Abstract
A gradual alteration in the mechanisms underlying reproduction and fertility characterizes the aging process in human females. These changes culminate in menopause, conventionally defined as a cessation of menstrual cycles that marks the end of reproductive capacity. In fact, a central and defining event in menopause is the discontinuation of ovulation, which is correlated with a number of structural and functional changes in the reproductive axis. Despite several decades of research, a degree of uncertainty remains as to whether nonhuman primates undergo menopause, and whether they are suitable models of human reproductive senescence. We review some of the controversies that have clouded our understanding of reproductive aging in nonhuman primates, including issues of definition, timing, comparability of data from wild versus captive populations, and cross-species comparisons. The existing data support the view that menopause occurs in a number of primate species and is not unique to humans.
Keywords: aging, ape, climacteric, evolution, hormones, human, menopause, menstrual cycle, monkey, nonhuman primates, ovary, primate, reproductive senescence
Menopause occurs in many species of nonhuman primates
INTRODUCTION
As a natural consequence of aging, human females gradually lose the ability to reproduce. This loss in fertility is signaled by a disruption in the normal cyclicity of menstrual periods and eventually the complete cessation of ovulation and menstrual bleeding. The functional and structural changes in the hypothalamic-pituitary-ovarian axis that underlie menopause have been the subject of considerable research [1–4]. For example, in an early study of hormonal changes across the human life span, Sherman and Korenman [5] reported that as females age, intermenstrual intervals increase, a phenomenon thought to result directly from lower serum estradiol (E2) levels and elevated follicle-stimulating hormone (FSH) concentrations. In addition to endocrine changes, the senescent ovary shrinks in size [6, 7], undergoes vascular changes [8, 9], decreases the rate of folliculogenesis [10], reorganizes morphologically [6], and increases androgen output [11–13]. These alterations in reproductive parameters have been linked to other markers of aging, including diminished bone density [14–16], cognition [17–21], and cardiovascular health [22, 23]. The physiological and behavioral consequences of aging, particularly in postmenopausal females, are far reaching and significant, and as a result, much effort has been devoted to achieving a better understanding of the menopausal process.
Because of their biological similarities to humans, nonhuman primates have been studied in an attempt to find a suitable model for menopause. One impediment to this research has been a paucity of normal aged primates for study. In addition, uncertainties about the life span of various primate species, definitions used to establish the markers of reproductive aging, measurements used to verify menopause, the comparability of data derived from captive versus free-ranging animals, as well as the similarities and differences among species, have hindered progress in our understanding of the biological mechanisms underlying reproductive senescence in primates. The resulting confusion has led to several assertions that menopause is uniquely human [24–26]. Here, we attempt to dispel some of the uncertainties surrounding the comparative analysis of menopause in primates by examining the commonalities and differences in established markers of menopause among primate species. We conclude that menopause is not unique to humans.
DEFINITION OF MENOPAUSE
Perhaps the most salient source of misunderstanding in comparative studies is the definition of menopause, which originated to describe obvious changes that occur in humans. The Oxford English Dictionary defines menopause as the “permanent cessation of menstruation; the period of a woman's life when this occurs...” [27]. This definition draws heavily from the historical characterization of menopause almost exclusively in terms of the termination of menstruation (e.g., “12 months of amenorrhea following the final menstrual period” [28]). From a physiological perspective, however, menopause can be defined as “the cessation of ovarian steroid hormone secretion as a result of the depletion of oocytes and surrounding follicular apparatus” [29]. By the first two definitions, only those species that menstruate—that is, exhibit cyclic vaginal discharge of blood—can experience menopause. The third definition, however, emphasizes the pivotal role of ovarian changes in the termination of reproductive viability, of which the discontinuation of menstruation is only one component. We agree with vom Saal and Finch [30] that an “underlying and unifying framework” for understanding female reproductive senescence is needed, and argue that a rigorous definition of menopause, focusing on its essential physiological features, will permit a more meaningful comparison of reproductive senescence across species.
Menstrual bleeding is an easily observable marker of the underlying ovarian and neuroendocrine phenomena that govern reproductive viability in humans (see vom Saal and Finch [30]). However, not all primates exhibit regular patterns of menstrual bleeding [31, 32], and menstruation is only one of many relevant biological events that define reproductive potential. A definition of menopause that encompasses hormonal changes and their anatomical substrates as key determinants of the age-associated loss of fertility provides a more fundamental view of reproductive senescence, inasmuch as studies of primates strongly suggest that ovarian exhaustion is central to this process [30]. The decline of ovarian function is not the only cause of hormonal change, however, and there is evidence in women that other age-associated neuroendocrine alterations may occur independently of ovarian influences [2]. Since not all primates menstruate, and because menstruation is only one facet of the complex physiological mechanism that governs reproductive cyclicity, we believe that a meaningful comparative analysis of female reproductive senescence should focus on the anatomical, physiological and biochemical changes that are essential to the cessation of ovarian cyclicity, regardless of whether menstrual bleeding is present. For these reasons, in the following comparative analysis, we define menopause in primates as the permanent, nonpathologic, age-associated cessation of ovulation. To infer this event, a variety of biological parameters, such as menstrual bleeding, perineal swelling, follicular depletion, and hormonal changes, can be useful. However, it should be emphasized that a change in any one of these phenomena cannot be considered an exclusionary criterion for the underlying physiological event.
TIMING OF MENOPAUSE IN THE LIFE SPAN
An important issue regarding comparative research on menopause involves the timing of menopause relative to average (mean) and maximum life span. Humans may be unique among primates in that the females have a long postreproductive survival potential [33]. The mean age at menopause for humans is approximately 50 yr [34–36]; if we consider that the maximum life span for humans is about 122 yr, a human female could spend nearly 60% of her life in a postreproductive state. This issue arises in the debate over whether nonhuman primates experience menopause, and if so, when this event occurs relative to death; one of the prevailing arguments against accepting nonhuman primates as models for human menopause emphasizes this very point [37]. For example, rhesus monkeys (Macaca mulatta) cease menstruating at approximately 25 yr of age [38–41]. In captivity, the known maximum life span of rhesus monkeys is around 40 yr [42, 43]. Hence, at most, about 40% of the maximum life span of rhesus monkeys is postreproductive.
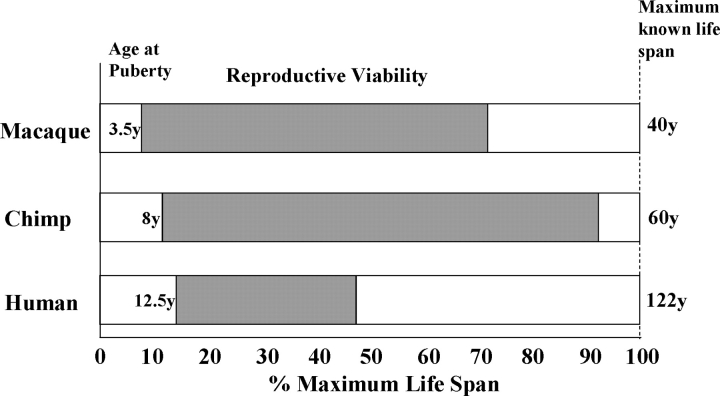
It can be difficult to compare life span across species due to differences in living environments, medical care, etc., but another potentially useful measure of longevity is the average life span (usually the mean or median life expectancy). Table 1 depicts current estimates of average (mean) and maximum life span for humans and a selection of nonhuman primates, as well as the approximate age of menopause and the proportion of the average and maximum life spans spent in a nonreproductive state. Species differences in these biological markers are evident, even among the great apes. A schematic representation of interspecies variability in key reproductive parameters is depicted in Figure 1. This diagram underscores the relatively small proportion of the known life span during which humans are reproductively viable compared with either chimpanzees or macaques. These cross-species comparisons provide a conceptual framework for understanding the timing of reproductive senescence in primates, and they support the view that humans have a uniquely extended postmenopausal life expectancy. Postreproductive survival, however, is a separate issue from the existence of menopause per se, as we discuss below in the section on operational definitions.
