Abstract
Bladder inflammation resulting from intravesical administration of zymosan significantly enhances the visceromotor reflex (VMR) evoked by urinary bladder distension (UBD). The present study examined whether intrathecal (i.t.) administration of receptor antagonists to either noreprinephrine (NE) or serotonin (5-HT) altered this enhancement effect. I.t. administration of the non-specific 5-HT receptor antagonist methysergide (30 μg), the 5-HT3 receptor antagonist ondansetron, or the 5-HT1A receptor antagonist WAY 100635 eliminated the enhancement effect produced by intravesical zymosan and also tended to reduce EMG responses to UBD in non-inflamed rats. I.t. administration of either the non-specific NE receptor antagonist phentolamine (30 μg) or the α1 antagonist WB4101 also eliminated the enhancement effect, whereas i.t. administration of the α2 antagonist yohimbine failed to significantly affect the enhancement effect. The effects of phentolamine and methysergide were not mediated by changes in bladder compliance. This is the first study to demonstrate that bladder hypersensitivity resulting from bladder inflammation is partly mediated by 5-HT and NE facilitatory effects. Based on these and previous findings we conclude that the net nociceptive response to bladder distension under conditions of bladder inflammation represents a complex interaction of facilitatory influences of spinal 5-HT and NE, and inhibitory influences of spinal opioids.
Keywords: bladder, serotonin, norepinephrine, visceromotor reflex, pain, facilitation
Introduction
Intravesical administration of zymosan in adult rats leads to a time-dependent increase in urinary bladder inflammation [20] and a significant enhancement of abdominal muscle electromyographic (EMG) and arterial blood pressure (ABP) responses to urinary bladder distension (UBD) when compared to controls [5,20,21]. This inflammation-induced bladder hypersensitivity is being suppressed by endogenous opioids because either intraperitoneal (i.p.) or intrathecal (i.t.) administration of naloxone at the time of UBD testing significantly increases the magnitude of EMG responses when compared to controls [5]. The opioid inhibitory effect may be under descending control of the RVM since electrical stimulation of the RVM inhibits the VMR to UBD and is partially mediated by spinal opioids. [19].
The RVM and other brainstem regions, including the A5, A6, and A7 cell groups, are also well established as final common pathways involved in the spinal release of 5-HT and NE. [c.f., 9,14,16]. These systems are primarily known for promoting descending spinal inhibitory influences and have figured prominently in our theoretical views about modulation of spinal nociceptive transmission [8]. However, these same regions and transmitters are now recognized as mediating descending spinal facilitatory influences on spinal nociceptive transmission, particularly contributing to hyperalgesic states [1,23,28,29,34-37].
In the present studies, we examined whether 5-HT and NE exerted either inhibitory or facilitatory roles in the bladder hypersensitivity produced by bladder inflammation. This was accomplished by using i.t. administration of both non-specific receptor antagonists (methysergide and phentolamine) and more selective receptor antagonists to 5-HT and NE (ondansetron, WAY 100635, yohimbine and WB4101).
Materials and methods
Animals and animal care
One hundred and eighteen adult female Sprague-Dawley rats were obtained from Harlan (Prattville, AL) and maintained in separate cages. The light-dark cycle for all rats was 6:00-6:00. At the time of adult testing, no attempt was made to control for phase of estrous cycle. All studies were approved by the University of Alabama at Birmingham Animal Care and Use Committee and conformed to the NIH guidelines for the care and use of laboratory animals.
General Surgical and Testing Procedures
Rats were pretreated with 30 min of exposure to either intravesical zymosan (1% solution in saline; 0.5 ml) under isoflurane/oxygen anesthesia (5% induction followed by 2%) or isoflurane/oxygen anesthesia alone (5% induction followed by 2%). The zymosan was administered via a 22-gauge angiocatheter. After 30 min the zymosan solution was drained and the angiocatheter removed. Each animal was administered 20 mg of ampicillin and awakened.
