Abstract
Peripheral inflammation evokes functional and biochemical changes in the periphery and spinal cord which result in central sensitization and hypersensitivity. Inhibitory control systems from the rostral ventromedial medulla (RVM) are also activated. The present study investigates whether endogenous kappa-opioid receptor (KOPr) systems contribute to these neuroadaptations. Inflammation was induced by intraplantar injection of complete Freund's adjuvant (CFA) into one hindpaw. Mechanical and thermal thresholds were determined using the Von Frey and radiant heat tests, respectively. KOPr gene deletion in mice or systemic administration of the long-acting KOPr antagonist, norbinaltorphimine (norBNI) significantly exacerbated mechanical and thermal hyper-sensitivity of the ipsilateral, inflamed paw. Thermal and mechanical thresholds of the non-inflamed, contralateral hindpaw were unaffected by CFA treatment. However, gene deletion as well as norBNI treatment resulted in mechanical, but not thermal hyper-sensitivity of the non-inflamed paw. Similar results were obtained when norBNI was administered intrathecally or into the RVM in rats. These data demonstrate a previously unrecognized role of endogenous KOPr systems in inhibiting hyperalgesia during inflammation. Furthermore, they demonstrate that decreased KOPr activity in either the spinal cord or RVM not only enhances mechanical and thermal hyperalgesia of the inflamed limb but also leads to an unmasking of mechanical hyperalgesia at a site remote from inflammation. The differential effects of KOPr antagonism on mechanical versus thermal thresholds for the non-inflamed paw support the notion that distinct neuroanatomical or neurochemical mechanisms modulate the processing of thermal versus mechanical stimuli.
Keywords: Inflammation, Kappa-opioid receptor, Rostral ventromedial medulla, Knockout, Spinal cord, CFA, Norbinaltorphimine, Gene deletion, Rat, Mouse
1. Introduction
Kappa-opioid receptor (KOPr) agonists are effective in reducing hyperalgesia, i.e. the increased response or heightened sensitivity to an innocuous or noxious stimulus, in animal models of localized peripheral inflammation [16,25,29] and arthritis [1,50,54].
Fundamental questions, however, exist regarding the role of endogenous KOP systems in the modulation of inflammatory nociception. Electrophysiology data showing that a subset of dorsal horn neurons are subject to an increased endogenous inhibitory KOP tone during the acute phase of inflammation [71,73] are consistent with plasticity of endogenous KOP systems during inflammation. Messenger RNA expression and peptide levels of the endogenous KOPr ligand, dynorphin [11], are markedly increased in the ipsilateral dorsal horn during peripheral inflammation [28,58]. The physiological consequence of dynorphin upregulation is, however, unclear, since both pro- and antinociceptive effects of dynorphin peptide and/or its degradation products have been described [8,70]. Furthermore, consistent changes in spinal cord KOPr number or mRNA expression are not seen [28,40].
Studies examining the influence of KOPr blockade in animal models of inflammatory pain are contradictory. Using the formalin test, Ossipov et al. reported that the selective blockade of spinal cord KOPr increases phase II nocifensive behavior [57]. However, no effect [82] or a decrease [13] have been observed as well. To date, only one study examined the influence of KOPr blockade in an animal model of prolonged inflammation. Millan and coworkers showed that subcutaneous (s.c.) administration of MR2266 further decreased mechanical thresholds of the ipsilateral paw in the complete Freund's adjuvant (CFA) model of unilateral peripheral inflammation [48,49]. Since, however, MR2266 blocks multiple opioid receptors and the nociceptin (ORL-1) receptor [36,55,51], the receptor(s) mediating the increased MR2266-evoked hyperalgesia remain(s) unclear. Furthermore, whether the effects observed result from the blockade of spinal or supraspinal KOPr is unknown.
The present study used pharmacological and gene deletion techniques to examine the role of endogenous KOPr systems in the modulation of nociception in the CFA model of unilateral peripheral inflammation.
Microinjection studies examined the influence of nor-BNI infused into either the spinal cord or the rostral ventromedial medulla (RVM) on nociception during inflammation. The RVM was selected for study due to its documented role in descending inhibition of pain during inflammation [77], and recent work showing that inflammatory pain is associated with an enhancement of the antinociceptive effects of a synthetic KOPr agonist infused into this region [64].
2. Methods
2.1. Animals
The generation of the KOPr knockout (KO) mice has been described elsewhere [12,27,65]. Heterozygous KOPr knockout (KO) mice, maintained on a C57BL/6J × 129S6 background, were backcrossed onto a pure C57BL/6J background for 10 generations. Mice (2−3 months of age) derived from wildtype wildtype (WT) and knockout × knockout matings (C8 generation) were used. The rat strain employed was Sprague–Dawley (Charles River, MD, USA), and rats weighted 300−375 g at the commencement of experiments. All animals were housed 2−3 per cage in a temperature-and humidity-controlled environment under a 12 h light/dark cycle for at least 2 weeks before experiments. They were maintained in facilities accredited by the American Association for the Accreditation of Laboratory Animal Care. Food and water were available ad libitum. All experiments were approved by the Institutional Care and Use Committee of the National Institute on Drug Abuse, National Institutes of Health and conducted in accordance with The Ethical Guidelines for Investigation of Experimental Pain in Conscious Animals established by The Committee for Research and Ethical Issues of the International Association for the Study of Pain [87].
2.2. Induction of peripheral inflammation
Mice received an intraplantar injection of 10 μl of a 1:1 dilution of CFA (Calbiochem, San Diego, CA, USA) in sterile water (5 μg Mycobacterium butyricum) into the right hindpaw under brief isoflurane anesthesia. Rats were injected with 20 μl undiluted CFA (20 μg Mycobacterium butyricum) [72]. Some animals were anesthetized but were not injected and are referred to as controls. The right paw was inoculated with CFA and is referred to as the ipsilateral paw throughout the text. The left paw, contralateral to the site of CFA injection, is referred to as the contralateral paw.
2.3. Experimental designs
Studies were conducted first to assess the selectivity of KOPr gene deletion in mice. For that purpose, the antinociceptive effects of the selective KOPr agonist, U69593, the selective mu-opioid receptor (MOPr) agonist, fentanyl, and the selective delta-opioid receptor agonist (DOPr), SNC80, were assessed in both KOPr WT and KO mice using the Hargreaves method [3,6,30,36] (NIDA Drug Supply, Bethesda, MD, USA). Opiates were dissolved in sterile saline (0.9%) and administered s.c. (n = 5−8 per treatment group). H3PO4 (1 M) was used to facilitate the dissolution of U69593 and SNC80. The pH of drug solutions was verified and titrated with NaOH (1 M) to pH 7. Paw withdrawal latencies (PWLs) were determined before and 10 and 15 min after fentanyl or 15 and 20 min following U69593 and SNC80 administration. These time points were selected in view of previous work in our laboratory showing maximal analgesic effect.
The effects of pharmacological KOPr blockade were examined using the selective KOPr antagonist, norBNI [9] (NIDA Drug Supply, Bethesda, MD, USA). Previous studies have shown that acute norBNI administration results in the long-lasting (≥3 weeks) blockade of KOPr [20,26,69]. Since MOPr mediated actions of norBNI have been reported during the first 4 h after its administration, a 24-h pretreatment interval was employed [4,15]. A control experiment was carried out to confirm the opioid receptor selectivity and duration of antagonism of norBNI. In this study, rats received a bolus injection of either saline or norBNI (10 mg/kg). The analgesic effects of U69593 (10 mg/kg, s.c.) or fentanyl (12 μg/kg, s.c.) to thermal stimuli were then determined 1 day, 1, 2 and 3 weeks after norBNI administration. Analgesia was assessed 15−20 min (U69593) and 8−13 min (fentanyl) after opiate agonist administration. All rats received either U69593 or fentanyl. Treatment groups thus included a saline/U69593 (n = 7), norBNI/U69593 (n = 6), saline/fentanyl (n = 6), and norBNI/fentanyl (n = 7) group. The volume of injection was 1.0 ml/kg.
To investigate the effects of persistent KOPr blockade on mechanical and thermal thresholds in mice, WT strains received an acute intraperitoneal (i.p.) injection of saline (n = 4), or norBNI (n = 4). The volume of injection was 10.0 ml/kg. The dose of norBNI employed (10 mg/kg) was that previously shown to be KOPr-selective [5,15,45,66,83]. Mice received an intraplantar injection of CFA under isoflurane anesthesia 24 h after i.p. injection. The Von Frey method was employed to determine mechanical thresholds and the Hargreaves test was used to assess thermal sensitivity at different time points 4 h–3 weeks post-CFA administration.
The effects of systemic norBNI on thermal and mechanical sensitivity in rats were determined using four experimental treatment groups: (1) i.p. saline/control, (2) i.p. norBNI/control, (3) i.p. saline/CFA, and (4) i.p. norBNI/CFA with n = 6−11 rats/group.
Drug and vehicle injections into the rat spinal cord were made using the method described by Hole and coworkers [74]. Rats were anesthetized with isoflurane and a blunt 20-gauge needle was inserted between the L5 and L6 vertebrae into the subarachnoid space. Sterile, stretched PE-10 catheter, containing a teflon-coated stainless steel guide wire (Braintree Scientific, Inc., Braintree, MA, USA), was inserted and advanced 3 cm rostrally beyond the tip of the needle. The guide wire was removed and 10 nmol norBNI (7.35 μg of the dihydrochloride salt) in 10 μl saline or an equivalent volume of saline was then manually injected over a 30 s period. The dose of norBNI used was selected on the basis of previous studies [21,35,56,86] and was administered 24 h before CFA administration. Four experimental groups were employed: (1) intrathecal saline/control, (2) intrathecal norBNI/control, (3) intrathecal saline/CFA, and (4) intrathecal norBNI/CFA with n = 7−12 rats/group. Animals were housed individually after surgery.
