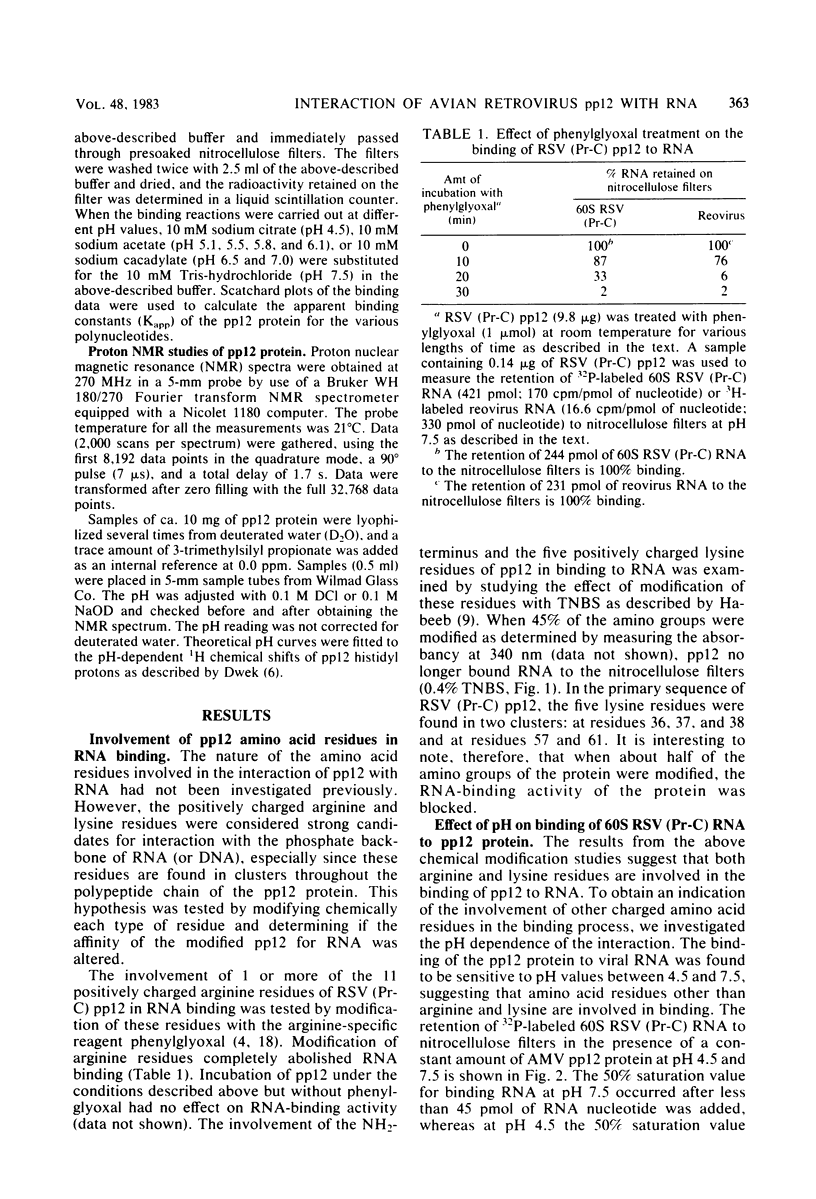
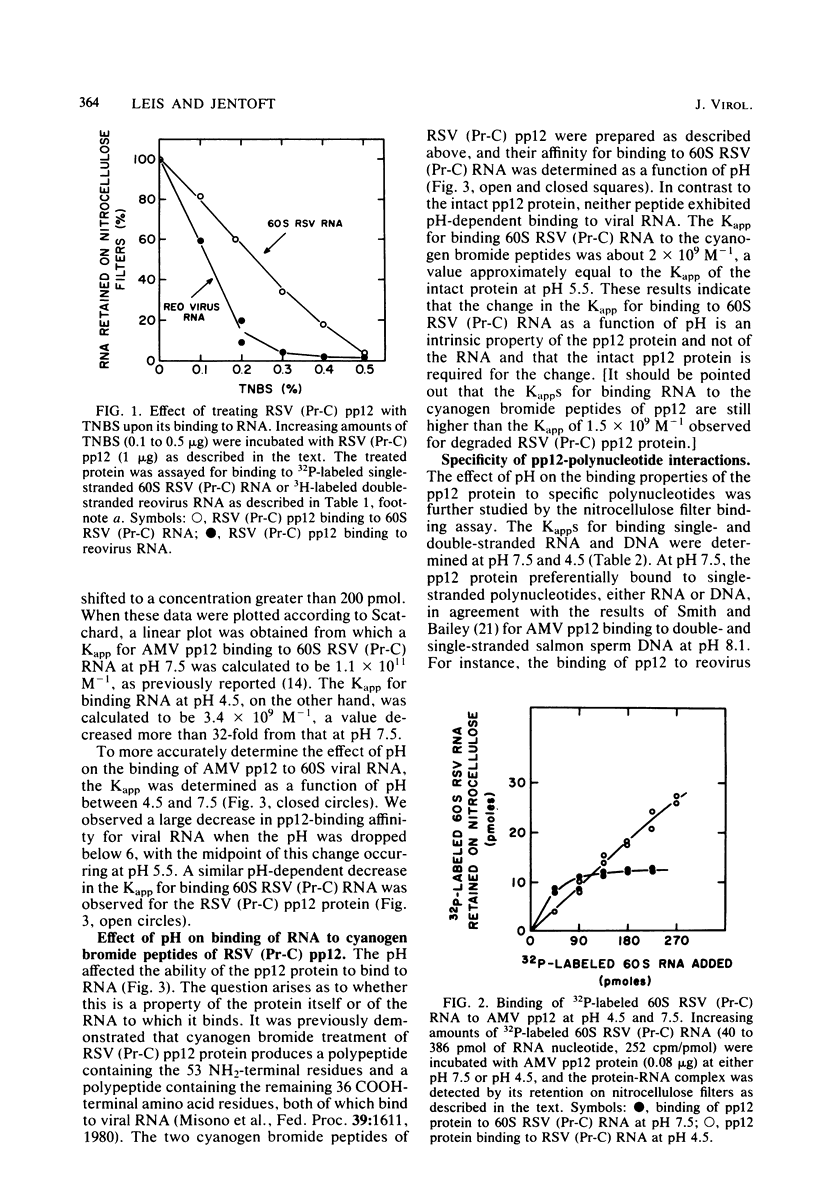
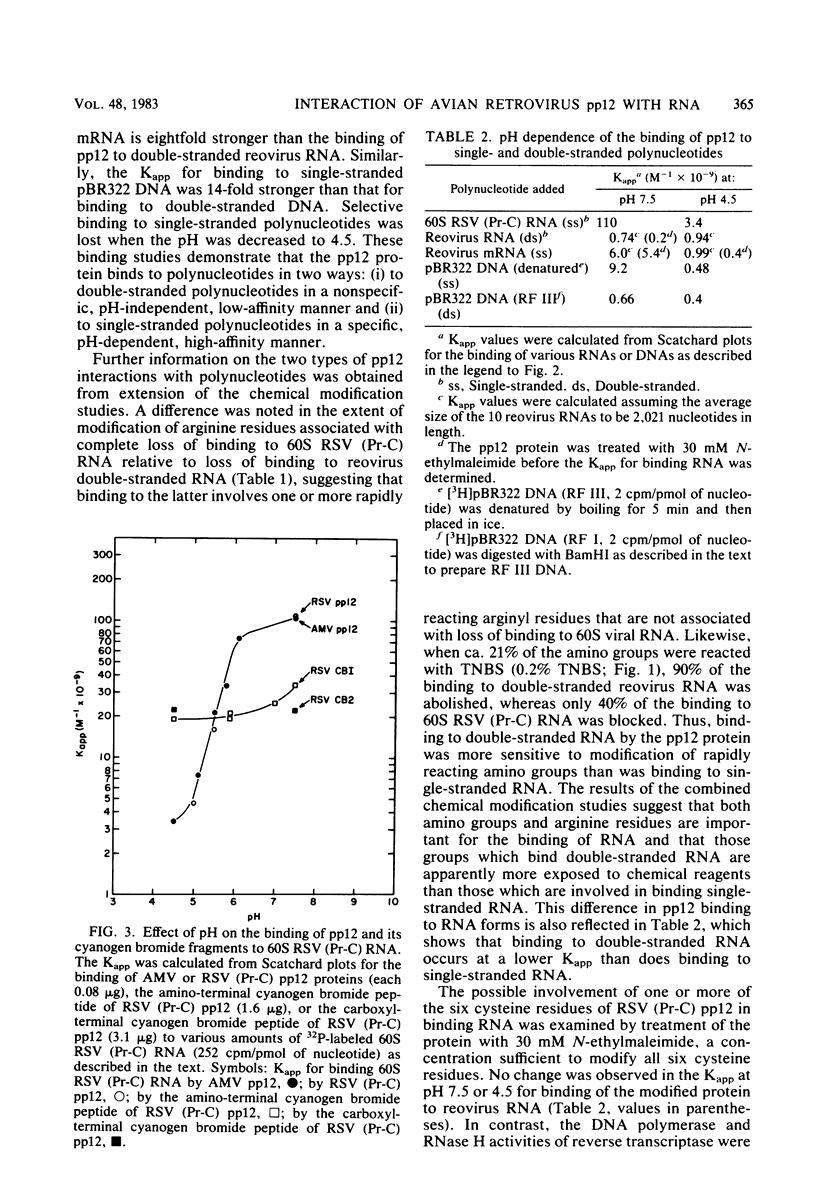
Abstract
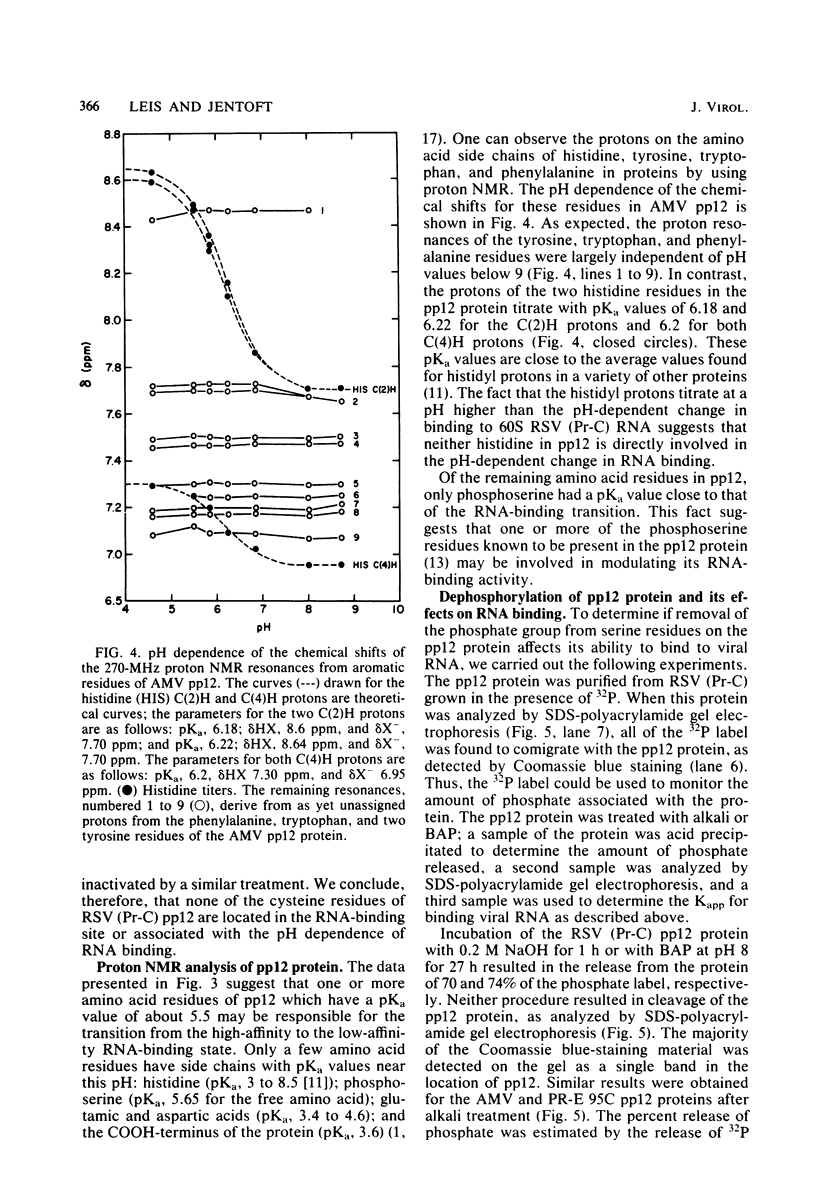
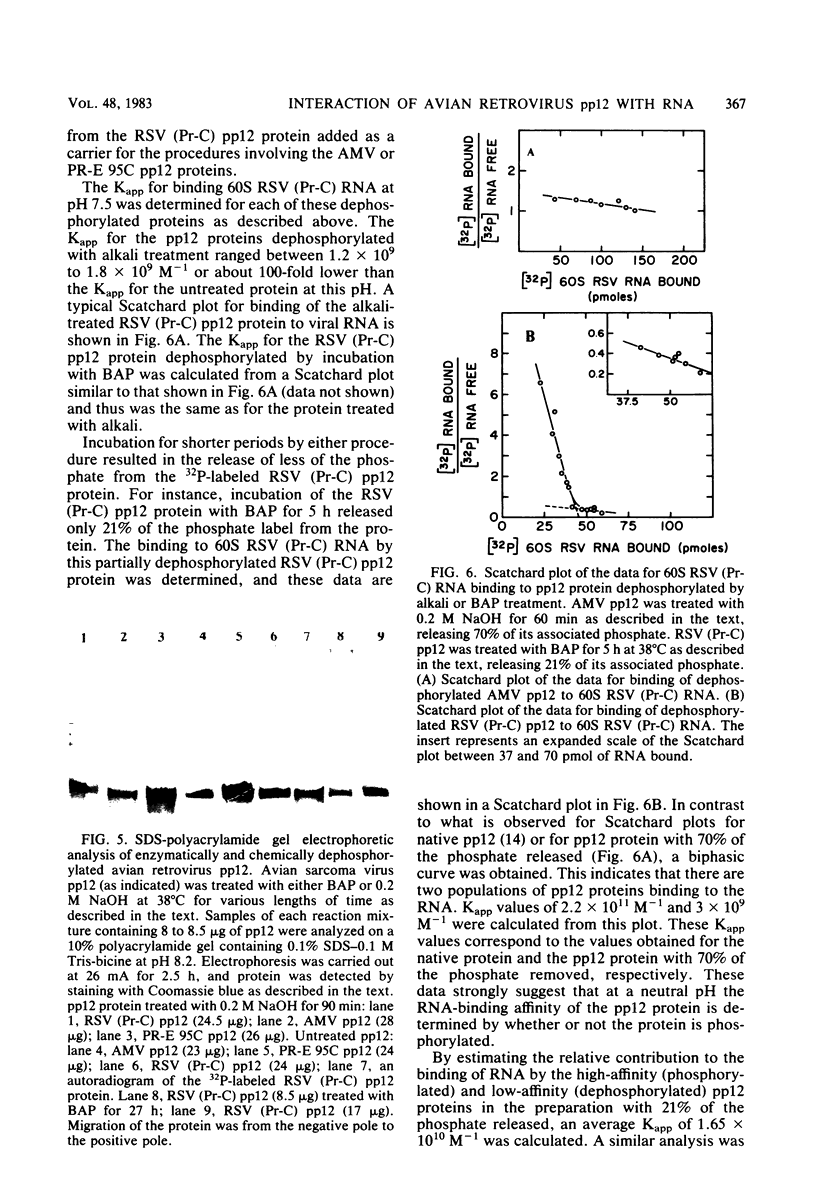
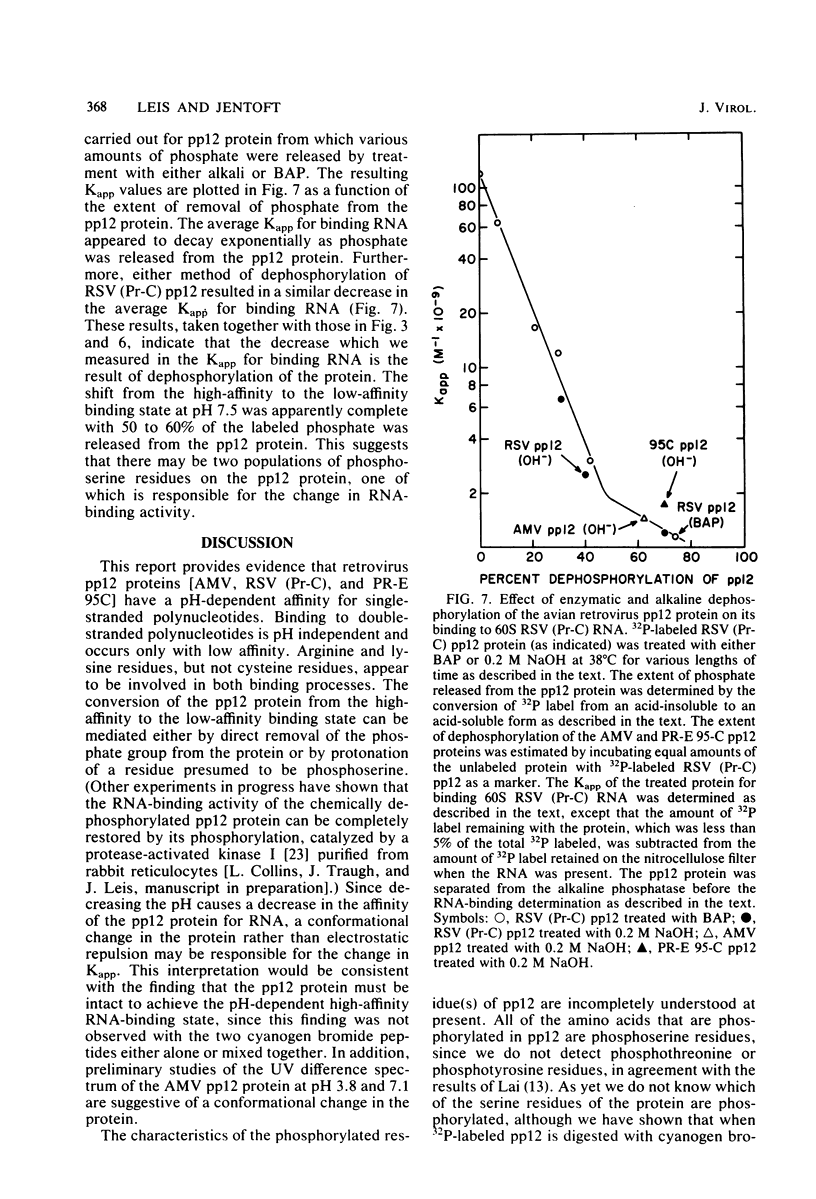
We investigated the interaction of the avian retrovirus pp12 protein with viral RNA to assess its possible role in virion assembly. Using chemical modification techniques, we found that reagents specific for lysine or arginine residues inactivated the RNA-binding capacity of the protein. The binding of pp12 to 60S viral RNA was also strongly affected by pH (pKapp of 5.5); the affinity for viral RNA decreased by as much as 40-fold after protonation of one or more titratable groups on the protein. When the protein was cleaved by cyanogen bromide, each of the two polypeptide products bound to RNA (with low affinity), but pH dependence was lost. Thus, an intact protein was required for this effect. Since histidine and phosphoserine residues have pKa values close to the pKapp of the pp12-RNA interaction, they