Abstract
Lipid A of Rhizobium leguminosarum, a nitrogen-fixing plant endosymbiont, displays several significant structural differences when compared with Escherichia coli. An especially striking feature of R. leguminosarum lipid A is that it lacks both the 1- and 4′-phosphate groups. Distinct lipid A phosphatases that attack either the 1 or the 4′ positions have previously been identified in extracts of R. leguminosarum and Rhizobium etli but not Sinorhizobium meliloti or E. coli. Here we describe the identification of a hybrid cosmid (pMJK-1) containing a 25-kb R. leguminosarum 3841 DNA insert that directs the overexpression of the lipid A 1-phosphatase. Transfer of pMJK-1 into S. meliloti 1021 results in heterologous expression of 1-phosphatase activity, which is normally absent in extracts of strain 1021, and confers resistance to polymyxin. Sequencing of a 7-kb DNA fragment derived from the insert of pMJK-1 revealed the presence of a lipid phosphatase ortholog (designated LpxE). Expression of lpxE in E. coli behind the T7lac promoter results in the appearance of robust 1-phosphatase activity, which is normally absent in E. coli membranes. Matrix-assisted laser-desorption/time of flight and radiochemical analysis of the product generated in vitro from the model substrate lipid IVA confirms the selective removal of the 1-phosphate group. These findings show that lpxE is the structural gene for the 1-phosphatase. The availability of lpxE may facilitate the re-engineering of lipid A structures in diverse Gram-negative bacteria and allow assessment of the role of the 1-phosphatase in R. leguminosarum symbiosis with plants. Possible orthologs of LpxE are present in some intracellular human pathogens, including Francisella tularensis, Brucella melitensis, and Legionella pneumophila.
Lipopolysaccharide (LPS)1 is a macromolecular glycolipid found in the outer membranes of Gram-negative bacteria (1–4). The structure of LPS consists of three domains: the lipid A moiety that serves as the hydrophobic anchor, a nonrepeating core oligosaccharide, and a highly immunogenic, distal O-antigen polysaccharide (1–4). LPS acts as an efficient barrier to antibiotics (5, 6) and helps bacterial cells resist complement-mediated lysis (7). Lipid A (endotoxin) is essential for viability in almost all Gram-negative bacteria (3, 8, 9), and it is the active component of LPS that is responsible for some of the effects associated with severe Gram-negative infections and septic shock (4, 10–12).
The structure of lipid A in common Gram-negative animal pathogens, such as Escherichia coli, Salmonella typhimurium, or Pseudomonas aeruginosa, can vary slightly, but most of its distinguishing structural features are conserved (1, 3, 13). In contrast, the lipid A from the nitrogen-fixing Gram-negative endosymbionts, Rhizobium leguminosarum and Rhizobium etli CE3, is strikingly different (14–17). R. leguminosarum and R. etli lipid A species lack the usual 1- and 4′-phosphate groups (14–17), and a galacturonic acid residue is present in place of the 4′-phosphate moiety (14–17). The proximal glucosamine 1-phosphate unit may be replaced with 2-aminogluconate (14–17). R. leguminosarum lipid A also lacks the secondary laurate and myristate residues present in E. coli lipid A (18–21) but instead is acylated with a secondary 27-hydroxyoctacosanoate chain (16, 17, 22) (see Fig. 1).
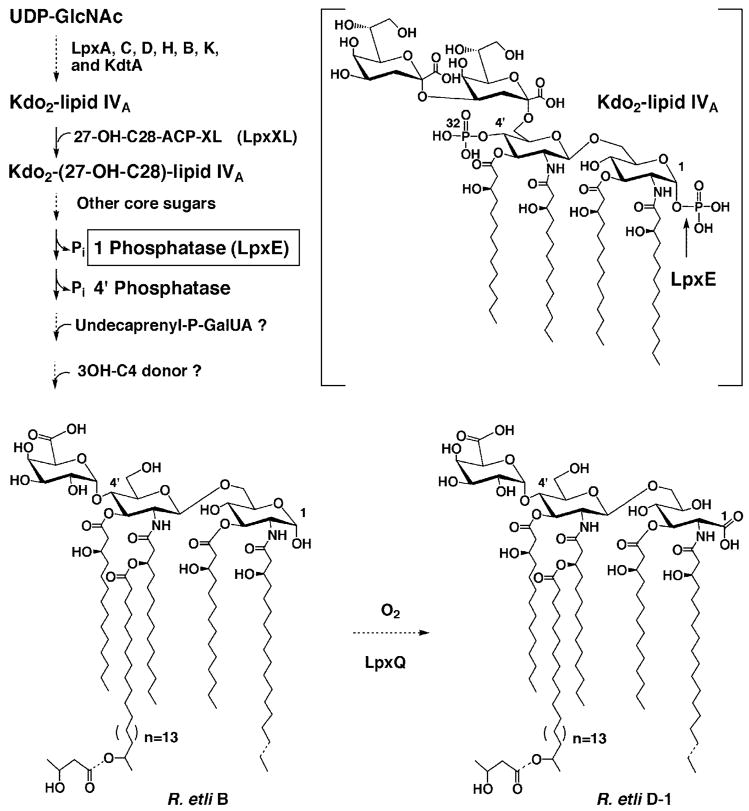
Fig. 1. Structure and biosynthesis of lipid A molecules in R. etli CE3.
The major lipid A species B and D-1 (16, 17, 114) contain no phosphate moieties in R. etli or R. leguminosarum. However, the seven enzymes that make Kdo2-lipid IVA in E. coli are also found in extracts of R. leguminosarum and R. etli (37). With the possible exception of lpxH (3, 115), the genes encoding these enzymes are present in single copy. Although very active with the model substrate Kdo2-[4′-32P]lipid IVA (structure in brackets), the LpxE phosphatase (cleavage site indicated by arrow) does not require the Kdo moiety for activity. However, LpxE normally functions after the secondary acyl chain and additional core sugars have been added (39) and is predicted to have a periplasmic active site. Most of the other enzymes needed to generate component B have previously been identified in R. leguminosarum and R. etli (22,37–39,43) with the exception of those that incorporate the 4′-galacturonic acid and β-hydroxybutyrate residues. Following the formation of component B and transport to the outer membrane, the LpxQ oxidase (43, 44) converts the proximal glucosamine residue to the 2-aminogluconate unit in an oxygen dependent manner to generate D-1. Both B and D-1 may also be 3-O-deacylated to form the minor components C and E (not shown) respectively.
Recent pharmacological studies have shown that both phosphate groups and the correct number of fatty acyl chains are crucial for the cytokine-inducing activities of lipid A molecules in animal systems, reflecting the selectivity of the TLRs of the host (10, 23–27). These signaling proteins recognize conserved surface components present in pathogens, like the lipid A moiety of LPS, which specifically activates TLR-4 (12, 28–30). Engagement of the TLR receptors directly stimulates the mammalian innate immune system (12, 30–32). The TLR proteins are characterized by the presence of leucine-rich repeats in their extracellular domains (12, 30–32), which may be responsible for ligand binding in conjunction with other accessory proteins (33).
In the past year, a plant receptor-like kinase has been identified that is distantly related to the TLR family (34, 35) and is required for symbiotic nodule development in legumes. Considering the functions of the TLR receptors in animals (12, 30–32), a carbohydrate-based mechanism for recognizing microbes in plants, inducing either a symbiotic or a pathogenic response, might be the underlying basis for nodule accommodation and development (36). Given that R. leguminosarum lipid A (16, 17) lacks all of the structural features thought to be necessary for the stimulation of the innate immune system in animals (2, 10), it is conceivable that the unique structural features of R. leguminosarum lipid A might somehow play a role during symbiotic nodule formation.
In spite of the structural diversity of their lipid A molecules, both E. coli and R. leguminosarum use the same seven enzymes to generate the conserved, phosphate containing precursor, Kdo2-lipid IVA (37). Several additional enzymes exist in R. leguminosarum that catalyze the further conversion of Kdo2-lipid IVA to R. leguminosarum lipid A. We have previously identified a 4′-phosphatase (38), a 1-phosphatase (39), a long chain acyltransferase (22, 40), a mannosyl transferase (39, 41, 42), a galactosyl transferase (39, 41, 42), and an atypical Kdo transferase (42) that are involved in the distinct metabolism of Kdo2-lipid IVA in extracts of R. leguminosarum but not in E. coli. Recently, we have also discovered a novel oxidase that acts on 1-dephosphorylated lipid A species to generate the unique 2-aminogluconate moiety found in the proximal unit of R. leguminosarum lipid A (43, 44) (see Fig. 1).
We now report the expression cloning of the R. leguminosarum gene that encodes the lipid A 1-phosphatase (see Fig. 1). A hybrid cosmid capable of directing the overexpression of 1-phosphatase activity was identified by assaying cell lysates of individual clones of a R. leguminosarum 3841 genomic DNA library (45) harbored in R. etli CE3 (46). The 1-phosphatase, which can be detected with either E. coli Kdo2-[4′-32P]lipid IVA or its precursor [4′-32P]lipid IVA as substrates (see Fig. 1) (39), is associated with the inner membrane. Sequencing of a 7-kb DNA insert derived from the hybrid cosmid permitted the identification of the structural gene (designated lpxE from the German eins) encoding the 1-phosphatase (see Fig. 1). The R. leguminosarum lpxE gene, which encodes a protein of 244 amino acid residues, can be overexpressed in E. coli behind the T7lac promoter, and the enzyme is catalytically active. The expression of lpxE in Sinorhizobium meliloti confers resistance to polymyxin. The availability of the lpxE gene should facilitate the re-engineering of lipid A structures in Gram-negative bacteria and enhance our understanding of the biological functions of 1-dephosphorylated lipid A species.
EXPERIMENTAL PROCEDURES
Chemicals and Materials
[γ-32P]ATP was obtained from Perkin-Elmer Life Sciences. Silica gel 60 thin layer plates (0.25 mm) were purchased from EM Separation Technology (Merck). Triton X-100 and bicinchoninic acid were from Pierce. Yeast extract and tryptone were purchased from Difco. All other chemicals were reagent grade and were obtained from either Sigma or Mallinckrodt. All of the restriction enzymes were from Invitrogen or New England Biolabs. PCR reagents were purchased from Stratagene. Shrimp alkaline phosphatase was purchased from U.S. Biochemical Corp. Seaplaque low temperature melting agarose was purchased from FMC Bioproducts (Rockland, ME). A “Dark Reader,” utilized for DNA manipulations, was purchased from Epicenter Technologies Corporation. Custom primers and T4 DNA ligase were from Invitrogen. All other molecular biology reagents and enzymes were purchased from either Roche Applied Science or Invitrogen.