On the next day, a 7.8 cm i.t. catheter made of PE10 tubing was inserted via the atlanto-occipital membrane and threaded down to the L6-S1 region via the spinal column under deep isoflurane/oxygen anesthesia (4%). The trachea was cannulated for artificial respiration and a 22-gauge angiocatheter was inserted into the urinary bladder via the urethra and held in place by a tight suture placed around the distal urethral orifice. The animal was then moved to a recording area, placed on a ventilator, and remained on 4% isoflurane/oxygen while platinum wire EMG electrodes were placed into the left external oblique musculature for differential amplification and recording of EMG activity. The EMG of the abdominal external oblique muscle was used to index the VMR to graded UBD. Rats were not restrained in any fashion and body temperature was maintained using a heating pad. The anesthesia was reduced to 0.75% and remained at this level for the rest of testing.
Each rat then received an i.t. injection of a receptor antagonist or saline vehicle followed by UBD testing 15 min later. At that time, three 60 mmHg distensions (20 s duration) of the urinary bladder were administered to overcome the initial period of bladder sensitization [4] and were followed by a sequence of 20 sec duration graded distensions of the bladder at pressures of 10, 20, 30, 40, 50, 60, 70 and 80 mmHg, respectively (3 min ITI). Receptor antagonists were methysergide maleate (30 μg), phentolamine HCl (30 μg), ondansetron (10 ug), WAY 100635 (10 ug), yohimbine (30 ug), WB4101 (30 ug) or saline vehicle. These drug doses were based on previous studies demonstrating their effectiveness in a variety of situations [10]. All drugs were administered in 7.5 μl of saline except for yohimbine which was administered in 15 μl of saline. Each drug administration was followed by a 10 μl saline flush.
A subset of rats that had received either the zymosan (N=5) or anesthesia (N=6) pretreatment and the i.t. saline treatment and UBD testing were given a second series of graded UBD trials after administering 30 μg i.t. phentolamine. This provided a pre (saline i.t.) / post (phentolamine i.t.) within-subject comparison to check our between-subjects design for phentolamine.
To ensure that any changes observed with the methysergide and phentolamine were not due to an influence on spinal neuronal control of bladder compliance, an additional three groups of rats (N=4/group) received pre-treatment with anesthesia alone 24 hours prior to CMG testing. At the time of CMG testing, these animals received placement of an i.t. catheter and i.t. drug administration (methysergide, phentolamine, or saline) as described above followed by a 40 min slow infusion (.05 ml/min) of room-temperature saline with continuous recording of intravesical pressure through an in-line pressure transducer.
I.t. catheters are routinely used in our laboratory. The placement of i.t. catheters are examined with dye injections in any animal where the experimenter reported difficulty in placing the catheter and are also examined through random sampling of animals. No animals were eliminated based on these examinations.
Data Analysis and Statistics
All data were saved on a computer using Spike-2 software and associated hardware (Micro 1401; CED, Cambridge, UK). The EMG response was defined as: (rectified EMG activity during UBD - rectified baseline EMG prior to UBD) / rectified baseline EMG as described previously [20,21]. Groups are designated as pretreatment (Z for zymosan and A for anesthesia) / i.t. drug. Group Z/saline served as the primary experimental comparison group. We continued to add subjects to this group with each additional order from the animal supplier in order to control for possible batch effects because we processed each receptor antagonist group in succession. This resulted in a total of 13 subjects in this group. Group data are presented as means ± S.E.M. An overall ANOVA was performed followed by analysis of simple main effects and post-hoc contrasts of mean using Holm’s procedure [11] to maintain family-wise α at 0.05. Statistical significance was defined as p values ≤0.05.
Results
Figure 1 presents the mean EMG responses for the various groups split into four panels for ease of data presentation. The control functions (groups Z/saline and A/saline) are reproduced in each panel. An ANOVA of all groups revealed significant effects for groups, UBD pressure, and groups × UBD pressure (all p’s < 0.002). Follow-up ANOVAs were then performed for group Z/saline versus group A/saline, and for group Z/saline versus each group receiving a receptor antagonist. Group Z/saline showed a robust EMG response to UBD that was significantly greater than that of group A/saline demonstrating that zymosan pretreatment produced an enhancement effect. Group Z/methysergide (Panel A) and group Z/phentolamine (Panel B) had significantly reduced EMG responses when compared to group Z/saline and the EMG responses were not significantly different from group A/saline. There also was a clear trend towards decreased EMG responses of groups A/methysergide and A/phentolamine when compared to group A/saline suggesting possible tonic facilitatory effects of 5-HT and NE under non-inflamed conditions, but the ANOVAs were not significant in either case. Panel C shows groups Z/ondansetron and group Z/WAY 100635 also had significantly reduced EMG responses compared to group Z/saline and achieved stimulus-response functions comparable to group A/saline and also comparable to those achieved with methysergide. Panel D shows that group Z/WB 4101, but not group Z/yohimbine, had significantly reduced the EMG responses of rats and achieved stimulus response functions equivalent to group A/saline.
