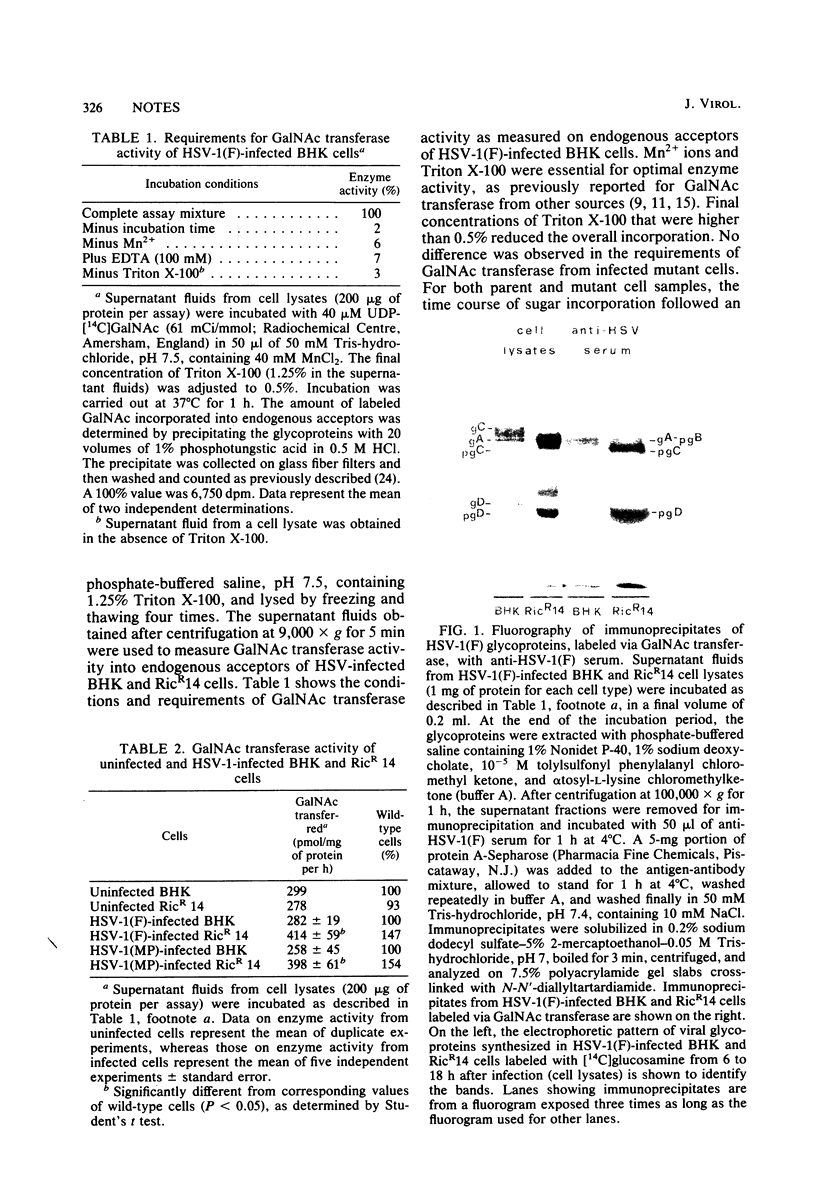
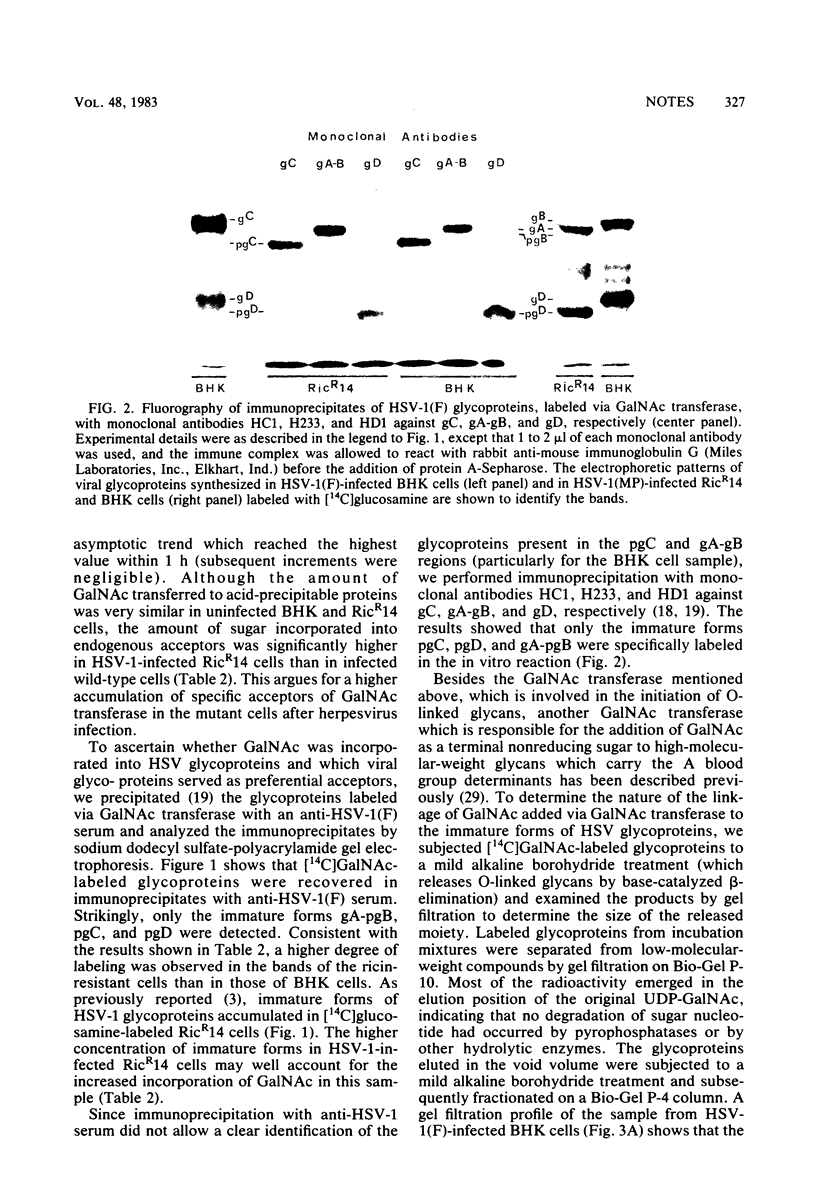
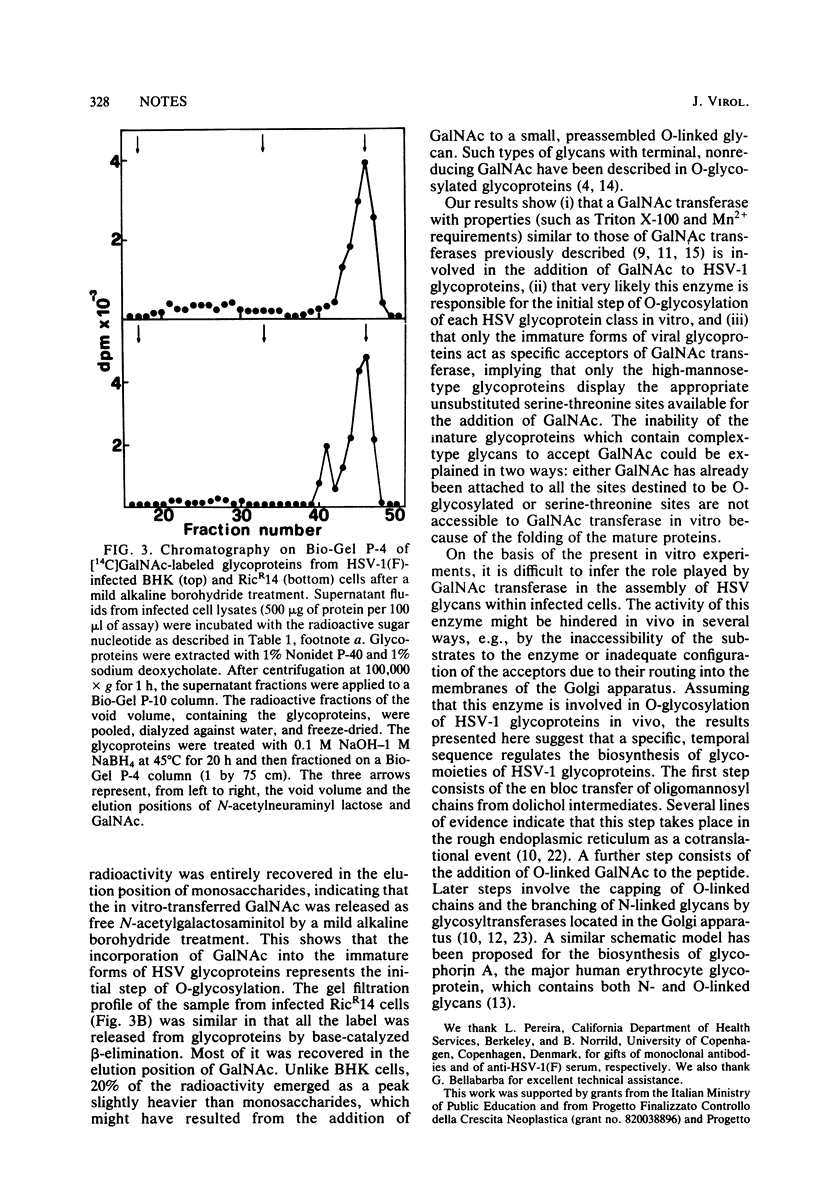
Abstract
We report on N-acetylgalactosaminyltransferase (UDPacetylgalactosamine--protein acetylgalactosaminyltransferase; EC 2.4.1.41) activity in herpes simplex virus type 1 (HSV-1)-infected BHK and RicR14 cells, a line of ricin-resistant BHK cells defective in N-acetylglucosaminyltransferase I. The enzyme catalyzed the transfer of [14C]N-acetylgalactosamine (GalNAc) from UDP-[14C]GalNAc into HSV glycoproteins, as identified by immunoprecipitation. The sugar was selectively incorporated into the immature forms of herpesvirus glycoproteins pgC, pgD, and gA-pgB, which are known to contain N-linked glycans of the high-mannose type. The high incorporation of [14C]GalNAc into endogenous acceptors of HSV-1-infected RicR14 cells was consistent with the accumulation of immature forms of HSV glycoproteins which occurs in these cells. Mild alkaline borohydride treatment of glycoproteins labeled via GalNAc transferase showed that the transferred GalNAc was O-linked and represented the first sugar added to the peptide backbone.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baucke R. B., Spear P. G. Membrane proteins specified by herpes simplex viruses. V. Identification of an Fc-binding glycoprotein. J Virol. 1979 Dec;32(3):779–789. doi: 10.1128/jvi.32.3.779-789.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer T. A., Sadler J. E., Rearick J. I., Paulson J. C., Hill R. L. Glycosyltransferases and their use in assessing oligosaccharide structure and structure-function relationships. Adv Enzymol Relat Areas Mol Biol. 1981;52:23–175. doi: 10.1002/9780470122976.ch2. [DOI] [PubMed] [Google Scholar]
- Campadelli-Fiume G., Poletti L., Dall'Olio F., Serafini-Cessi F. Infectivity and glycoprotein processing of herpes simplex virus type 1 grown in a ricin-resistant cell line deficient in N-acetylglucosaminyl transferase I. J Virol. 1982 Sep;43(3):1061–1071. doi: 10.1128/jvi.43.3.1061-1071.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson D. M. Structures and immunochemical properties of oligosaccharides isolated from pig submaxillary mucins. J Biol Chem. 1968 Feb 10;243(3):616–626. [PubMed] [Google Scholar]
