Abstract
Complicated grief (CG) occurs when an individual experiences prolonged, unabated grief. The neural mechanisms distinguishing CG from noncomplicated grief (NCG) are unclear, but hypothesized mechanisms include both pain-related activity (related to the social pain of loss) and reward-related activity (related to attachment behavior). Bereaved women (11 CG, 12 NCG) participated in an event-related functional magnetic resonance imaging scan, during grief elicitation with idiographic stimuli. Analyses revealed that whereas both CG and NCG participants showed pain-related neural activity in response to reminders of the deceased, only those with CG showed reward-related activity in the nucleus accumbens (NA). This NA cluster was positively correlated with self-reported yearning, but not with time since death, participant age, or positive/negative affect. This study supports the hypothesis that attachment activates reward pathways. For those with CG, reminders of the deceased still activate neural reward activity, which may interfere with adapting to the loss in the present.
There is no pain so great as the memory of joy in present grief.
~ Aeschylus, founder of Greek tragedy
Introduction
Grief is one of life’s most painful experiences. When suffering the loss of a loved one, one can feel as if the attendant sadness and longing will last indefinitely. Although successful adaptation to the loss is the most frequent response (Bonnano et al., 2002), grief does not abate in a substantial minority; rather, it develops into Complicated Grief (CG) (Ott, Lueger, Kelber, & Prigerson, 2007). CG, previously known as chronic, pathological or traumatic grief, includes debilitating recurrent pangs of painful emotions, with intense yearning, longing and searching for the deceased, and preoccupation with thoughts of the loved one. This syndrome has now been defined by an empirically-derived set of criteria (Boelen & van den Bout, 2005) and is being considered for inclusion in the DSM-V.
The neurocognitive mechanisms involved in CG are currently unknown. Some have hypothesized that attachment may activate reward pathways and that this neural response may have addiction-like properties (Insel, 2003; Panksepp, Knutson, & Burgdorf, 2002). We hypothesize that a major neurocognitive difference between CG and noncomplicated grief (NCG) is that reminders of the deceased may still activate neural rewards for those with CG. This reward activation may interfere with adapting to the loss in the present. Supporting this idea are subjective reports from CG patients indicating pleasurable reveries about the lost love (during which the reality of the loss is ignored) in addition to painful yearning (Shear, Frank, Houck, & Reynolds, 2005). These self-report findings suggest the involvement of both reward and pain networks.
The nucleus accumbens (NA) is the region most commonly associated with reward (Knutson, Adams, & Fong, 2001). NA has also been shown to play a role in social attachment, such as sibling and maternal affiliation, via neurotransmitters and peptides (e.g., dopamine and oxytocin) (Young, Lim, Gingrich, & Insel, 2001). The pain network, by contrast, including the dorsal anterior cingulate cortex (dACC), insula, and periaqueductal gray (PAG) has been previously implicated in both physical (Rainville, 2002) and social pain (Eisenberger, Lieberman, & Williams, 2003; Rainville, 2002). The present analysis specifically investigates whether the CG group had greater activity occurred in the brain’s reward or pain networks than the NCG group.
Methods and Materials
Participants
Women (11 CG, 12 NCG) who had experienced the death of a mother or sister to breast cancer in the past 5 years were recruited to participate in an event-related fMRI study. Participants were recruited from a clinic for women at familial risk of breast cancer and from the community. Sixty-four persons were screened by telephone, and 35 had an initial interview. Participants were excluded for current Axis I disorder (including major depression), as evidenced by a structured clinical interview (Spitzer, Williams, Gibbons, & First, 1994). CG was diagnosed in a structured clinical interview (Prigerson & Jacobs, 2001) using empirically-derived criteria. The mean age of participants was 43.70 (SD = 10.00), they had an average of 16.57 (SD = 2.35) years of school and 78.26% were white. The two groups did not differ on any demographic characteristics as assessed by ANOVA and chi-square analyses. The Institutional Review Board at UCLA approved the study and all participants gave written informed consent.
Procedure
Each participant provided a photograph of their deceased loved one and these photos were matched with photos of a stranger. Participants also shared an autobiographical narrative of the death event and 15 grief-related idiographic words were chosen from it. These words were matched for part of speech, number of letters and frequency of usage in the English language with 15 neutral words. These photos and words were made into 60 composites (Figure 1), each consisting of one picture (deceased or stranger) and one word (grief-related or neutral). Empirical support for the grief-eliciting task has been shown previously using both skin conductance methodology and participants’ subjective reports (Gündel, O'Connor, Littrell, Fort, & Lane, 2003). Participants viewed composites through goggles in randomized order. Each participant was given the same set of instructions: “Focus on any thoughts, feelings or memories you have to the combination of the picture and the word on the slide. Let yourself respond emotionally to each slide by being aware of your feelings without trying to alter them”.
