Abstract
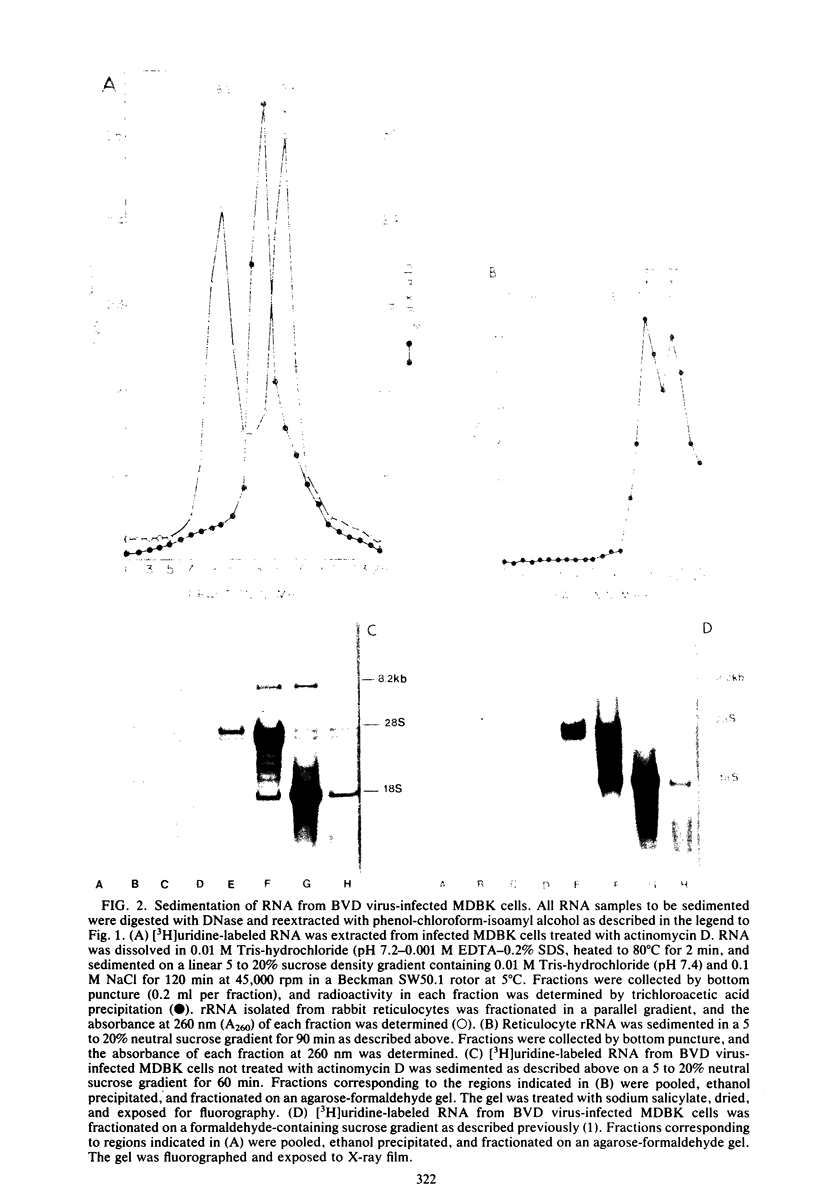
Infection of bovine kidney cells with bovine viral diarrhea virus resulted in the synthesis of a single species of virus-specific RNA. Electrophoresis of this RNA on agarose-urea and agarose-formaldehyde gels indicated that it had a molecular weight of 2.9 X 10(6), corresponding to 8,200 bases (8.2 kilobases). This 8.2-kilobase RNA was resistant to RNase A treatment at 1 microgram/ml but was digested at higher concentrations of RNase (10 micrograms/ml). Sedimentation on neutral sucrose gradients indicated that the majority of this RNA (98%) sedimented at 21S, with a small amount sedimenting at 33S. Sedimentation on formaldehyde-containing sucrose gradients resulted in the conversion of all of the RNA to the faster-sedimenting form. At no time after infection were we able to detect virus-specific RNA species of lower molecular weight than the 8.2-kilobase RNA. The implications of these findings with respect to the means of replication of various togaviruses are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arif B. M., Faulkner P. Genome of Sindbis virus. J Virol. 1972 Jan;9(1):102–109. doi: 10.1128/jvi.9.1.102-109.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- HERMODSSON S., DINTER Z. Properties of bovine virus diarrhoea virus. Nature. 1962 Jun 2;194:893–894. doi: 10.1038/194893a0. [DOI] [PubMed] [Google Scholar]
- Hafez S. M., Liess B. Studies on bovine viral diarrhea-mucosal disease virus. II. Stability and some physico-chemical properties. Acta Virol. 1972 Sep;16(5):399–408. [PubMed] [Google Scholar]
- Lai M. M., Patton C. D., Stohlman S. A. Replication of mouse hepatitis virus: negative-stranded RNA and replicative form RNA are of genome length. J Virol. 1982 Nov;44(2):487–492. doi: 10.1128/jvi.44.2.487-492.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Pritchett R., Manning J. S., Zee Y. C. Characterization of bovine viral diarrhea virus RNA. J Virol. 1975 Jun;15(6):1342–1347. doi: 10.1128/jvi.15.6.1342-1347.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchio A. F., Fareed G. C. Transformation of human embryonic kidney cells by human papovarirus BK. J Virol. 1979 Feb;29(2):763–769. doi: 10.1128/jvi.29.2.763-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards O. C., Ehrenfeld E. Heterogeneity of the 3' end of minus-strand RNA in the poliovirus replicative form. J Virol. 1980 Nov;36(2):387–394. doi: 10.1128/jvi.36.2.387-394.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B., Goran D., Cancedda R., Schlesinger S. Defective interfering passages of Sindbis virus: nature of the intracellular defective viral RNA. J Virol. 1974 Nov;14(5):1189–1198. doi: 10.1128/jvi.14.5.1189-1198.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengler G., Wengler G., Gross H. J. Studies on virus-specific nucleic acids synthesized in vertebrate and mosquito cells infected with flaviviruses. Virology. 1978 Sep;89(2):423–437. doi: 10.1016/0042-6822(78)90185-x. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. B., Sandeen D. The ribonuclease activity of crystallized pancreatic deoxyribonuclease. Anal Biochem. 1966 Feb;14(2):269–277. doi: 10.1016/0003-2697(66)90137-0. [DOI] [PubMed] [Google Scholar]