Abstract
The multipotential cytokine transforming growth factor-β (TGF-β) is secreted in a latent form. Latency results from the noncovalent association of TGF-β with its processed propeptide dimer, called the latency-associated peptide (LAP); the complex of the two proteins is termed the small latent complex. Disulfide bonding between LAP and latent TGF-β–binding protein (LTBP) produces the most common form of latent TGF-β, the large latent complex. The extracellular matrix (ECM) modulates the activity of TGF-β. LTBP and the LAP propeptides of TGF-β (isoforms 1 and 3), like many ECM proteins, contain the common integrin-binding sequence RGD. To increase our understanding of latent TGF-β function in the ECM, we determined whether latent TGF-β1 interacts with integrins. A549 cells adhered and spread on plastic coated with LAP, small latent complex, and large latent complex but not on LTBP-coated plastic. Adhesion was blocked by an RGD peptide, and cells were unable to attach to a mutant form of recombinant LAP lacking the RGD sequence. Adhesion was also blocked by mAbs to integrin subunits αv and β1. We purified LAP-binding integrins from extracts of A549 cells using LAP bound to Sepharose. αvβ1 eluted with EDTA. After purification in the presence of Mn2+, a small amount of αvβ5 was also detected. A549 cells migrated equally on fibronectin- and LAP-coated surfaces; migration on LAP was αvβ1 dependent. These results establish αvβ1 as a LAP-β1 receptor. Interactions between latent TGF-β and αvβ1 may localize latent TGF-β to the surface of specific cells and may allow the TGF-β1 gene product to initiate signals by both TGF-β receptor and integrin pathways.
INTRODUCTION
The transforming growth factor-βs (TGF-βs)1 are a family of cytokines that affect cell proliferation, extracellular matrix (ECM) synthesis, integrin expression, immune function, and development (Massague, 1990). In mammals, three closely related TGF-β isoforms exist: β1, 2, and 3. Each isoform is a 25-kDa homodimer derived from a precursor protein. TGF-β is usually released from cells in a latent form that results from the continued extracellular noncovalent association of TGF-β with latency-associated peptide (LAP), which is a homodimer of the processed TGF-β propeptide. TGF-β bound to LAP is called the small latent complex (SLC). LAP can disulfide bond to members of another protein family, the latent TGF-β–binding proteins (LTBPs); the latent form of TGF-β thus formed is called the large latent complex (LLC).
TGF-β is secreted predominantly in the LLC form, and it is well established that the LLC can be covalently incorporated into the ECM (Taipale et al., 1994). In cell culture, this can occur by transglutaminase-mediated cross-linking of LTBP-1 to proteins in the ECM (Nunes et al., 1997). Moreover, LTBP-1, either by itself or as part of the LLC, associates specifically with matrix fibrils (Dallas et al., 1995), and LTBP-1 can associate directly with fibronectin (FN) (Taipale et al., 1996). Human LTBP-1 and the β1 and β3 LAP isoforms contain the common integrin recognition sequence RGD, suggesting that LLC is one of a number of ECM proteins recognized by integrins.
The constitutive secretion of latent TGF-β by many cell types in culture suggests that there are extracellular mechanisms to control the activity of this potent cytokine. One such control mechanism is the regulated activation of the latent complex (reviewed by Munger et al., 1997). The ECM and its components participate in the activation process. For example, there is evidence suggesting that the LLC must be incorporated into the ECM before free TGF-β can be formed: in a latent TGF-β activation system consisting of cocultures of endothelial and vascular smooth-muscle cells, activation is dependent on transglutaminase and LTBP-1 (Flaumenhaft et al., 1993; Kojima et al., 1993). Also, several ECM proteins can bind active TGF-β and either enhance or neutralize its activity (Yamaguchi, 1990; Hildebrand et al., 1994; McCaffrey et al., 1994; Takeuchi, 1994). The extracellular adhesive protein thrombospondin-1 is unique in that it both activates latent TGF-β and binds TGF-β in an active form (Schultz-Cherry and Murphy-Ullrich, 1993).
Integrins are heterodimeric cell surface proteins consisting of α and β subunits (Hynes, 1992). The 22 known α/β dimers (at least 15 of which recognize ECM proteins) have distinct and often partially overlapping ligand specificities, and many recognize the tripeptide sequence RGD (Pierschbacher and Ruoslahti, 1984). Activation of integrins can result in signaling events that affect cell growth, differentiation, and survival (Giancotti, 1997). TGF-β is a potent regulator of integrin–substrate interactions. For example, treatment of cells with TGF-β results in up-regulation of several integrin subunits and a more adhesive phenotype (Ignotz and Massague, 1987). Also, TGF-β up-regulates the expression of ECM proteins that are recognized by integrins (e.g., collagens, FN, and laminin) (Ignotz and Massague, 1986). The combined effect of these changes probably accounts for the early observation that TGF-β–treated fibroblasts acquire the ability to form colonies in soft agar (Todaro et al., 1980).
Because latent TGF-β contains RGD sequences and is a constituent of the ECM, we hypothesized that latent TGF-β is an integrin ligand. In this paper we present evidence that some, but not all, cell types adhere and spread on LAP in an RGD-dependent manner, and that the major integrin mediating binding in the cells studied is αvβ1.
MATERIALS AND METHODS
Antibodies and Reagents
Recombinant SLC and LLC (both containing human TGF-β1, LLC also containing human LTBP-1) and human LTBP-1, purified from transfected CHO cells, were gifts of Drs. H. Ohashi and H. Tsumara (Kirin Brewery, Pharmaceutical Division, Gunma, Japan). The SLC is homogeneous as judged by Coomassie brilliant blue stains of SDS-PAGE gels. In the LLC material, there are some proteolytic fragments of LTBP. GRGDSP and GRGESP peptides were from Calbiochem (San Diego, CA). The following mAbs against extracellular integrin domains were used: TS2/7 (anti-α1) from American Type Culture Collection (Rockville, MD); P1E6 (anti-α2), P1B5 (anti-α3), P1D6 (anti-α5), and P1F6 (anti-αvβ5) from Life Technologies (Gaithersburg, MD); GoH3 (anti-α6) from Immunotech (Westbrook, ME); 4B4 (anti-β1) from Coulter (Miami, FL); and LM609 (anti-αvβ3) from D. Cheresh (Scripps Research Institute, La Jolla, CA). All of these mAbs have been characterized as capable of blocking adhesion mediated by the corresponding integrin or integrin subunit and are routinely used and active in our laboratories in immunoprecipitation and blocking experiments. The control mAb MOPC 21 (mouse IgG1, κ) was from Sigma (St. Louis, MO). Rabbit antisera raised against synthetic peptide sequences contained within cytoplasmic domains of αv, α3, α5, β1, β3, and β5 (Klein et al., 1993) were used for immunoprecipitations. Polyclonal rabbit antibody LT-2 against latent TGF-β1, which recognizes LAP, was a gift of C.-H. Heldin (Ludwig Institute for Cancer Research, Uppsala, Sweden). mAb VB3A9 (IgG1) was prepared in our laboratory after immunization of mice with recombinant LAP (TGF-β isoform 1) that was purified to homogeneity after production in a baculovirus system as described below; it binds human and simian LAP (TGF-β isoform 1) in reduced and nonreduced immunoblots, ELISAs, and immunoprecipitations. FN and vitronectin (VN) were purchased from Collaborative Biomedical Products (Bedford, MA) and stored frozen as 1-mg/ml aliquots in PBS. The simian LAP (TGF-β isoform 1) cDNA with a C33S mutation was a gift of R. Derynck (University of California at San Francisco, San Francisco, CA).
