Abstract
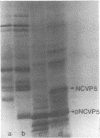
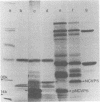
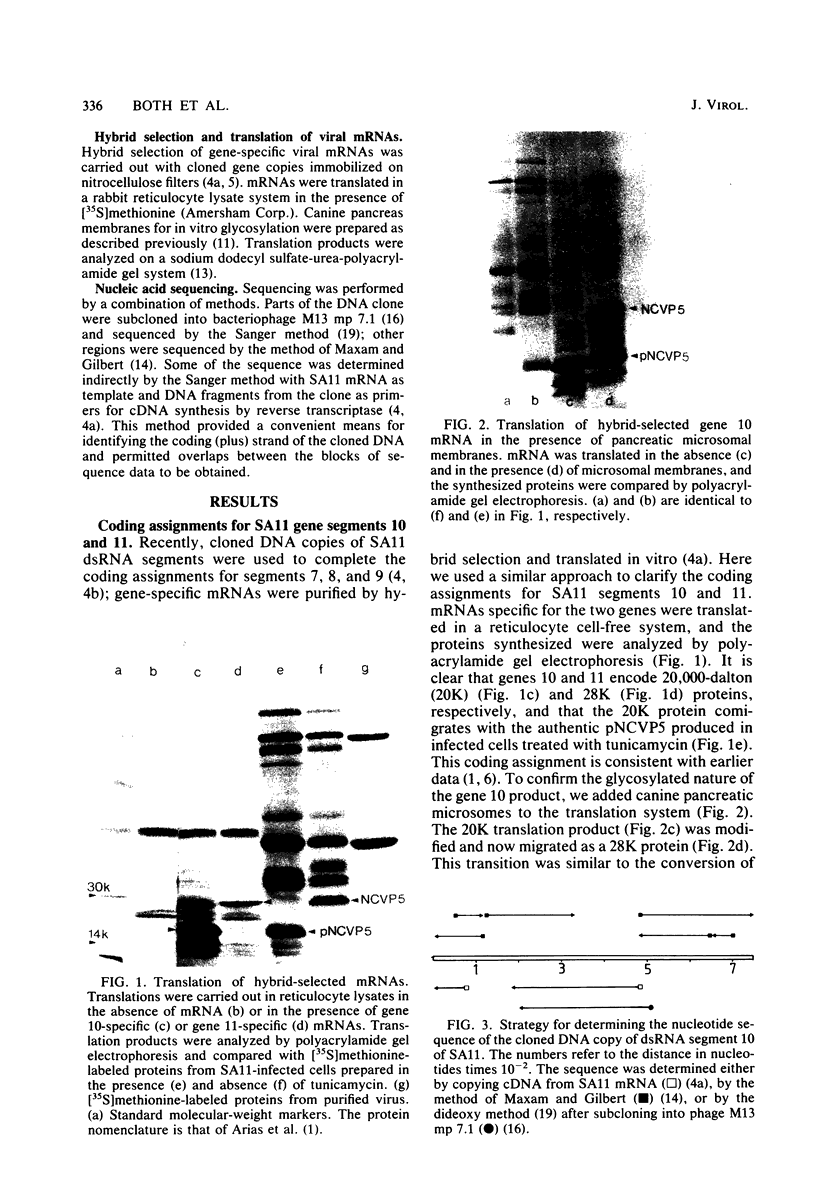
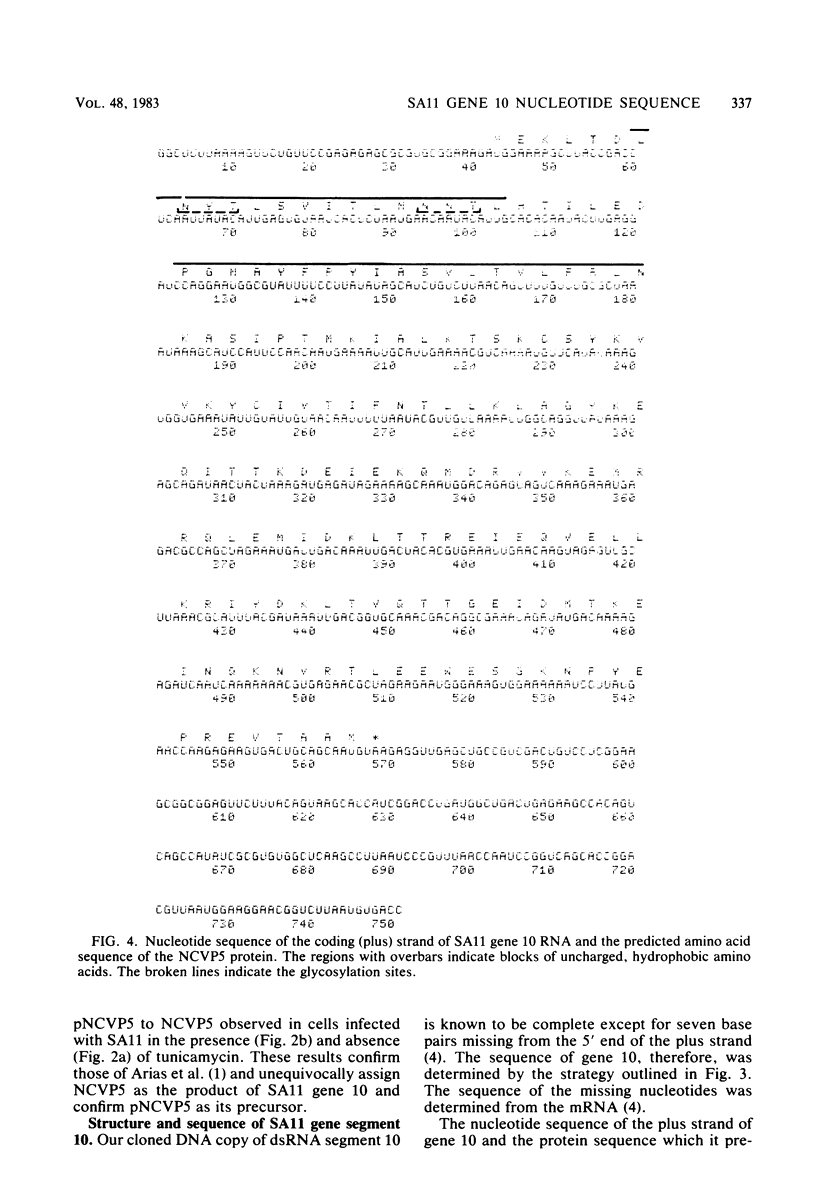
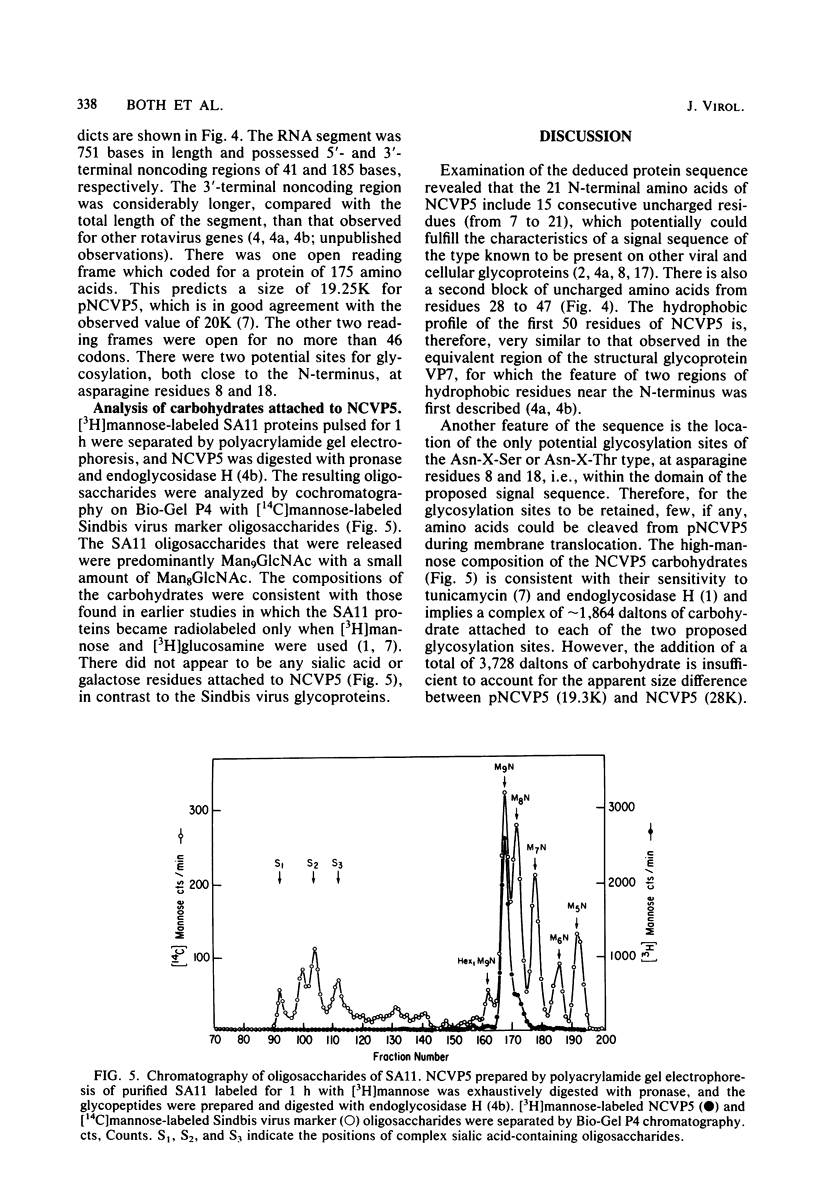
A cloned DNA copy of simian rotavirus SA11 genomic segment 10 was used to confirm the assignment of the nonstructural glycoprotein NCVP5 to this gene. Determination of the nucleotide sequence for gene 10 indicated that NCVP5 is 175 amino acids in length and has an N-terminal hydrophobic region with the characteristics of a signal sequence for membrane translocation. Unexpectedly, this region was also the location for the only two potential glycosylation sites within the molecule, asparagine residues 8 and 18. The carbohydrates carried by NCVP5 were of the high-mannose type, Man9GlcNAc and Man8GlcNAc, with the mannose 9 species predominating; no complex oligosaccharides were present. If these asparagine residues are the sites for carbohydrate attachment, this implies that cleavage of the putative signal peptide does not occur during the maturation of this nonstructural glycoprotein.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arias C. F., López S., Espejo R. T. Gene protein products of SA11 simian rotavirus genome. J Virol. 1982 Jan;41(1):42–50. doi: 10.1128/jvi.41.1.42-50.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Walter P., Chang C. N., Goldman B. M., Erickson A. H., Lingappa V. R. Translocation of proteins across membranes: the signal hypothesis and beyond. Symp Soc Exp Biol. 1979;33:9–36. [PubMed] [Google Scholar]
- Bonatti S., Blobel G. Absence of a cleavable signal sequence in Sindbis virus glycoprotein PE2. J Biol Chem. 1979 Dec 25;254(24):12261–12264. [PubMed] [Google Scholar]
