Abstract
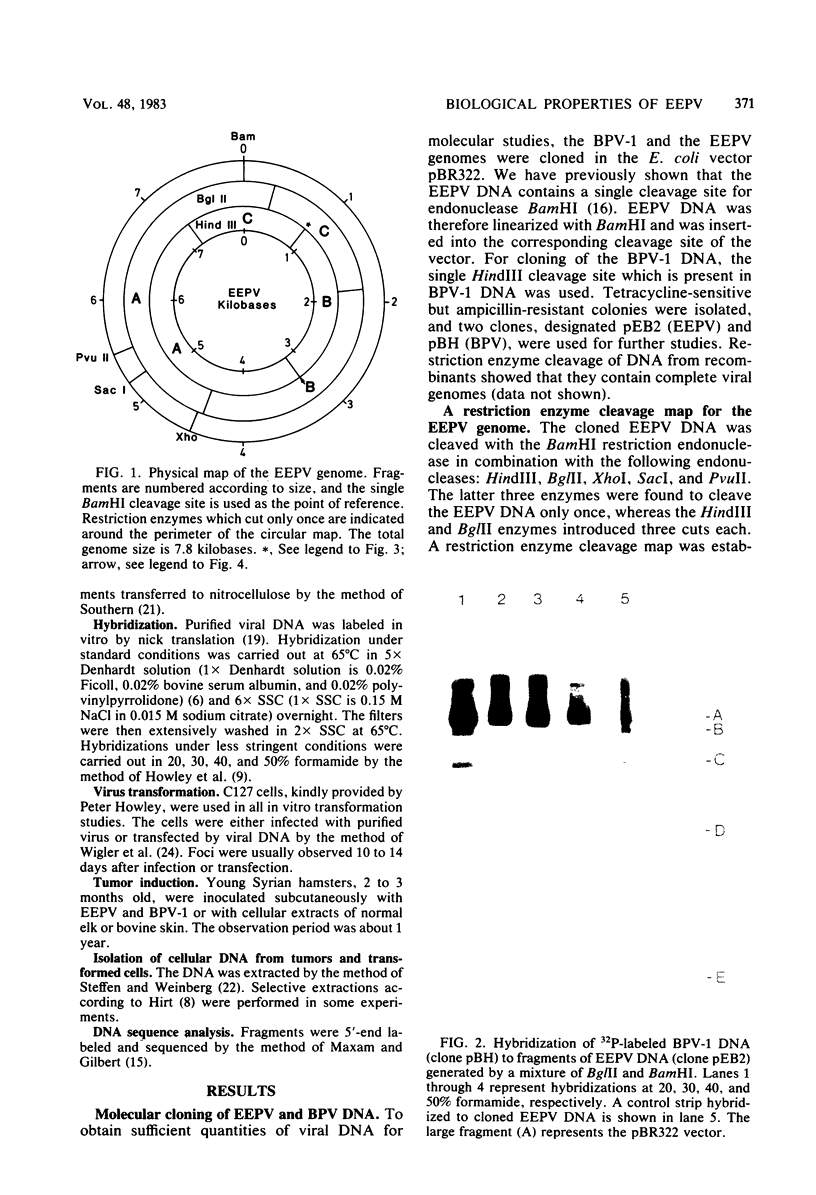
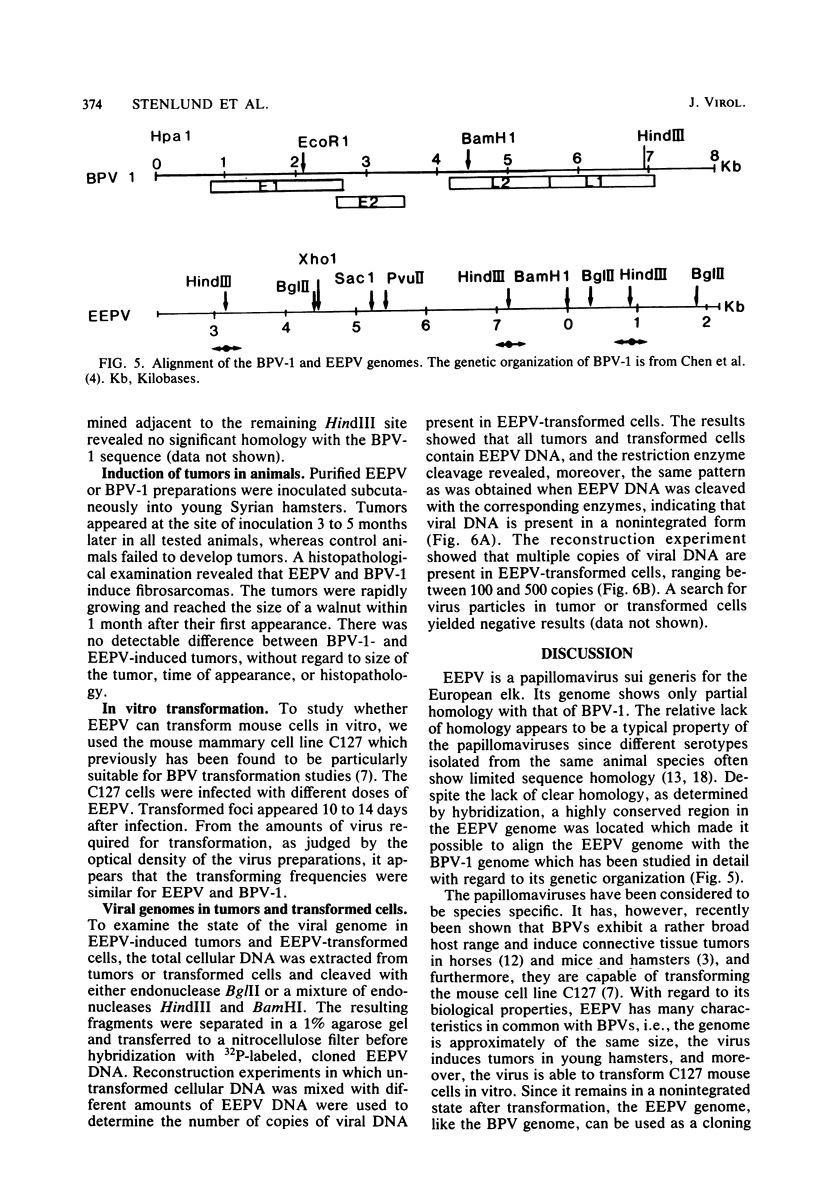
The European elk papillomavirus (EEPV) genome was cloned in the BamHI cleavage site of the pBR322 vector. The cloned genome was used for construction of a physical map, employing restriction endonucleases BamHI, BglII, HindIII, PvuII, SacI, and XhoI. The sequence homology between the EEPV and bovine papillomavirus type 1 genomes was elucidated by performing hybridizations in different concentrations of formamide. Sequence homology could only be revealed under less stringent conditions, i.e., Tm - 43 degrees C. Nucleotide sequence information was also collected from the regions which lie adjacent to the three HindIII sites that are present in the EEPV genome. The results made it possible to align the EEPV and bovine papillomavirus type 1 genomes. Transformation by EEPV was demonstrated with the C127 mouse cell line, and fibrosarcomas were induced in young hamsters after subcutaneous injection. The transformed cells and the tumors contain multiple, nonintegrated copies of the EEPV genome. Virus particles could not be detected either in tumors or in transformed cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahola H., Stenlund A., Moreno-Lopez J., Pettersson U. Sequences of bovine papillomavirus type 1 DNA--functional and evolutionary implications. Nucleic Acids Res. 1983 May 11;11(9):2639–2650. doi: 10.1093/nar/11.9.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amtmann E., Müller H., Sauer G. Equine connective tissue tumors contain unintegrated bovine papilloma virus DNA. J Virol. 1980 Sep;35(3):962–964. doi: 10.1128/jvi.35.3.962-964.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOIRON M., LEVY J. P., THOMAS M., FRIEDMANN J. C., BERNARD J. SOME PROPERTIES OF BOVINE PAPILLOMA VIRUS. Nature. 