Figure 1.
Example of picture/word composites presented to subject.
fMRI Methodology
Data were acquired on a Siemens Allegra 3T scanner. For each participant, a high-resolution structural T2-weighted echo-planar image (spin-echo; TR = 5000 ms; TE=33 ms; matrix size 128 × 128; 36 axial slices; FOV = 20cm; 3-mm thick, skip 1-mm) was acquired coplanar with the functional scan. A functional scan, lasting 5 minutes and 30 seconds, was acquired (echo planar T2*-weighted gradient-echo, TR = 2500 ms, TE = 25 ms, flip angle = 90, matrix size 64 × 64, 36 axial slices, FOV = 20cm; 3-mm thick, skip 1-mm).
The imaging data were analyzed using statistical parametric mapping (SPM’99; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). Images were realigned, normalized, and smoothed with an 8mm Gaussian kernel, full width at half maximum. Analysis used the general linear model in an event-related analysis. Effects at each voxel were estimated using linear contrasts to compare regionally specific effects. The contrasts for the individual subjects were aggregated for single-group analysis and for between-group analysis according to the random effect model in SPM. All group comparison analyses (e.g., CG > NCG) were thresholded using an uncorrected p-value of .005 combined with a cluster size threshold of 10 voxels. Where indicated, parameter estimates were extracted from group comparisons and reported for each group separately. All coordinates are reported in MNI format.
Results
Reward Activation
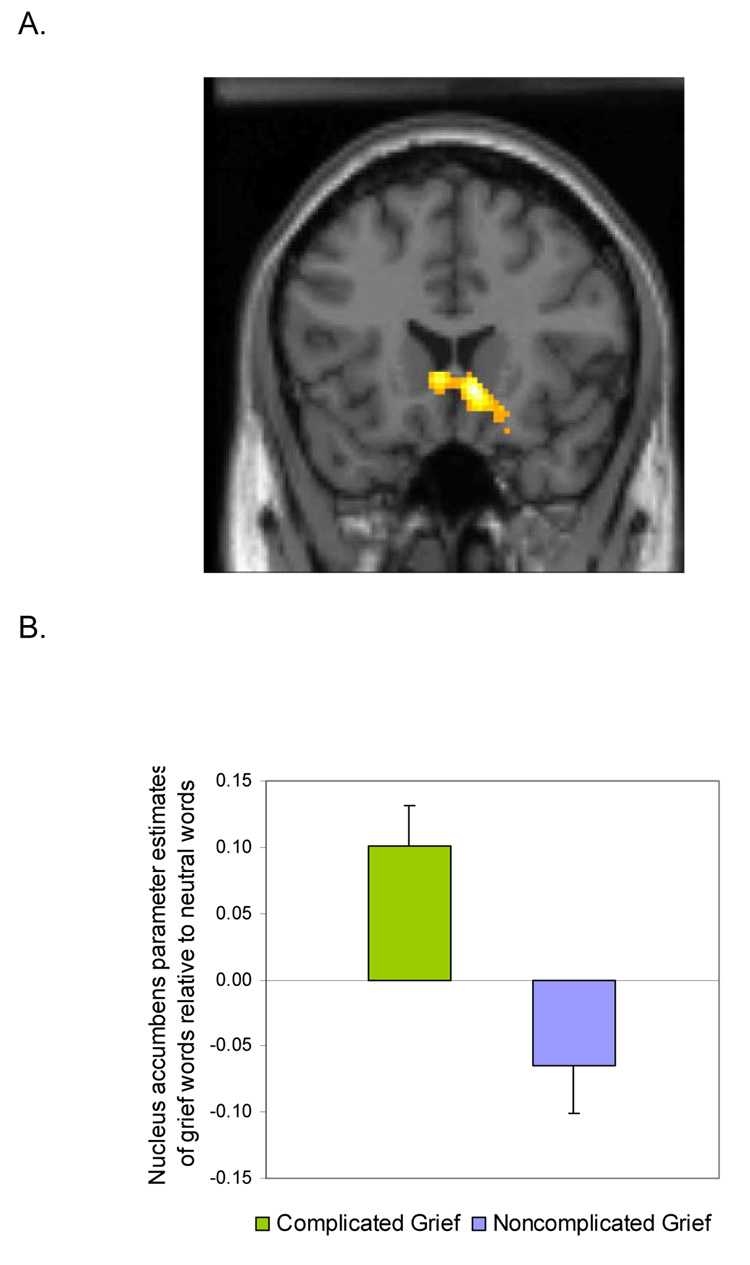
Greater reward-related activity occurred in the CG relative to the NCG individuals. Notably, the NA (x=10, y=20, z=−6; Figure 2A) was the only region of the brain that was more active in response to grief-related words than neutral words among those with CG compared to NCG (t=3.51, p<0.001, 15 voxels). Parameter estimates were extracted from this region and reported separately for each group as shown in Figure 2B. CG individuals produced increased NA activity in response to grief words (t=3.36, p<.01), whereas the NCG individuals did not (t=−1.82, p<.10). A comparison of the deceased and stranger photos did not produce NA activity. This may have been due to habituation effects as 15 different grief words were used, but only a single deceased photograph was shown repeatedly. To examine this possibility, we compared deceased and stranger photos from the first third of the trials only in order to isolate pre-habituation activity. Here, as in the grief words comparison, there was greater activation in the NA (x=−14, y=4, z=−16) for the CG group than the NCG group (t=3.68, p<.001, 18 voxels).