were studied to determine whether they were involved in this process. Each of the two histidyl residues in pp12 had pKa values of 6.2, as determined by proton nuclear magnetic resonance titrations, values too high to account for the pKapp of binding. The involvement of phosphoserine residues, which have pKa values similar to the pKapp, was investigated by removal of phosphate from pp12. When phosphate groups were chemically or enzymatically removed from the avian myeloblastosis virus, Rous sarcoma virus (Pr-C), and PR-E 95C virus pp12 proteins, the Kapp for binding 60S viral RNA was reduced 100-fold at pH 7.5. Thus, it seems possible that phosphorylation of the pp12 protein could favor viral nucleocapsid formation by increasing its affinity for the viral RNA genome. Dephosphorylation could provide for its release from the viral RNA during reverse transcription after viral infection of cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Leis J. P., Smith M. S., Faras A. J. Unwinding-like activity associated with avian retrovirus RNA-directed DNA polymerase. J Virol. 1978 May;26(2):498–509. doi: 10.1128/jvi.26.2.498-509.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daemen F. J., Riordan J. F. Essential arginyl residues in Escherichia coli alkaline phosphatase. Biochemistry. 1974 Jul 2;13(14):2865–2871. doi: 10.1021/bi00711a014. [DOI] [PubMed] [Google Scholar]
- Dittmar K. J., Moelling K. Biochemical properties of p15-associated protease in an avian RNA tumor virus. J Virol. 1978 Oct;28(1):106–118. doi: 10.1128/jvi.28.1.106-118.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson E., Brugge J. S., Erikson R. L. Phosphorylated and nonphosphorylated forms of avian sarcoma virus polypeptide p19. Virology. 1977 Jul 1;80(1):177–185. doi: 10.1016/0042-6822(77)90390-7. [DOI] [PubMed] [Google Scholar]
- Ghysdael J., Neil J. C., Vogt P. K. Cleavage of four avian sarcoma virus polyproteins with virion protease p15 removes gag sequences and yields large fragments that function as tyrosine phosphoacceptors in vitro. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5847–5851. doi: 10.1073/pnas.78.9.