TABLE 1.
Average (mean) life span (LS), maximum LS, average age at menopausal onset, and proportion of life spent in a nonreproductive state as a function of currently known average or maximum life spans in a variety of primate species.
FIG. 1.
Reproductive life span in female macaques (Macaca spp.), chimpanzees (Pan troglodytes), and humans (Homo sapiens) depicting approximate ages at puberty, menopause, and maximum life span. The following numbers were used: macaque: 3.5 yr [58], 25 yr [42], 40 yr [57]; chimpanzee: 8 yr [59], 50 yr [47], 60 yr [50]; and human: 12.5 yr [60], 80 yr [44], 122 yr [45].
The timing of the transition into menopause, known as perimenopause, also is highly variable in human females. Age at onset of perimenopause in humans ranges from the mid-30s to the early 50s [12, 35, 61–64]. This wide variability in timing has hindered efforts to understand the mechanisms that control the onset of menopause in humans. In nonhuman primates, the difficulties may be even more pronounced, since reproductive cessation occurs so late in the life span that relatively few animals live to ages approaching the maximum. Despite these obstacles, burgeoning data support the existence of a perimenopausal state in nonhuman primates [37, 40, 41, 65, 66], a condition which, by definition, indicates a transitional stage between fertility and age-associated infertility.
ASSESSING MENOPAUSE IN WILD VERSUS CAPTIVE ANIMALS
The comparability of data derived from wild versus captive animals is a recurrent issue in studies of nonhuman primates [67]. Although important demographic, behavioral, and anthropological information has emerged from field studies of free-ranging nonhuman primates, data dealing with specific physiological mechanisms that govern the timing of menopause in the wild are understandably scarce. Anecdotal evidence suggests the existence of menopause in wild primates [68–71], but the difficulty in obtaining definitive data under the challenging conditions imposed by naturalistic observation diminishes the impact of these reports. Uncertainty about the subjects' ages (which are often estimated [53]) and the effects of predation pressures [72], limited survivability [73, 74], infant mortality [75], food availability and nutrition [76], and social dynamics [77] are a few of the factors that might compromise the investigation of menopause in free-ranging primates. Because of these constraints, field observations of menopause in nonhuman primates are perhaps best viewed as an important, but limited, complement to data derived from captive animals.
OPERATIONAL DEFINITIONS OF MENOPAUSE FOR COMPARATIVE ANALYSIS
Perhaps much of the misunderstanding of reproductive senescence in nonhuman primates results from the broad array of operational definitions for menopause (see Table 2 for a list of criterion-based operational definitions). While each of the parameters measured has merit as a potential marker of reproductive senescence, a piecemeal approach to defining menopause can often lead to contradictory claims, as illustrated by research on captive chimpanzees. For example, Gould et al. [47] measured urinary and serum hormones for 1 mo, menstrual cycle length and perineal swelling for 12 mo, the effects of a single GnRH challenge, and the status of ovarian follicles in two common chimpanzees (Pan troglodytes) and one bonobo (Pan paniscus) to provide the earliest evidence of menopause in these species. Graham et al. [81] documented reproductive capacity in chimpanzees throughout life using conception rate, cycle frequency, and cycle length, based on perineal swelling. These studies conclude that chimpanzees do not undergo menopause until around the age of 50 yr. More recently, however, Videan and colleagues [49] measured serum luteinizing hormone (LH), FSH, and E2, sampled biannually, as well as cycle length and perineal swelling, in 14 common chimpanzees ranging in age from 31 to 50 yr. The authors reported the cessation of perineal swelling in the oldest animals (>44 yr) as well as endocrine profiles similar to those in postmenopausal human females. They conclude that menopause occurs at 35–40 yr of age in chimpanzees. When menstrual cycle length, inferred from perineal swelling and menstrual bleeding, is used exclusively as the dependent variable for defining menopause in chimpanzees, however, Lacreuse and colleagues found little evidence for full menopause in chimpanzees in their 30s, 40s, and 50s, except in one female who continued to cycle until 57 yr of age, 2 yr prior to her death [48].
TABLE 2.
Criterion-based operational definitions and dependent variables used to establish the existence of menopause.

This definitional dilemma was recently illustrated in a study that dichotomized reproductive aging in chimpanzees using separate but interrelated phenomena: reproductive senescence, defined on the basis of “reduced reproductive performance,” and menopause, defined as “species typical patterns of reproductive senescence that significantly exceed the general aging trajectory and result in a postreproductive life stage” [74], thus emphasizing the timing of menopause in the life span as a defining feature, rather than the physiological changes that characterize menopause itself. This somewhat unorthodox definition allows the researchers to suggest that menopause is a singularly human phenomenon.
All of these studies provide important information on reproductive aging in chimpanzees, but the use of different criteria and dependent variables leaves the question open: do chimpanzees experience menopause, and if so, when? If one adopts the view that reproductive aging is a process and not a singular event, then perhaps the apparently discrepant findings from chimpanzees can be understood more clearly. Falling birth rates [81, 102], follicular depletion [81, 82], and increased anovulatory cycles [71] certainly bespeak a system in decline. With a more comprehensive approach to characterizing reproductive senescence in chimpanzees, one might be convinced that their reproductive system is indeed undergoing changes that parallel those seen in humans. Rather than dismissing chimpanzees as “poorly suited” [37] models for human menopause, or concluding that chimpanzees fail to exhibit menopause altogether [74, 78, 103], it may be more advantageous to refine and focus our definition of menopause in order to deepen our understanding of age-related changes in reproductive function.
Table 2 depicts many useful criteria for establishing the presence of menopause, although most are by no means definitive. For example, cycle irregularity could foretell the onset of menopause, but it could also result from social, pathologic, or environmental factors that influence the reproductive system independent of aging. The same could be said for hormonal correlates of menopause (e.g., growth hormone [GH], inhibin, insulinlike growth factor 1, etc.), because these endocrine indices may describe a correlate of the menopausal state without providing irrefutable documentation of menopause per se. What is evident from Table 2 is that many criteria are available for examining menopause, and some have more merit than others.
ACROSS-SPECIES COMPARISONS OF MENOPAUSAL SIGNS
With the rapid expansion of the aged human population, interest in the mechanisms underlying reproductive senescence has grown rapidly, along with a concomitant evolution in opinion regarding menopause in nonhuman primates. Originally, menopause was considered to be a uniquely human phenomenon, as there was little scientific support for the existence of menopause in other primates [24, 104]. However, as record keeping and research tools improved, evidence began to mount that menopause does, in fact, occur in a number of nonhuman primate species (see below), and that these animals can be useful for expanding our understanding of the mechanisms underlying menopause in humans.
Great Apes
Chimpanzees (Pan spp.).