Figure 1.
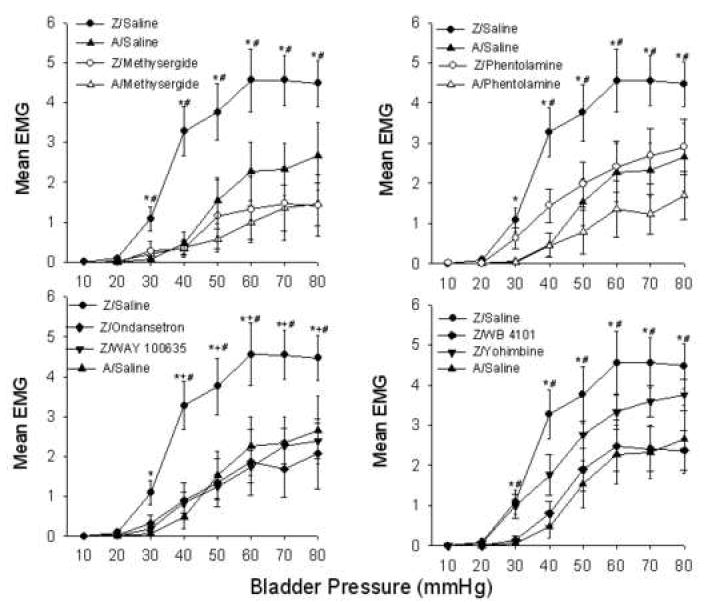
Group mean EMG responses during graded UBD. Groups received either intravesical zymosan (Z) or anesthesia alone (A) or 24 hrs prior to UBD testing. UBD testing occurred 15 minutes following i.t. administration of saline, methysergide, phentolamine, ondansetron, WAY 100635, WB 4101 or yohimbine. Panels A-D: group Z/saline significantly different from group A/saline (*p<0.05) and are reproduced in each panel as primary control comparisons. Panel A: group Z/saline significantly different from group Z/methysergide (#p<0.05). Panel B: group Z/Saline significantly different from group A/phentolamine (#p<0.05). Panel C: group Z/saline significantly different from groups Z/ondansetron (#p<0.05) and Z/Way 100635 (+p<0.05). Panel D: group Z/saline significantly different from group Z/WB 4101 (#p<0.05). All post-hoc tests were based on significant between-groups effects derived from the overall ANOVAs. N’s range from 8-13/group.
We also carried out some within-subject tests with phentolamine. Rats that initially received zymosan pretreatment followed by i.t. saline and UBD testing were administered i.t. phentolamine followed by a second set of UBD trials. We found that i.t. phentolamine exerted a significant reduction in the EMG responses to UBD in zymosan pretreated rats (F=11.76; p=0.027), but not in anesthesia pretreated rats in a manner virtually identical to that observed in the between-groups comparisons shown in Figure 1.
It is unlikely that any of these changes were due to drug-induced changes in neural control of bladder compliance because i.t. administration of the non-specific receptor antagonists, methysergide and phentolamine, failed to produce any significant differences in the intravesical pressures during slow saline infusions (F=0.57; p=.58; data not shown).
Discussion
Various brainstem regions, including the RVM and the A5, A6, and A7 NE cell groups are widely recognized to exert spinal inhibitory effects via release of 5-HT and NE [9, 14,26,36]. Activation of many of these same regions also can produce facilitatory or pronociceptive effects in either normal animals or animals with inflamed tissue that are also mediated by spinal release of 5-HT and NE [34-37,29]. Bee and Dickenson [1, c.f., 30,31] suggested that RVM-derived facilitatory influences may even predominate over RVM-derived inhibitory influences, based on studies of anesthetic blockade in both normal and spinal nerve injured rats. The purpose of the present studies was to determine whether spinal 5-HT and NE played a role in the bladder hypersensitivity produced by acute bladder inflammation.