Bacterial Strains and Growth Conditions
R. etli CE3 (46, 47), R. leguminosarum 3841 (40, 48) and S. meliloti 1021 (49) were grown as described previously (37, 40), and their properties are summarized in Table I.
Table I.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or description | Source or reference |
|---|---|---|
| Strains | ||
| R. etli CE3 | CFN42 Smr | Refs. 46 and 47 |
| R. leguminosarum 3841 | Wild type strain 300 biovar viciae Smr | Refs.40 and 48 |
| S. meliloti 1021 | SU47 Smr | Sharon Long (49) |
| E. coli | ||
| HB101 | hsdS20 supE44 ara14 galK2 lacY1 proA2 rpsL20 (Smr) xyl-5 mtl-1 recA13 mcrB thi-1 leuB6 | Invitrogen |
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac[F′ proAB laclqZDM15 Tn10 (Tetr)] | Stratagene |
| MT616 | pRK2013 Cmr, Km::Tn9 containing strain for tri-parental mating | Ref. 63 |
| Novablue (DE3) | E. coli host strain used for expression | Novagen |
| Plasmids | ||
| pLAFR-1 | Broad host range P-group cloning vector, mobilizable RK2 cosmid Tetr | Ref. 60 |
| pRK404a | Shuttle vector Tetr | Ref. 65 |
| pET-28a | E. coli expression vector | Novagen |
| pMJK-1 | pLAFR-1 derivative carrying a 25-kb fragment of R. leguminosarum 3841 = genomic DNA, which includes lpxE | This work |
| pLpxE-2 | pRK404a derivative carrying a 6.9-kb EcoRI fragment, which includes lpxE from pMJK-1 | This work |
| pLpxE-3 | pRK404a derivative carrying a 4.9-kb HindIII fragment, which includes lpxE from pMJK-1 | This work |
| pLpxE-4 | pET-28a derivative harboringlpxE behind the T7lac promoter | This work |
E. coli Novablue (DE3) strains (Novagen), harboring either the empty vector control pET-28a or the hybrid plasmid pLpxE-4 (containing the lpxE gene), were grown from a single colony in 200 ml of LB broth (10 g of tryptone, 5 g of yeast extract, and 10 g of NaCl per liter) (50) supplemented with kanamycin (15 μg/ml) at 37 °C until the A600 reached ~0.6. The culture was split into two equal portions, one of which was induced with 1 mM isopropyl-1-thio-β-D-galactopyranoside. Both cultures were further incubated with shaking at 225 rpm for an additional 4 h at 25 °C.
Preparation of Cell-free Extracts and Washed Membranes
All of the enzyme preparations were carried out at 0–4 °C. Protein concentration was determined by the bicinchoninic acid method (51) using bovine serum albumin as a standard (Pierce). Cell-free extracts, cytosol, and washed membranes were prepared as described previously (40) and stored in aliquots at −80 °C.
Preparation of Radiolabeled Substrates
The [4′-32P]lipid IVA was generated from [γ-32P]ATP and the appropriate tetraacyl-disaccharide 1-phosphate acceptor, using the overexpressed 4′-kinase present in membranes of E. coli BLR(DE3)/pLysS/pJK2 (52, 53). The Kdo2-[4′-32P]lipid IVA was then prepared from the [4′-32P]lipid IVA by the action of the purified E. coli Kdo transferase (KdtA) (53, 54). The Kdo2-[4′-32 P]lipid IVA and [4′-32P]lipid IVA were purified by preparative thin layer chromatography (53–55) and were stored as an aqueous dispersion at −20 °C in 10 mM Tris-HCl, pH 7.5, and 1 mM EDTA. Prior to use, all of the lipid substrates were dispersed by sonic irradiation for 1 min in a bath sonicator.
The substrate [32P]lipid X was prepared from 32Pi-labeled cells of E. coli strain MN7 (56, 57), as previously described (58, 59). Tetraacyl-disaccharide-1-32P was prepared from [32P]lipid X and UDP-2,3-diacylglucosamine using a highly purified preparation of the E. coli LpxB disaccharide synthase (59). Nonradioactive tetraacyl-disaccharide 1-phosphate carrier was prepared in the same way (59).
Assay of the 1-Phosphatase
Standard assay conditions for the 1-phosphatase activity are as follows. The reaction mixture (10–20 μl) contained 50 mM MES, pH 6.5, 1% Triton X-100, 10 mM NaCl, 2 mM dithiothreitol, and 5 μM Kdo2-[4′-32P]lipid IVA (3000–6000 cpm/nmol). Protein concentration was adjusted as described below. Dephosphorylation reactions were performed at 30 °C for 20 min or as indicated. The reactions were terminated by spotting 4-μl samples onto silica gel 60 TLC plates and dried under a cool air stream for 30 min. The TLC plates were developed in the solvent chloroform, pyridine, 88% formic acid, water (30:70:16:10 v/v/v/v). After drying and overnight exposure of the plate to a PhosphorImager screen, product formation was detected and quantified with a Molecular Dynamics Storm PhosphorImager equipped with ImageQuant software.
Transfer of a R. leguminosarum 3841 Library into R. etli CE3
A library of R. leguminosarum 3841 genomic DNA (~20–25 kb) (45), inserted into the cosmid pLAFR-1 (60) and harbored in E. coli 803 (61), was provided by Dr. J. Downie of the John Innes Institute (Norwich, UK). Because R. leguminosarum promoters are not usually recognized by E. coli RNA polymerase, colony lysates of the E. coli host cannot be assayed directly for production of 1-phosphatase. Accordingly, the entire library was transferred by tri-parental mating into R. etli CE3 (62), as described previously (44). E. coli strain 803 harboring the cosmid library served as the cosmid donor, and the helper strain E. coli MT 616 (63) provided the transfer functions (Table I).
Screening of the R. leguminosarum 3841 Library for 1-Phosphatase Activity
For screening, the glycerol stock of the mating mixture was thawed, appropriately diluted to obtain 50–100 colonies/plate, and spread onto TY agar containing 10 mM CaCl2, nalidixic acid (20 μg/ml), streptomycin (200 μg/ml), and tetracycline (12.5 μg/ml) to ensure selection of R. etli CE3 cells harboring a hybrid cosmid. The plates were incubated for 40 h at 30 °C. Individual colonies were picked with a sterile toothpick and inoculated into separate wells of a 96-well microtiter plate containing 150 μl of TY broth supplemented with 10 mM CaCl2, nalidixic acid (20 μg/ml), streptomycin (200 μg/ml), and tetracycline (12.5 μg/ml). Each microtiter plate was incubated at 30 °C with constant shaking for 40 h. To ensure consistency, growth was monitored until the A600 was greater than 0.5. Next, 50 μl from each well was transferred to another microtiter plate, adjusted to 20% glycerol, and stored as a stock at −80 °C for later use. The remaining 100 μl of cells were harvested by centrifugation at 3660 × g for 20 min at 4 °C. The supernatant was decanted, and the cell pellets were resuspended and washed in 50 μl of 50 mM HEPES, pH 7.5. The washed cells were harvested by centrifugation at 3660 × g for 20 min at 4 °C. The pellets were lysed by incubating with lysozyme (1 mg/ml) and EDTA (2 mM) for 30 min at room temperature, followed by a single freeze-thaw cycle.
Assay of the Pooled R. leguminosarum 3841 Library Lysates for 1-Phosphatase Activity
Four μl from each well of two microtiter plates were pooled into a single new microtiter dish (8 μl/well). The pooled cell lysates were assayed for their ability to metabolize Kdo2-[4′-32P]lipid IVA as follows. A 96-well microtiter plate was prepared with each well containing 2 μl of 250 mM MES buffer, pH 6.5, 0.5% Triton X-100, 10 mM EDTA, and 1.0 μM Kdo2-[4′-32P]lipid IVA (~1000 cpm/well), to which was added 8 μl of pooled cell lysate. Each plate was then incubated at 30 °C, and the reactions were terminated at the 10- and 20-min time points by spotting 5-μl portions onto a silica gel TLC plate with a multi-channel pipette. On each TLC plate, parallel reactions without enzyme or with R. leguminosarum CE3 crude cell lysates were also spotted as controls. The spots were dried with a stream of cool air for 20 min, and the plates were developed with the solvent chloroform, pyridine, 88% formic acid, water (30:70:16:10 v/v/v/v). Each TLC plate was exposed overnight to an imaging screen, and 1-phosphatase activity was quantified using a Molecular Dynamics model Storm PhosphorIm-ager equipped with ImageQuant software. About 200 colonies were assayed per week.
General Recombinant DNA Techniques
Plasmid and cosmid DNA were isolated using the Qiagen Spin Prep kit or the BIGGER prep kit (5 Prime → 3 Prime, Inc., Boulder, CO). Restriction endonucleases, shrimp alkaline phosphatase, and T4 DNA ligase were used according to the manufacturer’s instructions. DNA fragments were isolated from agarose gels using the Qiaex II gel extraction kit. All of the DNA fragment isolations were carefully performed with minimal UV irradiation to prevent mutations. All other techniques involving manipulation of DNA and cell transformation were done as described previously (64).
Plasmids or cosmids were introduced into R. etli CE3 by tri-parental mating, as outlined above for the library screening (62). E. coli strain 803 (61) or HB101 (Invitrogen) served as the plasmid donors, and E. coli MT616 (63) provided the transfer functions.
Subcloning of the ~25-kb Insert in pMJK-1 and DNA Sequencing
To increase the efficiency of subcloning, a low temperature melting agarose was used. The cosmid pMJK-1, isolated from R. etli CE3/pMJK-1, and the shuttle vector used for subcloning (pRK404a) were both subjected to restriction enzyme digestion with either EcoRI or HindIII. After incubation at 37 °C for 2 h, the restriction digests were heated at 65 °C for 20 min. The digested shuttle vector pRK404a was further subjected to shrimp alkaline phosphatase treatment for 1 h at 37 °C, and the reaction was heat-inactivated at 65 °C for 20 min.