Some of the earliest work on reproductive senescence in the great apes was that of Gould and colleagues [47] in three chimpanzees (two P. troglodytes [48 and 50 yr] and one P. paniscus [>40 yr]). This study reported similar menopausal signs in chimpanzees and in humans, particularly with regard to gonadotropin levels, FSH:LH ratios, and ovarian histology. In more recent work on a larger number of subjects, Lacreuse and colleagues [48] showed that many aged chimpanzees (P. troglodytes) continue to menstruate into their 50s. These workers also reported that cycle length in female chimpanzees increased from the age of 20 yr onward. Increasing cycle length with age has also been noted by Videan et al. [49], who supplemented this observation with hormonal data from biannual samples. They concluded that menopause occurs in this species at approximately 35–40 yr of age, a finding that concurs with data from wild chimpanzees in which 28% were found to cease cycling after the age of 30 [71].
Another analysis of chimpanzees in the wild, however, found that healthy chimpanzees remain reproductively viable well past the age of 40 yr [74]. The authors suggest that menopause occurs as a byproduct of ill health in this population. This conclusion was drawn from observations of a truncated (i.e., mortality-selected) distribution of wild chimpanzees, as the rate of survivability to 40 yr of age was only 7%. It should not be surprising that females who survive in the wild (or even in captivity) to advanced age are reproductively robust. Perhaps long-lived animals represent extraordinary females who manifest behavioral and constitutional traits that enhance health and survivability. In such females, a continuation of reproductive function, albeit at a diminished level, might simply be considered a marker for overall good health. This observation does not necessarily undermine the contention that female chimpanzees undergo menopause, but rather suggests that the onset of menopause may simply be delayed in relatively healthy, long-lived animals. In fact, chimpanzees show a number of other similarities to humans in terms of reproductive aging; both species show an increase in fetal loss as a function of advancing age [102, 105, 106], and the age-related depletion of ovarian follicles follows a remarkably similar trajectory in chimpanzees and humans [82]. Although some specifics regarding the precise timing of menopause-associated events remain uncertain, it seems clear that reproductive aging is remarkably similar in female chimpanzees and humans.
Orangutans (Pongo spp.).
Data on menopause from other great apes are much scarcer. Early reports from captive orangutans documented the endocrine characteristics of the menstrual cycle and illustrated similarities to the human cycle [107]. However, this work did not extend to an evaluation of aged females. An analysis of archival data examining live births and interbirth intervals across the life span showed a significant age-specific decline in fertility in captive orangutans (Pongo pygmaeus) [78]. Research on wild Sumatran orangutans (Pongo abelii) failed to document menopause, as inferred from increased interbirth intervals in animals whose ages were estimated [53]. However, data from wild animals are difficult to interpret because of countervailing factors, such as female rank, infant mortality, uncertain age, and ecological issues such as food availability. Rather than assuming that this species represents a “decoupling of reproductive and somatic aging” [69], it may be more pragmatic to focus on improving measurement techniques and methodologies used to assess menopause in the wild.
Gorillas (Gorilla gorilla).
Relatively little is known about reproductive senescence in female gorillas (Gorilla gorilla). Early work described the reproductive physiology of this species, correlating perineal tumescence with circulating hormones and reporting a pattern of cyclic hormone secretion similar to that in humans [108]. Cycle length (28–38 days) and urinary FSH levels also have been established in gorillas [109], with the authors concluding that this species provides a suitable model for human reproduction. More recently, fecal hormone determinations in two captive female gorillas (>40 yr old) revealed protracted luteal phases (as indicated by progestogen peaks and cyclic irregularities) typical of aging human females [51, 93]. An age-related decline in fertility was reported in wild mountain gorillas from the Virunga Volcano area in central Africa, although no evidence of a period of protracted postreproductivity in these females was found [89]. The paucity of data on gorillas and other great apes underscores the need for further research on reproductive senescence in this group of closely related species.
Baboons (Papio spp.).
Baboons have been studied extensively with regard to reproductive physiology because they are similar to humans in both menstrual cycle characteristics [56, 110, 111] and pregnancy [112–114]. Data regarding the occurrence of menopause in baboons (Papio spp.), however, are relatively meager. Based on menstrual cycle length (inferred from perineal turgescence), increased cyclic variation begins at approximately 19 yr of age, whereas total cessation of cycling occurs at ∼26 yr in captive baboons [56]. This finding supports an earlier report of menopause occurring in the mid-20s in this species [115]. Data from a wild troop of baboons confirm this conclusion, as inferred from age-related reductions in fecundity and increases in interbirth intervals [90]. Additional studies of wild baboons have reported increased cyclic variability with age and a complete loss of fertility by the age of 25 yr [116]. Thus, there is reasonably compelling evidence that baboons undergo age-linked alterations in reproductive function that are similar to those in humans.
Macaques
Rhesus monkeys (Macaca mulatta).
Reproductive senescence has been more thoroughly characterized in rhesus monkeys (Macaca mulatta) than in any other species of nonhuman primate. An early study of two captive rhesus monkeys found evidence of menopause, based on menstrual bleeding, in females older than 25 yr of age [38, 39]. Hodgen et al. [88] extended this observation with supporting endocrine data. They reported that a subset of animals older than 22 yr exhibited elevated gonadotropin concentrations and basal progesterone (P4) and E2 concentrations characteristic of human menopause. However, these original studies are difficult to interpret, because rhesus monkeys breed seasonally, and in controlled laboratory environments they exhibit natural anovulatory cycles that could be mistaken for menopause [117–120]. Furthermore, in a laboratory environment during the summer months, ovulatory cycles with abnormal luteal phases can occur [121, 122]. Summer corresponds to the nonbreeding season in outdoor-housed animals that are exposed to seasonal variations in daylight [123–127]. Because of seasonal variations in endocrine and menstrual parameters, it can be difficult to distinguish seasonal acyclicity and amenorrhea from reproductive senescence in captive females. This concern was addressed in two studies [40, 41] that documented menopause in female rhesus monkeys. Based on menstrual bleeding and serum E2 and LH levels analyzed over a 12-mo period, as well as ovarian histology, Walker [40] found evidence for menopause in six females ranging from 26 to 34 yr of age. This finding was corroborated by Gilardi et al. [41] in a group of 20- to 29-yr-old females using menstrual data and urinary steroid hormone levels. In this study, 13 animals with a mean age of 24 yr were considered to be perimenopausal, and two animals with a mean age of 29.5 yr were fully menopausal [37]. Shideler et al. [65] also examined the interaction of seasonality and aging by focusing on endocrine parameters. They confirmed earlier findings regarding the approximate age range for menopause, and reported a monotonic pattern of FSH secretion in these females. In addition, the anovulatory cycles characteristic of the nonbreeding season were found to immediately presage the onset of menopause. These observations were supported recently by Downs and Urbanski [66], who found that age-related changes in plasma FSH levels forecast menopause (no episodes of menses for ≥12 mo). Postmenopausal females showed diminished levels of circulating E2 and P4 and elevated FSH and LH. They further reported significant declines in anti-Mullerian hormone and inhibin B in postmenopausal females when compared to younger animals [66], indicating that these endocrine parameters may reflect changes in ovarian dynamics associated with menopause.
Few data from free-ranging rhesus monkeys exist. One exception is a study of provisioned, free-ranging monkeys by Johnson and Kapsalis that factored survivability into the assessment of the likelihood of reaching menopause [79]. The authors reported a median age of menopause >27 yr, but in a manner similar to Emery Thompson et al. [74], they concluded that reproductive senescence was highly correlated with general overall health. Perhaps in wild female primates, age-related declines in overall health may preclude the reliable observation of true menopause in many animals.