Cells and Cell Culture Conditions
A549 cells are derived from a human lung adenocarcinoma and were provided by R. Higgins (New York University, New York, NY). HT-1080 (human fibrosarcoma), MG-63 (human osteosarcoma), CHO K1, and 293 (human embryonic kidney epithelium) cells were from American Type Culture Collection. These cells were cultured in DMEM containing 10% heat-inactivated FCS. Hybridoma cell lines L230 (a gift of H. Chapman, Harvard Medical School, Boston, MA) and 9E10 (a gift of P. Cowen, New York University, New York, NY) were cultured in RPMI-1640 with 10% FCS. Bovine capillary endothelial cells were a gift of S. Klein (New York University, New York, NY) and were isolated and cultured as described (Klein et al., 1993). L230 is a mAb that recognizes αv and blocks αv-integrin–dependent binding (Weinacker et al., 1994); 9E10 is an anti-myc mAb used as a control. To produce hybridoma-conditioned medium, cells were washed two times and suspended in RPMI-1640 with 0.1% BSA and 20 mM HEPES at 106 cells/ml and cultured for 24 h. All media contained penicillin, streptomycin, and glutamine. Cells were cultured at 37°C in a humidified atmosphere of 95% air and 5% CO2.
Production of Recombinant LAP Using Insect Cells
The cDNA encoding simian LAP, with a mutation changing cysteine 33 to serine to prevent disulfide bonding to other peptides, was modified by PCR to add a sequence encoding a Factor Xa cleavage site (IEGR/N) to the 3′ end. A cDNA encoding a protein A–derived purification tag (from the pEZZ 18 vector; Pharmacia, Uppsala, Sweden) was then ligated in-frame to the new 3′ end. The resulting construct encodes a protein consisting of the entire LAP sequence, a Factor Xa cleavage site, and a C-terminal protein A tag. The fusion protein can be purified with IgG-Sepharose, and LAP can be released by treatment with Factor Xa. This construct was further modified by PCR mutagenesis to alter the codon encoding Asp-246 of LAP (GAC) to one encoding Glu (GAA). This allows expression of a LAP mutant in which the RGD site is changed to RGE. Both sequences were verified by sequencing. The full sequences and details of synthesis are available from the authors. Each construct was cloned into the baculovirus transfer vector pVL1392 (PharMingen, San Diego, CA). Sf9 insect cells were cotransfected with the plasmid and linearized AcNPV baculoviral DNA (PharMingen). The resulting viruses were plaque purified. Viral clones were screened by infecting Sf9 cells for 48 h and immunoblotting conditioned medium with anti-LAP mAb VB3A9. Clones were expanded by two rounds of Sf9 infection.
For production of recombinant LAP for adhesion assays, subconfluent Sf9 cells were infected with recombinant baculovirus and cultured for 3 d in normal growth medium (Hink’s TNM-FH with 10% heat-inactivated FCS). Medium was centrifuged to remove cells and incubated with IgG-Sepharose (Pharmacia) at 4°C while rotating overnight. Three to 5 ml of washed beads were used per liter of medium. The beads were washed with 10 vol of PBS, 20 vol of PBS with 1% Triton X-100, 20 vol of PBS, and 5 vol of Factor Xa buffer (20 mM Tris, pH 8, 100 mM NaCl, 2 mM CaCl2). The beads were suspended in 0.6 vol of Factor Xa buffer and treated with Factor Xa (60 μg/l of starting material; Boerhinger Mannheim, Indianapolis, IN) for 4 h at room temperature. The beads were removed by centrifugation, and factor Xa was removed by incubation with soybean trypsin inhibitor agarose beads (Sigma; 5-μl beads/μg of Factor Xa) at room temperature for 1 h.
To produce 20 mg of recombinant LAP for preparation of an affinity column, we used High Five insect cells (Invitrogen, Carlsbad, CA) because yields of LAP were two- to fivefold higher than from Sf9 cells. The procedure was identical, except that cells were cultured in EX-CELL 405 serum-free medium (JRH Biosciences, Lenexa, KS). Yields were 2–4 and 5–10 mg of LAP/l of medium for Sf9 and High Five cells, respectively. The insect cell–derived LAP consists of the entire modified simian LAP sequence and an additional four amino acids at the C terminus (IEGR, single-letter amino acid code) derived from the Factor Xa site.
Adhesion Assays
Ninety-six–well plates (Immulon plates; Dynatech Laboratories, Chantilly, VA) were coated with solutions of test proteins at concentrations up to 100 μg/ml in PBS for 1 h at 37°C and blocked with PBS and 1% BSA for at least 30 min at 37°C. In some experiments, anti-LAP mAb or control mAb was added at concentrations up to 100 μg/ml of solution in PBS for 1 h at 37°C after the coating and blocking steps. The wells were washed once with PBS just before addition of cells. Each experimental condition was tested in triplicate.
The cells to be tested were split 1:5 the day before assay. One hour before detaching the cells the medium was supplemented with 15 μg/ml cycloheximide. All subsequent buffers contained cycloheximide at the same concentration and 1 μM monensin. The cells were detached with PBS and 10 mM EDTA, washed three times in DMEM and 0.1% BSA, and suspended at 5.0–7.5 × 105 cells/ml in the same medium. One hundred microliters of cell suspension were added to each well. In experiments using antibodies or peptides, cells were incubated with the reagents for 5 min at room temperature before addition to the wells. Plates were incubated at 37°C for 1.5 h and washed twice with PBS to remove nonadherent cells. Cells were fixed with 3.7% paraformaldehyde in PBS for at least 30 min, permeabilized with 20% methanol for 20 min, and stained with 0.5% crystal violet in 20% methanol for 20 min. Excess stain was removed by rinsing with water. To quantitate cell binding, stain was eluted with 100 mM sodium citrate, pH 4, in 50% ethanol (60 μl/well), and the absorbance at 600 nm was measured. The absorbance of wells coated only with BSA before addition of cells was subtracted from each measurement. The results are expressed as the net absorbance multiplied by 1000 or as a ratio of the net absorbance to that of a control condition. For photomicrographs of adherent cells, cells were fixed as above and stained with Diff-Quik stain (Baxter Healthcare, Miami, FL).
ELISA
Ninety-six–well ELISA plates (Nunc Immunoplates; Nunc, Roskilde, Denmark) were coated with test proteins (LAP, FN, and VN) at 10 μg/ml in PBS at 37°C for 3 h. Wells were washed with PBS with 0.05% Tween (PBS/Tween) thrice. Dilutions of antibody (VB3A9 or MOPC 21) were added in 100 μl of PBS/Tween and incubated at 37°C for 1 h. Wells were washed with PBS/Tween thrice and incubated with secondary antibody (rabbit anti-mouse IgG conjugated with alkaline phosphatase, 1:5000) for 1 h at 37°C. Wells were washed three times with PBS/Tween and incubated with 1 mg/ml p-nitrophenyl phosphate in substrate buffer (Sigma) for 30 min at room temperature. Absorbances at 410 nm were measured in an ELISA plate reader. For each mAb concentration, the result for MOPC 21 was used as a blank. All antibody dilutions were tested with both VB3A9 and MOPC 21 in triplicate.
Affinity Purification of LAP-binding Integrins
An affinity column was prepared by coupling 20 mg of recombinant LAP (produced by High Five insect cells) to 3 ml of CNBr-activated Sepharose 4B (Pharmacia) according to the manufacturer’s instructions. The purification procedure was a modification of the method for the purification of the FN receptor (Pytela et al., 1987). Ten to 24 10-cm dishes of confluent A549 cells were washed with PBS. Cells were detached with PBS and 10 mM EDTA and washed twice in DMEM and 0.1% BSA, twice in Tris-buffered saline (TBS) with 1 mM CaCl2 (TBS/Ca). The cell pellet was suspended in an equal volume of TBS/Ca. Cell surface proteins were labeled with 1 mCi of Na125I and lactoperoxidase-H2O2 as described (Klein et al., 1993). Cells were washed five times in TBS/Ca with 0.02% sodium azide and lysed with 2 vol of 50 mM octyl-β-d-thioglucopyranoside (OSGP; Calbiochem) and 3 mM PMSF in TBS containing 1 mM each CaCl2, MgCl2, and (in one experiment) MnCl2 for 10 min at room temperature. Lysates were clarified by centrifugation at 16,000 × g for 15 min.