Facilitatory effects of 5-HT were found since i.t. administration of the non-specific (5-HT1/5-HT2) antagonist methysergide, the selective 5-HT1A antagonist WAY 100635, or the selective 5-HT3 antagonist ondansetron reduced the enhanced bladder sensitivity produced by intravesical zymosan to levels of control rats pretreated only with anesthesia and receiving i.t. saline at the time of testing. The effects of these 5-HT receptor antagonists may be due to blocking a direct spinal influence of a descending 5-HT system that enhances spinal nociceptive transmission from the bladder, e.g., a system originating in the RVM and recruited by bladder inflammation. Suzuki et al. [23,24] have emphasized a facilitatory role of 5-HT acting at 5-HT3 receptors localized on nerve terminals of a sub-population of small diameter primary afferents in enhancing neurotransmitter release in the dorsal horn. This view is consistent with our data and data from other studies showing facilitatory effects of spinal 5-HT and the mediation 5-HT2A and 5-HT3 receptors [23,24,25]. For example, the second phase of the formalin response or at-level mechanical allodynia produced by spinal cord injury are significantly attenuated by either 5,7-DHT depletion of spinal 5-HT or i.t. administration of the 5-HT3 antagonist ondansetron [17,18,25].
We also found effects of methysergide and the 5-HT1A antagonist WAY 100635 that were consistent with a facilitatory role of 5-HT acting at 5-HT1/5-HT2 receptors. The magnitude of these effects was comparable to those observed with the 5-HT3 antagonist ondansetron. Our 5-HT1A data are not in accord with the proposition of Kayser et al. [15] who suggested that 5-HT1B and to a lesser extent 5-HT1A receptors mediate endogenous inhibitory effects of 5-HT [c.f.,7,18]. These differences may reflect our analysis of inflamed visceral tissue, which has not been previously studied in this context. It should be noted, however, that there is a previous report of methysergide and the 5-HT1A receptor antagonist NAN-190 blocking secondary hyperalgesia produced by formalin injection in the paw [3] supporting a facilitatory role for 5-HT acting at a 5-HT1A receptor.
5-HT could also be acting in the spinal cord to modulate another spinal system in producing facilitation. Song, Chen, and Marvizon [22] used μ receptor internalization to show that spinal 5-HT acts at 5-HT1A receptors to inhibit opioid release from a subpopulation of spinal opioid-containing terminals. In this manner, 5-HT inhibition of spinal opioid release would have a facilitatory influence. One would expect that either methysergide or the specific 5-HT1A receptor antagonist WAY 100635 to block this facilitatory influence as was found in the present experiments.
Facilitatory effects of NE were also found since i.t. administration of either the non-specific NE antagonist phentolamine or the selective α1 antagonist WB 4101 reduced the bladder hypersensitivity produced by intravesical zymosan to levels of rats pretreated with anesthesia only and receiving i.t. saline vehicle. The selective α2 antagonist yohimbine was not effective in this. In general, all the NE antagonists appeared somewhat less effective the 5-HT antagonists. The NE-mediated facilitatory effects may derive from bladder inflammation-induced activation of any of the brainstem NE cell groups (A5, A6, and A7) that provide descending modulation of spinal nociceptive transmission, or the RVM, which has reciprocal connections with these NE cells groups and whose effects are in part mediated by spinal release of NE [9]. While inhibitory effects are more typically attributed to activation of these NE cells groups and activation of α2 spinal adrenoreceptors, facilitatory effects have been demonstrated for NE acting at α1 adrenoreceptors on dorsal horn neurons excited by either glutamate or noxious stimulation [2]. Further, activation of A7 neurons may exert both pro- and antinociceptive effects acting through spinal α1 and α2-adrenoreceptors, respectively [c.f., 10]. The α1 adrenoreceptor agonist phenylephrine also promotes release of substance P from capsaicin-sensitive sensory neurons from the urinary bladder and mobilizes intracellular Ca++ in cultured lumbar and sacral dorsal root ganglia innervating the bladder [27], an effect which is enhanced by inflaming the bladder with cyclophosphamide. Thus, bladder pain signaled by capsaicin-sensitive nociceptive afferents and enhanced by bladder inflammation is enhanced by NE acting primarily at α1 adrenoreceptors.