For intra-RVM infusions, rats were anesthetized (30 mg/kg pentobarbital, 120 mg/kg chloral hydrate, i.p.) and then placed in a Kopf stereotaxic apparatus (David Kopf, Tujunga, CA, USA). A small hole was drilled in the skull to enable insertion of a 26-gauge guide cannula (C315 G, Plastics One, Ranaoke, VA, USA) above the RVM. The stereotaxic coordinates were: AP: 10.8 mm caudal from bregma, L: 0.0 and V: 9.8 mm ventral from the skull surface [59]. The guide cannula was attached to the skull using dental acrylic cement and two small screws inserted into the parietal bone. Microinjections (0.3 μl) were made manually by inserting a 30-gauge injection cannula (C315 I, Plastics One) into the guide cannula. NorBNI (1 nmol; 735 ng of the dihydrochloride salt) or saline was injected using PE tubing (0.2 mm ID, 1.75 mm OD) attached to a 1.0 μl syringe (Hamilton, Reno, NV, USA). Solutions were infused over a 30-s period and the injection cannula was left in place for an additional 30 s to prevent backflow. The volume and dose of norBNI used has previously been reported to block the antinociceptive effect produced by intra-RVM infusion of U69593 but not of DAMGO [64].To determine whether blockade of RVM KOPr affects the development of mechanical hypersensitivity, rats were microinjected with norBNI or vehicle at the time of surgery. Rats then received an intraplantar injection of CFA 24 h later. Control animals were briefly anesthetized but did not receive a CFA injection. For studies examining the influence of KOPr blockade on the expression of mechanical hypersensitivity, animals were briefly anesthetized with isoflurane and injected intraplantar with CFA 5 days following stereotaxic surgery. Control rats were anesthetized but not injected with CFA. Separate groups of animals then received an intra-RVM infusion of nor-BNI or saline either 4 days or 2 weeks later, using the same injection procedure as described above. After surgery, rats were housed individually. For each norBNI time point tested, four treatment groups were tested: (1) RVM saline/control, (2) RVM norBNI/control, (3) RVM saline/CFA, and (4) RVM norBNI/CFA with n = 5−9 rats/group. Upon completion of all experiments, animals were euthanized by i.p. injection of chloral hydrate (1 g/kg) for anatomical confirmation of injection cannula placements. Injection cannulae were dipped briefly in cresyl violet and inserted into the guide cannula, allowing for visualization of placement on serial coronal sections of the brainstem (20 μm) using the atlas of Paxinos and Watson as a reference [59].
2.4. Assessment of nociceptive thresholds and paw thickness
On the day of testing, rats or mice were transported to the behavioral testing room and allowed at least a 1-h acclimatization period. The animals were then placed in the experimental test chambers for 30 min (rats) or 2−3 h (mice) prior to determination of mechanical thresholds. Mechanical thresholds of the plantar surface of the ipsilateral and contralateral hindpaw were determined with the Von Frey method [38] using the 0.6, 1.0, 2.0, 4.0, 6.0, 8.0, 15.0 and 26.0 g monofilaments (Stoelting, Wood Dale, IL, USA) for rats and the 0.04, 0.07, 0.16, 0.4, 0.6, 1.0, 2.0, 6.0 g filaments for mice. Testing was conducted in chambers (rats: 13 (W) × 15 (L) × 13 (H) cm; mice: 10 (diameter) × 15 cm (H)) composed of acrylic and a wire-mesh floor. The up and down method [10] was used for the computation of the 50% response threshold to mechanical stimuli. Thresholds were determined before, and at various time points (1 day–3 weeks) after administration of norBNI or saline.
Thermal-evoked thresholds of the plantar surface of the inflamed and non-inflamed hindpaw were measured using the Hargreaves method [31], at least 2−3 h after determination of mechanical thresholds in CFA-treated or control animals. Tests were conducted in acrylic transparent chambers (rats: 22 (W) × 13 (L) × 11(H) cm, mice: 13 (diameter) × 15 (H) cm). A high-intensity light beam, providing the radiant heat (IITC Life Science Inc., Woodland Hills, CA), was focused on the plantar surface of the hindpaw. The surface supporting the animals was composed of transparent acrylic material (Lexan XL10, GE Plastics, Pittsfield, MA) to avoid the heat-sink effect attributed to glass [24]. The time interval needed for the rodent to remove its hindpaw away from the thermal stimulus was defined as the paw withdrawal latency (PWL).
In control experiments performed to ascertain the selectivity of KOPr deletion, the analgesic effects of selective opioid receptor agonists were assessed in KOPr KO and WT mice using the Hargreaves test. The intensity of the light source of the Hargreaves apparatus was set to obtain baseline PWLs of approximately 7.0 s; the cutoff time was 15.0 s to prevent tissue damage. Three baseline measurements for each paw were obtained prior to agonist administration. Post-drug administration, two measurements of each paw were taken with at least 3 min separating each measurement. Averages of the 6 PWLs before and of the 4 PWLs after drug administration were computed and used for subsequent data analysis.
In studies assessing the duration of action and selectivity of norBNI in rats, PWLs were measured, as in mice, before and after opiate administration. However, the intensity of the beam of light was set to obtain basal PWLs of 10.0 s; the cutoff was 20.0 s.
Finally, for studies examining the effects of KOPr hypo-function in mice or rats with unilateral inflammation, eight replicates of the thermal threshold of each paw were obtained before and 4 h–3 weeks after CFA administration. If a withdrawal response did not occur within 20.0 s, the test was terminated to prevent tissue injury and the rat was assigned this latency. The means of the five last response latencies were computed and used for statistical analyses.
Paw volumes were estimated by measuring the dorsal-plantar foot thickness using a caliper (Mitutoyo Corporation, Kawasaki, Japan). Measurements were taken at the mid-plantar level at each occasion after completion of nociceptive testing.
2.5. Data analysis
PWL data from dose–response curves were converted to %MPE, defined as [(PWL post-drug – PWL pre-drug)/(cutoff – PWL pre-drug)] × 100. Dose–response curves were analyzed and compared using Graphpad Prism version 4 (GraphPad Software Inc., San Diego, CA) using a polynomial equation [14]. A Student's two-tailed t test was used to compare the dose–response curve of opioid agonists in KOPr WT and KO strains. A 2-factor ANOVA with repeated measures over time was employed to analyze the influence of: (i) norBNI on opioid receptor agonist-evoked analgesia in rats, (ii) gene deletion or KOPr blockade in mice, and (iii) the effects of norBNI administered to rats after the induction of inflammation. Mechanical and thermal thresholds of rats treated with norBNI or saline before the induction of inflammation were analyzed using a 3-factor ANOVA with drug pretreatment (norBNI versus saline) and intraplantar treatment (CFA versus control) as between-subject factors and time as the repeated measure. Post-hoc analyses were performed using the Tukey test. Data are reported as means ± SEM. P < 0.05 was considered statistically significant in all tests.
3. Results
3.1. Opiate analgesia in KOPr WT and KO mice
Fentanyl and SNC80 produced dose-related analgesia in both WT and KO mice. Importantly, regardless of the dose tested, no difference between genotypes in the potency of the MOPr and DOPr agonist was seen (fentanyl: t68 = 1.6, P = 0.2; SNC80: t38 = 0.6, P = 0.6; Fig. 1). This is reflected in the ED50 values presented in Table 1. Maximal responses (100% MPE) were obtained in both strains for equivalent doses of drug. The KOPr agonist U69593 elicited analgesia when injected s.c. in mice, however, a 50.5-fold decrease in potency was observed in KO mice (t46 = 3.0, P < 0.01).
Fig. 1.

Effects of systemically administered opioid receptor agonists on thermal withdrawal thresholds in KOPr WT and KO mice. Experiments were carried out using the Hargreaves method in naïve mice. MOPr and DOPr agonist-mediated analgesic effects were similar in KOPr KO and WT mice. The analgesic effects of the selective KOPr agonist U69593 were, however, reduced 50.5-fold in KO mice as compared to WT littermates (P < 0.05).
Table 1.
ED50 values of the analgesic effect of prototype opioid agonists in KOPr WT and KO mice
| ED50 | KOPr WT | KOPr KO |
|---|---|---|
| Fentanyl | 36.3 (26.3−50.1) μg/kg | 28.1 (22.3−35.4) μg/kg |
| SNC80 | 25.1 (11.0−57.3) mg/kg | 18.0 (8.0−40.3) mg/kg |
| U69593 | 0.68 (0.05−9.8) mg/kg | 34.2 (24.2−48.6)a mg/kg |
The analgesic effects of the MOPr agonist fentanyl and the DOPr agonist SNC80 did not differ between WT and KO mice.
U69593-evoked analgesia was decreased 50.5-fold in KO mice (P < 0.004).
3.2. Effect of constitutive KOPr deletion on nociceptive thresholds in mice with persistent inflammation
3.2.1. Mechanical thresholds
Mechanical thresholds of the ipsilateral, inflamed hindpaw of WT and KO mice are depicted in Fig. 2A. Baseline thresholds before induction of inflammation did not differ between genotypes (WT: 0.60 ± 0.03 g; KO: 0.60 ± 0.04 g; Tukey post-hoc P = 0.9). Injection of CFA decreased mechanical thresholds in WT and KO mice. However, this effect varied as a function of both genotype (F(1,10) = 8.1, P = 0.02) and time post-CFA injection (F(6,60) = 74.0, P < 0.01). A significant time × genotype interaction (F(6,60) = 4.7, P < 0.01) was also seen. Post-hoc analysis indicated a significantly greater CFA-evoked decrease in mechanical thresholds of the ipsilateral hindpaw in KO relative to WT mice. The difference between genotypes was first apparent 4 days post-CFA injection and persisted throughout the 2-week test period (P < 0.01 for all pairwise comparisons). Thus, the hypersensitivity following CFA administration was exacerbated in mice lacking the gene encoding KOPr.
Fig. 2.

Mechanical and thermal thresholds before and during unilateral peripheral inflammation in KOPr KO and WT mice and in norBNI-treated WT mice. (A) Mechanical thresholds of the inflamed paw were significantly decreased in both WT and KO mice relative to baseline values; the magnitude of the decrease was significantly greater in KO mice. (B) Mechanical thresholds of the paw contralateral to the site of inflammation were not affected by CFA injection in WT mice whereas a significant decrease was observed in KO mice. (C and D) Similar results as in (A) and (B) were obtained in WT mice that received norBNI i.p., 24 h after CFA administration. (E and F) CFA treatment decreased thermal thresholds of both the inflamed and non-inflamed paw. (E) The magnitude of decrease was significantly greater in the inflamed paw in KO mice as compared to WT littermates. (F) Thresholds of the contralateral paw were similar in both genotypes. *Significantly lower thresholds between groups (Tukey post-hoc, P < 0.05).
Baseline mechanical thresholds of the contralateral, non-inflamed, paw prior to inflammation (Fig. 2B) did not differ between WT (0.63 ± 0.04 g) and KO mice (0.61 ± 0.04 g, P = 0.8). ANOVA of thresholds following CFA injection revealed no effect of genotype (F(1,10) = 3.8, P = 0.08). However, a main effect of time post-CFA injection (F(6,60) = 5.1, P < 0.01) and a significant genotype time interaction (F(6,60) = 3.8, P = 0.03) were found. CFA did not affect mechanical thresholds of the contralateral paw in WT mice (P > 0.05). In contrast, significant mechanical hypersensitivity was present in KOPr KO mice 4 days–2 weeks post-CFA injection (P < 0.05 for all paired comparisons). These data demonstrate that constitutive KOPr deletion exacerbates mechanical hypersensitivity of the ipsilateral paw and induces hypersensitivity of the contralateral paw.