The LAP affinity column was equilibrated in the same buffer used for lysis, with the exception that the OSGP concentration was 25 mM (wash buffer). Lysate was loaded onto the column over a 1-h period. The column was washed until eluted radioactivity reached a minimum (requiring 15–40 ml of wash buffer), eluted with 8 ml of PBS with 25 mM OSGP and 10 mM EDTA for 1 h (collected in 1-ml fractions), and further eluted with 6 M urea. Fifty-microliter aliquots of each fraction were analyzed by SDS-PAGE and autoradiography. In the purification done in the presence of MnCl2, the remainder of selected pooled fractions was made 1% Triton X-100, 0.01% BSA and dialyzed against TBS/Ca before immunoprecipitation.
Immunoprecipitation of Affinity-purified Integrins
Five microliters of antiserum or nonimmune serum were added to ∼800 μl of sample and incubated at 4°C for 1 h. Fifty microliters of protein A-agarose (Boerhinger Mannheim) were added to each sample and incubated with rotation for 1 h at 4°C. Pellets were washed four times with TBS/Ca and 1% Triton X-100 and boiled in sample buffer. Samples were separated by nonreducing SDS-PAGE and visualized by autoradiography.
Cell Motility Assay
Cell motility was assessed by substrate-specific migration through a filter in a Boyden chamber. Filters (Nucleopore polycarbonate filters, 13-mm diameter, 8-μm pores; Costar, Cambridge, MA) were coated with 50-μg/ml solutions of LAP or FN in PBS on one or both sides, or 1% BSA in PBS on one side, for 2 h at 37°C. Filters were washed briefly in DMEM and 0.1% BSA and placed in Boyden chambers, shiny side up and (where relevant) protein-coated side down. DMEM and 0.1% BSA were used in top and bottom chambers. A549 cells were detached and washed as in adhesion assays. Cells (105) in 150 μl of medium were added to the top chamber. After 6 or 16 h, filters were removed. Cells on the top of each filter were removed by rubbing with a cotton swab. Filters were rinsed briefly in PBS, fixed for 10 s in methanol, rinsed in PBS, and stained for 30 min with 0.5% toluidine blue and 3.7% paraformaldehyde in PBS. Filters were washed extensively with water and examined under the microscope. Migration was quantitated by eluting the retained dye with 300 μl of 10% acetic acid, measuring the absorbance at 600 nm of the resulting solution, and subtracting the absorbance at 600 nm obtained from the same procedure done with filters coated with BSA. The values displayed are the net absorbances multiplied by 1000.
RESULTS
A549, MG-63, and BCE Cells Attach and Spread on LAP, SLC, and LLC in an RGD-dependent Manner
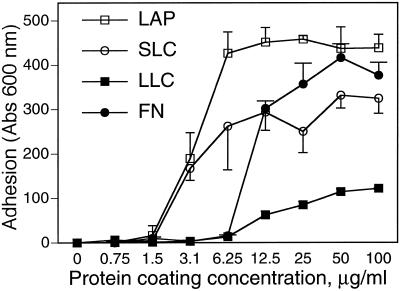
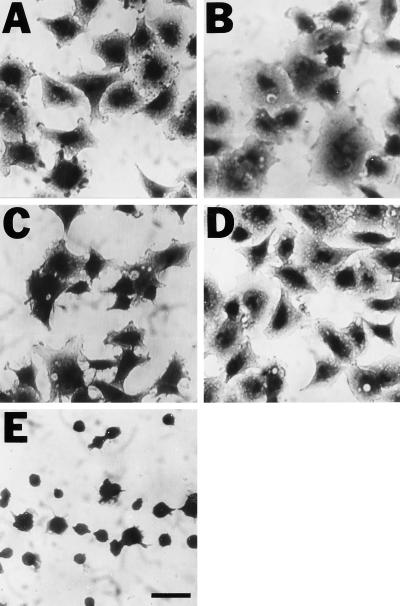
To test the hypothesis that one or more integrins can bind to latent TGF-β, we did cell adhesion assays in which cells are allowed to attach to proteins immobilized on plastic. In preliminary experiments we found that A549 cells adhered strongly and reproducibly to latent forms of TGF-β and therefore selected this cell type for detailed characterization. As shown in Figure 1, A549 cells adhered to LAP and SLC approximately as well as they adhered to FN. Binding to LLC was less avid. Because there is some degradation of the LTBP in the LLC preparation, we considered the possibility that a constituent of the sample had a toxic or antiadhesive effect. However, coating plastic with both SLC and LLC did not reduce the adhesion seen with the same amount of SLC coated alone (our unpublished results). In multiple experiments, we found no binding to immobilized LTBP-1 (our unpublished results). Adherence to LAP, SLC, and LLC was associated with cell spreading (Figure 2). Spreading was also seen in cells attached to FN but not in cells attached to poly-d-lysine (Figure 2).
Figure 1.
A549 cell adhesion to LAP, SLC, LLC, and FN. (A) Wells were coated with solutions of LAP, SLC, LLC, and FN, at various concentrations as shown, and blocked with BSA. A549 cells were allowed to adhere to the coated wells for 90 min, and nonadherent cells were washed off. Absorption at 600 nm indicates the amount of stain associated with adherent cells and is proportional to cells bound.
Figure 2.
A549 cells adhere and spread on plastic coated with LAP (A), SLC (B), LLC (C), and FN (D) but not on plastic coated with poly-d-lysine (E). Bar, 100 μm.
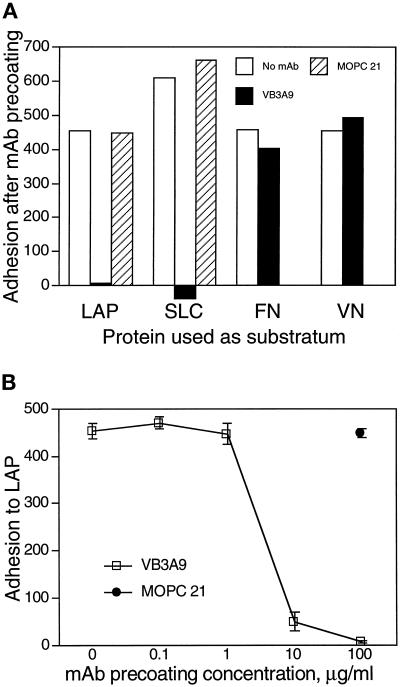
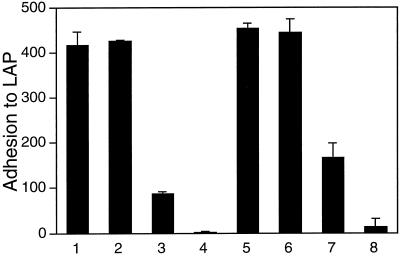
To show that adhesion to immobilized LAP and SLC is specifically attributable to LAP (and not to TGF-β1 or an impurity), we performed adhesion assays in which immobilized SLC was treated with a mAb against LAP before addition of the cells. Preincubation with the anti-LAP mAb VB3A9, but not with a control mAb, abolished A549 cell adhesion to LAP and SLC (Figure 3A), as well as to LLC (our unpublished results). We observed the same blocking effect using the polyclonal anti-LAP antibody LT-2 with immobilized SLC (our unpublished results). We did the same type of experiment using immobilized FN and VN. A549 adherence to FN and VN was not affected by preincubation with VB3A9 (Figure 3A), indicating that the blocking effect observed with immobilized LAP is not due to a nonspecific effect of VB3A9 on adhesion to RGD-containing proteins. The blocking effect of VB3A9 is dose dependent (Figure 3B).