Next, the samples were resolved on a 0.7% Seaplaque low melting agarose gel at 20–25 volts at 4 °C overnight. The bands were visualized with a “Dark Reader,” ensuring that no UV light (260 nm) was introduced. One EcoRI (7 kb) and one HindIII (4.9 kb) fragment derived from pMJK-1 were selected for ligation into the multiple cloning cassette of pRK404a (65). The desired bands were excised from the gel using a razor blade and carefully placed into conical plastic microcentrifuge tubes.
For ligation of selected DNA fragments derived from pMJK-1 with digested pRK404a, agarose plugs were melted at 70 °C for 10 min without denaturing the DNA. Melted gel slices were then combined as indicated and held at 37 °C, which keeps the agarose from solidifying. Typically, the melted agarose preparations (containing the vector or insert) were mixed in a 3:2 ratio, and water was added to bring the volume to 9 μl. Next, 11 μl of 2× concentrated ligase buffer (consisting of 30 μl of 10× ligase buffer (500 mM Tris-HCl, pH 7.5, 100 mM MgCl2, 100 mM dithiothreitol, 10 mM ATP, and 250 μg/ml bovine serum albumin), 20 μl of 400 units/μl T4 DNA ligase, and 10 μl of H2O) was added to the 9 μl DNA mixture. The reactions were incubated at 16 °C overnight.
The agarose in the ligase reaction mixtures was melted at 70 °C for 10 min before transformation into appropriate host strains. Typically, a 5–7-μl portion of each ligation mixture was added to a 50-μl aliquot of HB101 competent cells (Invitrogen), transformed, and plated on LB agar containing tetracycline (12.5 μg/ml). Resistant colonies were screened for the desired inserts by digestion with either EcoRI or HindIII. Subclones were designated pLpxE-2 (containing the 7-kb insert) or pLpxE-3 (containing the 4.9-kb insert), respectively.
Next, pLpxE-2 and pLpxE-3 were transferred into R. etli CE3 and S. meliloti 1021 by tri-parental mating. R. etli CE3 cells harboring pLpxE-2 and pLpxE-3 were selected on TY agar plates containing 10 mM CaCl2, nalidixic acid (20μg/ml), streptomycin (200 μg/ml), and tetracycline (12.5 μg/ml), whereas S. meliloti 1021 cells harboring pLpxE-2 and pLpxE-3 were selected on TY plates containing 10 mM CaCl2, nalidixic acid (20 μg/ml), streptomycin (200 μg/ml), and tetracycline (12.5 μg/ml). The cell lysates were prepared as described above and analyzed for 1-phosphatase activity.
The DNA inserts of pLpxE-2 and pLpxE-3 were purified with the Qiagen Plasmid Maxi-prep kit. DNA sequencing of both strands was done at Duke University using the Terminator Cycle sequencing system with AmpliTaq DNA polymerase and an ABI 377 PRISM DNA sequencing instrument.
Expression of the 1-Phosphatase Gene behind the T7lac Promoter
PCR-amplified lpxE DNA was cloned into the pET-28a vector behind the T7lac promoter. The forward PCR primer (5′-ACTTAGAGCTCATGCGGGCATTTTGG-3′) was designed with a clamp region, a SacI restriction site (underlined), and a match of the coding strand starting at the translation initiation site. The reverse primer (5′-ACTACTCGAGTTATGCAGTGCGGAAC-3′) was designed with a clamp region, a XhoI restriction site (underlined), and a match to the anti-coding strand that included the stop codon. The PCR was performed using Pfu polymerase. The hybrid cosmid pMJK-1 was used as the template. Amplification was carried out in a 100-μl reaction mixture containing 100 ng of template, 20 mM Tris-HCl, pH 8.8, 10 mM KCl, 10 mM (NH4)2SO4, 0.1% Triton X-100, 0.1% bovine serum albumin, 10 μM dNTPs, and 2 units of Pfu polymerase. The reaction was subjected to a hot start (1 min at 94 °C) followed by 25 cycles of denaturation (1 min at 94 °C), annealing (1 min at 55 °C), and extension (2 min at 72 °C). After the 25th cycle, a 10-min extension time was used. The reaction product was analyzed on a 1% agarose gel. The desired band was excised and gel-purified using a Qiagen gel extraction kit. The purified PCR product was digested using SacI and XhoI and ligated into the expression vector, pET-28a, that had been similarly digested. Ligase reactions were transformed into XL1-Blue cells (Stratagene) and screened for positive inserts. The desired construct was named pLpxE-4. The lpxE insert was confirmed by DNA sequencing. The plasmid pLpxE-4 was transformed into the E. coli expression strain Novablue (DE3).
Preparation of Membrane Fractions for SDS-PAGE Analysis
In the handling of membrane fractions, it was found that incubation of the sample in SDS at temperatures above 50 °C prior to electrophoresis led to LpxE aggregation in the stacking gel, a common phenomenon with membrane proteins (66). Consequently, the samples were prepared for SDS gel electrophoresis by addition of a 4× concentrated stock solution, consisting of 200 mM Tris, pH 6.8, 50% glycerol, 12% SDS, 200 mM dithiothreitol, and 0.02% bromphenol blue (66). Incubation for 30 min at 40 °C was optimal for LpxE membrane protein solubilization, and the samples were analyzed using 12% SDS-polyacrylamide gels.
Purification of the 1-Dephosphorylated Reaction Product Generated in Vitro from Lipid IVA
Briefly, two large scale reaction mixtures (10 ml each) were prepared as follows. The reaction mixture contained 50 mM MES, pH 6.5, 1% Triton X-100, 10 mM NaCl, 2 mM dithiothreitol, and 50 μM lipid IVA. Each reaction was initiated by addition of washed membranes prepared from Novablue(DE3)/pLpxE-4 so that the final protein concentration was 1 mg/ml. The mixtures were incubated at 30 °C for 18 h. Product formation was monitored by TLC, as described above for the assays, but the 1-phosphatase reaction product was detected by charring the plates after spraying with 10% sulfuric acid in ethanol. For product isolation, the aqueous reaction mixture was converted to a single-phase Bligh/Dyer mixture (67), consisting of chloroform/methanol/water (1:2:0.8, v/v). The sample was thoroughly mixed and centrifuged at 3,000 × g for 20 min at room temperature to remove precipitated proteins. The supernatant was then converted to a two-phase Bligh/Dyer mixture, consisting of chloroform/methanol/water (2: 2:1.8 v/v/v) by the addition of appropriate amounts of chloroform and water. After mixing, the phases were separated by centrifugation. The lower phase was recovered and dried by rotary evaporation. The sample was redissolved in ~5 ml of choloroform/methanol/H2O (2:3:1 v/v/v) and was loaded onto a 0.5-ml DEAE-cellulose column (Whatman DE-52), equilibrated as the acetate form in the same solvent mixture (68, 69). The column was washed with the same solvent mixture and then eluted with 5-ml portions of chloroform/methanol/aqueous ammonium acetate (2:3:1 v/v/v), in which the ammonium acetate concentration in the aqueous component was increased stepwise from 60, 120, 240, to 480 mM. The elution of the substrate and product was monitored by spotting 5 μl of each fraction onto a silica gel TLC plate, which was developed in the chloroform, pyridine, 88% formic acid, H2O (50:50:16:10 v/v/v/v). After drying, the lipids were detected by charring as described above. Fractions containing 1-dephosphorylated lipid IVA eluted with the 60 mM ammonium acetate component, whereas the remaining substrate eluted with 240 mM. To recover the product, the fractions containing the 1-dephosphorylated lipid IVA were pooled and were converted to a two-phase Bligh-Dyer mixture. The lower phases were dried under a stream of N2 and stored at −20°C until further analysis.
MALDI/TOF Analysis of 1-Dephophorylated Lipid IVA
Spectra were acquired in the negative and positive-ion linear modes using a Kratos Analytical (Manchester, UK) MALDI/TOF mass spectrometer, as described previously (43, 44).
Assay of Polymyxin Sensitivity
The outer membrane integrity of R. etli or S. meliloti cells harboring either control or lpxE expressing plasmids was evaluated by examining their sensitivity to the cationic antibacterial peptide polymyxin B sulfate (Sigma). The assay was performed by placing 6-mm filter discs containing varying amounts of polymyxin (2, 10, or 20 μg) onto a lawn of cells that had been freshly plated onto TY agar with a saturated cotton swab from a culture at A600 of ~0.2. After 40 h of growth at 30 °C, the diameters of the zones of clearing around each disc (itself 6 mm in diameter) were measured, providing an assessment of the relative polymyxin sensitivity.
RESULTS
Screening of a R. leguminosarum Genomic DNA Library for Clones Overexpressing Lipid A Phosphatase Activity
Previous attempts to clone the R. leguminosarum 1- and/or 4′-phosphatases by assaying ~4000 clones of a R. leguminosarum 3841 cosmid library harbored in S. meliloti 1021 were unsuccessful (40). The lipid A of Sinorhizobium strains normally contain phosphate groups at the 1 and 4′ positions (70, 71),2 and it may be that the R. leguminosarum lipid A phosphatases are toxic to S. meliloti under some conditions. Consequently, an alternative expression cloning strategy described under “Experimental Procedures” was developed. The entire R. leguminosarum 3841 genomic DNA library (20–25-kb insert size) in the cosmid pLAFR-1, harbored in E. coli 803, was transferred into R. etli CE3 by tri-parental mating. Membranes of R. etli CE3 normally contain 1- and 4′-phosphatase activities, and CE3 lipid A is completely devoid of phosphate residues (14, 16, 17). Expression cloning with R. leguminosarum DNA cannot be carried out directly in E. coli, because E. coli RNA polymerase does not recognize R. leguminosarum promoters.
A clone that overexpresses the 1-phosphatase was identified by assaying pools of two extracts for their ability to shift E. coli Kdo2-[4′-32P]lipid IVA (Fig. 1) to a more rapidly migrating species (data not shown). Extract a in pool 692 was the active lysate. Of the ~1200 lysozyme/EDTA lysates screened, only one clone (R. etli CE3/pMJK-1) was identified that overexpressed the putative 1-phosphatase activity. This plasmid was used for all further studies. No clones overexpressing the 4′-phosphatase activity were identified.
Verification of 1-Phosphatase Overproduction in Membranes of R. etli CE3/pMJK-1
To confirm the initial screening results obtained with the above lysates, membranes of late log phase cultures of R. etli CE3/pLAFR-1 (the vector control) and R. etli CE3/pMJK-1 were prepared and assayed under conditions optimized for the 1-phosphatase (Fig. 2). In membranes of R. leguminosarum or R. etli CE3, the C28-dependent long chain acyltransferase (22) and the 1-phosphatase (22, 39) are the only known enzymes that can convert the substrate Kdo2-[4′-32P]lipid IVA to faster migrating compounds when analyzed by thin layer chromatography in the solvent chloroform, pyridine, 88% formic acid, water (30:70:16:10 v/v/v/v). However, the acyltransferase also requires the soluble factor C28-AcpXL (22, 40), which was not added in these experiments.