There was some trend in our data suggesting that 5-HT and NE were producing tonic facilitatory effects, but our statistical analyses failed to reveal any significant decreases in EMG responses relative to the anesthesia pretreated control animals. It is possible this reflects a floor effect in our response measures. However, we were unable to evaluate a possible floor effect by decreasing the level of anesthesia and elevating the stimulus response function to UBD in group A/saline because 0.75% isoflurane is the lowest safe level we can use in our preparation. Our cystometry data ruled out a possible influence of these receptor antagonists in differentially affecting bladder compliance (c.f., 6 for similar outcomes), but we can’t rule out the possibility that the facilitatory effects were the result of spinal excitatory influences of 5-HT and NE on motor neurons [32,33].
We have shown in a previous study that i.t. or i.p. administration of opioid receptor antagonist naloxone at the time of UBD testing significantly increases the magnitude of the hypersensitivity produced by bladder inflammation [5], indicating an inhibitory influence of endogenous opioids. Thus, both facilitatory (5-HT and NE) and inhibitory (opioid) effects are recruited by bladder inflammation Overall, the present data indicate that bladder hypersensitivity resulting from bladder inflammation can be thought of as the net result of activating several independent and dependent processes, including peripheral sensitization of primary afferents, central sensitization of second-order dorsal horn neurons, and inflammation-induced activation of opioid inhibitory, and 5-HT and NE-facilitatory modulation of spinal nociceptive transmission from the bladder.
Acknowledgments
This research was supported by NIH R01-DK073218 and NIH R01-DK078655 grants to A. Randich.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bee LA, Dickenson AH. Rostral ventromedial medulla control of spinal sensory processing in normal and pathophysiological states. Neurosci. 2007;147:786–793. doi: 10.1016/j.neuroscience.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Budai D, Harasawa I, Fields HL. Midbrain periaqueductal gray (PAG) inhibits nociceptive inputs to sacral dorsal horn nociceptive neurons through α2-adrenergic receptors. J Neurophysiol. 1998;80:2244–2254. doi: 10.1152/jn.1998.80.5.2244. [DOI] [PubMed] [Google Scholar]
- 3.Calejesan AA, Ch’ang MH-C, Zhuo M. Spinal serotonergic receptors mediate facilitation of a nociceptive reflex by subcutaneous formalin injection into the hindpaw in rats. Brain Res. 1998;798:46–54. doi: 10.1016/s0006-8993(98)00394-1. [DOI] [PubMed] [Google Scholar]
- 4.Castroman P, Ness TJ. Vigor of visceromotor responses to urinary bladder distension in rats increases with repeated trials and stimulus intensity. Neurosci Letters. 2001;306:97–100. doi: 10.1016/s0304-3940(01)01886-9. [DOI] [PubMed] [Google Scholar]
- 5.DeBerry P, Ness TJ, Robbins MT, Randich A. Inflammation-induced enhancement of the visceromotor reflex to urinary bladder distension: modulation by endogenous opioids and the effects of early-in-life experience with bladder inflammation. J Pain. 2007;8(12):914–23. doi: 10.1016/j.jpain.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durant PAC, Lucas PC, Yaksh TL. Micturition in the unanesthetized rat: spinal vs. peripheral pharmacology of the adrenergic system. J Pharmacol Exp Therap. 1988;245:426–435. [PubMed] [Google Scholar]
- 7.El-Yassir N, Fleetwood-Walker SM. A 5-HT1-type receptor mediates the antinociceptive effect of nucleus raphe magnus stimulation in the rat. Brain Res. 1990;523:92–99. doi: 10.1016/0006-8993(90)91639-x. [DOI] [PubMed] [Google Scholar]