Studies of the neuroendocrine axis also reveal changes associated with somatic aging. Gore and colleagues [100] examined four aged rhesus monkeys (age range, 26.9–30.6 yr) that were implanted with intracranial push-pull cannulae for the measurement of pulsatile GnRH concentrations. The researchers found that aged animals continue to secrete GnRH in a pulsatile fashion, although absolute levels of this hormone are higher than in younger females. Similarly, whereas circulating levels of GH and GH pulse amplitude decline with age in monkeys, pituitary responsiveness to GHRH and GHRP-2 (GH-releasing peptide 2) is similar in young and aged monkeys [99]. Pulsatile LH release, however, is significantly higher in aged females, as is pulse amplitude [99]. These studies support the view that neuroendocrine changes in senescent monkeys are consistent with those found in humans [128–131].
Ovarian changes underlying menopause in rhesus females also have been characterized [40, 84, 97]. For example, gonadotropin stimulation produces significantly fewer oocytes in older (age range, 16–26 yr) rhesus monkeys, a finding reported earlier in humans [132]. When evaluated over much of the life span (from ages 1 to 25 yr), ovarian morphology changes substantially, with increasing evidence of atretic follicles and fewer primordial follicles in aged animals [84]. These studies indicate that rhesus monkeys undergo ovarian changes as a function of aging that are similar to those seen in humans [133, 134] and chimpanzees [82].
Pigtail macaques (Macaca nemestrina).
Few data exist describing the relationship between aging and reproduction in pigtail macaques (Macaca nemestrina). Two studies of ovarian changes concur that there is a decline in folliculogenesis with age. Graham et al. [81] examined ovaries from this species and reported a reduction in developing follicles after the age of 20 yr, and Miller et al. [83] extended this work by reporting a bilateral, linear decrease in primordial follicles up to the age of 12.5 yr (the oldest age analyzed). Observations from archival data show an age-associated decline in fertility, and in one group, 26% of the females ceased reproductive activity prior to death [78]. Whereas no endocrine data are available to clarify the mechanisms underlying these changes, it seems evident that pigtail macaques undergo a process of reproductive senescence consistent with menopause.
Japanese macaques (Macaca fuscata).
Both captive and free-ranging Japanese macaques (Macaca fuscata) experience reproductive decline and a period of infertility prior to death as well. Studies of free-ranging animals indicate that fertility rates diminish with age, and that by the age of 25 yr, females cease to reproduce [135–137]. Some captive females have been found to maintain normal menstrual cycles (based on periodic menses) despite loss of fertility [98], a finding similar to that in humans [138]. This decline in fertility may result from a suboptimal uterine or hormonal environment, fetal chromosomal abnormalities, or simply less sexual behavior. Luteinizing hormone levels are higher in aged females [98], and an E2 challenge elicits an LH surge, suggesting that this neuroendocrine mechanism is intact, as is the case in humans [139]. The ovaries of aged Macaca fuscata also show evidence of decline, as judged by increased sclerosis and abnormal follicles [98]. Although Pavelka and Fedigan [91] concluded that reproductive termination in Japanese macaques does not model human menopause, they used a nonclinical definition for the cessation of reproduction based on a strict classification using the time lag between last parturition and death. They conclude that “reproductive termination” is a certainty by the age of 25 yr in Japanese macaques, but are reluctant to call this “menopause” because of its timing in the life span of the animal. As discussed above, this concern begs the issue by focusing on the timing of the event within the life span rather than the underlying physiological process of menopause per se. The key point is that the similarities in reproductive senescence in humans and Japanese macaques greatly exceed the differences.
Barbary macaques (Macaca sylvanus).
Data from the Barbary macaque (Macaca sylvanus), though sparse, parallel those from other macaque species. Paul et al. [87] reported an age-related decline in fertility and an increase in interbirth interval, concluding that reproduction in Macaca sylvanus terminates at appropriately 25 yr of age. The authors indicate that a postreproductive period of greater than 5 yr is not uncommon in the Barbary macaque.
Other macaques (Macaca spp.).
Studies from other species of the genus Macaca generally confirm that senescent macaque females experience a period of waning fertility and cessation of ovulation characteristic of menopause. As summarized in Paul et al. [87], despite individual variability, the postreproductive life span of macaque females can range up to 8 yr. These authors cite the observations of reduced fertility in crab-eating macaques (Macaca fascicularis [140]), bonnet macaques (Macaca radiata [141]), and toque macaques (Macaca sinica [142]). A recent analysis of Macaca fascicularis [96] substantiated earlier observations in this species by demonstrating a postmenopausal neuroendocrine pattern similar to that in humans (elevated FSH and androgens, diminished estrogens).
Tamarins (Saguinus spp.)
Little research has examined aging and reproduction in New World primates. Two subspecies of tamarins (Saguinus oedipus and S. fuscicollis) were found to experience reduced fertility at approximately 17 yr of age, based on plasma and urinary hormone measurements [92]. However, ovarian histology of older females suggests that large luteal masses continue to process steroids, despite the absence of normal folliculogenesis. This phenomenon would distinguish these animals from Old World primates and humans, although further research on reproductive senescence in New World primates is clearly needed.
CONCLUSIONS
Menopause is a naturally occurring process in which the permanent cessation of ovulation is associated with a variety of physiological and structural changes in aging female primates. One obstacle to the acceptance of nonhuman primates as models of human menopause has been the tendency to focus on differences rather than commonalities. For example, it is apparent that the onset of reproductive decline occurs relatively later in the life span of nonhuman primates than of humans, resulting in a more protracted postreproductive period in humans [33, 143, 144]. Although postreproductive survival is important from an evolutionary standpoint, menopause is fundamentally a physiological process, and a gradual decline and eventual cessation of female reproductive capacity, based on a host of criteria, appears to be a consistent feature of aging in every primate species that has been examined.
We draw the following conclusions:
The multitude of definitions for menopause has contributed to a general confusion about its occurrence among nonhuman primate species. A uniform definition would facilitate our understanding of the way in which menopause reflects species-specific patterns of reproductive senescence.
We define menopause in primates as the permanent, nonpathlogic, age-associated cessation of ovulation. Menopause is associated with concomitant structural and functional changes, including (in species that exhibit menstrual bleeding) the termination of menstruation.
Whereas humans have a uniquely extended postmenopausal life expectancy, other primate species do experience a postreproductive period. The length of this period is not necessarily related to the existence of menopause per se.
Many of the apparent discrepancies in reports regarding the occurrence of menopause in nonhuman primates may be accounted for by environmental and social factors that distinguish captive versus wild animals, and by a paucity of data from sufficiently powered studies.
By almost any generally accepted definition, several species of nonhuman primates experience menopause.
Acknowledgments
The authors would like to thank Lary C. Walker for his critical input and insightful comments.
Footnotes
Supported by National Institutes of Health grants P51RR000165 and P01AG026423 to the Yerkes National Primate Research Center.