- Cohen G. H., Long D., Eisenberg R. J. Synthesis and processing of glycoproteins gD and gC of herpes simplex virus type 1. J Virol. 1980 Nov;36(2):429–439. doi: 10.1128/jvi.36.2.429-439.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Hydrean-Stern C., Cohen G. H. Structural analysis of precursor and product forms of type-common envelope glycoprotein D (CP-1 antigen) of herpes simplex virus type 1. J Virol. 1979 Sep;31(3):608–620. doi: 10.1128/jvi.31.3.608-620.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagopian A., Bosmann H. B., Eylar E. H. Glycoprotein biosynthesis: the localization of polypeptidyl: N-acetylgalactosaminyl, collagen: glucosyl, and glycoprotein:galactosyl transferases in HeLa cell membrane fractions. Arch Biochem Biophys. 1968 Nov;128(2):387–396. doi: 10.1016/0003-9861(68)90045-3. [DOI] [PubMed] [Google Scholar]
- Hagopian A., Eylar E. H. Glycoprotein biosynthesis: the purification and characterization of a polypeptide. N-acetylgalactosaminyl transferase from bovine submaxillary glands. Arch Biochem Biophys. 1969 Feb;129(2):515–524. doi: 10.1016/0003-9861(69)90209-4. [DOI] [PubMed] [Google Scholar]
- Hanover J. A., Lennarz W. J. Transmembrane assembly of membrane and secretory glycoproteins. Arch Biochem Biophys. 1981 Oct 1;211(1):1–19. doi: 10.1016/0003-9861(81)90423-9. [DOI] [PubMed] [Google Scholar]
- Hill H. D., Jr, Schwyzer M., Steinman H. M., Hill R. L. Ovine submaxillary mucin. Primary structure and peptide substrates of UDP-N-acetylgalactosamine:mucin transferase. J Biol Chem. 1977 Jun 10;252(11):3799–3804. [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. Monensin inhibits the processing of herpes simplex virus glycoproteins, their transport to the cell surface, and the egress of virions from infected cells. J Virol. 