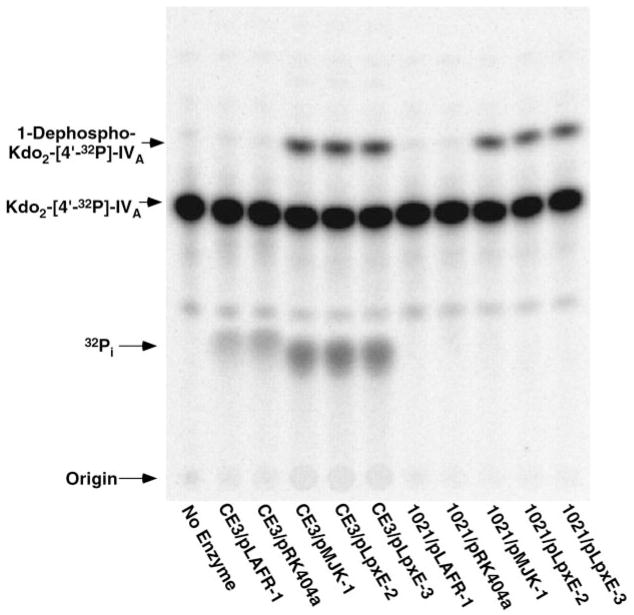
Fig. 2. pMJK-1-driven overexpression of 1-phosphatase in membranes of R. etli CE3.
The 1-phosphatase activity present in membranes of R. etli CE3/pMJK-1 was compared with that of membranes from R. etli CE3 harboring the vector pLAFR-1. The reactions (typically 20 μl) were carried out at 30 °C with 5 μM Kdo2-[4′-32P]lipid IVA as the substrate. Product formation was detected by TLC and PhosphorImager analysis.
As shown in Fig. 2, the vector pLAFR-1 has no effect on the chromosomal 1-phosphatase activity of CE3 membranes, but the hybrid cosmid pMJK-1 directs significant overexpression of the enzyme. At a CE3/pMJK-1 membrane protein concentration of 0.5 mg/ml, considerable conversion of 5 μM Kdo2-[4′-32P]lipid IVA to the putative 1-dephosphorylated Kdo2-[4′-32P]lipid IVA is observed after only 20 min (Fig. 2). Like the wild type 1-phosphatase of R. etli or R. leguminosarum (39), the overexpressed 1-phosphatase activity in CE3/pMJK-1 membranes can dephosphorylate lipid A disaccharide substrates lacking the Kdo moiety; the enzyme is completely membrane-bound and does not require cytosolic factors (data not shown). The optimal in vitro assay conditions for the activity overexpressed in R. etli CE3/pMJK-1, such as the Triton X-100 optimum of 1%, are very similar to those for the 1-phosphatase present in membranes of wild type R. leguminosarum or R. etli (39). Sucrose density gradient centrifugation revealed exclusive localization of the activity to the inner membranes (data not shown).
Similar levels of 4′-phosphatase activity are seen in membranes of R. etli CE3 cells harboring the empty vector control or the hybrid cosmid (pMJK-1) (Fig. 2), consistent with previous biochemical evidence that distinct enzymes catalyze 1- and 4′-dephosphorylation (38, 39).
Subcloning of the pMJK-1 ~25-kb Insert for the Gene Encoding LpxE Activity
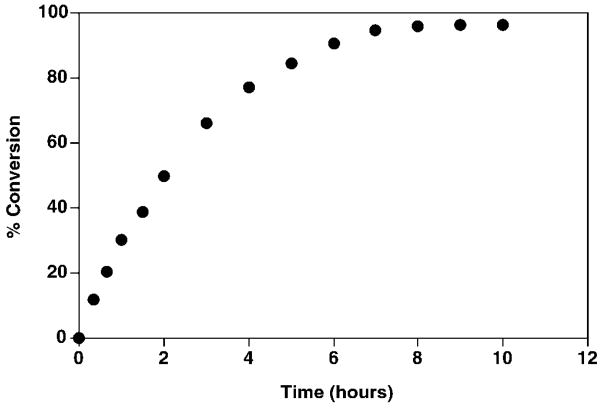
Restriction fragments of the ~25-kb R. leguminosarum 3841 genomic DNA insert in pMJK-1 were subcloned into the shuttle vector pRK404a (see “Experimental Procedures”). Plasmids obtained in this manner were individually transferred by tri-parental mating from an E. coli host into both R. etli CE3 and S. meliloti 1021. The latter normally lacks 1-phosphatase activity. Only the membranes of S. meliloti 1021 harboring pLpxE-2 or pLpxE-3, containing DNA inserts of ~7 and 4.9 kb, respectively, exhibited high levels of 1-phosphatase (Fig. 3). Further analysis of the pLpxE-2-directed phosphatase demonstrated that it is Kdo-independent and membrane-associated (data not shown). As shown in Fig. 4, 1-dephosphorylation of 5 μM Kdo2-[4′-32P]lipid IVA by 0.5 mg/ml S. meliloti 1021/pLpxE-2 membranes is linear with time for about 1 h at 30 °C and nearly goes to completion after 7 h. The initial rate of dephosphorylation is proportional to membrane protein concentrations up to 1.5 mg/ml (data not shown). About 5–7 fold higher 1-phosphatase specific activity (Table II) is detected in washed membranes of S. meliloti 1021/pMJK-1, S. meliloti 1021/pLpxE-2, or S. meliloti 1021/pLpxE-3 than wild type R. leguminosarum 3841. The 1-phosphatase activity of CE3 membranes is significantly lower than that of 3841 membranes (Table II), but the lipid A molecules isolated from both strains entirely lack phosphate residues (16, 17).
Fig. 3. pMJK-1, pLpxE-2, and pLpxE-3 directed 1-phosphatase over-expression in R. etli CE3 or S. meliloti 1021 membranes.
The 1-phosphatase activity present in membranes of R. etli CE3 or S. meliloti 1021 containing the indicated hybrid plasmids or vector controls (65) was assayed with 5 μM Kdo2-[4′-32P]lipid IVA as described under “Experimental Procedures.”
Fig. 4. Time course of dephosphorylation of Kdo2-[4′-32P]lipid IVA by S. meliloti 1021/pLpxE-2 membranes.
The 1-phosphatase reaction (40 μl) was performed under the standard conditions with membranes of S. meliloti/pLpxE-2 at 0.5 mg/ml.
Table II.
Overexpression of membrane-bound 1-phosphatase in R. etli CE3 or S. meliloti 1021 harboring pMJK-1
| Strain | Specific activity |
|---|---|
| nmol/min/mg | |
| R. etli CE3 | 0.002 |
| R. leguminosarum 3841 | 0.014 |
| R. etli CE3/pMJK-1 | 0.098 |
| S. meliloti 1021 | NDa |
| S. meliloti 1021/pLAFR-1 | ND |
| S. meliloti 1021/pMJK-1 | 0.059 |
ND, no detectable phosphatase activity is present in membranes. The specific activity of the 1-phosphatase was measured using washed membranes from the indicated strains of Rhizobium.
Identification of the Putative lpxE Gene
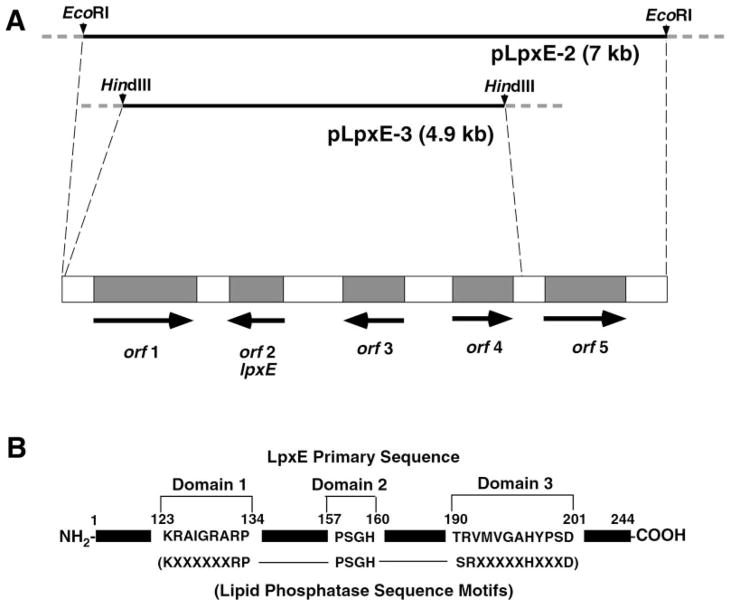
Sequencing of the DNA inserts in pLpxE-2 and pLpxE-3 revealed the presence of five putative open reading frames (Fig. 5A), which were identified using the programs DNA Strider and ORF Finder (72) (www.ncbi.nlm.nih.gov/gorf/gorf.html). Factors considered in determining the locations of lpxE and other genes on the insert include homology to sequences of known proteins, as well as the presence of possible promoter sequences and ribosomal binding sites. The complete nucleotide sequence relevant to the present paper has been submitted to GenBank™ under accession number AY371492.
Fig. 5. Genes near lpxE and location of the conserved lipid phosphatase motif within LpxE.
A, fragments of the ~25-kb DNA insert present in pMJK-1 were subcloned to make pLpxE-2 and pLpxE-3, both of which were sequenced. The dotted lines in the upper part of the figure mark the ends of the sequenced regions of the insert in pMJK-1. Five predicted open reading frames are present, and their directions of transcription are indicated. The lengths of the inserts and the genes are drawn approximately to scale. Some homologies and possible functions of these open reading frames are in Table IV. Orf2 (LpxE) is the only plausible candidate for the 1-phosphatase structural gene. Its sequence is: MRAFWASLDRRWRRSGVGMPPLRWQACLFVTLNAVILSMLLFDAPIGASKAPAPVKHLGELLTGFGDSAWLIYTSILLFFQGRAGYKLLKTARSKAQALYVSWIGAYLFFTVVFSGLLANLLKRAIGRARPDHFHDYGMFSFAPFSGHSAFESFPSGHSTTVGAFFAAFALLFPRYRVAFIACAIWLGMTRVMVGAHYPSDVIAGLAFGAWFSLLTAIVFARCGLLFKLAPDGWPLAKRLFRTA. B, the conserved tri-partite active site motif of the lipid phosphatase family (73) and the corresponding residues present in LpxE are indicated. The numbers at the top of the panel correspond to the amino acid residues of the predicted LpxE protein.