REFERENCES
- Gosden RG. The Biology of Menopause: The Causes and Consequences of Ovarian Aging. New York:: Academic Press;; 1985. [Google Scholar]
- Hall JE.Neuroendocrine changes with reproductive aging in women. Sem Reprod Med 2007; 25: 344–351. [DOI] [PubMed] [Google Scholar]
- Prior JC.Perimenopause: the complex endocrinology of the menopausal transition. Endocrine Rev 1998; 19: 397–428. [DOI] [PubMed] [Google Scholar]
- Santoro N, Banwell T, Tortoriello D, Lieman H, Adel T, Skurnick J.Effects of aging and gonadal failure on the hypothalamic-pituitary axis in women. Am J Obstet Gynecol 1998; 178: 732–741. [DOI] [PubMed] [Google Scholar]
- Sherman BM, Korenman SG.Hormonal characteristics of the human menstrual cycle throughout reproductive life. J Clin Invest 1975; 55: 699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement PB.Histology of the ovary. Am J Surg Pathol 1987; 11: 277–303. [DOI] [PubMed] [Google Scholar]
- Giacobbe M, Pinto-Neto AM, Costa-Paiva LH, Martinez EZ.Ovarian volume, age, and menopausal status. Menopause 2004; 11: 180–185. [DOI] [PubMed] [Google Scholar]
- Gonzales OV, Martinez NL, Rodriguez G, Ancer J.Pattern of vascular aging of the postmenopausal ovary. Ginecol Obstet Mex 1992; 60: 1–3. [PubMed] [Google Scholar]
- Kozik W.Arterial vasculature of ovaries in women of various ages in light of anatomic, radiologic and microangiographic examinations. Ann Acad Med Stetin 2000; 46: 25–34. [PubMed] [Google Scholar]
- Laszczynska M, Brodowska A, Starczewski A, Masiuk M, Brodowski J.Human postmenopausal ovary - hormonally inactive fibrous connective tissue or more? Histol Histopathol 2008; 23: 219–225. [DOI] [PubMed] [Google Scholar]
- Manieri C, Di Bisceglie C, Fornengo R, Grosso T, Zumpano E, Calvo F, Barardengo E, Volante M, Papotti M.Postmenopausal virilization in a woman with gonadotropin dependent ovarian hyperthecosis. J Endocrinol Invest 1998; 21: 128–132. [DOI] [PubMed] [Google Scholar]
- Burger HG, Dudley EC, Robertson DM, Dennerstein L.Hormonal changes in the menopause transition. Recent Prog Horm Res 2002; 57: 257–275. [DOI] [PubMed] [Google Scholar]
- Basaria S, Dobs AS.Clinical review: controversies regarding transdermal androgen therapy in postmenopausal women. J Clin Endocrinol Metab 2006; 91: 4743–4752. [DOI] [PubMed] [Google Scholar]
- Lindsay R, Hart DM, MacLean A, Clark AC, Kraszewski A, Garwood J.Bone response to termination of oestrogen treatment. Lancet 1978; 1: 1325–1327. [DOI] [PubMed] [Google Scholar]
- Christiansen C, Riis BJ.New methods for identifying “at risk” patients for osteoporosis. Clin Rheumatol 1989; 8: 52–55. [DOI] [PubMed] [Google Scholar]
- Heaney RP, Recker RR, Saville PD.Menopausal changes in bone remodeling. J Lab Clin Med 1978; 92: 964–970. [PubMed] [Google Scholar]
- Kampen DL, Sherwin BB.Estrogen use and verbal memory in healthy postmenopausal women. Obstet Gynecol 1994; 83: 979–983. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Sherwin BB.Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology 1992; 17: 485–495. [DOI] [PubMed] [Google Scholar]
- Sherwin BB.Sex hormones and psychological functioning in postmenopausal women. Exp Gerontol 1994; 29: 423–430. [DOI] [PubMed] [Google Scholar]
- Sherwin BB.Estrogen and cognitive functioning in women. Neuroscience 2006; 138: 1021–1026. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Henry JF.Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Front Neuroendocrinol 2007; 29: 88–113. [DOI] [PubMed] [Google Scholar]
- Gorodeski GI.Update on cardiovascular disease in post-menopausal women. Best Pract Res Clin Obstet Gynaecol 2002; 16: 329–355. [DOI] [PubMed] [Google Scholar]
- Vitale C, Miceli M, Rosano GM.Gender-specific characteristics of atherosclerosis in menopausal women: risk factors, clinical course and strategies for prevention. Climacteric 2007; 2: 16–20. [DOI] [PubMed] [Google Scholar]
- Lancaster JB, King BJ.An evolutionary perspective on menopause. Brown J, Kerns V.How Humans Adapt. A Biocultural Odyssey Washington, DC:Smithsonian Institution;1985: 14–37. [Google Scholar]
- Pavelka MS, Fedigan LN.Menopause: a comparative life history perspective. Yearb Phys Anthropol 1991; 34: 13–38. [DOI] [PubMed] [Google Scholar]
- Peccei JS.Menopause: adaptation or epiphenomenon? Evol Anthropol 2001; 10: 43–57. [Google Scholar]
- Oxford English Dictionary. 2nd ed. Oxford, UK:: Oxford University Press;; 1989. [Google Scholar]
- Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, Woods N.Executive summary: stages of reproductive aging workship (STRAW). Fertil Steril 2001; 76: 874–878. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Bairey-Merz CN, Braunstein GD, Berga SL, Bittner V, Hodgson TK, Gierach GI, Reis SE, Vido DA, Sharaf BI, Smith KM, Sopko G, et al. Determination of menopausal status in women: the NHLBI-sponsored women's ischemia syndrome evaluation. J Womens Health 2004; 13: 872–887. [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Finch CE.Reproductive senescence: phenomena and mechanisms in mammals and selected vertebrates. Knobil E, Neill J.The Physiology of Reproduction, vol. 2 New York:Raven Press;1988: 2351–2413. [Google Scholar]
- Schultz AH. Die Primaten. Wiesbaden:: R. Löwit;; 1971. [Google Scholar]
- Dukelow WR.Reproductive cyclicity and breeding in the squirrel monkey. Rosenblum LA, Coe CL.Handbook of Squirrel Monkey Research New York:Plenum Press;1985: 169–190. [Google Scholar]
- Hawkes K, O'Connell JF, Blurton Jones NG, Alvarez H, Charnov EL.Grandmothering, menopause, and the evolution of human life histories. Proc Natl Acad Sci U S A 1998; 95: 1336–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollman RF. The Menstrual Cycle. Philadelphia:: W.B. Saunders;; 1977. [Google Scholar]
- Treloar AE.Menstrual cyclicity and the pre-menopause. Maturitas 1981; 3: 49–64. [DOI] [PubMed] [Google Scholar]
- Abma J, Chandra A, Mosher W, Peterson L, Piccinino L.Fertility, family planning and women's health: new data from the 1995 National Survey of Family Growth. Vital Health Statistics 23: National Center for Health Statistics;1997. [PubMed] [Google Scholar]
- Bellino FL, Wise PM.Nonhuman primate models of menopause workshop. Biol Reprod 2003; 68: 10–18. [DOI] [PubMed] [Google Scholar]
- Van Wagenen G.Menopause in subhuman primate. Anat Rec 1970; 166: 392 [Google Scholar]
- Van Wagenen G.Vital statistics from a breeding colony: reproduction and pregnancy outcome in Macaca mulatta. J Med Primatol 1972; 1: 3–28. [PubMed] [Google Scholar]