To determine whether the decrease in thresholds observed in KO mice was a consequence of compensations resulting from constitutive KOPr deletion, experiments assessing the influence of systemic norBNI administration on thresholds were conducted in WT mice. Baseline thresholds prior to CFA injection did not differ in control animals and those that had received norBNI 24 h previously (saline: 0.67 ± 0.06 g; norBNI: 0.64 ± 0.08 g; Tukey post-hoc P = 0.9). Fig. 2C illustrates the effects of i.p. norBNI administration on thresholds of the ipsilateral hindpaw in WT mice. Mechanical thresholds of the ipsilateral paw varied as a function of norBNI treatment (F(1,6) = 82.3, P < 0.01) and time after CFA injection (F(6,36) = 27.0, P < 0.01); a significant interaction between norBNI treatment and time post-CFA was also present (F(6,36) = 3.1, P = 0.02). The mechanical thresholds of mice that had received norBNI 24 h prior to CFA injection were significantly lower than those of mice that had received CFA alone. This difference was apparent 1 day–3 weeks post-CFA injection (P < 0.01 for all pairwise comparisons).
Baseline thresholds of the contralateral hindpaw prior to CFA injection did not differ in control mice (0.66 ± 0.06 g) and those that had received norBNI 24 h previously (0.60 ± 0.07 g, P = 0.7, Tukey post-hoc). ANOVA indicated significant main effects of norBNI treatment (F(1,6) = 12.1, P = 0.01) and time (F(6,36) = 4.7, P < 0.01) as well as a significant norBNI time interaction (F(6,36) = 3.5, P = 0.01; Fig. 2D). In mice with unilateral inflammation, systemic administration of norBNI resulted in profound mechanical hypersensitivity of the contralateral hindpaw. Mechanical thresholds in nor-BNI-treated mice were significantly lower 1−3 weeks post-CFA inoculation as compared to mice that had received saline (P < 0.01 for all three pairwise comparisons).
3.2.2. Thermal thresholds
Thermal thresholds in WT and KO mice before and during peripheral inflammation are depicted in Fig. 2E and F. Baseline thresholds prior to inflammation did not differ between genotypes (WT: 19.4 ± 1.4 s; KO: 20.0 ± 1.2 s; Tukey post-hoc P = 0.9: Fig. 2E). Injection of CFA decreased PWLs in WT and KO mice. The magnitude of the decrease varied as a function of both geno-type (F(1,10) = 6.4, P = 0.03) and time post-CFA injection (F(5,44) = 26.4, P < 0.01). There was no significant time genotype interaction (F(5,44) = 1.7, P = 0.2). The CFA-evoked decrease in thermal thresholds was significantly greater in KO relative to WT mice, 2 days–1 week post-CFA administration (P ≤ 0.04 for all paired comparisons).
Baseline PWLs of the contralateral paw (Fig. 2F) did not differ between WT (19.0 ± 1.3 s) and KO mice (18.8 ± 0.9 s, P = 0.9). ANOVA of PWLs following CFA injection revealed no effect of genotype (F(1,10) = 1.1, P = 0.3) but a main effect of time post-CFA injection (F(5,44) = 4.3, P = 0.03) was found. No significant genotype × time interaction (F(5,44) = 0.4, P = 0.8) was observed, indicating that the hyperalgesia of the contralateral paw was quantitatively equal in both genotypes.
3.3. Lack of effect of constitutive KOPr gene deletion on paw volume and body weight gain in CFA-treated mice
CFA injection increased the volume of the ipsilateral paw. Statistical analysis revealed a significant main effect of time (F(6,60) = 44.3, P < 0.01) but no significant effect of genotype (F(1,10) = 1.0, P = 0.3) and no interaction effect (F(6,60) = 1.1, P = 0.4). Thus, the time course and magnitude of edema development was quantitatively similar in both genotypes. The maximal increase relative to pre-injection volumes was observed 1 day after CFA injection (WT: 120.3 ± 1.9%; KO: 123.4 ± 2.7%). Two weeks after CFA inoculation, the percentage increase in paw volumes was 102.1 ± 1.1% and 107.5 ± 1.5% for WT and KO mice, respectively.
Contralateral paw volumes of WT and KO mice remained within 102.5 ± 2.2% of baseline values throughout testing. Regardless of genotype (F(1,10) = 1.8, P = 0.2) or time post-CFA injection (F(6,60) = 0.8, P = 0.6), the volume of the contralateral paw was unaltered relative to pre-injection values. There was no significant interaction between time and treatment (F(6,60) = 1.3, P = 0.3).
CFA induced modest weight loss in both genotypes. An effect of genotype (F(1,10) = 5.9, P = 0.04) and time (F(6,60) = 20.5, P < 0.01) was observed, with KO mice losing relatively less weight as compared to WT litter-mates. However, there was no significant interaction effect between genotype and time (F(6,60) = 1.1, P = 0.4). Weight loss, expressed as a percentage of pre-injection values, was maximal 4 days post-CFA injection (WT: 94.2 ± 0.8%; KO: 96.6 ± 0.7%).
3.4. Effects of norBNI on MOPr and KOPr-mediated analgesia in rats
NorBNI administration did not alter the effects of fentanyl (12 μg/kg s.c.) in control rats (drug treatment F(1,11) = 0.7, P = 0.4; time post-drug treatment F(4,44) = 0.3, P = 0.9, interaction F(4,44) = 0.2, P = 0.9: Fig. 3). In contrast, a significant attenuation of U69593-evoked analgesia (10 mg/kg) was apparent after norBNI administration. ANOVA revealed a significant effect of norBNI pretreatment (F(1,11) = 144.7, P < 0.01), and of time (F(4,44) = 5.9, P < 0.01) as well as a significant interaction effect (F(4,44) = 5.9, P < 0.01). U69593-evoked analgesia was significantly reduced 24 h–3 weeks post-norBNI treatment. These data indicate that following a single i.p. bolus injection of norBNI in rats, KOPr agonists are blocked for at least 3 weeks.
Fig. 3.

Selective and persistent KOPr blockade in rats following norBNI administration. The antinociceptive effects of a MOPr and a KOPr agonist were determined in naïve rats using the Hargreaves method. NorBNI treatment (10 mg/kg, i.p.) did not attenuate analgesia evoked by MOPr selective agonist fentanyl (12 μg/kg, s.c.). The analgesic effects of U69593 (10 mg/kg, s.c.) were reduced by prior norBNI treatment for up to 3 weeks. *Significant difference between saline and norBNI-pretreated rats which were tested with U69593 (P < 0.05).
3.5. Effect of systemic norBNI administration prior to CFA inoculation in rats
3.5.1. Mechanical thresholds
Fig. 4A and B illustrates the effects of i.p. norBNI administration on mechanical thresholds of control and CFA-treated rats. Mechanical thresholds of the ipsilateral paw (Fig. 4A) varied as a function of CFA treatment (F(1,32) = 270.7, P < 0.01) and time after CFA injection (F(7,161) = 22.3, P < 0.01). Although there was no main effect of norBNI pretreatment (F(1,32) = 3.2, P = 0.1), a significant interaction between norBNI and CFA treatment (F(1,32) = 9.2, P = 0.01) was found. The mechanical thresholds of rats that had received norBNI 24 h prior to CFA injection were significantly lower than those of rats that had received saline prior to CFA. This difference was apparent 1−3 weeks post-CFA injection (P ≤ 0.04 for all pairwise comparisons). NorBNI did not alter mechanical thresholds of control (non CFA-treated) rats (P > 0.05).
Fig. 4.

Effect of norBNI on mechanical and thermal thresholds of rats. NorBNI (10 mg/kg, i.p.) was injected 24 h before intraplantar injection of CFA. (A) CFA injection decreased mechanical thresholds of the inflamed paw. This effect was significantly greater in rats that had received norBNI i.p. (B) CFA did not affect mechanical thresholds of the paw contralateral to the injection site in control (saline-treated) rats. In rats that had received norBNI, CFA significantly decreased mechanical thresholds. (C) CFA injection decreased thermal thresholds of the inflamed paw. This effect was significantly greater in rats that had received norBNI. (D) CFA did not affect thermal thresholds of the non-inflamed paw in control or norBNI-treated rats. *Significantly lower thresholds of norBNI/CFA-treated rats relative to saline/CFA-treated rats (Tukey post-hoc, P < 0.05).
ANOVA of mechanical thresholds of the contralateral hindpaw (Fig. 4B) indicated significant main effects of CFA (F(1,32) = 32.3, P < 0.01), norBNI treatment (F(1,32) = 9.5, P = 0.01), and time (F(7,161) = 10.9, P < 0.01) as well as a significant norBNI × CFA interaction (F(1,32) = 15.7, P = 0.01). NorBNI treatment did not affect mechanical thresholds of control (non CFA-treated) animals (P > 0.05). In animals with unilateral inflammation, systemic administration of norBNI resulted in significant hypersensitivity of the contralateral hindpaw. Regardless of the time post-CFA injection, mechanical thresholds of norBNI-treated rats were significantly lower than those of rats that had received CFA alone 4 h–3 weeks post-CFA administration (P ≤ 0.03 for all pairwise comparisons).
To further confirm that KOPr agonists are blocked 3 weeks after administration of a single dose of norBNI, a separate experiment was carried out in which rats with unilateral peripheral inflammation were injected with U69593 (1.2 mg/kg, s.c.) 3 weeks after norBNI or saline injection. A significant effect of norBNI pretreatment (F(1,12) = 15.6, P = 0.01), U69593 (F(1,12) = 12.8, P = 0.01), and their interaction (F(1,12) = 9.6, P = 0.01) were found at the inflamed paw. Mechanical thresholds of the inflamed paw increased from 2.5 ± 0.4 g (baseline) to 9.0 ± 1.4 g after U69593 treatment in saline pretreated rats (P < 0.01). The mean threshold of norBNI pretreated rats prior to U69593 administration was 2.6 ± 0.2 g. No effect of U69593 on mechanical thresholds was observed (3.0 ± 0.3 g, P = 0.8, data not shown).
Significant effects of norBNI pretreatment on U69593-evoked antihyperalgesic effects of the non-inflamed paw were found as well. ANOVA revealed significant effects of norBNI (F(1,12) = 113.0, P < 0.01), and U69593 (F(1,12) = 22.5, P < 0.01), as well as a significant interaction effect (F(1,12) = 12.6, P = 0.01). Mechanical thresholds of the non-inflamed paw increased 10 min after U69593 injection in saline-treated rats (baseline: 7.3 ± 1.1 g; post-U69593: 14.6 ± 0.7 g, P < 0.01). In nor-BNI-treated rats, U69593 administration did not increase thresholds of the contralateral paw (baseline: 3.6 ± 0.3 g; post-drug: 4.6 ± 0.5 g, P = 0.4: data not shown).
3.5.2. Thermal thresholds
Fig. 4C and D illustrates the effects of i.p. norBNI administration on thermal thresholds of control and CFA-treated rats. Thresholds of the ipsilateral paw (Fig. 4C) varied as a function of CFA injection (F(1,17) = 33.0, P < 0.00), time after CFA injection (F(7,98) = 4.0, P < 0.01), and norBNI pretreatment (F(1,17) = 6.5, P = 0.02). A significant interaction between norBNI and CFA treatment was also seen (F(1,17) = 5.1, P = 0.04). CFA decreased ipsilateral PWLs 3 and 4 days after inoculation in saline-treated rats (P < 0.05). PWLs were decreased 1 day–1 week in norBNI-treated animals (P < 0.04). In rats that had received CFA and norBNI, PWLs were significantly lower than those that had received CFA alone. This effect was significant 1−3 days post-CFA injection (P < 0.03 for all pairwise comparisons). NorBNI did not alter thermal thresholds of non CFA-treated rats (P > 0.05).