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeeb A. F. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal Biochem. 1966 Mar;14(3):328–336. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- Hizi A., Leis J. P., Joklik W. K. The RNA-dependent DNA polymerase of avian sarcoma virus B77. Binding of viral and nonviral ribonucleic acids to the alpha, beta2, and alphabeta forms of the enzyme. J Biol Chem. 1977 Oct 10;252(19):6878–6884. [PubMed] [Google Scholar]
- Johnson S. P., Veigl M., Vanaman T., Leis J. Cyanogen bromide digestion of the avian myeloblastosis virus pp19 protein: isolation of an amino-terminal peptide that binds to viral RNA. J Virol. 1983 Feb;45(2):876–881. doi: 10.1128/jvi.45.2.876-881.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. M. Phosphoproteins of Rous sarcoma viruses. Virology. 1976 Oct 15;74(2):287–301. doi: 10.1016/0042-6822(76)90336-6. [DOI] [PubMed] [Google Scholar]
- Leis J. P., McGinnis J., Green R. W. Rous sarcoma virus p19 binds to specific double-stranded regions of viral RNA: effect of p19 on cleavage of viral RNA by RNase III. Virology. 1978 Jan;84(1):87–98. doi: 10.1016/0042-6822(78)90220-9. [DOI] [PubMed] [Google Scholar]
- Leis J. P., Scheible P., Smith R. E. Correlation of RNA binding affinity of avian oncornavirus p19 proteins with the extent of processing of virus genome RNA in cells. J Virol. 1980 Sep;35(3):722–731. doi: 10.1128/jvi.35.3.722-731.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal B. K., Roy-Burman P. RNA tumor virus phosphoproteins: subvirion location of the multiple phosphorylated species. Virology. 1977 Dec;83(2):423–427. doi: 10.1016/0042-6822(77)90188-x. [DOI] [PubMed] [Google Scholar]
- Peters R. G., Jones W. C., Cromartie T. H. Inactivation of L-lactate monooxygenase with 2,3-butanedione and phenylglyoxal. Biochemistry. 1981 Apr 28;20(9):2564–2571. doi: 10.1021/bi00512a031. [DOI] [PubMed] [Google Scholar]
- Schulein M., Burnette W. N., August J. T. Stoichiometry and specificity of binding of Rauscher oncovirus 10,000-dalton (p10) structural protein to nucleic acids. J Virol. 1978 Apr;26(1):54–60. doi: 10.1128/jvi.26.1.54-60.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Smith B. J., Bailey J. M. The binding of an avian myeloblastosis virus basic 12,000 dalton protein to nucleic acids. Nucleic Acids Res. 1979 Dec 11;7(7):2055–2072. doi: 10.1093/nar/7.7.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. E., Nebes S., Leis J. Production of large amounts of 35S RNA and complementary DNA from avian RNA tumor viruses. Anal Biochem. 1977 Jan;77(1):226–234. doi: 10.1016/0003-2697(77)90308-6. [DOI] [PubMed] [Google Scholar]
- Tahara S. M., Traugh J. A. Cyclic Nucleotide-independent protein kinases from rabbit reticulocytes. Identification and characterization of a protein kinase activated by proteolysis. J Biol Chem. 1981 Nov 25;256(22):11558–11564. [PubMed] [Google Scholar]
- von der Helm K. Cleavage of Rous sarcoma viral polypeptide precursor into internal structural proteins in vitro involves viral protein p15. Proc Natl Acad Sci U S A. 1977 Mar;74(3):911–915. doi: 10.1073/pnas.74.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]