Figure 2.
A) Nucleus accumbens activity (10, 20, −6) in response to grief-related vs. neutral words that was significantly greater in the Complicated Grief group compared to the Noncomplicated Grief group (pictured at p < .05). B) Bar graph showing nucleus accumbens activity (10, 20, −6) in response to grief-related vs. neutral words for those with Complicated and Noncomplicated Grief.
Yearning
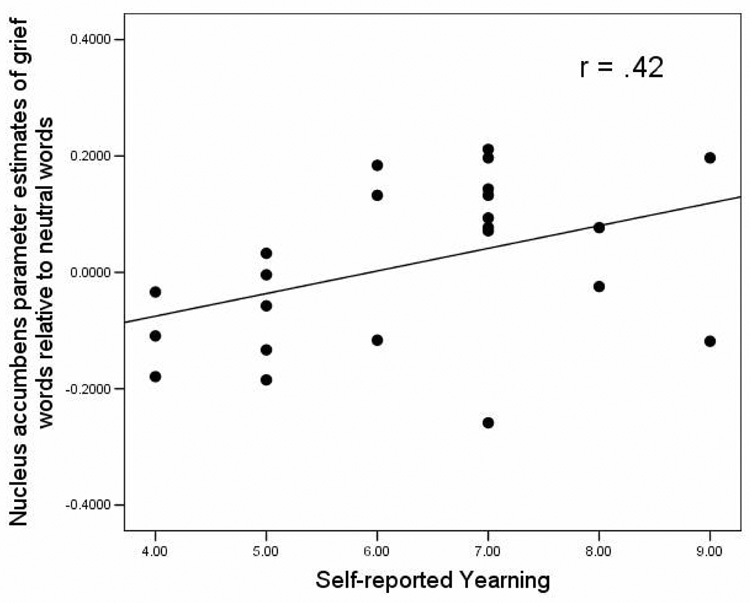
To clarify the functional role of NA activity in grief, correlational analyses were performed with parameter estimates of activity from the observed cluster (10, 20, −6). Greater NA activation in the grief words comparison correlated significantly with more self-reported yearning for the deceased (r=.42, p<.05) (Figure 3). No correlation was observed between NA activation and length of time since the death, participant age, or general positive or negative affect. Similarly, the NA activation observed during the early trials of the deceased photos comparison was also associated with yearning (r=.36, p<.05), and was not correlated with length of time since the death, participant age, or general positive or negative affect.
Figure 3.
Positive correlation between self-reported yearning and BOLD activity in the NA (10, 20, −6; N=23) for each subject.
Pain Network Activation
To examine pain-related neural activity, we examined: a) whether each group showed neural activity in three regions previously linked to pain processes (anterior cingulate cortex (ACC), insula, periaqueductal gray (PAG) (Peyron, Laurent & Garcia- Larrea, 2000); and b) whether there were significant differences between the groups in pain-related neural activity in those regions (p < .005, 10 voxels). If one group showed significant activation in a region (p< .005, 10 voxels), we reported the activation from that same coordinate in the other group (Table 1). Each group showed significant activity in pain-related regions (dACC, insula, PAG) in the comparisons of the deceased vs. stranger pictures and grief vs. neutral words (Table 1), as has been previously shown in NCG (Gündel et al., 2003). The only activity in pain-related regions that differed between groups was in the left insula (x=−34, y=14, z=18), which was more active in the NCG group during the viewing of grief-related vs. neutral words (t=4.20, p<0.001, 87 voxels).
