed] [Google Scholar]
- Driever W, Nüsslein-Volhard C. The bicoid protein determines position in the Drosophila embryo in a concentration-dependent manner. Cell. 1988;54:95–104. doi: 10.1016/0092-8674(88)90183-3. [DOI] [PubMed] [Google Scholar]
- Ephrussi A, Dickinson LK, Lehmann R. Oskar organizes the germ plasm and directs localization of the posterior determinant nanos. Cell. 1991;66:37–50. doi: 10.1016/0092-8674(91)90137-n. [DOI] [PubMed] [Google Scholar]
- Ferrandon D, Elphick L, Nüsslein-Volhard C, St. Johnston D. Staufen protein associates with the 3-UTR of bicoid mRNA to form particles that move in a microtubule-dependent manner. Cell. 1994;79:1221–1231. doi: 10.1016/0092-8674(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Goslin K, Banker G. Rat hippocampal neurons in low-density culture. In: Banker G, Goslin K, editors. Culturing Nerve Cells. Cambridge, MA: MIT Press; 1997. pp. 251–281. [Google Scholar]
- Haubensack W, Narz F, Heumann R, Leßmann V. BDNF-GFP containing secretory granules are localized in the vicinity of synaptic junctions of cultured cortical neurons. J Cell Sci. 1998;111:1483–1493. doi: 10.1242/jcs.111.11.1483. [DOI] [PubMed] [Google Scholar]
- Hazelrigg T. The destinies and destinations of RNAs (meeting review) Cell. 1998;95:451–460. doi: 10.1016/s0092-8674(00)81613-x. [DOI] [PubMed] [Google Scholar]
- Hoek K, Kidd GJ, Carson JH, Smith R. hnRNP A2 selectively binds the cytoplasmic transport sequence of myelin basic protein mRNA. Biochemistry. 1998;37:7021–7029. doi: 10.1021/bi9800247. [DOI] [PubMed] [Google Scholar]
- Holmes E, Hermanson G, Cole R, de Vellis J. Developmental expression of glial-specific mRNAs in primary cultures of rat brain visualized by in situ hybridization. J Neurosci. 1988;19:389–396. doi: 10.1002/jnr.490190402. [DOI] [PubMed] [Google Scholar]
- Kaether C, Salm T, Glombik M, Almers W, Gerdes H-H. Targeting of GFP to neuroendocrine secretory granules: a new tool for real time studies of regulated protein secretion. Eur J Cell Biol. 1997;74:133–142. [PubMed] [Google Scholar]
- Kiebler M, Hemraj I, Verkade P, Köhrmann M, Fortes P, Marión RM, Ortín J, Dotti CG. The mammalian Staufen protein localizes to the somatodendritic domain of cultured hippocampal neurons: implications for its involvement in mRNA transport. J Neurosci. 1999;19:288–297. doi: 10.1523/JNEUROSCI.19-01-00288.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles RB, Kosik KS. Neurotrophin-3 signals redistribute RNA in neurons. Proc Natl Acad Sci USA. 1997;94:14804–14808. doi: 10.1073/pnas.94.26.14804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles RB, Sabry JH, Martone ME, Deerinck TJ, Ellisman MH, Bassell GJ, Kosik KS. Translocation of RNA granules in living neurons. J Neurosci. 1996;16:7812–7820. doi: 10.1523/JNEUROSCI.16-24-07812.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl D, Skehel P. Dendritic localization of mRNAs. Curr Opin Neurobiol. 1998;8:600–606. doi: 10.1016/s0959-4388(98)80087-1. [DOI] [PubMed] [Google Scholar]
- Marión RM, Fortes P, Beloso A, Dotti CG, Ortín J. A human sequence homologue of staufen is an RNA-binding protein that is associated with polysomes and localizes to the rough ER. Mol Cell Biol. 1999;19:2212–2219. doi: 10.1128/mcb.19.3.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayford M, Baranes D, Podsypanina K, Kandel ER. The 3′-untranslated region of CaMKII alpha is a cis-acting signal for the localization and translation of mRNA in dendrites. Proc Natl Acad Sci USA. 1996;93:13250–13255. doi: 10.1073/pnas.93.23.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muslimov IA, Santi E, Homel P, Perini S, Higgins D, Tiedge H. RNA transport in dendrites: a cis-acting targeting element is contained within neuronal BC1 RNA. J Neurosci. 1997;17:4722–4733. doi: 10.1523/JNEUROSCI.17-12-04722.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AF, Oleynikov Y, Kislauskis EH, Taneja KL, Singer RH. Characterization of a β-actin mRNA zipcode-binding protein. Mol Cell Biol. 1997;17:2158–2165. doi: 10.1128/mcb.17.4.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, De Strooper B, Multhaup G, Tienari PJ, Dotti CG, Beyreuther K. Amyloidogenic processing of the human amyloid precursor protein in primary cultures of rat hippocampal neurons. J Neurosci. 1996;16:899–908. doi: 10.1523/JNEUROSCI.16-03-00899.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector I, Shochet NR, Blasberger D, Kashman Y. Latrunculins—novel marine macrolides that disrupt microfilament organization and affect cell growth: I. comparison with cytochalasin D. Cell Motil Cytoskeleton. 1989;13:127–144. doi: 10.1002/cm.970130302. [DOI] [PubMed] [Google Scholar]
- St. Johnston D. The intracellular localization of mRNAs. Cell. 1995;81:161–170. doi: 10.1016/0092-8674(95)90324-0. [DOI] [PubMed] [Google Scholar]
- St. Johnston D, Beuchle D, Nuesslein-Volhard C. staufen, a gene required to localize maternal RNAs in the Drosophila egg. Cell. 1991;66:51–63. doi: 10.1016/0092-8674(91)90138-o. [DOI] [PubMed] [Google Scholar]
- Tiedge H, Bloom FE, Richter D. RNA, whither goest thou? Science. 1999;283:186–187. doi: 10.1126/science.283.5399.186. [DOI] [PubMed] [Google Scholar]
- Wacker I, Kaether C, Krömer A, Migala A, Almers W, Gerdes H-H. Microtubule-dependent transport of secretory vesicles visualized in real time with a GFP-tagged secretory protein. J Cell Sci. 1997;110:1453–1463. doi: 10.1242/jcs.110.13.1453. [DOI] [PubMed] [Google Scholar]
- Wallace CS, Lyford GL, Worley PF, Steward O. Differential intracellular sorting of immediate early gene mRNAs depends on signals in the mRNA sequence. J Neurosci. 1998;18:26–35. doi: 10.1523/JNEUROSCI.18-01-00026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Hazelrigg T. Implications for bcd mRNA localization from spatial distribution of exu protein in Drosophila oogenesis. Nature. 1994;369:400–403. doi: 10.1038/369400a0. [DOI] [PubMed] [Google Scholar]
- Wickham L, Duchaîne T, Luo M, Nabi IR, DesGroseillers L. Mammalian Staufen is a double-stranded RNA- and tubulin-binding protein which localizes to the rough ER. Mol Cell Biol. 1999;19:2220–2230. doi: 10.1128/mcb.19.3.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm JE, Vale RD. RNA on the move: the mRNA localization pathway. J Cell Biol. 1993;123:269–274. doi: 10.1083/jcb.123.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.