1964 Jan 25;201:423–424. doi: 10.1038/201423a0. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Howley P. M., Levinson A. D., Seeburg P. H. The primary structure and genetic organization of the bovine papillomavirus type 1 genome. Nature. 1982 Oct 7;299(5883):529–534. doi: 10.1038/299529a0. [DOI] [PubMed] [Google Scholar]
- Danos O., Engel L. W., Chen E. Y., Yaniv M., Howley P. M. Comparative analysis of the human type 1a and bovine type 1 papillomavirus genomes. J Virol. 1983 May;46(2):557–566. doi: 10.1128/jvi.46.2.557-566.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dvoretzky I., Shober R., Chattopadhyay S. K., Lowy D. R. A quantitative in vitro focus assay for bovine papilloma virus. Virology. 1980 Jun;103(2):369–375. doi: 10.1016/0042-6822(80)90195-6. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Howley P. M., Israel M. A., Law M. F., Martin M. A. A rapid method for detecting and mapping homology between heterologous DNAs. Evaluation of polyomavirus genomes. J Biol Chem. 1979 Jun 10;254(11):4876–4883. [PubMed] [Google Scholar]
- Lancaster W. D. Apparent lack of integration of bovine papillomavirus DNA in virus-induced equine and bovine tumor cells and virus-transformed mouse cells. Virology. 1981 Jan 30;108(2):251–255. doi: 10.1016/0042-6822(81)90433-5. [DOI] [PubMed] [Google Scholar]
- Lancaster W. D., Olson C., Meinke W. Bovine papilloma virus: presence of virus-specific DNA sequences in naturally occurring equine tumors. Proc Natl Acad Sci U S A. 1977 Feb;74(2):524–528. doi: 10.1073/pnas.74.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster W. D. Physical maps of bovine papillomavirus type 1 and type 2 genomes. J Virol. 1979 Nov;32(2):684–687. doi: 10.1128/jvi.32.2.684-687.