Table 1.
Effects in three regions of the pain network for both CG (N=11) and NCG (N=12) in response to pictures of the deceased and grief-related words. Significant activations in one group (p < .005, 10 voxels) were examined and reported for the other group. T and p values are listed for each group separately. Cluster sizes were determined at the threshold of .005, 10 voxels (if the significance did not reach this threshold, cluster sizes were not comparable and therefore not listed). dACC = dorsal anterior cingulate cortex, PAG = periaqueductal gray
| Region | Group | MNI Coordinates (x,y,z) | T | p-value (voxels) | ||
|---|---|---|---|---|---|---|
| Grief-related > Neutral (words): | ||||||
| ACC | ||||||
| NCG | −2 | 34 | 8 | 5.12 | 0.001(140) | |
| CG | −2 | 34 | 8 | 2.74 | 0.02 | |
| Insula | ||||||
| NCG | −36 | 18 | 18 | 4.73 | 0.001(44) | |
| CG | ----- | ----- | ----- | |||
| PAG | ||||||
| NCG | ----- | ----- | ----- | |||
| CG | ----- | ----- | ----- | |||
| Deceased > Stranger (pictures): | ||||||
| dACC | ||||||
| NCG | −2 | 24 | 24 | 4.17 | 0.001(309) | |
| CG | −2 | 24 | 24 | 1.87 | 0.05 | |
| Insula | ||||||
| NCG | −40 | 8 | −2 | 4.76 | 0.001(235) | |
| 48 | 10 | 0 | 4.47 | 0.001(277) | ||
| CG | −40 | 8 | −2 | 1.70 | 0.02 | |
| 48 | 10 | 0 | 2.31 | 0.03 | ||
| PAG | ||||||
| NCG | −10 | −18 | −16 | 9.74 | 0.0001(55) | |
| CG | −10 | −18 | −16 | 2.37 | 0.02 | |
Discussion
Two models of grief have been hypothesized: a detachment model and a reunion model (Bowlby, 1980). In the detachment model, the grief emotion is believed to play a role in the acceptance of the reality of the death and therefore assist in recovery from the loss. In the reunion model, the grief is a form of protest against the separation from the deceased, and serves to promote reunion with the lost person, not detachment. After the death, cues of the deceased (such as memories, photos, etc.) persist, triggering yearning and grief. Coping with these salient cues is a major task in adjusting to the death (Freed & Mann, 2007). Freed and Mann hypothesize that if the detachment model is correct, the pangs of grief would occur with reduced NA activity over time, as the salience of the cues decreases and acceptance of the reality leads to detachment. If the reunion model is correct, that the pangs of grief would continue to occur with NA activity, with reward activity in response to the cues motivating reunion with the deceased. This study demonstrates that each of these models may be accurate for distinct subgroups of bereaved individuals: relative to non-grief eliciting stimuli, those with CG produced significant NA activations, whereas those with NCG produced NA reductions during grief elicitation.
Findings suggest that both CG and NCG groups may have felt pain upon presentation of grief-related stimuli, but only those with CG also activated an area important for reward processing when viewing cues of the deceased. The association between NA activity and yearning, but not time since death, participant age, or positive/negative affect provides convergent and discriminative validity. The addiction-relevant aspect of this neural response (Knutson et al., 2001) may help to explain why it is hard to resist engaging in pleasurable reveries about the deceased even though engaging in these reveries may prevent those with CG from adjusting the to the realities of the present. Many who suffer from addiction-like disorders experience them as afflictions; similarly we are not suggesting that reveries about the deceased are emotionally satisfying, but rather may serve as craving responses that may make adapting to the reality of the loss more difficult.
Prior work has demonstrated a relationship between autonomic arousal and posterior cingulate (PCC) activity during grief-elicitation (O’Connor et al., 2007). O’Connor and colleagues hypothesized a role for PCC in those with CG, given that those with CG may have greater arousal. However, there were no significant differences in PCC activity between the NCG and CG groups in the present study. Future work is needed to more carefully explore whether the PCC plays a role in CG in a larger sample. In addition, connectivity analyses in the prior study showed that PCC activation was associated with subgenual ACC activation. Similarly, future studies should investigate connectivity between NA and prefrontal or limbic regions important in emotion regulation.