- Both G. W., Bellamy A. R., Street J. E., Siegman L. J. A general strategy for cloning double-stranded RNA: nucleotide sequence of the Simian-11 rotavirus gene 8. Nucleic Acids Res. 1982 Nov 25;10(22):7075–7088. doi: 10.1093/nar/10.22.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Mattick J. S., Bellamy A. R. Serotype-specific glycoprotein of simian 11 rotavirus: coding assignment and gene sequence. Proc Natl Acad Sci U S A. 1983 May;80(10):3091–3095. doi: 10.1073/pnas.80.10.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Dyall-Smith M. L., Holmes I. H. Gene-coding assignments of rotavirus double-stranded RNA segments 10 and 11. J Virol. 1981 Jun;38(3):1099–1103. doi: 10.1128/jvi.38.3.1099-1103.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson B. L., Graham D. Y., Mason B. B., Estes M. K. Identification, synthesis, and modifications of simian rotavirus SA11 polypeptides in infected cells. J Virol. 1982 Jun;42(3):825–839. doi: 10.1128/jvi.42.3.825-839.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H., Frischauf A. M., Simons K., Lehrach H., Delius H. Nucleotide sequence of cdna coding for Semliki Forest virus membrane glycoproteins. Nature. 1980 Nov 20;288(5788):236–241. doi: 10.1038/288236a0. [DOI] [PubMed] [Google Scholar]
- Holmes I. H. Viral gastroenteritis. Prog Med Virol. 1979;25:1–36. [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Katz F. N., Rothman J. E., Lingappa V. R., Blobel G., Lodish H. F. Membrane assembly in vitro: synthesis, glycosylation, and asymmetric insertion of a transmembrane protein. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3278–3282. doi: 10.1073/pnas.74.8.