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M. F., Lancaster W. D., Howley P. M. Conserved polynucleotide sequences among the genomes of papillomaviruses. J Virol. 1979 Oct;32(1):199–207. doi: 10.1128/jvi.32.1.199-207.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M. F., Lowy D. R., Dvoretzky I., Howley P. M. Mouse cells transformed by bovine papillomavirus contain only extrachromosomal viral DNA sequences. Proc Natl Acad Sci U S A. 1981 May;78(5):2727–2731. doi: 10.1073/pnas.78.5.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Lopez J., Pettersson U., Dinter Z., Philipson L. Characterization of a papilloma virus from the European elk (EEPV). Virology. 1981 Jul 30;112(2):589–595. doi: 10.1016/0042-6822(81)90304-4. [DOI] [PubMed] [Google Scholar]
- Morgan D. M., Murphy M. F., Abraham J. M., Meinke W. J. A restriction map of bovine papillomavirus type-1 DNA. J Gen Virol. 1981 Sep;56(Pt 1):213–217. doi: 10.1099/0022-1317-56-1-213. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steffen D., Weinberg R. A. The integrated genome of murine leukemia virus. Cell. 1978 Nov;15(3):1003–1010. doi: 10.1016/0092-8674(78)90284-2. [DOI] [PubMed] [Google Scholar]
- Stenlund A., Perricaudet M., Tiollais P., Pettersson U. Construction of restriction enzyme fragment libraries containing DNA from human adenovirus types 2 and 5. Gene. 1980 Jun;10(1):47–52. doi: 10.1016/0378-1119(80)90142-0. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Condylomata acuminata and human genital cancer. Cancer Res. 1976 Feb;36(2 Pt 2):794–794. [PubMed] [Google Scholar]
- zur Hausen H. Human papillomaviruses and their possible role in squamous cell carcinomas. Curr Top Microbiol Immunol. 1977;78:1–30. doi: 10.1007/978-3-642-66800-5_1. [DOI] [PubMed] [Google Scholar]