1982 Sep;43(3):1102–1112. doi: 10.1128/jvi.43.3.1102-1112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. O-linked oligosaccharides are acquired by herpes simplex virus glycoproteins in the Golgi apparatus. Cell. 1983 Mar;32(3):987–997. doi: 10.1016/0092-8674(83)90083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokinen M., Gahmberg C. G., Andersson L. C. Biosynthesis of the major human red cell sialoglycoprotein, glycophorin A, in a continuous cell line. Nature. 1979 Jun 14;279(5714):604–607. doi: 10.1038/279604a0. [DOI] [PubMed] [Google Scholar]
- McAuliffe F. M., Kabat E. A. Immunochemical studies on blood groups. Structures and immunochemical properties of oligosaccharides from two fractions of blood group substance from human ovarian cyst fluid differing in B, I, and i activities and reactivity toward concanavalin A. Arch Biochem Biophys. 1976 Jul;175(1):90–113. doi: 10.1016/0003-9861(76)90488-4. [DOI] [PubMed] [Google Scholar]
- McGuire E. J., Roseman S. Enzymatic synthesis of the protein-hexosamine linkage in sheep submaxillary mucin. J Biol Chem. 1967 Aug 25;242(16):3745–3747. [PubMed] [Google Scholar]
- Niemann H., Klenk H. D. Coronavirus glycoprotein E1, a new type of viral glycoprotein. J Mol Biol. 1981 Dec 25;153(4):993–1010. doi: 10.1016/0022-2836(81)90463-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson S., Blomberg J., Lycke E. O-glycosidic carbohydrate-peptide linkages of Herpes simplex virus glycoproteins. Arch Virol. 1981;70(4):321–329. doi: 10.1007/BF01320247. [DOI] [PubMed] [Google Scholar]
- Pereira L., Dondero D., Norrild B., Roizman B. Differential immunologic reactivity and processing of glycoproteins gA and gB of herpes simplex virus types 1 and 2 made in Vero and HEp-2 cells. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5202–5206. doi: 10.1073/pnas.78.8.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Klassen T., Baringer J. R. Type-common and type-specific monoclonal antibody to herpes simplex virus type 1. Infect Immun. 1980 Aug;29(2):724–732. doi: 10.1128/iai.29.2.724-732.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person S., Kousoulas K. G., Knowles R. W., Read G. S., Holland T. C., Keller P. M., Warner S. C. Glycoprotein processing in mutants of HSV-1 that induce cell fusion. Virology. 1982 Mar;117(2):293–306. doi: 10.1016/0042-6822(82)90470-6. [DOI] [PubMed] [Google Scholar]