Understanding the reward processes activated in those with CG could substantially change treatment of this disorder. Therapies such as behavioral interventions that target reward processes may confer benefit and preferentially aid in adapting to the loss (Shear et al., 2005). Likewise, dopaminergic interventions that alter reward sensitivity could theoretically be more effective in treating CG than serotonergic interventions, which have failed to alter grief intensity (Zygmont et al., 1998). Addressing the continued craving of past relationships may assist those with CG in adapting to the loss.
ACKNOWLEDGMENTS
We would like to thank the UCLA Brain Mapping Center for their assistance. This research was supported by funds from the California Breast Cancer Research Program of the University of California, Grant Number 10IB-0048. This work was also supported in part by grant T32-MH19925, the Cousins Center for Psychoneuroimmunology and the Friends of the Semel Institute for Neuroscience and Human Behavior.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Boelen PA, van den Bout J. Complicated Grief, Depression, and Anxiet as Distinct Postloss Syndromes: A Confirmatory Factor Analysis Study. American Journal of Psychiatry. 2005;162(11):2175–2177. doi: 10.1176/appi.ajp.162.11.2175. [DOI] [PubMed] [Google Scholar]
- Bonnano GA, Wortman CB, Lehman DR, Tweed RG, Haring M, Sonnega J, et al. Resilience to loss and chronic grief: A prospective study from preloss to 18-months postloss. Journal of Personality & Social Psychology. 2002;83(5):1150–1164. doi: 10.1037//0022-3514.83.5.1150. [DOI] [PubMed] [Google Scholar]
- Bowlby J. Attachment and loss: Vol 3. Loss, sadness and depression. New York: Basic Books; 1980. [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302(5643):290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Gündel H, O'Connor M-F, Littrell L, Fort C, Lane RD. Functional neuroanatomy of grief: An fMRI study. American Journal of Psychiatry. 2003;160(11):1946–1953. doi: 10.1176/appi.ajp.160.11.1946. [DOI] [PubMed] [Google Scholar]
- Insel TR. Is social attachment an addictive disorder? Physiology & Behavior. 2003;79(3):351. doi: 10.1016/s0031-9384(03)00148-3. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams C, Fong GH, D Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience. 2001 doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor M-F, Gündel H, McRae K, Lane RD. Functional neuroanatomical correlates of emotional arousal during grief. Neuropsychopharmacology. 2007;32:2184–2189. doi: 10.1038/sj.npp.1301342. [DOI] [PubMed] [Google Scholar]
- Ott CH, Lueger RJ, Kelber ST, Prigerson HG. Spousal bereavement in older adults: Common, resilient, and chronic grief with defining characteristics. Journal of Nervous and Mental Disorders. 2007;195(4):332–341. doi: 10.1097/01.nmd.0000243890.93992.1e. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Knutson B, Burgdorf J. The role of brain emotional systems in addictions: a neuro-evolutionary perspective and new "self-report" animal model. Addiction. 2002;97(4):459–469. doi: 10.1046/j.1360-0443.2002.00025.x. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis. Clinical Neurophysiology. 2000;30(5):263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Prigerson HG, Jacobs SC. Traumatic grief as a distinct disorder: A rationale, consensus criteria, and preliminary empirical test. In: Stroebe MS, Hansson RO, Stroebe W, Schut H, editors. Handbook of Bereavement Research: Consequences, Coping and Care. Washington, D.C.: American Psychological Association; 2001. pp. 613–645. [Google Scholar]
- Rainville P. Brain mechanisms of pain affect and pain modulation. Current Opinion in Neurobiology. 2002;12(2):195. doi: 10.1016/s0959-4388(02)00313-6. [DOI] [PubMed] [Google Scholar]
- Shear K, Frank E, Houck PR, Reynolds CF., III Treatment of Complicated Grief: A Randomized Controlled Trial. Journal of the American Medical Association. 2005;293(21):2601–2608. doi: 10.1001/jama.293.21.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbons M, First MD. Structured Clinical Interview of the DSM-IV. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- Young L, Lim M, Gingrich B, Insel T. Cellular mechanisms of social attachment. Hormones and Behavior. 2001 doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- Zygmont M, Prigerson H, Houck P, Miller M, Shear M, Jacobs S, et al. A post hoc comparison of paroxetine and nortriptyline for symptoms of traumatic grief. Journal of Clinical Psychiatry. 1998;59(5):241–245. doi: 10.4088/jcp.v59n0507. [DOI] [PubMed] [Google Scholar]