Thermal thresholds of the non-inflamed hindpaw (Fig. 4D) did not vary as a function of CFA treatment (F(1,17) = 0.1, P = 1.0), norBNI pretreatment (F(1,17) = 1.2, P = 0.3), or time (F(7,98) = 1.4, P = 0.5). There was no significant norBNI × CFA interaction (F(1,17) = 0.8, P = 0.4). Thus, in contrast to the inflamed paw, neither CFA nor norBNI treatment affected PWLs of the non-inflamed paw.
3.6. Effects of systemic norBNI administration post-CFA inoculation in rats
To determine whether administration of norBNI could reverse CFA-induced mechanical or thermal hypersensitivity once expressed, norBNI was administered 4 days after CFA injection.
Mechanical thresholds of the inflamed paw were reduced in rats which had received norBNI after the induction of inflammation (data not shown). ANOVA indicated a significant effect of time (F(10,90) = 116.0, P < 0.01), drug treatment (F(1,9) = 7.6, P = 0.02), and an interaction effect (F(10,90) = 2.6, P < 0.01). Ipsilateral Von Frey thresholds were significantly lower 1−3 weeks post-CFA administration (3, 10 and 17 days post-nor-BNI, P < 0.05) in rats treated with norBNI as compared to saline controls.
ANOVA of the non-inflamed paw revealed significant effects of time post-CFA injection (F(10,90) = 16.0, P < 0.01), drug treatment (F(1,9) = 13.4, P < 0.01), and an interaction effect (F(10,90) = 5.7, P < 0.01). Contralateral mechanical thresholds were significantly lower 1−3 weeks post-CFA in norBNI as compared to i.p. saline-treated rats (P < 0.01, data not shown).
NorBNI reduced thermal thresholds of the inflamed paw further post-CFA injection. ANOVA revealed significant effects of time (F(10,90) = 10.0, P < 0.01), norBNI (F(1,9) = 7.7, P = 0.03), and an interaction effect (F(10,90) = 2.3, P = 0.02). PWLs were further decreased 1−3 weeks post-CFA in norBNI-treated rats as compared to i.p. saline controls (data not shown).
Thresholds of the contralateral paw were not affected by norBNI treatment (effect of time F(10,90) = 10.9, P < 0.01; effect norBNI F(1,9) = 0.4, P = 0.6; interaction F(10,90) = 0.6, P = 0.8: data not shown).
3.7. Lack of effect of systemic norBNI administration on paw volume or body weight gain in rats with persistent inflammation
Systemic norBNI administration did not affect the increase in volume of the inflamed paw (CFA treatment: F(1,18) = 0.1, P = 0.9; norBNI: F(1,18) = 0.1, P = 1.0; interaction: F(1,18) = 0.0, P = 0.9). Rats gained weight at an equal rate, regardless of CFA or norBNI treatment (time: F(6,108) = 94.6, P < 0.01, CFA: F(1,18) = 0.1, P = 0.9, norBNI: F(1,18) = 0.1, P = 0.8, interaction: F(1,18) = 0.1, P = 0.8: data not shown).
3.8. Effects of intrathecal administration of norBNI to rats prior to CFA injection
3.8.1. Mechanical thresholds
The effects of intrathecal administration of norBNI (10 nmol) on mechanical thresholds are shown in Fig. 5A and B. ANOVA of mechanical thresholds of the ipsilateral hindpaw (Fig. 5A) revealed significant effects of CFA treatment (F(1,29) = 135.3, P < 0.01), and time (F(7,203) = 13.7, P < 0.01), but no main effect of norBNI pretreatment (F(1,29) = 1.8, P = 0.2). However, a significant CFA × norBNI treatment interaction was found (F(1,29) = 4.5, P = 0.04). NorBNI treatment did not alter mechanical thresholds of control (non CFA-treated) rats. CFA injection decreased mechanical thresholds of the ipsilateral hindpaw. This effect was potentiated in rats that had received intrathecal norBNI 24 h prior to the induction of inflammation. Mechanical thresholds of norBNI-treated rats were significantly reduced 1−3 weeks post-CFA as compared to rats which received saline prior to CFA injection (P < 0.05 for all pairwise comparisons).
Fig. 5.

Effect of intrathecal norBNI on mechanical thresholds in control and CFA-treated rats. NorBNI (10 nmol) was injected intrathecally 24 h before intraplantar CFA injection. (A) CFA inoculation decreased mechanical thresholds of the inflamed paw. This effect was significantly greater in rats that had received norBNI. (B) CFA did not alter contralateral mechanical thresholds in control animals but significantly decreased thresholds in norBNI-treated rats. (C) CFA decreased thermal thresholds of the inflamed paw. This effect was significantly greater in rats that had received norBNI. (D) CFA did not alter contralateral thermal thresholds in either control or norBNI-treated animals. *Significantly lower thresholds post-CFA injection of norBNI-treated rats in comparison with saline-treated rats (Tukey post-hoc, P < 0.05).
Analysis of mechanical thresholds of the contralateral paw (Fig. 5B) revealed significant effects of time (F(7,203) = 10.0, P < 0.01), CFA (F(1,29) = 40.9, P < 0.01), and norBNI pretreatment (F(1,29) = 10.0, P < 0.01) and a significant CFA × norBNI treatment interaction (F(1,29) = 8.7, P < 0.01). Analogous to the ipsilateral paw, post-hoc analysis indicated significantly lower mechanical thresholds of CFA-treated rats that had received norBNI prior to the induction of inflammation. This effect was apparent 4 h post-CFA injection and persisted for 3 weeks (P < 0.05). These data demonstrate that during prolonged inflammation, long-term blockade of KOPr in the spinal cord increases sensitivity of the contralateral paw to mechanical stimuli.
3.8.2. Thermal thresholds
The effects of intrathecal administration of norBNI (10 nmol) on thermal thresholds are shown in Fig. 5C and D.
ANOVA of ipsilateral PWLs revealed significant effects of time (F(7,140) = 32.4, P < 0.01), as well as significant effects of CFA (F(1,19) = 234.3, P < 0.01), and norBNI pretreatment (F(1,19) = 26.8, P < 0.01). A significant CFA × norBNI treatment interaction was seen (F(1,19) = 33.5, P < 0.01). Importantly, the effect of CFA was potentiated in rats that had received intrathecal norBNI. In these animals, a significantly greater reduction in PWLs was observed 2 days (P = 0.02) and 1−3 weeks post-CFA-injection (P < 0.01 for all pairwise comparisons). NorBNI treatment did not alter thermal thresholds of rats which did not receive CFA (P > 0.05), indicating that KOPr hypofunction results in an ipsilateral exacerbation of thermal hypersensitivity.
Analysis of data from the contralateral paw (Fig. 5D) revealed no significant effect of CFA (F(1,19) = 0.4, P = 0.6), time (F(7,140) = 1.7, P = 0.2) or norBNI (F(1,19) = 2.5, P = 0.1) and no significant CFA × nor-BNI interaction (F(1,19) = 4.2, P = 0.1). These data indicate that norBNI and CFA do not affect thermal thresholds of the contralateral paw.
3.9. Effects of intra-RVM norBNI administration to rats prior to CFA inoculation
3.9.1. Mechanical thresholds
Mechanical thresholds of rats that received an acute intra-RVM infusion of norBNI (1 nmol) 1 day prior to the induction of unilateral peripheral inflammation are shown in Fig. 6A and B.
Fig. 6.

Effect of intra-RVM infusion of norBNI (1 nmol) on mechanical and thermal thresholds in control and CFA-treated rats. NorBNI was injected 24 h before CFA administration. (A) CFA decreased mechanical thresholds of the inflamed paw and this effect was significantly greater in rats which received norBNI. (B) Contralateral mechanical thresholds were not affected by CFA treatment in control animals but were significantly decreased in rats that had received norBNI. (C and D) When norBNI was injected 1.0 mm dorsal to the RVM, 24 h after treatment with CFA, it did not affect mechanical thresholds of the inflamed (C) or non-inflamed paw (D). (E) CFA injection decreased PWLs of the inflamed paw and this effect was significantly greater in rats which received norBNI. (F) Contralateral thermal thresholds were not affected by CFA treatment in control animals or in rats that had received norBNI. *Significantly lower thresholds post-CFA injection of norBNI-treated rats in comparison with saline-treated rats (Tukey post-hoc, P < 0.05).
ANOVA revealed significant effects of CFA treatment (F(1,25) = 346.7, P < 0.01) and time (F(7,175) = 19.6, P < 0.01) on mechanical thresholds of the ipsilateral paw (Fig. 6A). Although no main effect of norBNI was found (F(1,25) = 2.0, P = 0.2), there was a significant CFA × norBNI interaction (F(1,25) = 7.9, P = 0.01). Post-hoc analysis revealed no effect of nor-BNI in control animals (P > 0.05). Mechanical thresholds of CFA-treated rats were significantly decreased relative to non CFA-injected rats. In rats that had received intra-RVM infusion of norBNI, a significantly greater decrease in thresholds was observed 2−3 weeks post-CFA injection (P ≤ 0.04 for both comparisons).
Fig. 6B shows mechanical thresholds of the contralateral hindpaw. Significant effects of CFA treatment (F(1,25) = 38.6, P = 0.01), norBNI administration (F(1,25) = 12.3, P < 0.01), and time (F(7,175) = 13.1, P < 0.01) as well as a significant norBNI × CFA interaction (F(1,25) = 11.0, P < 0.01) were seen. In rats that received intra-RVM saline 24 h prior to CFA injection, mechanical thresholds of the contralateral paw were unaltered relative to control (non CFA-injected) animals. Intra-RVM infusion of norBNI did not affect contralateral paw thresholds of control animals. In rats, that had received intra-RVM norBNI, however, a significant decrease in mechanical thresholds of the contralateral paw was seen 4 h–4 days post-CFA injection as well as 2−3 weeks post-injection (P ≤ 0.03 for all pairwise comparisons).
NorBNI was microinjected into the medial longitudinal fasciculus, a site 1.0 mm dorsal to the RVM, to confirm that the effects of intra-RVM norBNI result from the KOPr blockade in this specific region (Fig. 6C and D). In contrast to RVM injection, the dorsal microinjection site did not alter thresholds of either the ipsilateral (Fig. 6C) or contralateral (Fig. 6D) hindpaw of CFA-injected rats. ANOVA revealed a significant effect of time post-CFA injection (ipsilateral: F(7,70) = 34.2, P < 0.01; contralateral: F(7,70) = 3.2, P = 0.01) but no effects of nor-BNI treatment (ipsilateral: F(1,10) = 0.0, P = 0.9, contralateral: F(1,10) = 4.8, P = 0.1) and no significant time norBNI interactions (ipsilateral: F(7,70) = 0.5, P = 0.8; contralateral: F(7,70) = 0.5, P = 0.8). The significant effect of time for the contralateral paw was due to random threshold variations that occurred during the 3-week test period. Placements of cannulae are shown in Fig. 7.