Figure 3.
A mAb to LAP blocks adhesion of A549 cells to LAP and SLC. Adhesion assays were done as described in Figure 1 using wells coated with 50-μg/ml solutions of LAP, SLC, FN, and VN. After being coated with a specific protein and blocked with BSA, wells were incubated with 0.1- to 100-μg/ml solutions of the mAbs VB3A9 (anti-LAP) and MOPC 21 (irrelevant control) and then washed with PBS and used for measuring cell adhesion. In A, mAbs were used at 100 μg/ml. (Binding to SLC was measured in a separate experiment; the greater binding to SLC than to LAP in the absence of mAb likely represents a difference in the number of cells added.) (B) The ability of VB3A9 preincubation to block adhesion to LAP is dose dependent.
These results suggest that VB3A9 recognizes an epitope specific to LAP and not an RGD-related epitope present in other RGD-containing proteins. To further demonstrate the specificity of the VB3A9 mAb, we did ELISAs to measure the interaction of soluble VB3A9 with immobilized LAP, FN, and VN. Concentrations of VB3A9 as low as 62 ng/ml gave an easily discernable signal with immobilized LAP, but VB3A9 concentrations as high as 2 μg/ml resulted in no measurable signal using immobilized VN and FN (our unpublished results).
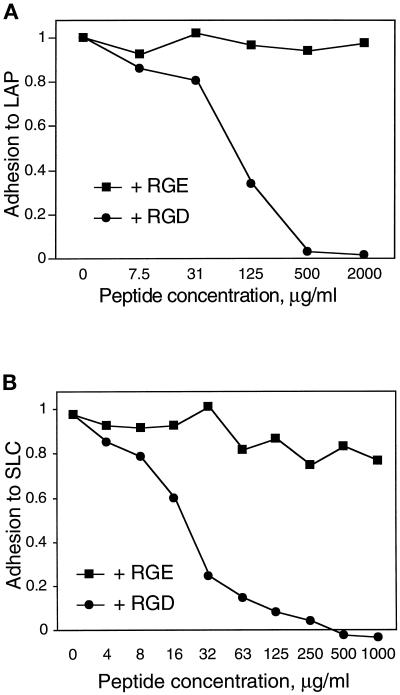
If the observed adhesion was due to integrin binding to the RGD sequence in LAP, adhesion should be blocked by competing concentrations of soluble RGD peptide. Indeed, an RGD-containing peptide (GRGDSP) blocked adhesion of A549 cells to LAP (Figure 4A) and SLC (Figure 4B), whereas a control peptide (GRGESP) had no effect.
Figure 4.
An RGD-containing peptide blocks adhesion of A549 cells to LAP and SLC. Adhesion of A549 cells to wells coated with 33 μg/ml LAP (A) or SLC (B) was measured as described in Figure 1. Cells were incubated with the indicated concentrations of an RGD-containing peptide or control (RGE) peptide during the adhesion period. Adhesion is normalized to cells bound to poly-d-lysine in the absence of peptide.
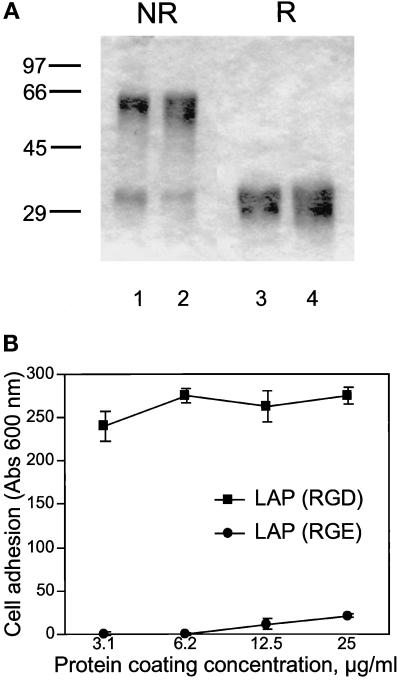
To confirm that the observed binding requires the RGD site in LAP, we prepared a modified form of LAP in which the RGD sequence is mutated to RGE. The two forms of purified recombinant LAP are homogeneous and appear identical on SDS-PAGE (Figure 5A). When run nonreduced, most of the protein migrates as ∼60-kDa dimers, with a small amount of monomer migrating at ∼30 kDa. There are two species of slightly different mass (29 and 30 kDa) seen in the reduced lanes. These likely represent nonglycosylated and glycosylated forms of the monomer; the predicted mass of the monomer (including the four–amino acid extension at the C terminus derived from the Factor Xa cleavage site) is 29 kDa. Both forms are functional in that they neutralize TGF-β1 activity in a bioassay (McMahon et al., 1996; our unpublished results). However, in cell adhesion assays the RGE mutant form is unable to support A549 cell adhesion (Figure 5B).
Figure 5.
A549 cells do not bind to a mutant form of recombinant LAP lacking the RGD site. (A) Recombinant LAP is homogeneous by SDS-PAGE. LAP was produced in insect cells and purified by affinity chromatography as described in MATERIALS AND METHODS. One form consists of the simian LAP sequence with a C33S mutation (lanes 2 and 4); the other form is identical except that the RGD sequence is changed to RGE (lanes 1 and 3). Three micrograms were loaded per lane. Samples were run reduced (R) or nonreduced (NR) and stained with Coomassie brilliant blue. (B) The two forms of recombinant LAP were coated on plastic microtiter wells and used for adhesion assays with A549 cells, as described in Figure 1.
We performed similar adhesion assays using additional cell types. Bovine capillary endothelial cells and MG-63 osteosarcoma cells adhered to LAP in an RGD-dependent manner (our unpublished results). However, HT-1080 fibrosarcoma cells and human lung fibroblasts (explanted from a normal adult lung) adhered poorly or not at all to LAP and SLC (our unpublished results). Several other cell types (bovine aortic endothelial cells, bovine smooth-muscle cells, UMR-106 osteosarcoma cells, and H441 lung adenocarcinoma cells) also adhered to SLC but were not tested further.
A549 Cell Attachment Is Blocked by Antibodies to αv- and β1-Integrin Subunits
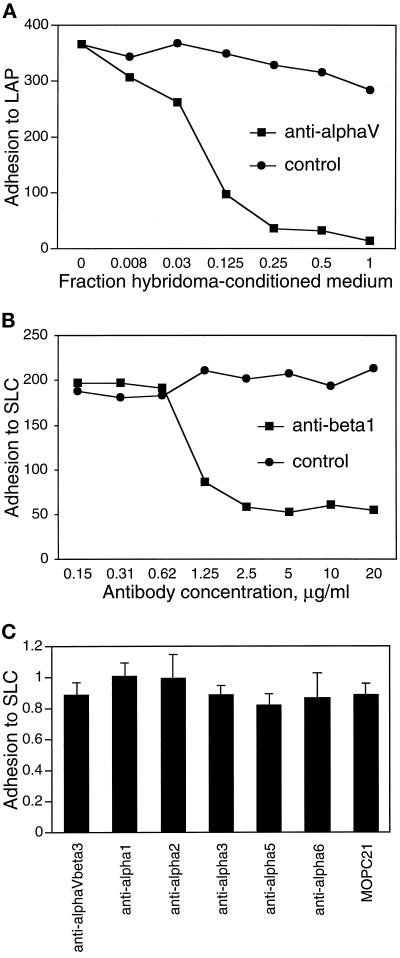
We tested the ability of several anti-integrin blocking mAbs to interfere with A549 cell adherence to LAP. A549 cells have been shown to express the αvβ3, αvβ5, and α3β1 integrins (Falcioni et al., 1994) and may express others. Anti-αv mAb L230 completely blocked adhesion to LAP (Figure 6A) and SLC (our unpublished results). Anti-β1 mAb 4B4 blocked 75% of the adhesion to SLC (Figure 6B). In contrast, mAbs recognizing αvβ3 and α subunits 1, 2, 3, 5, and 6 (Figure 6C) had no effect on A549 cell adhesion to SLC. These results suggest that the integrin αvβ1 is the predominant integrin in A549 cells that mediates binding to LAP. Because the 293 human kidney epithelial cell line has been shown to express the αvβ1 integrin (Bodary and McLean, 1990), we tested the binding of these cells to LAP. 293 cells adhered and spread on plastic coated with a 25-μg/ml solution of LAP, and adhesion was blocked completely by mAbs against αv and β1 but not by mAbs against αvβ3 or αvβ5 (our unpublished results).