- Walker ML.Menopause in female rhesus monkeys. Am J Primatol 1995; 35: 59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilardi KVK, Shideler SE, Valverde CR, Roberts JA, Lasley BL.Characterization of the onset of menopause in the rhesus macaque. Biol Reprod 1997; 57: 335–340. [DOI] [PubMed] [Google Scholar]
- Tigges J, Gordon TP, McClure HM, Hall EC, Peters A.Survival rate and life span of rhesus monkeys at the Yerkes Regional Primate Center. Am J Primatol 1988; 15: 263–273. [DOI] [PubMed] [Google Scholar]
- Ramsey JJ, Laatsch JL, Kemnitz JW.Age and gender differences in body composition, energy expenditure, and glucoregulation of adult rhesus monkeys. J Med Primatol 2000; 29: 11–19. [DOI] [PubMed] [Google Scholar]
- Arias E, MacDorman MF, Strobino DM, Guyer B.Annual summary of vital statistics–2002. Pediatrics 2003; 112: 1215–1230. [DOI] [PubMed] [Google Scholar]
- Robine JM, Allard M. Jeanne Calment: validation of the duration of her life. In: Jeune B, Vaupel JW, editors. Validation of Exceptional Longevity. Odense, Denmark:: Odense University Press;; 1999. [Google Scholar]
- Wallis J.A survey of reproductive parameters in the free-ranging chimpanzees of Gombe National Park. J Reprod Fertil 1997; 109: 297–307. [DOI] [PubMed] [Google Scholar]
- Gould KG, Flint M, Graham CE.Chimpanzee reproductive senescence: a possible model for evolution of the menopause. Maturitas 1981; 3: 157–166. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Chennareddi L, Johnson J, Gould KG, Hawkes K, Wijayawardana S, Chen J, Herndon JG.Menstrual cycles continue into advanced old age in the common chimpanzee (Pan troglodytes). Biol Reprod 2008; 79: 407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videan EN, Fritz J, Heward CB, Murphy J.The effects of aging on hormone and reproductive cycles in female chimpanzees (Pan troglodytes). Comp Med 2006; 56: 291–299. [PubMed] [Google Scholar]
- Nowak RM. Walker's Primates of the World. Baltimore:: Johns Hopkins University Press;; 1999. [Google Scholar]
- Margulis SW, Atsalis S, Bellem A, Wielebnowski N.Assessment of reproductive behavior and hormonal cycles in geriatric western Lowland gorillas. Zoo Biol 2007; 26: 117–139. [DOI] [PubMed] [Google Scholar]
- Rowe N. The Pictorial Guide to the Living Primates. East Hampton, NY:: Pogonias Press;; 1996. [Google Scholar]
- Wich SA, Utami-Atmoko SS, Setia TM, Rijksen HD, Schürmann C, van Hooff JA, van Schaik CP.Life history of wild Sumatran orangutans (Pongo abelii). J Hum Evol 2004; 47: 385–398. [DOI] [PubMed] [Google Scholar]
- Bronikowski AM, Alberts SC, Altmann J, Packer C, Carey KD, Tatar M.The aging baboon: comparative demography in a non-human primate. Natl Acad Sci U.S.A 2002; 99: 9591–9595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge DS, Carey JR.Post-reproductive life predicted by primate patterns. J Gerontol A Biol Sci Med Sci 2000; 55: B201–B209. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Carey KD, Comuzzie AG.Variations in menstrual cycle length and cessation of menstruation in captive raised baboons. Mech Ageing Dev 2003; 124: 865–871. [DOI] [PubMed] [Google Scholar]
- Cork LC, Walker LC.Age-related lesions, nervous system. Jones TC, Mohr U, Hunt RD.Nonhuman Primates II New York:Springer-Verlag;1993: 173–183. [Google Scholar]
- van Wagenen G.Age at menarche of the laboratory rhesus monkey. Anat Rec 1952; 112: 436 [Google Scholar]
- Young WC, Yerkes RM.Factors influencing the reproductive cycle in the chimpanzee; the period of adolescent sterility and related problems. Endocrinology 1943; 33: 121–154. [Google Scholar]
- Anderson SE, Dallal GE, Must A.Relative weight and race influence average age at menarche: results from two nationally representative surveys of US girls studied 25 years apart. Pediatrics 2003; 111: 844–850. [DOI] [PubMed] [Google Scholar]
- McKinlay SM, Brambilla DJ, Posner JG.The normal menopause transition. Maturitas 1992; 14: 103–115. [DOI] [PubMed] [Google Scholar]
- Santoro N, Rosenberg Brown J, Adel T, Skurnick JH.Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab 1996; 81: 1495–1501. [DOI] [PubMed] [Google Scholar]
- Hidayer NM, Sharaf SA, Aref SR, Tawfik TA, Moubarak II.Correlates of natural menopause: a community-based study in Alexandria. Mediterr Health J 1999; 5: 307–319. [PubMed] [Google Scholar]
- Zapantis G, Santoro N.The menopausal transition: characteristics and management. Best Pract Res Clin Endocrinol Metab 2003; 17: 33–52. [DOI] [PubMed] [Google Scholar]
- Shideler SE, Gee NA, Chen J, Lasley BL.Estrogen and progesterone metabolites and follicle-stimulating hormone in the aged macaque female. Biol Reprod 2001; 65: 1718–1725. [DOI] [PubMed] [Google Scholar]
- Downs JL, Urbanski HF.Neuroendocrine changes in the aging reproductive axis of female rhesus macaques (Macaca mulatta). Biol Reprod 2006; 75: 539–546. [DOI] [PubMed] [Google Scholar]
- Zihlman A, Bolter D, Boesch C.Wild chimpanzee dentition and its implications for assessing life history in immature hominin fossils. Proc Natl Acad Sci U S A 2004; 101: 10541–10543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waser PM.Postreproductive survival and behavior in a free-ranging female mangabey. Folia Primatol 1978; 29: 142–160. [DOI] [PubMed] [Google Scholar]
- Blaffer Hrdy S."Nepotists" and "altruists": the behavior of old females among macaques and langur monkeys. Amoss PT, Harrell S.Other Ways of Growing Old Stanford, CA:Stanford University Press;1981: 59–76. [Google Scholar]
- Goodall J. Patterns of Behavior. Cambridge, MA:: Harvard University Press;; 1986. The Chimpanzees of Gombe. [Google Scholar]
- Nishida T, Corp N, Hamai M, Hasegawa Y, Hiraiwa-Hasegawa M, Hosaka K, Hunt KD, Itoh N, Kawanaka K, Matsumoto-Oda A, Mitani JC, Nakamura M, et al. Demography, female life history, and reproductive profiles among the chimpanzees of Mahale. Am J Primatol 2003; 59: 99–121. [DOI] [PubMed] [Google Scholar]
- Hamilton WD, III, Busse C, Smith KS.Adoption of infant orphan chacma baboons. Anim Behav 1982; 30: 29–34. [Google Scholar]
- Clutton-Brock TH, Isvaran K.Sex differences in ageing in natural populations of vertebrates. Proc R Soc B 2007; 274: 3097–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery Thompson M, Jones JH, Pusey AE, Brewer-Marsden S, Goodall J, Marsden D, Matsuzawa T, Nishida T, Reynolds V, Sugiyama Y, Wrangham RW.Aging and fertility patterns in wild chimpanzees provide insights into the evolution of menopause. Curr Biol 2007; 17: 2150–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teleki G, Hunt EE, Pfifferling JH.Demographic observations (1963–1973) on the chimpanzees of Gombe National Park, Tanzania. J Hum Evol 1976; 5: 559–598. [Google Scholar]