- 8.Gebhart GF. Descending modulation of pain. Neurosci Biobehav Rev. 2004;7(8):729–37. doi: 10.1016/j.neubiorev.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Hammond DL, Yaksh TL. Antagonism of stimulation-produced antinociception by intrathecal administration of methysergide or phentolamine. Brain Res. 1984;298:329–337. doi: 10.1016/0006-8993(84)91432-x. [DOI] [PubMed] [Google Scholar]
- 10.Holden JE, Schwartz EJ, Proudfit HK. Microinjection of morphine in the A7 catecholamine cell group produces opposing effects on nociception that are mediated by a1 and a2-adrenoreceptors. Neurosci. 1999;91:979–990. doi: 10.1016/s0306-4522(98)00673-3. [DOI] [PubMed] [Google Scholar]
- 11.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 12.Jensen TS, Yaksh TL. Spinal monoamine and opiate systems partly mediate the antinociceptive effects produced by glutamate at brainstem sites. Brain Res. 1984;321:287–289. doi: 10.1016/0006-8993(84)90181-1. [DOI] [PubMed] [Google Scholar]
- 13.Jensen TS, Yaksh TL., II Examination of spinal monoamine receptors through which brainstem opiate-sensitive systems act in the rat. Brain Res. 1986;363:114–127. doi: 10.1016/0006-8993(86)90663-3. [DOI] [PubMed] [Google Scholar]
- 14.Jones SL, Gebhart GF. Inhibition of spinal nociceptive transmission from the midbrain, pons and medulla in the rat: activation of descending inhibition by morphine, glutamate and electrical stimulation. Brain Res. 1988;460:281–296. doi: 10.1016/0006-8993(88)90373-3. [DOI] [PubMed] [Google Scholar]
- 15.Kayser V, Elfassi IE, Aubel B, Melfort M, Julius D, Gingrich JA, Hamon M, Bourgoin S. Mechanical, thermal, and formalin-induced nociception is differentially altered in 5-HT1A -/-, 5-HT1B -/-,5-HT2A -/-, 5-HT3A -/-, and 5-HTT -/- knock-out male mice. Pain. 2007;130:235–248. doi: 10.1016/j.pain.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Loomis CW, Jhamandas K, Milne B, Cervenko F. Monoamine and opioid interactions in spinal analgesia and tolerance. Pharmacol Biochem Behav. 1987;26:445–451. doi: 10.1016/0091-3057(87)90146-8. [DOI] [PubMed] [Google Scholar]
- 17.Oatway MA, Chen Y, Weaver LC. The 5-HT3 receptor facilitates at-level mechanical allodynia following spinal cord injury. Pain. 2004;110:259–268. doi: 10.1016/j.pain.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 18.Oyama T, Ueda M, Kuraishi Y, Akaike A, Satoh M. Dual effect of serotonin on formalin-induced nociception in the rat spinal cord. Neurosci Res. 1996;25:129–135. doi: 10.1016/0168-0102(96)01034-6. [DOI] [PubMed] [Google Scholar]
- 19.Randich A, Mebane H, DeBerry J, Ness TJ. Rostral ventral medulla modulation of the visceromotor reflex evoked by urinary bladder distension in female rats. J Pain. doi: 10.1016/j.jpain.2008.05.011. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Randich A, Uzzell T, Cannon R, Ness TJ. Inflammation and enhanced nociceptive responses to bladder distension produced by intravesical zymosan in the rat. BMC Urol. 2006a;6:2. doi: 10.1186/1471-2490-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Randich A, Uzzell T, DeBerry JJ, Ness TJ. Neonatal urinary bladder inflammation produces adult bladder hypersensitivity. J Pain. 2006b;7(7):468–479. doi: 10.1016/j.jpain.2006.01.450. [DOI] [PubMed] [Google Scholar]