Fig. 7.
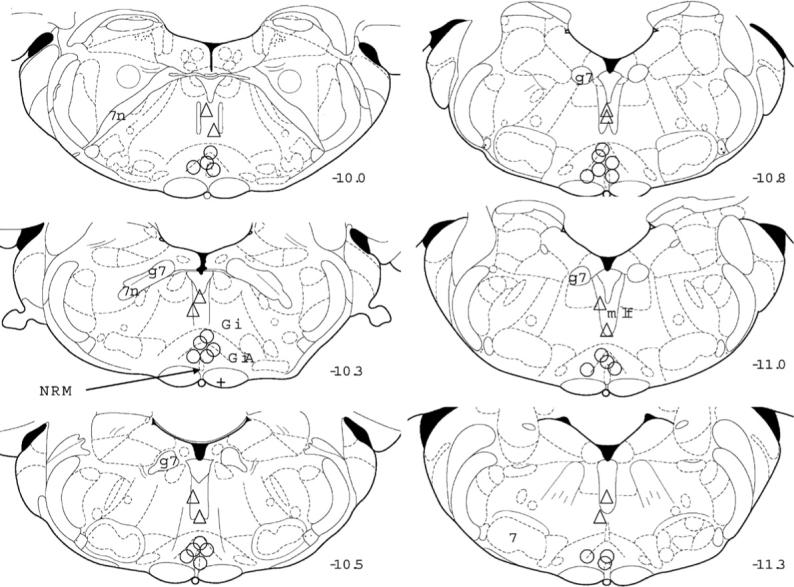
Placement of cannulae in the RVM and in controls. The localization of the injection cannulae is recorded on representative coronal diagrams from [59]. Circles represent injection sites aimed at the RVM in rats which received either saline or norBNI 4 days post-CFA administration; the + sign represents an outlier. Triangles refer to injection sites aimed 1.0 mm dorsal to the RVM in rats that received CFA 24 h after norBNI. Numbers to the right of each section refer to the distance caudal to bregma. 7, facial nerve nucleus; n, nerve nucleus or root; g, genu; GiA, nucleus reticularis gigantocellularis pars alpha; mlf, medial longitudinal lemniscus; NRM, nucleus raphe magnus.
3.9.2. Thermal thresholds
Thermal thresholds of rats that received an acute intra-RVM infusion of norBNI (1 nmol) 1 day prior to the induction of inflammation are shown in Fig. 6E and F.
ANOVA of thresholds of the inflamed paw revealed significant effects of CFA treatment (F(1,17) = 125.5, P < 0.01), time (F(6,102) = 4.0, P < 0.01) and norBNI (F(1,17) = 20.5, P < 0.01), as well as a significant CFA × norBNI interaction (F(1,17) = 35.9, P < 0.01, Fig. 6E). CFA decreased thermal thresholds of saline-treated rats 1−4 days post-inoculation (P < 0.05). In rats that had received intra-RVM infusion of norBNI, however, a significantly greater decrease in thresholds was observed 2 days–2 weeks post-CFA injection (P < 0.01 for all pairwise comparisons). No effect of norBNI was observed in non CFA-treated animals (P > 0.05).
Fig. 6F shows thermal thresholds of the non-inflamed hindpaw. ANOVA indicated significant effects of time (F(6,102) = 5.3, P < 0.01) and of CFA-treatment (F(1,17) = 9.3, P = 0.01), but no effect of norBNI administration (F(1,17) = 0.1, P = 0.9) and no interaction effect (F(1,17) = 0.1, P = 0.8).
3.10. Effects of intra-RVM norBNI administration to rats when administered after the induction of inflammation
3.10.1. Mechanical thresholds
To investigate whether RVM KOPr modulates the expression of mechanical hypersensitivity once developed, norBNI (1.0 nmol) or saline was microinjected into the RVM 4 days following the induction of inflammation (Fig. 8). The effects of intra-RVM infusion of saline and norBNI on nociceptive thresholds were also examined in control (non CFA-treated) rats.
Fig. 8.

Effect of norBNI (1 nmol) microinjection into the RVM on mechanical and thermal thresholds in control and CFA-treated rats. NorBNI or saline was microinjected 4 days after CFA administration (see arrows). Thresholds were determined 1 hour (+1 h), 3 days (1w post-CFA), 10 days (2w post-CFA) and 17 days (3w post-CFA) after norBNI or saline injection as indicated in the figures. (A) CFA injection decreased mechanical thresholds and this effect was significantly greater in rats that had received intra-RVM norBNI during inflammation. (B) Contralateral mechanical thresholds did not differ between control and CFA-treated rats. In CFA-treated rats, norBNI elicited a significant decrease in thresholds. (C) CFA injection decreased thermal thresholds of the inflamed paw and this effect was significantly greater in rats which received norBNI. (D) Contralateral thermal thresholds were not affected by CFA or norBNI treatment. *Lower thresholds post-CFA injection of norBNI-treated rats in comparison with saline-treated rats (Tukey post-hoc, P < 0.05).
ANOVA of ipsilateral mechanical thresholds (Fig. 8A) showed significant main effects of CFA treatment (F(1,18) = 133.8, P < 0.01), and time (F(6,108) = 11.5, P < 0.01), but no main effect of norBNI treatment (F(1,18) = 2.8, P = 0.1). A significant interaction effect between CFA and norBNI treatment (F(1,18) = 6.2, P = 0.02) was found. NorBNI was without effect in control animals. However, in CFA-treated rats, thresholds were significantly lower in norBNI as compared to saline-treated rats. This effect was significant 1 h and 3−17 days post-norBNI infusion (P ≤ 0.03 for all pairwise comparisons), i.e. at 4 days and 1−3 weeks post-CFA injection.
Analysis of contralateral paw thresholds (Fig. 8B) revealed significant effects of CFA (F(1,18) = 6.1, P = 0.02) and norBNI treatment (F(1,18) = 6.8, P = 0.02), as well as a significant effect of time (F(6,108) = 10.6, P < 0.01) and a significant CFA × nor-BNI treatment interaction (F(1,18) = 8.1, P = 0.01). As observed for the ipsilateral paw, norBNI did not affect contralateral thresholds in control rats. However, in CFA-treated rats, thresholds were significantly lower in norBNI as compared to saline-treated rats. This effect was significant 1 h and 3−17 days post-norBNI infusion (P ≤ 0.02 for all pairwise comparisons), i.e. at 4 days and 1−3 weeks post-CFA injection.
Similar results were obtained when norBNI was administered 2 weeks post-CFA injection. ANOVA indicated significant main effects of CFA treatment (F(1,21) = 110.6, P < 0.01), and time (F(6,126) = 13.8, P < 0.01), but no main effect of norBNI treatment (F(1,21) = 2.4, P = 0.1). A significant interaction effect between CFA and norBNI treatment (F(1,21) = 4.7, P = 0.04) was found. Thresholds were significantly lower in norBNI as compared to saline-treated rats 1 h–2 weeks post-norBNI injection (P ≤ 0.04 for all pairwise comparisons), i.e. at 2−4 weeks post-CFA injection (data not shown).
Analysis of contralateral hindpaw thresholds also revealed significant effects of CFA (F(1,21) = 6.5, P = 0.02) and time (F(6,126) = 10.9, P < 0.01), but not of norBNI treatment (F(1,21) = 1.8, P = 0.1). There was, however, a significant interaction between CFA and norBNI treatment (F(1,21) = 5.6, P = 0.03). Thresholds were significantly lower in norBNI as compared to saline-treated rats 1 h–2 weeks post-norBNI treatment (P < 0.01 for all pairwise comparisons), i.e. 2−4 weeks post-CFA injection (data not shown). Collectively, these data demonstrate a role of endogenous RVM KOPr systems in the bilateral descending inhibition of mechanical nociception.
3.10.2. Thermal thresholds
Fig. 8C and D illustrates the effects of intra-RVM norBNI administration 4 days post-CFA administration on thermal thresholds.
Thermal thresholds of the ipsilateral paw (Fig. 8C) varied as a function of CFA (F(1,17) = 51.2, P < 0.01), and time (F(6,102) = 22.3, P < 0.01). A significant effect of nor-BNI treatment (F(1,17) = 6.4, P = 0.02), and a significant norBNI × CFA interaction (F(1,17) = 3.7, P = 0.04) were found. Thermal thresholds of rats that had received nor-BNI 4 days post-CFA were significantly lower than those of rats treated with CFA alone. This difference was apparent 3, 10 and 17 days post-norBNI administration at 1−3 weeks post-CFA injection (P ≤ 0.04 for all pairwise comparisons). NorBNI did not alter mechanical thresholds of control (non CFA-treated) rats (P > 0.05). Micro-injection of norBNI into the RVM did not alter thermal thresholds of the non-inflamed paw (time: F(6,102) = 5.7, P < 0.01, norBNI: F(1,17) = 0.5, P = 0.5, interaction: F(1,17) = 0.6, P = 0.4: Fig. 8D).
4. Discussion
The present study demonstrates that in the CFA model of prolonged unilateral inflammation, KOPr gene deletion or pharmacological inactivation increases mechanical and thermal hyperalgesia of the inflamed paw and induces mechanical hyperalgesia of the contra-lateral paw. Furthermore these effects were observed following the selective inactivation of KOPr in either the spinal cord or RVM.
In KO mice, MOPr and DOPr analgesia was preserved and quantitatively comparable to WT littermates. ED50 values for fentanyl and SNC80 were within the range of those specified previously [3,46]. Similarly, KOPr analgesia in WT mice was comparable to previous reports [47,81]. In KO mice, however, a 50.5-fold diminished analgesic effect was found for U69593. We conclude that KOPr gene deletion resulted in a selective KOPr analgesia KO phenotype.
In agreement with a previous report, KOPr gene deletion did not alter mechanical or thermal thresholds in naïve animals [44]. Lack of KOPr, however, was associated with an exacerbation of CFA-evoked mechanical and thermal hypersensitivity of the ipsilateral paw. Furthermore, in contrast to WT mice, hypersensitivity of the non-inflamed paw was seen to mechanical stimuli. Edema and body weight loss were similar in both WT and KO mice during inflammation, indicating that the enhanced hypersensitivity of KO mice cannot be attributed to an increase in local inflammation or to malaise.