- van Noordwijk MA, van Schaik CO.Development of ecological competence in Sumatran orangutans. Am J Phys Anthropol 2005; 127: 79–94. [DOI] [PubMed] [Google Scholar]
- Machatschke IH, Wallner B, Dittami J.Impact of social environment on female chimpanzee reproductive cycles. Horm Behav 2006; 50: 126–131. [DOI] [PubMed] [Google Scholar]
- Caro TM, Sellen DW, Parish A, Frank R, Brown DM, Voland E, Borgerhoff Mulder M.Termination of reproduction in nonhuman and human female primates. Int J Primatol 1995; 16: 205–220. [Google Scholar]
- Johnson RL, Kapsalis E.Menopause in free-ranging rhesus macaques: estimated incidence, relation to body condition, and adaptive significance. Int J Primatol 1998; 19: 751–765. [Google Scholar]
- Wolfe LD, Noyes MJS.Reproductive senescence among female Japanese macaques (Macaca fuscata fuscata). J Mammal 1981; 62: 698–705. [Google Scholar]
- Graham CE, Kling OR, Steiner RA.Reproductive senescence in female nonhuman primates. Bowden DM.Aging in Nonhuman Primates New York:Van Nostrand Reinhold;1979: 183–202. [Google Scholar]
- Jones K, Walker L, Anderson D, Lacreuse A, Robson SL, Hawkes K.Depletion of ovarian follicles with age in chimpanzees: similarities to humans. Biol Reprod 2007; 77: 247–251. [DOI] [PubMed] [Google Scholar]
- Miller PB, Charleston JS, Battaglia DE, Klein NA, Soules MR.Morphometric analysis of primordial follicle number in pigtailed monkey ovaries: symmetry and relationship with age. Biol Reprod 1999; 61: 553–556. [DOI] [PubMed] [Google Scholar]
- Nichols SM, Bavister BD, Brenner CA, Didier PJ, Harrison RM, Kubisch HM.Ovarian senescence in the rhesus monkey (Macaca mulatta). Hum Reprod 2005; 20: 79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki M, Yamashita K, Shimuzu K.Age-related changes in ovarian morphology from birth to menopause in the Japanese monkey, Macaca fuscata fuscata. Primates 1997; 38: 89–100. [Google Scholar]
- Johnson RL, Kapsalis E.Ageing, infecundity and reproductive senescence in free-ranging rhesus monkeys. J Reprod Fertil 1995; 105: 271–278. [DOI] [PubMed] [Google Scholar]
- Paul A, Kuester J, Podzuweit D.Reproductive senescence and terminal investment in female Barbary macaques (Macaca sylvanus) at Salem. Int J Primatol 1993; 14: 105–124. [Google Scholar]
- Hodgen GD, Goodman AL, O'Connor A, Johnson DK.Menopause in rhesus monkeys: model for study of disorders in the human climacteric. Am J Obstet Gynec 1977; 127: 581–584. [DOI] [PubMed] [Google Scholar]
- Robbins AM, Robbins MM, Gerald-Steklis N, Steklis HD.Age-related patterns of reproductive success among female mountain gorillas. Am J Phys Anthropol 2006; 131: 511–521. [DOI] [PubMed] [Google Scholar]
- Strum SC, Western JD.Variations in fecundity with age and environment in olive baboons (Papio anubis). Am J Primatol 1982; 3: 61–76. [DOI] [PubMed] [Google Scholar]
- Pavelka MSM, Fedigan LM.Reproductive termination in female Japanese monkeys: a comparative life history perspective. Am J Phys Anthropol 1999; 109: 455–464. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Ziegler TE.Features of female reproductive senescence in tamarins (Saguinus spp.), a New World primate. J Reprod Fertil 1992; 94: 411–421. [DOI] [PubMed] [Google Scholar]
- Atsalis S, Margulis SW, Bellem A, Wielebnowski N.Sexual behavior and hormonal estrus cycles in captive aged lowland gorillas (Gorilla gorilla). Am J Primatol 2004; 62: 123–132. [DOI] [PubMed] [Google Scholar]
- Hubbard GB, Carey KD, Levine H, Bachmann GA.Evaluation of a vaginal moisturizer in baboons with decreasing ovarian function. Lab Animal Sci 1997; 47: 36–39. [PubMed] [Google Scholar]
- McKinney KA, Duell BP, Wheaton DL, Hess DL, Patton PE, Spies HG, Burry KA.Differential effects of subcutaneous estrogen and progesterone on low-density lipoprotein size and susceptibility to oxidation in postmenopausal rhesus monkeys. Fertil Steril 1997; 68: 525–530. [DOI] [PubMed] [Google Scholar]
- Kavanagh K, Koudy Williams J, Wagner JD.Naturally occurring menopause in cynomolgus monkeys: changes in hormone, lipid, and carbohydrate measures with hormonal status. J Med Primatol 2005; 34: 171–177. [DOI] [PubMed] [Google Scholar]
- Schramm RD, Paprocki AM, Bavister BD.Features associated with reproductive ageing in female rhesus monkeys. Hum Reprod 2002; 17: 1597–1603. [DOI] [PubMed] [Google Scholar]
- Nozaki M, Mitsunaga F, Shimizu K.Reproductive senescence in female Japanese monkeys (Macaca fuscata): age- and season-related changes in hypothalamic-pituitary-ovarian functions and fecundity rates. Biol Reprod 1995; 52: 1250–1257. [DOI] [PubMed] [Google Scholar]
- Woller MJ, Everson-Binotto G, Nichols E, Acheson A, Keen KL, Bowers CY, Terasawa E.Aging-related changes in release of growth hormone and luteinizing hormone in female rhesus monkeys. J Clin Endocrinol Metab 2002; 87: 5160–5167. [DOI] [PubMed] [Google Scholar]
- Gore AC, Windsor-Engnell BM, Terasawa E.Menopausal increases in pulsatile gonadotropin-releasing hormone release in a nonhuman primate (Macaca mulatta). Endocrinology 2004; 145: 4653–4659. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Kemnitz JW, Lane MA, Abbott DH, Binkley N.Skeletal effects of aging and menopausal status in female rhesus macaques. J Clin Endocrinol Metab 1999; 84: 4144–4148. [DOI] [PubMed] [Google Scholar]
- Roof KA, Hopkins WD, Izard MK, Hook M, Schapiro SJ.Maternal age, parity, and reproductive outcome in captive chimpanzees (Pan trogodytes). Am J Primatol 2005; 67: 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE, Sapolsky R.The evolution of Alzheimer disease, the reproductive schedule, and apoE isoforms. Neurobiol Aging 1999; 20: 407–428. [DOI] [PubMed] [Google Scholar]
- Flint M.Does the chimpanzee have a menopause? Am J Phys Anthropol 1976; 44: 178–179. [Google Scholar]
- Wood JW. Dynamics of Human Reproduction: Biology, Biometry, Demography. New York:: Aldine de Gruyter;; 1994. [Google Scholar]
- Holman DJ, Wood JW.Pregnancy loss and fecundity in women. Ellison PT.Reproduction, Ecology, and Human Evolution New York:Aldine de Gruyter;2001: 15–38. [Google Scholar]
- Collins DC, Graham CE, Preedy JR.Indentification and measurement of urinary estrone, estradiol-17-beta, estriol, pregnanediol and androsterone during the menstrual cycle of the orangutan. Endocrinology 1975; 96: 93–101. [DOI] [PubMed] [Google Scholar]
- Nadler RD.Reproductive physiology and behavior of gorillas. J Reprod Fertil 1980; 28: 79–89. [PubMed] [Google Scholar]