Figure 6.
mAbs against integrin subunits αv and β1 block adhesion of A549 cells to LAP. Adhesion of A549 cells to wells coated with 50 μg/ml LAP or SLC was determined as described in Figure 1, in the presence of the indicated concentrations of mAbs against integrins αv (A) and β1 (B). L230 (anti-αv) was used as dilutions of hybridoma-conditioned medium; medium conditioned by the irrelevant hybridoma 9E10 was used as a control. MOPC 21 served as a control for the anti-β1 mAb 4B4. (C) Blocking mAbs against other integrins or integrin subunits had no significant effect on A549 cell adhesion to SLC. Anti-αvβ3 was used at 20 μg/ml; all other mAbs were used at 25 μg/ml. Control attachment levels for the different conditions were similar. The mAbs used are listed in MATERIALS AND METHODS.
Integrins αvβ1 and αvβ5 Bind to a LAP Affinity Column
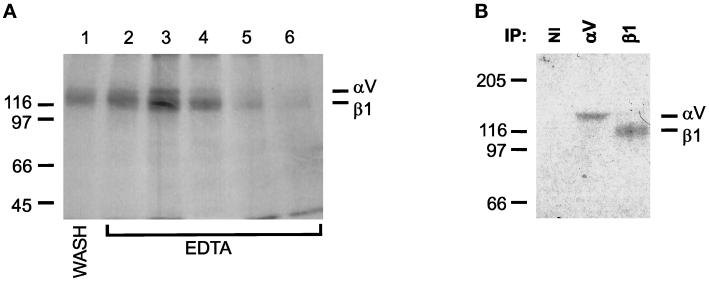
To isolate the integrins that bind to LAP, we used a LAP affinity column to purify LAP-binding proteins from A549 cells. We chose binding conditions originally defined for isolation of the FN receptor (Pytela et al., 1985). Under these conditions (which include 1 mM concentrations of Ca2+ and Mg2+), two bands were eluted from the column by EDTA (Figure 7A). The bands migrated at the expected positions for αv (top band) and β1 (bottom band). Immunoprecipitation of the eluate with antisera against the cytoplasmic domains of αv and β1 in each case recovered the respective bands, whereas nonimmune serum did not (Figure 7B). This result indicates that αvβ1 bound to the column and was eluted with EDTA. However, the yield of eluted integrin in this experiment was poor, and some integrin apparently was released from the column in the wash before EDTA elution (Figure 7A, lane 1).
Figure 7.
Affinity purification of LAP-binding integrins. Recombinant LAP was coupled to Sepharose 4B beads as described in MATERIALS AND METHODS. A549 cells were surface labeled with 125I and extracted with OSGP. Extracts were loaded on the LAP column and washed in buffer containing 1 mM Ca2+ and Mg2+. Retained proteins were eluted with 10 mM EDTA and collected in 1-ml fractions. (A) EDTA-eluted integrins. Aliquots of each fraction were separated by SDS-PAGE and autoradiographed. Lane 1, last wash fraction; lanes 2–6, elution with EDTA. (B) Immunoprecipitation with polyclonal Abs against cytoplasmic domains of αv and β1. Fractions 2–6 were pooled; equal volumes were immunoprecipitated with antisera to αv and β1 or with nonimmune serum (NI). Immunoprecipitates were separated by SDS-PAGE and autoradiographed. Gels were 8% and nonreducing. The positions of protein standards are shown to the side of each gel (masses in kilodaltons).
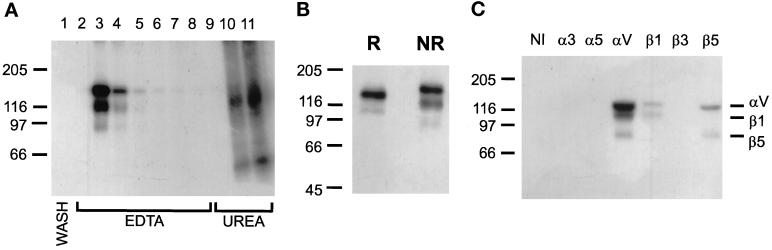
Because manganese can increase the affinity of integrin binding (Gailit and Ruoslahti, 1988), we performed an experiment in which Mn2+ was added to the lysis and wash buffers. Under these conditions, there was no detectable protein in the last wash fraction (Figure 8A, lane 1), and more iodinated protein of the same molecular weights observed previously was eluted with EDTA. However, we observed a faint third iodinated band in the EDTA elution fractions, migrating below the αv and β1 positions at the expected position for β3 or β5 (Figure 8A). Because some integrin subunits are disulfide linked, we compared the migration of the eluted integrins under reducing and nonreducing conditions (Figure 8B). The αv and β1 bands comigrated under reducing conditions, a result reported by others (Pytela et al., 1985; Nesbitt et al., 1993). After dialysis against CaCl2-containing buffer to promote heterodimer formation and stability, we immunoprecipitated the eluted proteins with antisera against α and β subunits as indicated in Figure 8C. Antisera against αv and β1 precipitated the top two bands; anti-αv serum also precipitated the bottom band. The antiserum against β5 precipitated the top and bottom bands. Thus, in the presence of Ca2+, Mg2+, and Mn2+, αvβ1 and αvβ5 bound to LAP.
Figure 8.
Affinity purification of LAP-binding integrins in the presence of Mn2+. LAP-Sepharose affinity chromatography was done as described in Figure 8, except that buffers contained 1 mM Ca2+, Mg2+, and Mn2+. (A) Autoradiograph of fractions from the LAP affinity column, run nonreduced on a 7% gel. Lane 1: last wash fraction; lanes 2–8, elution with EDTA; lanes 9–11, elution with 6 M urea. (B) The EDTA-eluted fractions shown in B were pooled. Aliquots were electrophoresed under reducing (R) and nonreducing (NR) conditions on 7% gels and autoradiographed. (C) The same sample was divided into equal aliquots and immunoprecipitated with polyclonal antisera to cytoplasmic domains of the indicated integrin subunits or with nonimmune rabbit serum (NI). Immunoprecipitates were separated nonreduced on 7% gels and autoradiographed.