Table III lists some of the closest homologs in the nonredundant data base of the polypeptides encoded by the five predicted open reading frames in the ~7-kb insert of pLpxE-2. Of particular interest is the distant, yet highly significant similarity of the predicted product of orf2 to enzymes that dephosphorylate various phospholipids. Orf2, which is predicted to be 244 amino acid residues long (Fig. 5 legend), contains the conserved, tri-partite lipid phosphatase catalytic motif (Fig. 5B) (73), and it is also related to various other acid phosphatases and glucose 6-phosphatases.
Table III.
Possible functions of genes in the vicinity of LpxE based on similarities to selected gene products
| Predicted protein (length) | Related protein with characterized function if any (length) | Accession number | Homologya | E value |
|---|---|---|---|---|
| Orf1 (628) | Integral inner membrane metabolite transport protein, MtbA, Bradyrhizobium japonicum (555) | AAF78796 | 254/301/343 | 10−132 |
| LpxE (244) | Putative phosphatidic acid phosphatase, Lactococcus lactis (218) | AAC45388 | 43/56/125 | 0.001 |
| Orf3 (370) | β-Isopropylmalate dehydrogenase, LeuB, A. tumefaciens (370) | AAA22089 | 326/344/370 | 10−175 |
| Orf4 (313) | L-Serine dehydratase, SdhL, Rattus norvegicus (327) | CAA68721 | 144/196/313 | 10−57 |
| Orf5 (201) | 3-Isopropylmalate dehydratase small subunit, LeuD, Azotobacter vinelandii (215) | CAA722150 | 107/135/201 | 2 × 10−53 |
Homology is given as the number of identities/number of positives/number of residues (including gaps) in the related segment. The gapped Blastp algorithm (86) was used to identify possible orthologs using the predicted R. leguminosarum proteins as the probe.
Orf2 is predicted to have six transmembrane helices (data not shown). The topography strongly suggests that the residues of the tri-partite active site motif face the periplasmic surface of the inner membrane. The closest Orf2 orthologs (Table IV) are found in Agrobacterium tumefaciens, S. meliloti, Mesorhizobium loti, and Francisella tularensis. Although not extensively - studied, the first three of these bacteria are thought to synthesize fully phosphorylated lipid A molecules (70, 71, 74).2 However, it may be that their 1-phosphatase orthologs are induced under special growth conditions. F. tularensis has recently been shown to contain lipid A molecules lacking the 1-phosphate moiety (75). We propose that orf2 be named lpxE (from the German eins for 1), given its function as the structural gene of the lipid A 1-phosphatase.
Table IV. Selected proteins related to R. leguminosarum LpxE.
Possible orthologs in the NCBI database as of May 30, 2003 were identified with the PSI-Blast algorithm (86), using the predicted R. leguminosarum LpxE protein sequence of 244 residues as the probe. The low complexity filter was removed. The Legionella and Franciscella orthologs were found using the tBLASTn algorithm at www.ncbi.nlm.nih.gov/BLAST/ or artedi.ebc.uu.se/Projects/Francisella/blast/, respectively. A putative protein identical to our LpxE sequence is also predicted by tBLASTn searching of the R. leguminosarum genome at www.sanger.ac.uk/Projects/Microbes/.
| Organism | Homology (Gaps)a | Approximate E values |
|---|---|---|
| A. tumefaciensb | 84/125/214 (4) | 5 × 10− 30 |
| S. melilotic | 87/123/222 (2) | 6 × 10− 26 |
| M. loti | 83/108/223 | 8 × 10− 26 |
| F. tularensis | 51/65/112 (1) | 1 × 10− 21 |
| Legionella pneumophila | 55/72/149 | 3 × 10− 16 |
| Magnetospirillum magnetotacticum | 69/101/209 (18) | 4 × 10− 14 |
| Brucella melitensis 16M | 40/61/119 | 4 × 10− 11 |
Homology is given as the number of identities/number of positives/number of residues (including gaps) in the related segment when compare with R. leguminosarum LpxE, a hypothetical protein of 244 amino acid residues.
There are two separately sequenced A. tumefaciens strains. This is the strain sequenced by the University of Washington (88). The strain sequenced by the Cereon group contains the same ortholog (87).
This organism contains an LpxE ortholog and a cluster of genes similar to that present in R. leguminosarum 3841.
Heterologous Expression of the lpxE Gene in E. coli and Characterization of the Recombinant Enzyme
Unequivocal evidence that lpxE encodes the lipid A 1-phosphatase is provided by heterologous expression in E. coli, extracts of which normally have no phosphatase activity against lipid IVA and other lipid A precursors (38, 39). A phosphatase that attacks the 1 position would release 32Pi from [1-32P]lipid IVA. E. coli membranes prepared from cells containing the vector control pET-28a did not catalyze any dephosphorylation of [1-32P]lipid IVA (Fig. 6B). However, E. coli membranes from induced cells containing the plasmid pLpxE-4 rapidly dephosphorylated the 1 position of [1-32P]lipid IVA, as judged by the appearance of radiolabeled inorganic phosphate with time (Fig. 6B). The 1-phosphatase in the membranes of E. coli Novablue (DE3)/pLpxE-4 has a pH optimum and Triton X-100 dependence similar to what is observed with the native R. leguminosarum 1-phosphatase (39).
Fig. 6. Expression and assay of LpxE in E. coli Novablue (DE3) membranes.

A, the SDS-PAGE analysis of the membrane proteins prepared from iso-propyl-1-thio-β-D-galactopyranoside induced E. coli Novablue (DE3) cells, harboring either pLpxE-4 or the vector control pET-28a, was performed using 10-μg samples of membrane protein/lane in parallel with the indicated molecular mass standards. Lanes 1 and 2, vector control, uninduced and induced, respectively; lanes 3 and 4, vector with lpxE insert, uninduced and induced, respectively. B, washed membranes from cells harboring either the pET-28a vector or pLpxE-4 were assayed with [1-32P]lipid IVA as the substrate and with 0.2 mg/ml membrane protein. In this case the 1-phosphatase activity was quantified based on the release of 32Pi.
Considering the high apparent level of protein expression (Fig. 6A), the estimated specific activity of the recombinant 1-phosphatase in E. coli Novablue (DE3)/pLpxE-4 membranes seems low at 0.1 nmol/min/mg. The low specific activity may be due in part to incorrect folding or inclusion body formation. Extraction of the 1-phosphatase with Triton X-100 increases its specific activity to about 1.6 nmol/min/mg, suggesting inhibitory substances in the crude membrane fraction.
Mass Spectrometry of the Product Generated by the Recombinant 1-Phosphatase
The reaction product generated from lipid IVA by the 1-phosphatase expressed in E. coli was isolated and analyzed by mass spectrometry. The MALDI/TOF mass spectrum in the negative mode is shown in Fig. 7A. The structure of the proposed 1-dephosphorylated lipid IVA product is provided. The peak at m/z 1325.1 is interpreted as (M −H)− of the 1-phosphatase reaction product. The observed (M − H) − confirms that a single phosphate group is removed from lipid IVA by recombinant LpxE. The positive ion spectrum of the reaction product (Fig. 7B) shows that the distal residue of lipid IVA is not dephosphorylated, as revealed by the presence of the B1+ ion at m/z 695.1, which is the same as what is seen with lipid IVA.
Fig. 7. MALDI/TOF mass spectrometry of the 1-phosphatase reaction product generated from lipid IVA.
The reaction product was purified by DEAE cellulose chromatography. The spectra were acquired in the negative mode (A) and the positive mode (B). The molecular weight of lipid IVA is 1405.72 and that of 1-dephospho lipid IVA is 1325.73.
Expression of lpxE in S. meliloti 1021 Renders Cells Polymyxin-resistant
The lipid A of S. meliloti 1021 differs from that of R. etli and R. leguminosarum in that it is fully phosphorylated at the 1 and 4′ positions when grown under laboratory conditions (16, 17).2 Interestingly, S. meliloti 1021 is somewhat more sensitive to polymyxin at 10 or 20 μg (Table V) than is R. etli CE3 (Table V) or R. leguminosarum (not shown). To determine whether or not dephosphorylation at the 1 position might be playing a role, we tested the polymyxin sensitivity of S. meliloti 1021 harboring recombinant lpxE or the appropriate vector control (Table V). There was a significant decrease in the sensitivity to polymyxin when lpxE was expressed in S. meliloti, but not in R. etli, as judged by disc diffusion assays (Table V). Presumably, the reduced negative charge of the resulting 1-dephosphorylated lipid A molecules in S. meliloti harboring lpxE lowers the affinity of the outer membrane for polymyxin, preventing the drug from reaching the inner membrane and killing the cells (76).
Table V.
Reduced polymyxin sensitivity of S. meliloti cells expressing lpxE
| Zone of clearing diameter
|
|||
|---|---|---|---|
| Strain | 2 μg of polymyxin | 10 μg of polymyxin | 20 μg of polymyxin |
| mm | |||
| R. etli CE3/pRK404a | 8 | 12.5 | 14 |
| R. etli CE3/pLpxE-2 | 10 | 13 | 15 |
| R. etli CE3/pLpxE-3 | 10 | 13 | 15 |
| S. meliloti 1021/pRK404a | 9 | 17 | 18 |
| S. meliloti 1021/pLpxE-2 | <6 | <6 | 7 |
| S. meliloti 1021/pLpxE-3 | <6 | <6 | 7 |
DISCUSSION
The lipopolysaccharides of some Gram-negative bacteria, including R. etli (14–17), R. leguminosarum (14–17), F. tularensis (75), Aquifex pyrophilus (77), Helicobacter pylori (78), and Porphyromonas gingivalis (79), contain lipid A moieties that are dephosphorylated at the 1 and/or 4′ positions. In R. etli and R. leguminosarum, the structures of such phosphate-deficient lipid A molecules are well characterized (Fig. 1) (16, 17). Enzymatic studies with R. etli and R. leguminosarum membranes have also demonstrated the presence of distinct 1- and 4′-phosphatases in these organisms (38, 39), supporting the proposed pathway shown in Fig. 1. R. etli and R. leguminosarum first synthesize a lipid A structure resembling that of E. coli but then modify the resulting substance further with additional enzymes not present in E. coli. Many of the enzymes that are unique to R. etli and R. leguminosarum may be located on the outer surface of the inner membrane, as suggested by the sequence of LpxE (Fig. 5 legend) or in the outer membrane as previously shown for LpxQ (Fig. 1) (43, 44).