- Dahl KD, Czekala NM, Lim P, Hsueh AJ.Monitoring the menstrual cycle of humans and lowland gorillas based on urinary profiles of bioactive follicle-stimulating hormone and steroid metabolites. J Clin Endocrinol Metab 1987; 64: 486–493. [DOI] [PubMed] [Google Scholar]
- Hendrickx AG.The menstrual cycle of the baboon as determined by the vaginal smear, vaginal biopsy, and perineal swelling. Vagtborg H.The Baboon in Biomedical Research. Proceedings of the 2nd International Symposium on the Baboon and Its Use as an Experimental Animal, vol. 11 Austin, TX:University of Texas Press;1967: 437–459. [Google Scholar]
- Stevens VC.Some reproductive studies in the baboon. Hum Reprod Update 1997; 3: 533–540. [DOI] [PubMed] [Google Scholar]
- Albrecht ED, Townsley JD.Serum estradiol in mid and late gestation and estradiol/progesterone ratio in baboons near parturition. Biol Reprod 1978; 18: 247–250. [DOI] [PubMed] [Google Scholar]
- Albrecht ED.A role for estrogen in progesterone production during baboon pregnancy. Am J Obstet Gynecol 1980; 136: 569–574. [DOI] [PubMed] [Google Scholar]
- Albrecht ED, Pepe GJ.Placental steroid hormone biosynthesis in primate pregnancy. Endocr Rev 1990; 11: 124–150. [DOI] [PubMed] [Google Scholar]
- Lapin BA, Krilova RI, Cherkovich GM, Asanov NS.Observations from Sukhumi. Bowden DB.Aging in Nonhuman Primates New York:Van Nostrand Reinhold;1979: 14–37. [Google Scholar]
- Packer C, Tatar M, Collins A.Reproductive cessation in female mammals. Nature 1998; 392: 807–811. [DOI] [PubMed] [Google Scholar]
- Hartman CG.Studies in the reproduction of the monkey Macacus (Pithecus) rhesus with special references to menstruation and pregnancy. Contrib Embryol 1932; 134: 3–132. [Google Scholar]
- Van Wagenen G.Optimal mating time for pregnancy in the monkey. Endocrinology 1945; 34: 307–311. [DOI] [PubMed] [Google Scholar]
- Keverne EB, Richael RP.Annual changes in menstruation of rhesus monkeys. J Endocrinol 1970; 48: 669–670. [DOI] [PubMed] [Google Scholar]
- Riesen JW, Meyer RK, Wolf RC.The effect of season on the occurrence of ovulation in the rhesus monkey. Biol Reprod 1970; 5: 111–114. [DOI] [PubMed] [Google Scholar]
- Daily RA, Neill JD.Season variation in reproductive hormones of rhesus monkeys: anovulatory and short luteal phase menstrual cycles. Biol Reprod 1981; 25: 560–567. [DOI] [PubMed] [Google Scholar]
- Hutz RJ, Dierschke DJ, Wolf RC.Seasonal effects on ovarian folliculogenesis in rhesus monkeys. Biol Reprod 1985; 33: 653–659. [DOI] [PubMed] [Google Scholar]
- Lindburg DG.The rhesus monkey in north India: an ecological and behavioral study. Rosenblum LA.Primate Behavior New York:Academic Press;1971: 1–106. [Google Scholar]
- Vandenbergh JG.Environmental influences on breeding in rhesus monkeys. Symposium of the IVth International Congress of Primatology: Primate Reproductive Behavior Basel, Switzerland;1973: 1–19. [Google Scholar]
- Neville MK.Ecology and activity of Himalayan foothill rhesus monkeys (Macaca mulatta). Ecology 1978; 49: 110–123. [Google Scholar]
- Gordon TP.Reproductive behavior in the rhesus monkey: social and endocrine variables. Am Zool 1981; 21: 185–195. [Google Scholar]
- Walker ML, Wilson ME, Gordon TP.Endocrine control of the seasonal occurrence of ovulation in rhesus monkeys housed outdoors. Endocrinology 1984; 114: 1074–1081. [DOI] [PubMed] [Google Scholar]
- Finkelstein JW, Roffwarg HP, Boyar RM, Kream J, Hellman I.Age-related change in the twenty-four-hour spontaneous secretion of growth hormone. J Clin Endocrinol Metab 1972; 35: 665–670. [DOI] [PubMed] [Google Scholar]
- Corpas E, Harman SM, Pineyro MA, Roberson R, Blackman NR.Growth hormone (GH)-releasing hormone-(1–29) twice daily reverses the decreased GH and insulin-like growth factor-I levels in old men. J Clin Endocrinol Metab 1992; 75: 530–535. [DOI] [PubMed] [Google Scholar]
- Matt DW, Kauma SW, Pincus SM, Veldhuis JD, Evans WS.Characteristics of luteinizing hormone secretion in younger versus older premenopausal women. Am J Obstet Gynecol 1998; 178: 504–510. [DOI] [PubMed] [Google Scholar]
- Gill S, Sharpless JL, Rado K, Hall JE.Evidence that GnRH decreases with gonadal steroid feedback but increases with age in postmenopausal women. J Clin Endocrinol Metab 2002; 87: 2290–2296. [DOI] [PubMed] [Google Scholar]
- Janny L, Menezo YJ.Maternal age effect on early human embryonic development and blastocyst formation. Mol Reprod Dev 1996; 45: 31–37. [DOI] [PubMed] [Google Scholar]
- Richardson SJ, Senikas V, Nelson JF.Follicular depletion during the menopausal transition: evidence for accelerated loss and ultimate exhaustion. J Clin Endocrinol Metab 1987; 65: 1231–1237. [DOI] [PubMed] [Google Scholar]
- Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF.Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod 1992; 7: 1342–1346. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Mori A, Kawai M.Characteristic features of the reproduction of Koshima monkeys. Primates 1992; 33: 1–32. [Google Scholar]
- Koyama N, Takahata Y, Huffman MA, Norikoshi K, Suzuki H.Reproductive parameters of female Japanese macaques: thirty years data from Arashiyama troops, Japan. Primates 1992; 33: 33–47. [Google Scholar]
- Itogawa N, Tanaka T, Ukai N, Fujii H, Kurokawa T, Ando A, Watanabe Y, Imakawa S.Demography and reproductive parameters of a free-ranging group of Japanese macaques (Macaca fuscata) at Katsuyama. Primates 1992; 33: 49–68. [Google Scholar]
- von Saal FS, Finch CE.Reproductive senescence: phenomena and mechanisms in mammals and selected vertebrates. Knobil E, Neill J.The Physiology of Reproduction New York:Raven Press;1988: 2351–2413. [Google Scholar]
- Odell WD, Swerdloff RS.Progesterone-induced luteinizing and follicle-stimulating hormone surge in post-menopausal women: a simulated ovulatory peak. Proc Natl Acad Sci U S A 1968; 61: 529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Noordwijk MA, van Schaik CP.Competition among adult female long-tailed macaques. Anim Behav 1987; 35: 577–589. [Google Scholar]
- Jensen GD, Blanton FL, Gribbles DH.Older monkeys' (Macaca radiata) response to new group formation: behavior, reproduction and mortality. Exp Gerontol 1980; 15: 399–406. [DOI] [PubMed] [Google Scholar]
- Dittus WPJ.Population dynamics of the toque monkey, Macaca sinica. Tuttle RH.Sociobiology and Psychology of Primates The Hague, Switzerland:Mouton;1975: 125–152. [Google Scholar]
- Sherman PW.The evolution of menopause. Nature 1998; 392: 759–761. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB.Evolution of aging. Mech Ageing Dev 2002; 123: 737–745. [DOI] [PubMed] [Google Scholar]