Antibodies to β1-Integrins and αvβ5 Have an Additive Effect on Inhibition of A549 Cell Binding to LAP
The affinity purification experiments indicated that both αvβ1 and αvβ5 can bind to LAP. To clarify the relative contribution of these two integrins in the adhesion of A549 cells to LAP, we performed adhesion experiments with blocking antibodies against β1 and αvβ5, singly or combined. Compared with control adhesion of A549 cells to LAP (Figure 9, bar 1), anti-αvβ5 mAb did not block adhesion, whereas anti-β1 blocked most, but not all, adhesion (Figure 9, bars 2 and 3, respectively). However, the two antibodies combined eliminated the residual binding seen with anti-β1 alone (Figure 9, bar 4). The results of these adhesion assays suggest that weak adhesion attributable to αvβ5 is revealed only when αvβ1 adhesion is eliminated. The same pattern of changes in adherence was seen when the cells adhered in the presence of Mn2+ (Figure 9, bars 5–8). However, the β1-independent adherence was increased slightly by Mn2+ (Figure 9, bar 3 compared with bar 7), suggesting that Mn2+ increased the affinity of the LAP–αvβ5 interaction. The same relative amounts of binding, including the small increase in β1-independent adherence in the presence of Mn2+, was seen in each of the three independent experiments we performed.
Figure 9.
Effect of αvβ5 blocking antibody on A549 cell adhesion to LAP. Adhesion of A549 cells to immobilized LAP was measured as described in Figure 1, with the addition of mAbs to β1 (4B4, 10 μg/ml), αvβ5 (P1F6, 1:1000 dilution of ascites fluid), or both during the adhesion period. Adhesion was tested with or without addition of 0.5 mM MnCl2 to the medium. Additions are indicated in the bottom panel.
To confirm that the P1F6 mAb was active in these experiments, we did control binding experiments with CHO K1 cells. Although P1F6 was generated using human αvβ5 as immunogen, it functions across species and is able to block CHO K1 cell adhesion to VN (Weinacker et al., 1994). Our results show that the P1F6 used in these experiments is active; P1F6 concentrations of 1:1000 dilution or greater blocked 70–90% of CHO K1 adhesion to VN (our unpublished results).
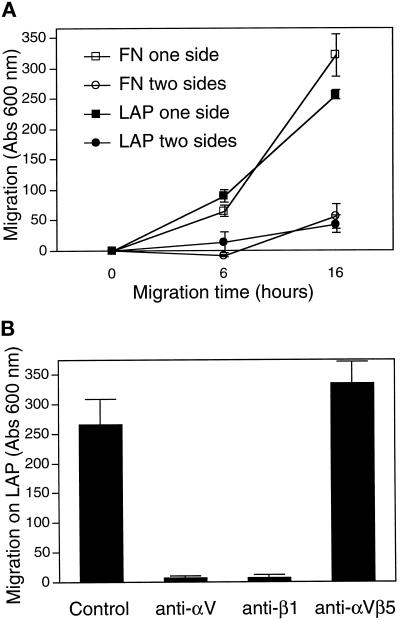
A549 Cells Are Able to Migrate on LAP
The function of αvβ1 is not known. αvβ1 expressed in CHO cells can bind FN but cannot by itself promote cell migration on a FN-coated surface (Zhang et al., 1993). It is not known whether this lack of migration is due to an intrinsic deficiency of the αvβ1 integrin in promoting migration or due to a deficiency in FN as a ligand for αvβ1. To clarify this issue, we did similar motility assays to see whether A549 cells could migrate on LAP using the αvβ1 integrin. The assay measures the ability of cells to migrate through a porous filter; the cells start from a nonadhesive side and pass to the second side, which is coated with a test protein. Motility of A549 cells on LAP and FN was similar (Figure 10A). To confirm that migration on LAP was due to the αvβ1 integrin, we did motility assays in the presence of blocking mAbs. Migration was blocked by mAbs against αv and β1 but not by mAb against αvβ5 (Figure 10B). Because the 4B4 mAb stock solution contains sodium azide, we did controls to show that A549 cell migration on LAP was not affected by either heat-inactivated 4B4 or by sodium azide alone at the same final concentration (our unpublished results).
Figure 10.
A549 cells migrate equally on LAP and FN. (A) Cell motility assays were performed in Boyden chambers as described in MATERIALS AND METHODS. The results indicate the number of cells that migrated from the top to the bottom of a porous filter that was coated on the bottom or on both sides with a test protein. (B) Cells were allowed to migrate on LAP as in A for 16 h, in the presence or absence of mAbs to αv (50% L230 hybridoma medium), β1 (10 μg/ml 4B4), or αvβ5 (1:500 dilution of P1F6 ascites fluid).
DISCUSSION
Because it affects both integrin expression and matrix molecule synthesis, TGF-β is a major regulator of cell attachment. In addition, in both its latent and free forms TGF-β is itself a matrix-associated molecule (Taipale and Keski-Oja, 1997). Isoforms 1 and 3 of LAP contain the RGD sequence, suggesting that latent TGF-β may be an integrin ligand. In this paper, we show that LAP isoform 1 is a ligand for the αvβ1 integrin. This conclusion is based on the observations that A549 cells bind LAP (or latent TGF-β complexes containing LAP), that adhesion is blocked by anti-LAP mAb, RGD peptide, and antibodies to integrin subunits αv and β1, and that αvβ1 can be purified from cell extracts with a LAP affinity column. The inability of cells to adhere to a mutant LAP lacking the RGD site indicates that the integrin binding is indeed to the RGD site of LAP and not to another site on LAP or to an impurity.
The avidity of cell binding to LAP or latent complexes is LAP > SLC > LLC. This order suggests that the integrin binding site, the RGD sequence located 33 amino acids from the COOH terminus of LAP, is less exposed for integrin interactions when LAP is complexed with TGF-β and/or LTBP than when LAP is in the unbound state. A change in the exposure of the RGD region related to complex formation is consistent with the fact that LAP undergoes a major conformational change upon binding TGF-β (McMahon et al., 1996). We cannot exclude the possibility that LAP-binding integrins recognize only non-TGF-β–bound LAP for two reasons. First, ∼5–10% of the TGF-β in the recombinant SLC used in these experiments is active, so that some free LAP is present. Second, it is possible that when latent TGF-β binds to plastic, the latent complex conformation of LAP is altered in a way that exposes the RGD site.
To our knowledge these are the first data directly showing a specific integrin–LAP interaction, although Yang et al. (1997) have found RGD-dependent FET cell adhesion to recombinant LAP produced in bacteria. Grainger et al. (1995) reported that human platelets contain both SLC and LLC and that SLC is specifically retained within blood clots and can be liberated by incubation with RGD peptide. One explanation for the the observations reported by Grainger et al. (1995) is that LAP is bound by one or more platelet integrins (e.g., αIIbβ3) at the RGD site. If this explanation is correct, the fact that SLC is bound preferentially is consistent with our result that SLC is a better integrin ligand than is LLC.
Human LTBP-1 contains an RGD sequence within the ninth EGF-like domain. However, we found no evidence that this sequence functions as an integrin ligand in A549 cells. Purified LTBP-1 did not support A549 cell adhesion, and adhesion to LLC was completely blocked by an anti-LAP mAb. These facts, and the observation that murine LTBP-1 does not contain an RGD sequence, suggest that the RGD sequence in human LTBP-1 is not related to integrin binding.
In A549 cells, αvβ1 is responsible for most of the adhesion to LAP. Two observations indicate that αvβ5 interacts only weakly with LAP. First, αvβ5 was detected after LAP affinity chromatography only in the presence of Mn2+, whereas αvβ1 was isolated in its absence. Second, when used alone anti-αvβ5 mAb did not inhibit cell binding but inhibited only the small amount of β1-independent cell binding. However, the weak αvβ5 effect in the cells we tested might also be due simply to low levels of this integrin relative to αvβ1. Thus, our results do not rule out a role for αvβ5 in binding LAP in other systems. Also, it is possible that other RGD-binding integrins not expressed in A549 cells may bind LAP or LTBP.