We now report the expression cloning and characterization of a gene, designated lpxE, that encodes the lipid A 1-phosphatase of R. leguminosarum. This enzyme normally is not present in extracts of S. meliloti or E. coli (39). However, both S. meliloti 1021 and E. coli can express the R. leguminosarum 1-phosphatase when lpxE is introduced on an appropriate vector (Figs. 3, 6, and 7). Heterologous expression of lpxE behind the T7lac promoter in E. coli is especially effective (Fig. 6 and Table II) and provides definitive evidence that lpxE is the structural gene for the 1-phosphatase.
An examination of the COG data base (72) shows that three distant orthologs of LpxE are present in E. coli, which include the phosphatidylglycerol-phosphate phosphatase PgpB (80, 81), a putative additional phosphatidylglycerol-phosphate phosphatase that can be assayed in mutants lacking pgpB (82), and the as yet uncharacterized undecaprenyl-diphosphate phosphatase involved in peptidoglycan assembly (83, 84). Like PgpB and many of the eucaryotic phosphatases that act on glycerophospholipids (73, 81, 85), the R. leguminosarum lipid A 1-phosphatase is membrane-bound and requires detergent for activity. Compared with E. coli PgpB or the periplasmic alkaline phosphatase, the lipid A 1-phosphatase is distinct and highly selective for the 1 position of lipid A (Figs. 6 and 7). Sequence comparisons using PSI-BLAST (86) indicate that LpxE shares key conserved active site residues with PgpB and related lipid phosphatases (Fig. 5B), but this limited similarity likely reflects a shared catalytic mechanism and not substrate specificity. The putative catalytic residues of LpxE (Fig. 5B) are predicted to face the periplasmic surface of the inner membrane (see www.cbs.dtu.dk/services/TMHMM-2.0/). LpxE must now be purified to homogeneity, characterized with regard to lipid substrate specificity and topography, and subjected to site-directed mutagenesis. However, the remarkable selectivity of LpxE for the 1 position of Kdo2-lipid IVA or lipid IVA (Figs. 3, 6, and 7) argues against the idea that this enzyme is a nonspecific lipid phosphatase. Conversely, when commercially available, soluble phosphatases from calf intestine or shrimp were tested under the conditions optimized for the R. leguminosarum 1-phosphatase, no dephosphorylation of Kdo2-[4′-32P]lipid IVA was observed (data not shown).
The presence of closely related LpxE orthologs in A. tumefaciens (87, 88), M. loti (89), and S. meliloti (49) (Table IV) is unexpected and especially intriguing. Limited available structural information suggests the presence of phosphate-containing lipid A molecules in these organisms (70, 71, 74).2 Under our standard assay conditions there is no endogenous 1-phosphatase activity in membranes of S. meliloti 1021 grown on TY broth (Fig. 3). The M. loti lpxE (Table IV) does in fact encode a 1-phosphatase, as judged by heterologous expression of 1-phosphatase activity in E. coli.3 It is therefore plausible that the orthologs shown in Table IV are induced under special environmental circumstances that necessitate the dephosphorylation of lipid A in these organisms.
PSI-BLAST analysis (86), using R. leguminosarum LpxQ oxidase (Fig. 1) (44) as the probe, reveals the presence of a very similar protein in A. tumefaciens (44). This variant is fully functional as a lipid A oxidase when expressed in E. coli (44). Considering the confirmed function of A. tumefaciens LpxQ as a lipid A oxidase, it seems probable that the A. tumefaciens LpxE ortholog (Table IV) functions as the lipid A 1-phosphatase required for generating the LpxQ substrate (Fig. 1), although this remains to be demonstrated experimentally. The structural analysis of A. tumefaciens lipid A and a systematic search for an induced lipid A 1-phosphatase under special growth conditions might reveal how the LpxE and LpxQ orthologs of this organism are regulated.
Enteric Gram-negative bacteria detect changes in environmental conditions using two-component regulatory systems (90). Although no two-component systems in R. leguminosarum or S. meliloti are known to be involved in the modification of lipid A, this possibility cannot be excluded. There is in fact evidence that the LPS of R. leguminosarum 3841 is altered under the conditions that would be encountered during symbiosis (91, 92). When comparing LPS of R. leguminosarum 3841 isolated from pea bacteroids to LPS from strains grown in the laboratory, distinct epitopes are observed by immunochemical methods (91–94). These epitopes can be induced ex planta by growth at low pH and/or at low oxygen concentrations (95, 96), indicating that LPS may be modified in a regulated manner. However, lipid A structure was not examined in these studies (95, 96). Various covalent modifications of lipid A, induced by growth on low magnesium ion concentrations or low pH via the PhoP/Q or the PmrA/B two component regulatory systems, respectively, are known to occur in E. coli and S. typhimurium (97–104).
Many chemical studies have confirmed the importance of the phosphate groups of lipid A for stimulating mammalian immune cells (10). Although not yet well characterized in terms of their ability to respond to lipid A, plants have recently been shown to possess systems of innate immunity (105, 106) and to synthesize antibacterial peptides in a manner that is reminiscent of insects infected with bacteria or fungi (107, 108). The unusual lipid A of R. leguminosarum might conceivably help bacteroids evade the innate immune response of plants during symbiosis in root cells while still allowing the plant to defend itself against Gram-negative pathogens containing a more typical, phosphorylated lipid A disaccharide. Mutants of R. leguminosarum lacking the 1-phosphatase will be required to validate this hypothesis.
The identification of the 1-phosphatase gene from R. leguminosarum provides a new tool for the selective modification and preparation of interesting lipid A analogs. Given the structure-activity relationships of known lipid A derivatives (10), one would expect that many of the unusual features of R. leguminosarum lipid A would greatly reduce immune stimulation in animal systems. Recent studies with related preparations from Rhizobium sin-1 suggest that some of these substances might even be novel LPS antagonists (109). Current methods to prepare 1-dephosphorylated lipid A species from bacterial sources or from enzymatically synthesized molecules require acid hydrolysis at 100 °C (43). This treatment is non-specific in that it also cleaves the labile Kdo linkages in compounds like Kdo2-lipid IVA (110–112). Consequently, the availability of LpxE will enable the preparation of novel molecules, such as 1-dephospho-Kdo2-lipid IVA or 1-dephospho-Kdo2-lipid A, starting with Kdo2-lipid IVA (Fig. 1) or Kdo2-lipid A, which are readily accessible (54, 55, 113).
The expression of the lpxE gene behind the T7lac promoter in E. coli (Table II) will facilitate the purification of large amounts of the 1-phosphatase. With the pure enzyme, it will be possible to evaluate the functions of the conserved residues in the phosphatase motif (Fig. 5) and to examine the substrate selectivity of LpxE toward various lipids. It will also be possible to use lpxE to re-engineer lipid A structure in living Gram-negative bacteria and to investigate the effects of such structural modifications on outer membrane assembly, symbiosis, and pathogenesis. The induction of polymyxin resistance in S. meliloti by heterologous expression of R. leguminosarum lpxE (Table V) suggests that 1-dephosphorylation of lipid A can have important physiological consequences.
Acknowledgments
We thank Dr. J. A. Downie (John Innes Institute) and Dr. P. Poole (University of Reading) for providing the R. leguminosarum genomic DNA cosmid library. We also thank Dr. D. Borthakur (University of Hawaii) for the pRK404a plasmid shuttle vector. We thank Drs. Shib Sankar Basu and M. Stephen Trent for help with the preparation of this manuscript.
Footnotes
This work was supported by National Institutes of Health Grant R37-GM-51796 (to C. R. H. R.).
The abbreviations used are: LPS, lipopolysaccharide; MES, 2-(N-morpholino)-ethanesulfonic acid; Kdo, 3-deoxy-D-manno-octulosonic acid; MALDI/TOF, matrix-assisted laser-desorption/time of flight; TLR, toll-like receptor.
N. L. S. Que-Gewirth and C. R. H. Raetz, unpublished observations.
M. J. Karbarz and C. R. H. Raetz, unpublished observations.
The nucleotide sequence(s) reported in this paper has been submitted to the GenBank™/EBI Data Bank with accession number(s) AY371492.