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason B. B., Graham D. Y., Estes M. K. In vitro transcription and translation of simian rotavirus SA11 gene products. J Virol. 1980 Mar;33(3):1111–1121. doi: 10.1128/jvi.33.3.1111-1121.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick J. S., Zehner Z. E., Calabro M. A., Wakil S. J. The isolation and characterization of fatty-acid synthetase mRNA from rat mammary gland. Eur J Biochem. 1981 Mar;114(3):643–651. doi: 10.1111/j.1432-1033.1981.tb05192.x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae M. A., Joklik W. K. The nature of the polypeptide encoded by each of the 10 double-stranded RNA segments of reovirus type 3. Virology. 1978 Sep;89(2):578–593. doi: 10.1016/0042-6822(78)90199-x. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C. M., Strauss J. H. Nucleotide sequence of the 26S mRNA of Sindbis virus and deduced sequence of the encoded virus structural proteins. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2062–2066. doi: 10.1073/pnas.78.4.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger S. M., Holmes I. H. Comparison of the genomes of simian, bovine, and human rotaviruses by gel electrophoresis and detection of genomic variation among bovine isolates. J Virol. 1979 Jun;30(3):839–846. doi: 10.1128/jvi.30.3.839-846.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter I., Burstein Y., Zemell R., Ziv E., Kantor F., Papermaster D. S. Messenger RNA of opsin from bovine retina: isolation and partial sequence of the in vitro translation product. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2654–2658. doi: 10.1073/pnas.76.6.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]