Most known integrin ligands are ECM proteins, cell surface receptors of the Ig superfamily, or plasma proteins of the complement or coagulation systems (Hynes, 1992). Recent reports have described novel integrin ligands, including matrix metalloproteinase-2 (Brooks et al., 1996) and fibroblast growth factor-2 (Rusnati et al., 1997), both of which interact with αvβ3, and insulin-like growth factor–binding protein-1, which interacts with α5β1 (Jones et al., 1993). Our results define an additional “atypical” integrin ligand.
αvβ1 is an unusual integrin in that it is composed of two “promiscuous” subunits; that is, both αv and β1 form dimers with multiple partners. αvβ1 has been reported to bind FN, VN, and osteopontin at RGD sites (Bodary and McLean, 1990; Vogel et al., 1990; Hu et al., 1995). VN and osteopontin are also ligands of αvβ5. The β1 subunit can exist in several isoforms, one of which (β1B) has a dominant negative effect on cell adhesion (Balzac et al., 1994). Calcium abrogates the magnesium-promoted binding of αvβ1 to FN in cell-free assays (Kirchhofer et al., 1991); this phenomenon is consistent with the relatively poor binding of αvβ1 to the LAP affinity column in the presence of Ca2+ and Mg2+.
A specific function for αvβ1 has not been defined, and in fact the most detailed studies of its function suggest that it is nonfunctional in many respects. Zhang et al. (1993, 1995) reported that in cells transfected to express either αvβ1 or α5β1 as the sole FN receptor, only cells expressing α5β1 could migrate on FN, assemble an FN matrix, and survive serum deprivation when plated on FN, whereas both cell types could adhere to FN. The results of Zhang et al. (1993, 1995) could be due to the lower affinity of αvβ1 for FN or the inability of αvβ1 to localize to focal adhesions in these cells. In contrast, we found that A549 cells migrated on LAP- and FN-coated surfaces equally. Thus, although our experiments are not directly analagous to those of Zhang et al. (1993, 1995), our results show that there is no intrinsic defect in the ability of αvβ1 to mediate migration. The difference in αvβ1-mediated migration on LAP and FN may be due to differences in the cells used but could also be explained by a qualitative difference between the ligands: LAP, in contrast to FN, functions as an activating ligand.
Several possible consequences of αvβ1–LAP interactions can be envisioned. For example, it is possible that this interaction is involved in the activation of latent TGF-β. Work by our laboratory and others has suggested a model of activation in which latent TGF-β released from matrix is localized to the cell surface for activation (Munger et al., 1997); αvβ1 might serve this surface localization role, although the mannose-6-phosphate/insulin-like growth factor-2 receptor has already been implicated in this regard (Dennis and Rifkin, 1991). Expression of αvβ1 may not be sufficient for TGF-β activation, because media conditioned by A549 and MG-63 cells contain TGF-β1 only in the latent form (our unpublished results), but may be required under certain conditions. Experiments to detect more subtle activation of TGF-β occurring only at the cell surface, a process that might be influenced by integrin–LAP interactions, are in progress.
Another possible consequence of αvβ1–LAP interactions is the modulation of latent TGF-β incorporation into the ECM. Both LTBP and LLC associate with the ECM (Taipale et al., 1994; Dallas et al., 1995) and more recently have been shown to colocalize with FN and assemble into at least two distinct types of fibrillar structure (Taipale et al., 1996). Many questions remain about the assembly of LTBP and LLC into the ECM, including the relative amounts of LLC and free LTBP involved, the number of different structures or proteins with which LTBP and LLC can associate, and what the functional consequences of the various binding states are. αvβ1 might influence this poorly understood process by modulating the amount of LLC incorporated into the matrix (by analogy to the requirement for FN receptors for FN matrix formation) (Akiyama et al., 1989) and/or by favoring specific structural associations over others.
A final possibility is that integrin binding to LAP creates a signal that acts in addition to classical TGF-β signaling. The fact that A549 cells spread and migrate on LAP-coated surfaces implies outside-in signaling as a result of αvβ1–LAP binding. Because our data suggest that free LAP is a better integrin ligand than latent complexes, one can speculate that such an integrin-derived signal would increase after latent TGF-β activation. (This might depend on the mechanism of activation; for example, extracellular proteinases might degrade the binding site). Others have shown synergism between integrin and tyrosine kinase growth factor receptor signaling (reviewed by Giancotti, 1997). The extent of such relationships between TGF-β receptor and integrin signaling and the role integrin–LAP interactions in particular might play remain to be determined.
ACKNOWLEDGEMENTS
We acknowledge the Kirin Brewery, D. Cheresh, and H. Chapman for generous gifts of cells and reagents. We thank K. Wary for assistance with reagents, S. Klein and R. Mazzieri for discussions and ideas, K. Hubbard for editorial advice, and J. Lee for technical assistance. These studies were supported by National Institutes of Health (NIH) grants RO1CA23753 (D.B.R.) and RO1CA58976 (F.G.G). J.S.M. is the recipient of a Clinical Associate Physician award from the NIH and the New York University–Bellevue Hospital Center General Clinical Research Center (grant MO100096) and gratefully acknowledges support from the Inger-Ma Sonneborn Fund. J.G.H. is the recipient of grant GM07308 from the NIH.
Abbreviations used:
- ECM
extracellular matrix
- FN
fibronectin
- Ig
immunoglobulin
- LAP
latency-associated peptide
- LLC
large latent complex
- LTBP
latent TGF-β–binding protein
- OSGP
octyl-β-d-thioglucopyranoside
- SLC
small latent complex
- TBS
Tris-buffered saline
- VN
vitronectin
REFERENCES
- Akiyama SK, Yamada SS, Chen WT, Yamada KM. Analysis of fibronectin receptor function with monoclonal antibodies: roles in cell adhesion, migration, matrix assembly, and cytoskeletal organization. J Cell Biol. 1989;109:863–875. doi: 10.1083/jcb.109.2.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzac F, Retta SF, Albini A, Melchiorri A, Koteliansky VE, Geuna M, Silengo L, Tarone G. Expression of beta1B integrin isoform in CHO cells results in a dominant negative effect on cell adhesion and motility. J Cell Biol. 1994;127:557–565. doi: 10.1083/jcb.127.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodary SC, McLean JW. The integrin β1 subunit associates with the vitronectin receptor αv subunit to form a novel vitronectin receptor in a human embryonic kidney cell line. J Biol Chem. 1990;265:5938–5941. [PubMed] [Google Scholar]
- Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin αvβ3. Cell. 1996;85:683–693. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- Dallas SL, Miyazono K, Skerry TM, Mundy GR, Bonewald LF. Dual role for the latent transforming growth factor-β binding protein in storage of latent TGF-β in the extracellular matrix and as a structural protein. J Cell Biol. 1995;131:539–549. doi: 10.1083/jcb.131.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis PA, Rifkin DB. Cellular activation of latent transforming growth factor-β requires binding to the cation-independent mannose-6-phosphate/insulin-like growth factor type II receptor. Proc Natl Acad Sci USA. 1991;88:580–584. doi: 10.1073/pnas.88.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcioni R, Cimino L, Gentileschi MP, D’Agnano I, Zupi G, Kennel SJ, Sacchi A. Expression of β1, β3, β4, and β5 integrins by human lung carcinoma cells of different histiotypes. Exp Cell Res. 1994;210:113–122. doi: 10.1006/excr.1994.1017. [DOI] [PubMed] [Google Scholar]