References
- 1.Raetz CRH. Annu Rev Biochem. 1990;59:129–170. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- 2.Raetz CRH. In: Escherichia coli and Salmonella: Cellular and Molecular Biology. 2. Neidhardt FC, editor. Vol. 1. American Society for Microbiology; Washington, D. C: 1996. pp. 1035–1063. [Google Scholar]
- 3.Raetz CRH, Whitfield C. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brade H, Opal SM, Vogel SN, Morrison DC, editors. Endotoxin in Health and Disease. Marcel Dekker, Inc; New York: 1999. [Google Scholar]
- 5.Nikaido H. In: Escherichia coli and Salmonella: Cellular and Molecular Biology. 2. Neidhardt FC, editor. Vol. 1. American Society for Microbiology; Washington, D. C: 1996. pp. 29–47. [Google Scholar]
- 6.Vaara M. Antimicrob Agents Chemother. 1993;37:2255–2260. doi: 10.1128/aac.37.11.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roantree RJ. Annu Rev Microbiol. 1967;21:443–466. doi: 10.1146/annurev.mi.21.100167.002303. [DOI] [PubMed] [Google Scholar]
- 8.Galloway SM, Raetz CRH. J Biol Chem. 1990;265:6394–6402. [PubMed] [Google Scholar]
- 9.Onishi HR, Pelak BA, Gerckens LS, Silver LL, Kahan FM, Chen MH, Patchett AA, Galloway SM, Hyland SA, Anderson MS, Raetz CRH. Science. 1996;274:980–982. doi: 10.1126/science.274.5289.980. [DOI] [PubMed] [Google Scholar]
- 10.Rietschel ET, Kirikae T, Schade FU, Mamat U, Schmidt G, Loppnow H, Ulmer AJ, Zähringer U, Seydel U, Di Padova F, Schreier M, Brade H. FASEB J. 1994;8:217–225. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- 11.Parillo JE. N Engl J Med. 1993;328:1471–1477. doi: 10.1056/NEJM199305203282008. [DOI] [PubMed] [Google Scholar]
- 12.Aderem A, Ulevitch RJ. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 13.Zähringer U, Lindner B, Rietschel ET. In: Endotoxin in Health and Disease. Brade H, Opal SM, Vogel SN, Morrison DC, editors. Marcel Dekker, Inc; New York: 1999. pp. 93–114. [Google Scholar]
- 14.Bhat UR, Forsberg LS, Carlson RW. J Biol Chem. 1994;269:14402–14410. [PubMed] [Google Scholar]
- 15.Forsberg LS, Carlson RW. J Biol Chem. 1998;273:2747–2757. doi: 10.1074/jbc.273.5.2747. [DOI] [PubMed] [Google Scholar]
- 16.Que NLS, Lin S, Cotter RJ, Raetz CRH. J Biol Chem. 2000;275:28006–28016. doi: 10.1074/jbc.M004008200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Que NLS, Ribeiro AA, Raetz CRH. J Biol Chem. 2000;275:28017–28027. doi: 10.1074/jbc.M004009200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qureshi N, Takayama K, Mascagni P, Honovich J, Wong R, Cotter RJ. J Biol Chem. 1988;263:11971–11976. [PubMed] [Google Scholar]
- 19.Brozek KA, Raetz CRH. J Biol Chem. 1990;265:15410–15417. [PubMed] [Google Scholar]
- 20.Clementz T, Bednarski JJ, Raetz CRH. J Biol Chem. 1996;271:12095–12102. doi: 10.1074/jbc.271.20.12095. [DOI] [PubMed] [Google Scholar]
- 21.Clementz T, Zhou Z, Raetz CRH. J Biol Chem. 1997;272:10353–10360. doi: 10.1074/jbc.272.16.10353. [DOI] [PubMed] [Google Scholar]
- 22.Brozek KA, Carlson RW, Raetz CRH. J Biol Chem. 1996;271:32126–32136. doi: 10.1074/jbc.271.50.32126. [DOI] [PubMed] [Google Scholar]
- 23.Loppnow H, Brade H, Dürrbaum I, Dinarello CA, Kusumoto S, Rietschel ET, Flad HD. J Immunol. 1989;142:3229–3238. [PubMed] [Google Scholar]
- 24.Golenbock DT, Hampton RY, Qureshi N, Takayama K, Raetz CRH. J Biol Chem. 1991;266:19490–19498. [PubMed] [Google Scholar]
- 25.Lien E, Means TK, Heine H, Yoshimura A, Kusumoto S, Fukase K, Fenton MJ, Oikawa M, Qureshi N, Monks B, Finberg RW, Ingalls RR, Golenbock DT. J Clin Invest. 2000;105:497–504. doi: 10.1172/JCI8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poltorak A, Ricciardi-Castagnoli P, Citterio S, Beutler B. Proc Natl Acad Sci U S A. 2000;97:2163–2167. doi: 10.1073/pnas.040565397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Persing DH, Coler RN, Lacy MJ, Johnson DA, Baldridge JR, Hershberg RM, Reed SG. Trends Microbiol. 2002;10:S32–37. doi: 10.1016/s0966-842x(02)02426-5. [DOI] [PubMed] [Google Scholar]
- 28.Poltorak A, He X, Smirnova I, Liu MY, Huffel CV, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 29.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 30.Lien E, Ingalls RR. Crit Care Med. 2002;30:S1–S11. [PubMed] [Google Scholar]
- 31.Janeway CA, Jr, Medzhitov R. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 32.Takeda K, Akira S. Cell Microbiol. 2003;5:143–153. doi: 10.1046/j.1462-5822.2003.00264.x. [DOI] [PubMed] [Google Scholar]
- 33.Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Endre G, Kereszt A, Kevei Z, Mihacea S, Kalo P, Kiss GB. Nature. 2002;417:962–966. doi: 10.1038/nature00842. [DOI] [PubMed] [Google Scholar]
- 35.Stracke S, Kistner C, Yoshida S, Mulder L, Sato S, Kaneko T, Tabata S, Sandal N, Stougaard J, Szczyglowski K, Parniske M. Nature. 2002;417:959–962. doi: 10.1038/nature00841. [DOI] [PubMed] [Google Scholar]
- 36.Spaink HP. Nature. 2002;417:910–911. doi: 10.1038/417910a. [DOI] [PubMed] [Google Scholar]
- 37.Price NPJ, Kelly TM, Raetz CRH, Carlson RW. J Bacteriol. 1994;176:4646–4655. doi: 10.1128/jb.176.15.4646-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Price NJP, Jeyaretnam B, Carlson RW, Kadrmas JL, Raetz CRH, Brozek KA. Proc Natl Acad Sci U S A. 1995;92:7352–7356. doi: 10.1073/pnas.92.16.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brozek KA, Kadrmas JL, Raetz CRH. J Biol Chem. 1996;271:32112–32118. [PubMed] [Google Scholar]
- 40.Basu SS, Karbarz MJ, Raetz CRH. J Biol Chem. 2002;277:28959–28971. doi: 10.1074/jbc.M204525200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kadrmas JL, Brozek KA, Raetz CRH. J Biol Chem. 1996;271:32119–32125. [PubMed] [Google Scholar]
- 42.Kadrmas JL, Allaway D, Studholme RE, Sullivan JT, Ronson CW, Poole PS, Raetz CRH. J Biol Chem. 1998;273:26432–26440. doi: 10.1074/jbc.273.41.26432. [DOI] [PubMed] [Google Scholar]
- 43.Que-Gewirth NLS, Lin S, Cotter RJ, Raetz CRH. J Biol Chem. 2003;278:12109–12119. doi: 10.1074/jbc.M300378200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Que-Gewirth NLS, Karbarz MJ, Kalb SR, Cotter RJ, Raetz CRH. J Biol Chem. 2003;278:12120–12129. doi: 10.1074/jbc.M300379200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ronson CW, Astwood PM, Downie JA. J Bacteriol. 1984;160:903–909. doi: 10.1128/jb.160.3.903-909.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cava JR, Elias PM, Turowski DA, Noel KD. J Bacteriol. 1989;171:8–15. doi: 10.1128/jb.171.1.8-15.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noel KD, Sanchez A, Fernandez L, Leemans J, Cevallos MA. J Bacteriol. 1984;158:148–155. doi: 10.1128/jb.158.1.148-155.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnston AW, Beringer JE. J Gen Microbiol. 1975;87:343–350. doi: 10.1099/00221287-87-2-343. [DOI] [PubMed] [Google Scholar]
- 49.Galibert F, Finan TM, Long SR, Puhler A, Abola P, Ampe F, Barloy-Hubler F, Barnett MJ, Becker A, Boistard P, Bothe G, Boutry M, Bowser L, Buhrmester J, Cadieu E, Capela D, Chain P, Cowie A, Davis RW, Dreano S, Federspiel NA, Fisher RF, Gloux S, Godrie T, Goffeau A, Golding B, Gouzy J, Gurjal M, Hernandez-Lucas I, Hong A, Huizar L, Hyman RW, Jones T, Kahn D, Kahn ML, Kalman S, Keating DH, Kiss E, Komp C, Lelaure V, Masuy D, Palm C, Peck MC, Pohl TM, Portetelle D, Purnelle B, Ramsperger U, Surzycki R, Thebault P, Vandenbol M, Vorholter FJ, Weidner S, Wells DH, Wong K, Yeh KC, Batut J. Science. 2001;293:668–672. doi: 10.1126/science.1060966. [DOI] [PubMed] [Google Scholar]
- 50.Miller JR. Experiments in Molecular Genetics. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1972. [Google Scholar]
- 51.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 52.Garrett TA, Kadrmas JL, Raetz CRH. J Biol Chem. 1997;272:21855–21864. doi: 10.1074/jbc.272.35.21855. [DOI] [PubMed] [Google Scholar]
- 53.Basu SS, York JD, Raetz CRH. J Biol Chem. 1999;274:11139–11149. doi: 10.1074/jbc.274.16.11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Belunis CJ, Raetz CRH. J Biol Chem. 1992;267:9988–9997. [PubMed] [Google Scholar]
- 55.Brozek KA, Hosaka K, Robertson AD, Raetz CRH. J Biol Chem. 1989;264:6956–6966. [PubMed] [Google Scholar]
- 56.Nishijima M, Raetz CRH. J Biol Chem. 1979;254:7837–7844. [PubMed] [Google Scholar]
- 57.Nishijima M, Bulawa CE, Raetz CRH. J Bacteriol. 1981;145:113–121. doi: 10.1128/jb.145.1.113-121.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takayama K, Qureshi N, Mascagni P, Nashed MA, Anderson L, Raetz CRH. J Biol Chem. 1983;258:7379–7385. [PubMed] [Google Scholar]
- 59.Radika K, Raetz CRH. J Biol Chem. 1988;263:14859–14867. [PubMed] [Google Scholar]
- 60.Friedman AM, Long SR, Brown SE, Buikema WJ, Ausubel FM. Gene (Amst) 1982;18:289–296. doi: 10.1016/0378-1119(82)90167-6. [DOI] [PubMed] [Google Scholar]
- 61.Wood W. J Mol Biol. 1966;16:118–133. doi: 10.1016/s0022-2836(66)80267-x. [DOI] [PubMed] [Google Scholar]