- Pizer L. I., Cohen G. H., Eisenberg R. J. Effect of tunicamycin on herpes simplex virus glycoproteins and infectious virus production. J Virol. 1980 Apr;34(1):142–153. doi: 10.1128/jvi.34.1.142-153.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Lodish H. F. Synchronised transmembrane insertion and glycosylation of a nascent membrane protein. Nature. 1977 Oct 27;269(5631):775–780. doi: 10.1038/269775a0. [DOI] [PubMed] [Google Scholar]
- Schachter H. The subcellular sites of glycosylation. Biochem Soc Symp. 1974;(40):57–71. [PubMed] [Google Scholar]
- Serafini-Cessi F., Campadelli-Fiume G. Studies on benzhydrazone, a specific inhibitor of herpesvirus glycoprotein synthesis. Size distribution of glycopeptides and endo-beta-N-acetylglucosaminidase-H treatment. Arch Virol. 1981;70(4):331–343. doi: 10.1007/BF01320248. [DOI] [PubMed] [Google Scholar]
- Serafini-Cessi F. Sialyltransferase activity in regenerating rat liver. Biochem J. 1977 Sep 15;166(3):381–386. doi: 10.1042/bj1660381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shida H., Dales S. Biogenesis of vaccinia: carbohydrate of the hemagglutinin molecules. Virology. 1981 May;111(1):56–72. doi: 10.1016/0042-6822(81)90653-x. [DOI] [PubMed] [Google Scholar]
- Spear P. G. Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J Virol. 1976 Mar;17(3):991–1008. doi: 10.1128/jvi.17.3.991-1008.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vischer P., Hughes R. C. Glycosyl transferases of baby-hamster-kidney (BHK) cells and ricin-resistant mutants. N-glycan biosynthesis. Eur J Biochem. 1981 Jul;117(2):275–284. doi: 10.1111/j.1432-1033.1981.tb06334.x. [DOI] [PubMed] [Google Scholar]
- Watkins W. M. Biochemistry and Genetics of the ABO, Lewis, and P blood group systems. Adv Hum Genet. 1980;10:1-136, 379-85. doi: 10.1007/978-1-4615-8288-5_1. [DOI] [PubMed] [Google Scholar]
- Wenske E. A., Bratton M. W., Courtney R. J. Endo-beta-N-acetylglucosaminidase H sensitivity of precursors to herpes simplex virus type 1 glycoproteins gB and gC. J Virol. 1982 Oct;44(1):241–248. doi: 10.1128/jvi.44.1.241-248.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]