- Flaumenhaft R, Abe M, Sato Y, Miyazono K, Harpel J, Heldin C-H, Rifkin DB. Role of the latent TGF-β binding protein in the activation of latent TGF-β by co-cultures of endothelial and smooth muscle cells. J Cell Biol. 1993;120:995–1002. doi: 10.1083/jcb.120.4.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailit J, Ruoslahti E. Regulation of the fibronectin receptor affinity by divalent cations. J Biol Chem. 1988;263:12927–12933. [PubMed] [Google Scholar]
- Giancotti FG. Integrin signaling: specificity and control of cell survival and cell cycle progression. Curr Opin Cell Biol. 1997;9:691–700. doi: 10.1016/s0955-0674(97)80123-8. [DOI] [PubMed] [Google Scholar]
- Grainger DJ, Wakefield L, Bethell HW, Farndale RW, Metcalfe JC. Release and activation of platelet latent TGF-β in blood clots during dissolution with plasmin. Nat Med. 1995;1:932–937. doi: 10.1038/nm0995-932. [DOI] [PubMed] [Google Scholar]
- Hildebrand A, Romaris M, Rasmussen LM, Heinegard D, Twardzik DR, Border WA, Ruoslahti E. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor-β. Biochem J. 1994;302:527–534. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu DD, Lin ECK, Kovach NL, Hoyer JR, Smith JW. A biochemical characterization of the binding of osteopontin to integrins αvβ1 and αvβ5. J Biol Chem. 1995;270:26232–26238. doi: 10.1074/jbc.270.44.26232. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Ignotz RA, Massague J. Transforming growth factor-β stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986;261:4337–4345. [PubMed] [Google Scholar]
- Ignotz RA, Massague J. Cell adhesion protein receptors as targets for transforming growth factor-β action. Cell. 1987;51:189–197. doi: 10.1016/0092-8674(87)90146-2. [DOI] [PubMed] [Google Scholar]
- Jones JI, Gockerman A, Busby WHJ, Wright G, Clemmons DR. Insulin-like growth factor binding protein 1 stimulates cell migration and binds to the α5β1 integrin by means of its Arg-Gly-Asp sequence. Proc Natl Acad Sci USA. 1993;90:10553–10557. doi: 10.1073/pnas.90.22.10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhofer D, Grzesiak J, Pierschbacher MD. Calcium as a potential physiological regulator of integrin-mediated cell adhesion. J Biol Chem. 1991;266:4471–4477. [PubMed] [Google Scholar]
- Klein S, Giancotti FG, Presta M, Albelda SM, Buck CA, Rifkin DB. Basic fibroblast growth factor modulates integrin expression in microvascular endothelial cells. Mol Biol Cell. 1993;4:973–982. doi: 10.1091/mbc.4.10.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Nara K, Rifkin DB. Requirement for transglutaminase in the activation of latent transforming growth factor-β in bovine endothelial cells. J Cell Biol. 1993;121:439–448. doi: 10.1083/jcb.121.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J. The transforming growth factor-β family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- McCaffrey TA, Falcone DJ, Vicente D, Du B, Consigli S, Borth W. Protection of transforming growth factor-β1 activity by heparin and fucoidan. J Cell Physiol. 1994;159:51–59. doi: 10.1002/jcp.1041590108. [DOI] [PubMed] [Google Scholar]
- McMahon GA, Dignam JD, Gentry LE. Structural characterization of the latent complex between transforming growth factor-β1 and β1-latency-associated peptide. Biochem J. 1996;313:343–351. doi: 10.1042/bj3130343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger JS, Harpel JG, Gleizes P, Mazzieri R, Nunes I, Rifkin DB. Latent transforming growth factor-β: Structural features and mechanisms of activation. Kidney Int. 1997;51:1376–1382. doi: 10.1038/ki.1997.188. [DOI] [PubMed] [Google Scholar]
- Nesbitt S, Nesbit A, Helfrich M, Horton M. Biochemical characterization of human osteoclast integrins. Osteoclasts express αvβ3, α2β1 and αvβ1 integrins. J Biol Chem. 1993;268:16737–16745. [PubMed] [Google Scholar]
- Nunes I, Gleizes PE, Metz CN, Rifkin DB. Latent transforming growth factor-β binding protein domains involved in activation and transglutaminase-dependent cross-linking of latent transforming growth factor-β. J Cell Biol. 1997;136:1151–1163. doi: 10.1083/jcb.136.5.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309:30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Pytela R, Pierschbacher MD, Argraves S, Suzuki S, Ruoslahti E. Arginine-glycine-aspartic acid adhesion receptors. Methods Enzymol. 1987;144:475–489. doi: 10.1016/0076-6879(87)44196-7. [DOI] [PubMed] [Google Scholar]
- Pytela R, Pierschbacher MD, Ruoslahti E. Identification and isolation of a 140 kD cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985;40:191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- Rusnati M, Tanghetti E, Dell’Era P, Gualandris A, Presta M. αvβ3 Integrin mediates the cell-adhesive capacity and biological activity of basic fibroblast growth factor (FGF-2) in cultured endothelial cells. Mol Biol Cell. 1997;8:2449–2461. doi: 10.1091/mbc.8.12.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz-Cherry S, Murphy-Ullrich JE. Thrombospondin causes activation of latent transforming growth factor-β secreted by endothelial cells by a novel mechanism. J Cell Biol. 1993;122:923–932. doi: 10.1083/jcb.122.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale J, Keski-Oja J. Growth factors in the extracellular matrix. FASEB J. 1997;11:51–59. doi: 10.1096/fasebj.11.1.9034166. [DOI] [PubMed] [Google Scholar]
- Taipale J, Miyazono K, Heldin CH, Keski-Oja J. Latent transforming growth factor-β1 associates to fibroblast extracellular matrix via latent TGF-β binding protein. J Cell Biol. 1994;124:171–181. doi: 10.1083/jcb.124.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale J, Saharinen J, Hedman K, Keski-Oja J. Latent transforming growth factor-β1 and its binding protein are components of extracellular matrix microfibrils. J Histochem Cytochem. 1996;44:875–889. doi: 10.1177/44.8.8756760. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Kodama Y, Matsumoto T. Bone matrix decorin binds transforming growth factor-β and enhances its bioactivity. J Biol Chem. 1994;269:32634–32638. [PubMed] [Google Scholar]
- Todaro GJ, Fryling C, DeLarco JE. Transforming growth factors produced by certain human tumor cells: polypeptides that interact with epidermal growth factor receptors. Proc Natl Acad Sci USA. 1980;77:5258–5262. doi: 10.1073/pnas.77.9.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel BE, Tarone G, Giancotti FG, Gailit J, Ruoslahti E. A novel fibronectin receptor with an unexpected subunit composition (αvβ1) J Biol Chem. 1990;265:5934–5937. [PubMed] [Google Scholar]
- Weinacker A, Chen A, Agrez M, Cone RI, Nishimura S, Wayner E, Pytela R, Sheppard D. Role of the integrin αvβ6 in cell attachment to fibronectin. Heterologous expression of intact and secreted forms of the receptor. J Biol Chem. 1994;269:6940–6948. [PubMed] [Google Scholar]
- Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-β by the proteoglycan decorin. Nature. 1990;346:281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- Yang Y, Dignam JD, Gentry LE. Role of carbohydrate structures in the binding of β1-latency-associated peptide to ligands. Biochemistry. 1997;36:11923–11932. doi: 10.1021/bi9710479. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Morla AO, Vuori K, Bauer JS, Juliano RL, Ruoslahti E. The αvβ1 integrin functions as a fibronectin receptor but does not support fibronectin matrix assembly and cell migration on fibronectin. J Cell Biol. 1993;122:235–242. doi: 10.1083/jcb.122.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Vuori K, Reed JC, Ruoslahti E. The α5β1 integrin supports survival of cells on fibronectin and up-regulates Bcl-2 expression. Proc Natl Acad Sci USA. 1995;92:6161–6165. doi: 10.1073/pnas.92.13.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]