- 62.Glazebrook J, Walker GC. Methods Enzymol. 1991;204:398–418. doi: 10.1016/0076-6879(91)04021-f. [DOI] [PubMed] [Google Scholar]
- 63.Finan TM, Kunkel B, De Vos GF, Signer ER. J Bacteriol. 1986;167:66–72. doi: 10.1128/jb.167.1.66-72.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. John Wiley & Sons; New York: 1989. [Google Scholar]
- 65.Ditta G, Stanfield S, Corbin D, Helinski DR. Proc Natl Acad Sci U S A. 1980;77:7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.von Jagow G, Schagger H, editors. A Practical Guide to Membrane Protein Purification. Academic Press; New York: 1994. [Google Scholar]
- 67.Bligh EG, Dyer JJ. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 68.Raetz CRH, Kennedy EP. J Biol Chem. 1973;248:1098–1105. [PubMed] [Google Scholar]
- 69.Raetz CRH, Purcell S, Meyer MV, Qureshi N, Takayama K. J Biol Chem. 1985;260:16080–16088. [PubMed] [Google Scholar]
- 70.Gudlavalleti SK, Forsberg LS. J Biol Chem. 2003;278:3957–3968. doi: 10.1074/jbc.M210491200. [DOI] [PubMed] [Google Scholar]
- 71.Kanipes MI, Kalb SR, Cotter RJ, Hozbor DF, Lagares A, Raetz CRH. J Biol Chem. 2003;278:16365–16371. doi: 10.1074/jbc.M301256200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wheeler DL, Church DM, Federhen S, Lash AE, Madden TL, Pontius JU, Schuler GD, Schriml LM, Sequeira E, Tatusova TA, Wagner L. Nucleic Acids Res. 2003;31:28–33. doi: 10.1093/nar/gkg033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stukey J, Carman GM. Protein Sci. 1997;6:469–472. doi: 10.1002/pro.5560060226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Urbanik-Sypniewska T, Choma A, Kutkowska J, Kaminska T, Kandefer-Szerszen M, Russa R, Dolecka J. Immunobiology. 2000;202:408–420. doi: 10.1016/S0171-2985(00)80043-1. [DOI] [PubMed] [Google Scholar]
- 75.Vinogradov E, Perry MB, Conlan JW. Eur J Biochem. 2002;269:6112–6118. doi: 10.1046/j.1432-1033.2002.03321.x. [DOI] [PubMed] [Google Scholar]
- 76.Nikaido H, Vaara M. Microbiol Rev. 1985;49:1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Plotz BM, Lindner B, Stetter KO, Holst O. J Biol Chem. 2000;275:11222–11228. doi: 10.1074/jbc.275.15.11222. [DOI] [PubMed] [Google Scholar]
- 78.Moran AP, Lindner B, Walsh EJ. J Bacteriol. 1997;179:6453–6463. doi: 10.1128/jb.179.20.6453-6463.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ogawa T. FEBS Lett. 1993;332:197–201. doi: 10.1016/0014-5793(93)80512-s. [DOI] [PubMed] [Google Scholar]
- 80.Icho T, Raetz CRH. J Bacteriol. 1983;153:722–730. doi: 10.1128/jb.153.2.722-730.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Icho T. J Bacteriol. 1988;170:5117–5124. doi: 10.1128/jb.170.11.5117-5124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Funk CR, Zimniak L, Dowhan W. J Bacteriol. 1992;174:205–213. doi: 10.1128/jb.174.1.205-213.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Park JT. In: Escherichia coli and Salmonella typhimurium. Neidhardt F, editor. Vol. 1. American Society for Microbiology; Washington, D. C: 1987. pp. 663–671. [Google Scholar]
- 84.Bugg TD, Brandish PE. FEMS Microbiol Lett. 1994;119:255–262. doi: 10.1111/j.1574-6968.1994.tb06898.x. [DOI] [PubMed] [Google Scholar]
- 85.Carman GM. Biochim Biophys Acta. 1997;1348:45–55. doi: 10.1016/s0005-2760(97)00095-7. [DOI] [PubMed] [Google Scholar]
- 86.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goodner B, Hinkle G, Gattung S, Miller N, Blanchard M, Qurollo B, Goldman BS, Cao Y, Askenazi M, Halling C, Mullin L, Houmiel K, Gordon J, Vaudin M, Iartchouk O, Epp A, Liu F, Wollam C, Allinger M, Doughty D, Scott C, Lappas C, Markelz B, Flanagan C, Crowell C, Gurson J, Lomo C, Sear C, Strub G, Cielo C, Slater S. Science. 2001;294:2323–2328. doi: 10.1126/science.1066803. [DOI] [PubMed] [Google Scholar]
- 88.Wood DW, Setubal JC, Kaul R, Monks DE, Kitajima JP, Okura VK, Zhou Y, Chen L, Wood GE, Almeida NF, Jr, Woo L, Chen Y, Paulsen IT, Eisen JA, Karp PD, Bovee D, Sr, Chapman P, Clendenning J, Deatherage G, Gillet W, Grant C, Kutyavin T, Levy R, Li MJ, McClelland E, Palmieri A, Raymond C, Rouse G, Saenphimmachak C, Wu Z, Romero P, Gordon D, Zhang S, Yoo H, Tao Y, Biddle P, Jung M, Krespan W, Perry M, Gordon-Kamm B, Liao L, Kim S, Hendrick C, Zhao ZY, Dolan M, Chumley F, Tingey SV, Tomb JF, Gordon MP, Olson MV, Nester EW. Science. 2001;294:2317–2323. doi: 10.1126/science.1066804. [DOI] [PubMed] [Google Scholar]
- 89.Kaneko T, Nakamura Y, Sato S, Asamizu E, Kato T, Sasamoto S, Watanabe A, Idesawa K, Ishikawa A, Kawashima K, Kimura T, Kishida Y, Kiyokawa C, Kohara M, Matsumoto M, Matsuno A, Mochizuki Y, Nakayama S, Nakazaki N, Shimpo S, Sugimoto M, Takeuchi C, Yamada M, Tabata S. DNA Res. 2000;7:331–338. doi: 10.1093/dnares/7.6.331. [DOI] [PubMed] [Google Scholar]
- 90.Galperin MY, Nikolskaya AN, Koonin EV. FEMS Microbiol Lett. 2001;203:11–21. doi: 10.1111/j.1574-6968.2001.tb10814.x. [DOI] [PubMed] [Google Scholar]
- 91.VandenBosch KA, Brewin NJ, Kannenberg EL. J Bacteriol. 1989;171:4537–4542. doi: 10.1128/jb.171.9.4537-4542.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sindhu SS, Brewin NJ, Kannenberg EL. J Bacteriol. 1990;172:1804–1813. doi: 10.1128/jb.172.4.1804-1813.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kannenberg EL, Perotto S, Bianciotto V, Rathbun EA, Brewin NJ. J Bacteriol. 1994;176:2021–2032. doi: 10.1128/jb.176.7.2021-2032.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kannenberg EL, Brewin NJ. Trends Microbiol. 1994;2:277–283. doi: 10.1016/0966-842x(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 95.Kannenberg EL, Brewin NJ. J Bacteriol. 1989;171:4543–4548. doi: 10.1128/jb.171.9.4543-4548.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kannenberg EL, Rathbun EA, Brewin NJ. Mol Microbiol. 1992;6:2477–2487. doi: 10.1111/j.1365-2958.1992.tb01424.x. [DOI] [PubMed] [Google Scholar]
- 97.Guo L, Lim KB, Gunn JS, Bainbridge B, Darveau RP, Hackett M, Miller SI. Science. 1997;276:250–253. doi: 10.1126/science.276.5310.250. [DOI] [PubMed] [Google Scholar]
- 98.Guo L, Lim KB, Poduje CM, Daniel M, Gunn JS, Hackett M, Miller SI. Cell. 1998;95:189–198. doi: 10.1016/s0092-8674(00)81750-x. [DOI] [PubMed] [Google Scholar]
- 99.Gunn JS, Lim KB, Krueger J, Kim K, Guo L, Hackett M, Miller SI. Mol Microbiol. 1998;27:1171–1182. doi: 10.1046/j.1365-2958.1998.00757.x. [DOI] [PubMed] [Google Scholar]
- 100.Zhou Z, Lin S, Cotter RJ, Raetz CRH. J Biol Chem. 1999;274:18503–18514. doi: 10.1074/jbc.274.26.18503. [DOI] [PubMed] [Google Scholar]
- 101.Bishop RE, Gibbons HS, Guina T, Trent MS, Miller SI, Raetz CRH. EMBO J. 2000;19:5071–5080. doi: 10.1093/emboj/cdd507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou Z, Ribeiro AA, Lin S, Cotter RJ, Miller SI, Raetz CRH. J Biol Chem. 2001;276:43111–43121. doi: 10.1074/jbc.M106960200. [DOI] [PubMed] [Google Scholar]
- 103.Groisman EA. J Bacteriol. 2001;183:1835–1842. doi: 10.1128/JB.183.6.1835-1842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ohl ME, Miller SI. Annu Rev Med. 2001;52:259–274. doi: 10.1146/annurev.med.52.1.259. [DOI] [PubMed] [Google Scholar]
- 105.Medzhitov R, Janeway CA., Jr Curr Opin Immunol. 1998;10:12–15. doi: 10.1016/s0952-7915(98)80024-1. [DOI] [PubMed] [Google Scholar]
- 106.Borregaard N, Elsbach P, Ganz T, Garred P, Svejgaard A. Immunol Today. 2000;21:68–70. doi: 10.1016/s0167-5699(99)01570-4. [DOI] [PubMed] [Google Scholar]
- 107.Caaveiro JM, Molina A, Gonzalez-Manas JM, Rodriguez-Palenzuela P, Garcia-Olmedo F, Goni FM. FEBS Lett. 1997;410:338–342. doi: 10.1016/s0014-5793(97)00613-3. [DOI] [PubMed] [Google Scholar]
- 108.Boman HG. Scand J Immunol. 1998;48:15–25. doi: 10.1046/j.1365-3083.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 109.Vandenplas ML, Carlson RW, Jeyaretnam BS, McNeill B, Barton MH, Norton N, Murray TF, Moore JN. J Biol Chem. 2002;277:41811–41816. doi: 10.1074/jbc.M205252200. [DOI] [PubMed] [Google Scholar]
- 110.Qureshi N, Takayama K, Ribi E. J Biol Chem. 1982;257:11808–11815. [PubMed] [Google Scholar]
- 111.Kanipes MI, Lin S, Cotter RJ, Raetz CRH. J Biol Chem. 2001;276:1156–1163. doi: 10.1074/jbc.M009019200. [DOI] [PubMed] [Google Scholar]
- 112.Kanipes MI, Ribeiro AA, Lin S, Cotter RJ, Raetz CRH. J Biol Chem. 2003;278:16356–16364. doi: 10.1074/jbc.M301255200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brabetz W, Muller-Loennies S, Holst O, Brade H. Eur J Biochem. 1997;247:716–724. doi: 10.1111/j.1432-1033.1997.00716.x. [DOI] [PubMed] [Google Scholar]
- 114.Basu SS, White KA, Que NL, Raetz CRH. J Biol Chem. 1999;274:11150–11158. doi: 10.1074/jbc.274.16.11150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Babinski KJ, Ribeiro AA, Raetz CRH. J Biol Chem. 2002;277:25937–25946. doi: 10.1074/jbc.M204067200. [DOI] [PubMed] [Google Scholar]