Systemic administration of the selective KOPr antagonist, norBNI, to WT mice 24 h prior to CFA treatment also resulted in bilateral mechanical and ipsilateral thermal hypersensitivity. Identical results were obtained in rats. These data demonstrate that the phenotype of knockout mice cannot be attributed to lack of the KOPr gene during development. The results obtained in both mice and rats also provide initial evidence that endogenous KOPr systems inhibit the development of mechanical and thermal hypersensitivity at the site of inflammation. Furthermore, they indicate that the activity of KOPr systems is necessary for the prevention of mirror pain.
The findings of KOPr-dependent modulation of mechanical nociception during inflammation are in agreement with observations made by Millan et al. [48]. Using a unilateral peripheral inflammation model, they reported decreased mechanical thresholds of the inflamed hindpaw when a KOPr antagonist was administered 1 or 7 days post-CFA inoculation. However, the antagonist did not alter contralateral thresholds [48]. These findings contrast with those of the present study and most likely reflect differences in the methodology employed, e.g. different populations of afferent fibers could be activated using punc-tate (Von Frey) versus blunt (Randall Selitto) mechanical stimuli [19] and when using a low-intensity radiant heat stimulus (C-fibers) versus a warm water bath (Ad-fibers) [84,85]. Differences in the receptor selectivity of the antagonist employed [36,51,55], and the dependent measure tested (development versus expression of hypersensitivity) might add to the discrepancies observed between studies.
Intrathecal norBNI infusion did not alter nociceptive thresholds of control rats. However, in CFA-treated rats, analogous to systemic administration, intrathecal norBNI produced an enduring increase in mechanical and thermal hypersensitivity of the inflamed paw and an induction of mechanical hypersensitivity of the non-inflamed paw. These data are noteworthy in that they indicate that spinal KOPr systems do not modulate mechanical nociception in the absence of inflammation. However, they are necessary for the inhibition of nociceptive input originating from the inflamed paw and for the prevention of mirror pain.
Dynorphin immunoreactivity [28,48,53] is elevated in the dorsal horn during inflammation. Both antinociceptive and pronociceptive effects of intrathecal dynorphin have been reported. Increasing evidence, however, suggests that dynorphin antinociception is KOPr mediated, whereas its pronociceptive effects are elicited by binding of its enzymatic degradation peptide fragments to non-opioid receptors [8,37,61,79]. Neurophysiological studies have provided evidence of increased endogenous KOPr tone on ipsilateral spinal cord neurons during unilateral inflammation [73]. We hypothesize that during persistent inflammation dynorphin is released in the dorsal horn to activate KOPr and subsequently to inhibit nociception, as previously suggested in the formalin test [57].
The pronociceptive effects of intrathecal norBNI on mechanical thresholds were observed bilaterally. Although most studies have focused on ipsilateral changes in the dorsal horn during unilateral inflammation or nociceptive fiber stimulation, contralateral activation of dorsal horn neurons has been observed [22,23,43,80]. Similarly, bilateral increases in dynorphin immunoreactivity were reported in a model of peripheral unilateral inflammation [39] and in a model of peripheral neuropathy [78]. Commissural propriospinal interneurons [60] provide a potential anatomical substrate for communication between the ipsi- and contra-lateral dorsal horn and such interactions have been reported in studies of nerve injury [67,68]. One explanation for the present findings is that during inflammation both the ipsi- and contralateral dorsal horn are sensitized to mechanical stimuli and KOPr-dependent processes are activated to counteract these sensitization processes.
Neither systemic nor intrathecal administration of norBNI produced thermal hyperalgesia of the contra-lateral paw. This observation was unexpected. The most parsimonious explanation for the stimulus modality-dependent effect of norBNI is that different anatomical substrates mediate mechanical and thermal nociceptive processing during inflammation. Indeed, evidence in support of this hypothesis has been obtained during zymogen induced inflammation [62] and neuropathy [7,17,33,34,52]. Alternatively, the receptor systems mediating hypersensitivity to both stimulus modalities could be different, as is the case for intrathecal NMDA-induced thermal versus intrathecal AMPA-induced mechanical hypersensitivity [32]. Other receptor systems, e.g. MOP, N-methyl-d-asparate and Neurkinin-1 receptors, have been reported to modulate mechanical but not thermal hypersensitivity in various animal models of persistent injury [2,41,42,75]. Finally, KOPr-mediated inhibition of nociceptive processing and/or central sensitization may be greater in pathways encoding mechanical stimuli. One could hypothesize that the ongoing afferent barrage from the inflamed paw (spontaneous or evoked by ambulation) in pathways which subserve mechanical stimuli is suffcient to sensitize both the ipsi- and contralateral dorsal horn to mechanical stimuli whereas the afferent input from ‘‘thermal pathways” is insuffcient to sensitize the contralateral dorsal horn to this stimulus modality. The afferent barrage is known to be essential to the development of central sensitization during capsaicin-induced secondary hyperalgesia [76].
Intra-RVM infusion of norBNI either prior to or after CFA injection did not affect thresholds of either hindpaw in control rats. In CFA-treated rats, however, mechanical hypersensitivity of both paws was increased. These data suggest that RVM KOPr-mediated descending inhibition has a role in limiting both the development and expression of mechanical hyper-sensitivity during inflammation. The bilateral effects of RVM KOPr blockade on mechanical thresholds are consistent with the anatomical organization of the descending pathways of the RVM. Neurons in this region are believed to project bilaterally to the dorsal horn via the dorsolateral funiculus (DLF) [18,20,75]. Alternatively, changes in the contralateral dorsal horn could alter its sensitivity to the descending modulation of the RVM, the descending output being unaltered. Spinal cord transection or DLF lesions following the induction of unilateral inflammation increase dorsal horn neuron activity bilaterally [39,63,80], suggesting that peripheral inflammation activates the descending pathways from the RVM to inhibit nociceptive processing in both the ipsi- and contralateral dorsal horn.
Studies examining the influence of CFA on dynorphin release or prodynorphin gene expression in the RVM are lacking. Hammond and coworkers [58] found no alteration of tissue levels of dynorphin in this region. Since tissue concentrations reflect intracellular as well as extracellular peptide, it is unclear as to whether increased dynorphin release during inflammation underlies the effects of intra-RVM norBNI. Studies examining the influence of CFA on KOPr signal transduction, affnity and number are also lacking. Therefore, the mechanism mediating the effects of norBNI will require further study.
In conclusion, our data show that unilateral inflammation of the hindpaw increases the activity of KOPr systems at the level of the dorsal horn and the RVM. These increases serve an essential function in inhibiting mechanical and thermal hyperalgesia of the inflamed paw and preventing mechanical hyperalgesia of the non-inflamed paw. Furthermore, the concomitant activation of both RVM and spinal cord receptors is required for KOPr-mediated inhibition of nociceptive processing during inflammatory pain.
References
- 1.Antic J, Vasiljevic T, Stanojevic S, Vujic V, Kovacevic-Jovanovic V, Djergovic D, et al. Suppression of adjuvant arthritis by kappa-opioid receptor agonist: effect of route of administration and strain differences. Immunopharmacology. 1996;34:105–12. doi: 10.1016/0162-3109(96)00114-2. [DOI] [PubMed] [Google Scholar]
- 2.Bennett AD, Everhart AW, Hulsebosch CE. Intrathecal administration of an NMDA or a non-NMDA receptor antagonist reduces mechanical but not thermal allodynia in a rodent model of chronic central pain after spinal cord injury. Brain Res. 2000;859:72–82. doi: 10.1016/s0006-8993(99)02483-x. [DOI] [PubMed] [Google Scholar]
- 3.Bilsky EJ, Wang T, Lai J, Porreca F. Selective blockade of peripheral delta opioid agonist induced antinociception by intrathecal administration of delta receptor antisense oligodeoxynucleotide. Neurosci Lett. 1996;220:155–8. doi: 10.1016/s0304-3940(96)13262-6. [DOI] [PubMed] [Google Scholar]
- 4.Broadbear JH, Negus SS, Butelman ER, de Costa BR, Woods JH. Differential effects of systemically administered nor-binaltorphi-mine (nor-BNI) on kappa-opioid agonists in the mouse writhing assay. Psychopharmacology (Berl) 1994;115:311–9. doi: 10.1007/BF02245071. [DOI] [PubMed] [Google Scholar]
- 5.Butelman ER, Negus SS, Ai Y, de Costa BR, Woods JH. Kappa opioid antagonist effects of systemically administered nor-binaltorphimine in a thermal antinociception assay in rhesus monkeys. J Pharmacol Exp Ther. 1993;267:1269–76. [PubMed] [Google Scholar]
- 6.Calderon SN, Rothman RB, Porreca F, Flippen-Anderson JL, McNutt RW, Xu H, et al. Probes for narcotic receptor mediated phenomena. 19. Synthesis of (+)-4-[(alpha R)-alpha-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide (SNC 80): a highly selective, nonpeptide delta opioid receptor agonist. J Med Chem. 1994;37:2125–8. doi: 10.1021/jm00040a002. [DOI] [PubMed] [Google Scholar]
- 7.Campbell JN, Raja SN, Meyer RA, Mackinnon SE. Myelinated afferents signal the hyperalgesia associated with nerve injury. Pain. 1988;32:89–94. doi: 10.1016/0304-3959(88)90027-9. [DOI] [PubMed] [Google Scholar]