- 22.Song B, Chen W, Marvizon JCG. Inhibition of opioid release in the rat spinal cord by serotonin 5-HT1A receptors. Brain Res. 2007;1158:57–62. doi: 10.1016/j.brainres.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki R, Rahman W, Hunt SP, Dickenson AH. Descending facilitatory control of mechanically evoked responses is enhanced in deep dorsal horn neurons following peripheral nerve injury. Brain Res. 2004a;1019:68–76. doi: 10.1016/j.brainres.2004.05.108. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki R, Rygh LJ, Dickenson AH. Bad news from the brain: descending 5-HT pathways that control spinal pain processing. TRENDS in Pharmacol Sci. 2004b;25:613–617. doi: 10.1016/j.tips.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Svensson CI, Tran TK, Tizsimmons B, Yaksh TL, Hua XY. Descending serotonergic facilitation of spinal ERK activation and pain behavior. FEBS Lttrs. 2006;580:6629–6634. doi: 10.1016/j.febslet.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsuruoka M, Maeda M, Inoue T. Stimulation of the nucleus locus coeruleus/subcoeruleus suppresses visceromotor responses to colorectal distention in the rat. Neurosci Lttrs. 2005;381:97–101. doi: 10.1016/j.neulet.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Trevisani M, Campi B, Gatti R, Andre E, Materazzi S, Nicoletti P, Gassieri D, Geppetti P. The influence of alpha1-adrenoreceptors on neuropeptide release from primary sensory afferents of the lower urinary tract. Eur Urol. 2007;52:901–908. doi: 10.1016/j.eururo.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 28.Urban MO, Zahn PK, Gebhart GF. Descending facilitatory influences from the rostral medial medulla mediate secondary, but not primary hyperalgesia in the rat. Neurosci. 1999;90:349–352. doi: 10.1016/s0306-4522(99)00002-0. [DOI] [PubMed] [Google Scholar]
- 29.Urban MO, Gebhart GF. Supraspinal contributions to hyperalgesia. Proc Nat Acad Sci USA. 1999;96:7687–7692. doi: 10.1073/pnas.96.14.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vanegas H. To the descending pain-control system in rats, inflammation-induced primary and secondary hyperalgesia are two different things. Neurosci Lttrs. 2004;361:225–228. doi: 10.1016/j.neulet.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Vanegas H, Schaible H-G. Descending control of persistent pain: inhibitory or facilitatory? Brain Res Rev. 2004;46:295–309. doi: 10.1016/j.brainresrev.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 32.White SR, Neuman RS. Facilitation of spinal motoneurone excitability by 5-hydroxytryptamine and noradrenaline. Brain Res. 1979;188:119–127. doi: 10.1016/0006-8993(80)90561-2. [DOI] [PubMed] [Google Scholar]
- 33.White SR, Neuman RS. Pharmacological antagonism of facilitatory but not inhibitory effects of serotonin and norepinephrine on excitability of spinal motoneurons. Neuropharm. 1983;22:489–494. doi: 10.1016/0028-3908(83)90168-5. [DOI] [PubMed] [Google Scholar]
- 34.Zhuo M, Gebhart GF. Characterization of descending inhibition and facilitation from the nuclei reticularis gigantocellularis and gigantocellularis pars alpha in the rat. Pain. 1990;42:337–350. doi: 10.1016/0304-3959(90)91147-B. [DOI] [PubMed] [Google Scholar]
- 35.Zhuo M, Gebhart GF. Spinal serotonin receptors mediate descending facilitation of a nociceptive reflex from the nuclei reticularis gigantocellularis and gigantocellularis pars alpha in the rat. Brain Res. 1991;550:35–48. doi: 10.1016/0006-8993(91)90402-h. [DOI] [PubMed] [Google Scholar]
- 36.Zhuo M, Gebhart GF. Characterization of descending inhibition and facilitation of spinal nociceptive transmission from the nuclei reticularis gigantocellularis and gigantocellularis pars alpha in the rat. J Neurophysiol. 1992;67:1599–1614. doi: 10.1152/jn.1992.67.6.1599. [DOI] [PubMed] [Google Scholar]
- 37.Zhuo M, Gebhart GF. Facilitation and attenuation of a visceral nociceptive reflex from the rostroventral medulla in the rat. Gastroenterol. 2002;122:1007–1019. doi: 10.1053/gast.2002.32389. [DOI] [PubMed] [Google Scholar]
- 38.Zorman G, Belcher G, Adams JE, Fields HL. Lumbar intrathecal naloxone blocks analgesia produced by microstimulation of the ventromedial medulla in the rat. Brain Res. 1982;236:77–84. doi: 10.1016/0006-8993(82)90035-x. [DOI] [PubMed] [Google Scholar]