- 8.Caudle RM, Mannes AJ. Dynorphin: friend or foe? Pain. 2000;87:235–9. doi: 10.1016/S0304-3959(00)00360-2. [DOI] [PubMed] [Google Scholar]
- 9.Chang AC, Cowan A, Takemori AE, Portoghese PS. Aspartic acid conjugates of 2-(3,4-dichlorophenyl)-N-methyl-N-[(1S)-1(3-aminophenyl)-2-(1-pyrrolidinyl)ethyl]acetamide: kappa opioid receptor agonists with limited access to the central nervous system. J Med Chem. 1996;39:4478–82. doi: 10.1021/jm960459x. [DOI] [PubMed] [Google Scholar]
- 10.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 11.Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215:413–5. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- 12.Chefer VI, Czyzyk T, Bolan EA, Moron J, Pintar JE, Shippenberg TS. Endogenous kappa-opioid receptor systems regulate mesoaccumbal dopamine dynamics and vulnerability to cocaine. J Neurosci. 2005;25:5029–37. doi: 10.1523/JNEUROSCI.0854-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi SS, Han KJ, Lee HK, Han EJ, Suh HW. Possible antinociceptive mechanisms of opioid receptor antagonists in the mouse formalin test. Pharmacol Biochem Behav. 2003;75:447–57. doi: 10.1016/s0091-3057(03)00144-8. [DOI] [PubMed] [Google Scholar]
- 14.DeLean A, Munson PJ, Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose–response curves. Am J Physiol. 1978;235:E97–E102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- 15.Endoh T, Matsuura H, Tanaka C, Nagase H. Nor-binaltorphi-mine: a potent and selective kappa-opioid receptor antagonist with long-lasting activity in vivo. Arch Int Pharmacodyn Ther. 1992;316:30–42. [PubMed] [Google Scholar]
- 16.Endoh T, Tajima A, Suzuki T, Kamei J, Narita M, Tseng L, et al. Characterization of the antinociceptive effects of TRK-820 in the rat. Eur J Pharmacol. 2000;387:133–40. doi: 10.1016/s0014-2999(99)00815-8. [DOI] [PubMed] [Google Scholar]
- 17.Gardell LR, Ossipov MH, Vanderah TW, Lai J, Porreca F. Dynorphin-independent spinal cannabinoid antinociception. Pain. 2002;100:243–8. doi: 10.1016/S0304-3959(02)00173-2. [DOI] [PubMed] [Google Scholar]
- 18.Gogas KR, Levine JD, Basbaum AI. Differential contribution of descending controls to the antinociceptive actions of kappa and mu opioids: an analysis of formalin-evoked C-fos expression. J Pharmacol Exp Ther. 1996;276:801–9. [PubMed] [Google Scholar]
- 19.Greenspan JD, McGillis SL. Stimulus features relevant to the perception of sharpness and mechanically evoked cutaneous pain. Somatosens Mot Res. 1991;8:137–47. doi: 10.3109/08990229109144738. [DOI] [PubMed] [Google Scholar]
- 20.Grossman A, Clement-Jones V. Opiate receptors: enkephalins and endorphins. Clin Endocrinol Metab. 1983;12:31–56. doi: 10.1016/s0300-595x(83)80028-0. [DOI] [PubMed] [Google Scholar]
- 21.Guirimand F, Strimbu-Gozariu M, Willer JC, Le Bars D. Effects of mu, delta and kappa opioid antagonists on the depression of a C-fiber reflex by intrathecal morphine and DAGO in the rat. J Pharmacol Exp Ther. 1994;269:1007–20. [PubMed] [Google Scholar]
- 22.Herdegen T, Leah JD, Manisali A, Bravo R, Zimmermann M. c-JUN-like immunoreactivity in the CNS of the adult rat: basal and transynaptically induced expression of an immediate-early gene. Neuroscience. 1991;41:643–54. doi: 10.1016/0306-4522(91)90356-s. [DOI] [PubMed] [Google Scholar]
- 23.Herdegen T, Tolle TR, Bravo R, Zieglgansberger W, Zimmer-mann M. Sequential expression of JUN B, JUN D and FOS B proteins in rat spinal neurons: cascade of transcriptional operations during nociception. Neurosci Lett. 1991;129:221–4. doi: 10.1016/0304-3940(91)90466-7. [DOI] [PubMed] [Google Scholar]
- 24.Hirata H, Pataky A, Kajander K, LaMotte RH, Collins JG. A model of peripheral mononeuropathy in the rat. Pain. 1990;42:253–5. doi: 10.1016/0304-3959(90)91169-J. [DOI] [PubMed] [Google Scholar]
- 25.Ho J, Mannes AJ, Dubner R, Caudle RM. Putative kappa-2 opioid agonists are antihyperalgesic in a rat model of inflammation. J Pharmacol Exp Ther. 1997;281:136–41. [PubMed] [Google Scholar]
- 26.Horan P, Taylor J, Yamamura HI, Porreca F. Extremely long-lasting antagonistic actions of nor-binaltorphimine (nor-BNI) in the mouse tail-flick test. J Pharmacol Exp Ther. 1992;260:1237–43. [PubMed] [Google Scholar]
- 27.Hough LB, Nalwalk JW, Chen Y, Schuller A, Zhu Y, Zhang J, et al. Improgan, a cimetidine analog, induces morphine-like antinociception in opioid receptor-knockout mice. Brain Res. 2000;880:102–8. doi: 10.1016/s0006-8993(00)02776-1. [DOI] [PubMed] [Google Scholar]
- 28.Iadarola MJ, Brady LS, Draisci G, Dubner R. Enhancement of dynorphin gene expression in spinal cord following experimental inflammation: stimulus specificity, behavioral parameters and opioid receptor binding. Pain. 1988;35:313–26. doi: 10.1016/0304-3959(88)90141-8. [DOI] [PubMed] [Google Scholar]
- 29.Janson W, Stein C. Peripheral opioid analgesia. Curr Pharm Biotechnol. 2003;4:270–4. doi: 10.2174/1389201033489766. [DOI] [PubMed] [Google Scholar]
- 30.Janssen PA, Niemegeers CJ, Dony JG. The inhibitory effect of fentanyl and other morphine-like analgesics on the warm water induced tail withdrawal reflex in rats. Arzneimittelforschung. 1963;13:502–7. [PubMed] [Google Scholar]
- 31.Joris JL, Dubner R, Hargreaves KM. Opioid analgesia at peripheral sites: a target for opioids released during stress and inflammation? Anesth Analg. 1987;66:1277–81. [PubMed] [Google Scholar]
- 32.Kolhekar R, Meller ST, Gebhart GF. N-methyl-d-aspartate receptor-mediated changes in thermal nociception: allosteric modulation at glycine and polyamine recognition sites. Neuroscience. 1994;63:925–36. doi: 10.1016/0306-4522(94)90560-6. [DOI] [PubMed] [Google Scholar]
- 33.Koltzenburg M, Lundberg LE, Torebjork HE. Dynamic and static components of mechanical hyperalgesia in human hairy skin. Pain. 1992;51:207–19. doi: 10.1016/0304-3959(92)90262-A. [DOI] [PubMed] [Google Scholar]
- 34.Koltzenburg M, Torebjork HE, Wahren LK. Nociceptor modulated central sensitization causes mechanical hyperalgesia in acute chemogenic and chronic neuropathic pain. Brain. 1994;117:579–91. doi: 10.1093/brain/117.3.579. [DOI] [PubMed] [Google Scholar]
- 35.Kong LL, Yu LC. Involvement of mu- and delta-opioid receptors in the antinociceptive effects induced by AMPA receptor antagonist in the spinal cord of rats. Neurosci Lett. 2006;402:180–3. doi: 10.1016/j.neulet.2006.03.071. [DOI] [PubMed] [Google Scholar]
- 36.Lahti RA, Mickelson MM, McCall JM, Von Voigtlander PF. [3H]U-69593 a highly selective ligand for the opioid kappa receptor. Eur J Pharmacol. 1985;109:281–4. doi: 10.1016/0014-2999(85)90431-5. [DOI] [PubMed] [Google Scholar]
- 37.Lai J, Luo MC, Chen Q, Ma S, Gardell LR, Ossipov MH, et al. Dynorphin A activates bradykinin receptors to maintain neuropathic pain. Nat Neurosci. 2006;9:1534–40. doi: 10.1038/nn1804. [DOI] [PubMed] [Google Scholar]
- 38.Levin S, Pearsall G, Ruderman RJ. Von Frey's method of measuring pressure sensibility in the hand: an engineering analysis of the Weinstein-Semmes pressure aesthesiometer. J Hand Surg [Am] 1978;3:211–6. doi: 10.1016/s0363-5023(78)80084-7. [DOI] [PubMed] [Google Scholar]
- 39.MacArthur L, Ren K, Pfaffenroth E, Franklin E, Ruda MA. Descending modulation of opioid-containing nociceptive neurons in rats with peripheral inflammation and hyperalgesia. Neuroscience. 1999;88:499–506. doi: 10.1016/s0306-4522(98)00204-8. [DOI] [PubMed] [Google Scholar]
- 40.Maekawa K, Minami M, Masuda T, Satoh M. Expression of mu- and kappa-, but not delta-, opioid receptor mRNAs is enhanced in the spinal dorsal horn of the arthritic rats. Pain. 1996;64:365–71. doi: 10.1016/0304-3959(95)00132-8. [DOI] [PubMed] [Google Scholar]
- 41.Mansikka H, Sheth RN, DeVries C, Lee H, Winchurch R, Raja SN. Nerve injury-induced mechanical but not thermal hyperalgesia is attenuated in neurokinin-1 receptor knockout mice. Exp Neurol. 2000;162:343–9. doi: 10.1006/exnr.1999.7336. [DOI] [PubMed] [Google Scholar]
- 42.Mansikka H, Zhao C, Sheth RN, Sora I, Uhl G, Raja SN. Nerve injury induces a tonic bilateral mu-opioid receptor-mediated inhibitory effect on mechanical allodynia in mice. Anesthesiology. 2004;100:912–21. doi: 10.1097/00000542-200404000-00022. [DOI] [PubMed] [Google Scholar]
- 43.Mapp PI, Terenghi G, Walsh DA, Chen ST, Cruwys SC, Garrett N, et al. Monoarthritis in the rat knee induces bilateral and time-dependent changes in substance P and calcitonin gene-related peptide immunoreactivity in the spinal cord. Neuroscience. 1993;57:1091–6. doi: 10.1016/0306-4522(93)90051-g. [DOI] [PubMed] [Google Scholar]
- 44.Martin M, Matifas A, Maldonado R, Kieffer BL. Acute antinociceptive responses in single and combinatorial opioid receptor knockout mice: distinct mu, delta and kappa tones. Eur J Neurosci. 2003;17:701–8. doi: 10.1046/j.1460-9568.2003.02482.x. [DOI] [PubMed] [Google Scholar]
- 45.McCurdy CR, Sufka KJ, Smith GH, Warnick JE, Nieto MJ. Antinociceptive profile of salvinorin A, a structurally unique kappa opioid receptor agonist. Pharmacol Biochem Behav. 2006;83:109–13. doi: 10.1016/j.pbb.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 46.Meert TF, Vermeirsch HA. A preclinical comparison between different opioids: antinociceptive versus adverse effects. Pharmacol Biochem Behav. 2005;80:309–26. doi: 10.1016/j.pbb.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 47.Millan MJ, Colpaert FC. 5-Hydroxytryptamine (HT)1A receptors and the tail-flick response. III. Structurally diverse 5-HT1A partial agonists attenuate mu- but not kappa-opioid antinociception in mice and rats. J Pharmacol Exp Ther. 1991;256:993–1001. [PubMed] [Google Scholar]
- 48.Millan MJ, Czlonkowski A, Morris B, Stein C, Arendt R, Huber A, et al. Inflammation of the hind limb as a model of unilateral, localized pain: influence on multiple opioid systems in the spinal cord of the rat. Pain. 1988;35:299–312. doi: 10.1016/0304-3959(88)90140-6. [DOI] [PubMed] [Google Scholar]
- 49.Millan MJ, Czlonkowski A, Pilcher CW, Almeida OF, Millan MH, Colpaert FC, et al. A model of chronic pain in the rat: functional correlates of alterations in the activity of opioid systems. J Neurosci. 1987;7:77–87. doi: 10.1523/JNEUROSCI.07-01-00077.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Millan MJ, Morris BJ, Colpaert FC, Herz A. A model of chronic pain in the rat: high-resolution neuroanatomical approach identifies alterations in multiple opioid systems in the periaqueductal grey. Brain Res. 1987;416:349–53. doi: 10.1016/0006-8993(87)90917-6. [DOI] [PubMed] [Google Scholar]
- 51.Mundey MK, Ali A, Mason R, Wilson VG. Pharmacological examination of contractile responses of the guinea-pig isolated ileum produced by mu-opioid receptor antagonists in the presence of, and following exposure to, morphine. Br J Pharmacol. 2000;131:893–902. doi: 10.1038/sj.bjp.0703659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nagase H, Kawai K, Hayakawa J, Wakita H, Mizusuna A, Matsuura H, et al. Rational drug design and synthesis of a highly selective nonpeptide delta-opioid agonist, (4aS*,12aR*)-4a-(3-hydroxyphenyl)-2-methyl-1,2,3,4,4a,5,12,12a-octahydropyrido[3,4-b]acridine (TAN-67). Chem Pharm Bull (Tokyo) 1998;46:1695–702. doi: 10.1248/cpb.46.1695. [DOI] [PubMed] [Google Scholar]
- 53.Nahin RL, Hylden JL, Humphrey E. Demonstration of dynorphin A 1−8 immunoreactive axons contacting spinal cord projection neurons in a rat model of peripheral inflammation and hyperalgesia. Pain. 1992;51:135–43. doi: 10.1016/0304-3959(92)90254-9. [DOI] [PubMed] [Google Scholar]
- 54.Neil A, Kayser V, Gacel G, Besson JM, Guilbaud G. Opioid receptor types and antinociceptive activity in chronic inflammation: both kappa- and mu-opiate agonistic effects are enhanced in arthritic rats. Eur J Pharmacol. 1986;130:203–8. doi: 10.1016/0014-2999(86)90269-4. [DOI] [PubMed] [Google Scholar]
- 55.Nicholson JR, Paterson SJ, Menzies JR, Corbett AD, McKnight AT. Pharmacological studies on the ‘‘orphan” opioid receptor in central and peripheral sites. Can J Physiol Pharmacol. 1998;76:304–13. [PubMed] [Google Scholar]
- 56.Obara I, Mika J, Schafer MK, Przewlocka B. Antagonists of the kappa-opioid receptor enhance allodynia in rats and mice after sciatic nerve ligation. Br J Pharmacol. 2003;140:538–46. doi: 10.1038/sj.bjp.0705427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ossipov MH, Kovelowski CJ, Wheeler-Aceto H, Cowan A, Hunter JC, Lai J, et al. Opioid antagonists and antisera to endogenous opioids increase the nociceptive response to formalin: demonstration of an opioid kappa and delta inhibitory tone. J Pharmacol Exp Ther. 1996;277:784–8. [PubMed] [Google Scholar]
- 58.Parra MC, Nguyen TN, Hurley RW, Hammond DL. Persistent inflammatory nociception increases levels of dynorphin 1−17 in the spinal cord, but not in supraspinal nuclei involved in pain modulation. J Pain. 2002;3:330–6. doi: 10.1054/jpai.2002.125185. [DOI] [PubMed] [Google Scholar]
- 59.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- 60.Petko M, Antal M. Propriospinal afferent and efferent connections of the lateral and medial areas of the dorsal horn (laminae I–IV) in the rat lumbar spinal cord. J Comp Neurol. 2000;422:312–25. doi: 10.1002/(sici)1096-9861(20000626)422:2<312::aid-cne11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 61.Przewlocki R, Stala L, Greczek M, Shearman GT, Przewlocka B, Herz A. Analgesic effects of mu-, delta- and kappa-opiate agonists and, in particular, dynorphin at the spinal level. Life Sci. 1983;33:649–52. doi: 10.1016/0024-3205(83)90586-6. [DOI] [PubMed] [Google Scholar]
- 62.Randich A, Meller ST, Gebhart GF. Responses of primary afferents and spinal dorsal horn neurons to thermal and mechanical stimuli before and during zymosan-induced inflammation of the rat hindpaw. Brain Res. 1997;772:135–48. doi: 10.1016/s0006-8993(97)00883-4. [DOI] [PubMed] [Google Scholar]
- 63.Ren K, Ruda MA. Descending modulation of Fos expression after persistent peripheral inflammation. Neuroreport. 1996;7:2186–90. doi: 10.1097/00001756-199609020-00026. [DOI] [PubMed] [Google Scholar]
- 64.Schepers RJ, Mahoney JL, Shippenberg TS. Inflammation-induced changes in rostral ventromedial medulla mu and kappa opioid receptor mediated antinociception. Pain. 2007 doi: 10.1016/j.pain.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 65.Sharifi N, Diehl N, Yaswen L, Brennan MB, Hochgeschwender U. Generation of dynorphin knockout mice. Brain Res Mol Brain Res. 2001;86:70–5. doi: 10.1016/s0169-328x(00)00264-3. [DOI] [PubMed] [Google Scholar]
- 66.Sofuoglu M, Portoghese PS, Takemori AE. Maintenance of acute morphine tolerance in mice by selective blockage of kappa opioid receptors with norbinaltorphimine. Eur J Pharmacol. 1992;210:159–62. doi: 10.1016/0014-2999(92)90666-r. [DOI] [PubMed] [Google Scholar]
- 67.Sotgiu ML, Brambilla M, Valente M, Biella GE. Contralateral input modulates the excitability of dorsal horn neurons involved in noxious signal processes. Potential role in neuronal sensitization. Somatosens Mot Res. 2004;21:211–5. doi: 10.1080/08990220400012539. [DOI] [PubMed] [Google Scholar]
- 68.Sotgiu ML, Valente M, Caramenti GC, Biella GE. Heterotopic inputs facilitate poststimulus after discharges of spinal WDR neurons in rats with chronic nerve constriction. Brain Res. 2006;1099:97–100. doi: 10.1016/j.brainres.2006.04.130. [DOI] [PubMed] [Google Scholar]
- 69.Spanagel R, Almeida OF, Shippenberg TS. Evidence that norbinaltorphimine can function as an antagonist at multiple opioid receptor subtypes. Eur J Pharmacol. 1994;264:157–62. doi: 10.1016/0014-2999(94)00449-8. [DOI] [PubMed] [Google Scholar]
- 70.Stanfa L, Dickenson A. Spinal opioid systems in inflammation. Inflamm Res. 1995;44:231–41. doi: 10.1007/BF01782974. [DOI] [PubMed] [Google Scholar]
- 71.Stanfa LC, Sullivan AF, Dickenson AH. Alterations in neuronal excitability and the potency of spinal mu, delta and kappa opioids after carrageenan-induced inflammation. Pain. 1992;50:345–54. doi: 10.1016/0304-3959(92)90040-I. [DOI] [PubMed] [Google Scholar]
- 72.Stein C, Millan MJ, Herz A. Unilateral inflammation of the hindpaw in rats as a model of prolonged noxious stimulation: alterations in behavior and nociceptive thresholds. Pharmacol Biochem Behav. 1988;31:451–5. doi: 10.1016/0091-3057(88)90372-3. [DOI] [PubMed] [Google Scholar]
- 73.Stiller RU, Grubb BD, Schaible HG. Neurophysiological evidence for increased kappa opioidergic control of spinal cord neurons in rats with unilateral inflammation at the ankle. Eur J Neurosci. 1993;5:1520–7. doi: 10.1111/j.1460-9568.1993.tb00220.x. [DOI] [PubMed] [Google Scholar]
- 74.Storkson RV, Kjorsvik A, Tjolsen A, Hole K. Lumbar catheterization of the spinal subarachnoid space in the rat. J Neurosci Methods. 1996;65:167–72. doi: 10.1016/0165-0270(95)00164-6. [DOI] [PubMed] [Google Scholar]
- 75.Tokuyama S, Nagae R, Mashida E, Hamabe W. Involvement of kappa opioid receptors in formalin-induced inhibition of analgesic tolerance to morphine in mice. J Pharm Pharmacol. 2007;59:1109–15. doi: 10.1211/jpp.59.8.0008. [DOI] [PubMed] [Google Scholar]
- 76.Torebjork HE, Lundberg LE, LaMotte RH. Central changes in processing of mechanoreceptive input in capsaicin-induced secondary hyperalgesia in humans. J Physiol. 1992;448:765–80. doi: 10.1113/jphysiol.1992.sp019069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vanegas H, Schaible HG. Descending control of persistent pain: inhibitory or facilitatory? Brain Res Brain Res Rev. 2004;46:295–309. doi: 10.1016/j.brainresrev.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 78.Wagner R, DeLeo JA, Coombs DW, Willenbring S, Fromm C. Spinal dynorphin immunoreactivity increases bilaterally in a neuropathic pain model. Brain Res. 1993;629:323–6. doi: 10.1016/0006-8993(93)91339-t. [DOI] [PubMed] [Google Scholar]
- 79.Wang Z, Gardell LR, Ossipov MH, Vanderah TW, Brennan MB, Hochgeschwender U, et al. Pronociceptive actions of dynorphin maintain chronic neuropathic pain. J Neurosci. 2001;21:1779–86. doi: 10.1523/JNEUROSCI.21-05-01779.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wei F, Ren K, Dubner R. Inflammation-induced Fos protein expression in the rat spinal cord is enhanced following dorsolateral or ventrolateral funiculus lesions. Brain Res. 1998;782:136–41. doi: 10.1016/s0006-8993(97)01253-5. [DOI] [PubMed] [Google Scholar]
- 81.Wells JL, Bartlett JL, Ananthan S, Bilsky EJ. In vivo pharmacological characterization of SoRI 9409, a nonpeptidic opioid muagonist/delta-antagonist that produces limited antinociceptive tolerance and attenuates morphine physical dependence. J Pharmacol Exp Ther. 2001;297:597–605. [PubMed] [Google Scholar]
- 82.Wu HE, Hung KC, Mizoguchi H, Nagase H, Tseng LF. Roles of endogenous opioid peptides in modulation of nocifensive response to formalin. J Pharmacol Exp Ther. 2002;300:647–54. doi: 10.1124/jpet.300.2.647. [DOI] [PubMed] [Google Scholar]
- 83.Xu M, Petraschka M, McLaughlin JP, Westenbroek RE, Caron MG, Lefkowitz RJ, et al. Neuropathic pain activates the endogenous kappa opioid system in mouse spinal cord and induces opioid receptor tolerance. J Neurosci. 2004;24:4576–84. doi: 10.1523/JNEUROSCI.5552-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yeomans DC, Pirec V, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: behavioral evidence. Pain. 1996;68:133–40. doi: 10.1016/S0304-3959(96)03176-4. [DOI] [PubMed] [Google Scholar]
- 85.Yeomans DC, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: electrophysiological evidence. Pain. 1996;68:141–50. doi: 10.1016/S0304-3959(96)03177-6. [DOI] [PubMed] [Google Scholar]
- 86.Yu LC, Lu JT, Huang YH, Meuser T, Pietruck C, Gabriel A, et al. Involvement of endogenous opioid systems in nociceptin-induced spinal antinociception in rats. Brain Res. 2002;945:88–96. doi: 10.1016/s0006-8993(02)02743-9. [DOI] [PubMed] [Google Scholar]
- 87.Zimmerman M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]


