Abstract
The Prevention of Events with Angiotensin Converting Enzyme inhibition (PEACE) trial evaluated angiotensin-converting enzyme inhibition with trandolapril versus placebo added to conventional therapy in patients with stable coronary disease and preserved left ventricular function. The PEACE hemodynamic substudy evaluated effects of trandolapril on pulsatile hemodynamics. Hemodynamic studies were performed in 300 participants from 5 PEACE centers a median of 52 months (range, 25 to 80 months) after random assignment to trandolapril at a target dose of 4 mg per day or placebo. Central pulsatile hemodynamics and carotid–femoral pulse wave velocity were assessed by using echocardiography, tonometry of the carotid and femoral arteries, and body surface transit distances. Patients randomly assigned to trandolapril tended to be older (mean±SD: 64.2±7.9 versus 62.9±7.7 years; P=0.14), with a higher body mass index (28.5±4.0 versus 27.8±3.9 kg/m2; P=0.09) and lower ejection fraction (57.1±8.1% versus 58.7±8.4%; P<0.01). At the time of the hemodynamic substudy, the trandolapril group had lower mean arterial pressure (93.1±10.2 versus 96.3±11.3 mm Hg; P<0.01) and lower carotid-femoral pulse wave velocity (geometric mean [95% CI]: 10.4 m/s [10.0 to 10.9 m/s] versus 11.2 m/s [10.7 to 11.8 m/s]; P=0.02). The difference in carotid–femoral pulse wave velocity persisted (P<0.01) in an analysis that adjusted for baseline characteristics and follow-up mean pressure. In contrast, there was no difference in aortic compliance, characteristic impedance, augmentation index, or total arterial compliance. Angiotensin-converting enzyme inhibition with trandolapril produced a modest reduction in carotid–femoral pulse wave velocity, a measure of aortic wall stiffness, beyond what would be expected from blood pressure lowering or differences in baseline characteristics alone.
Keywords: angiotensin-converting enzyme, coronary artery disease, randomized clinical trial, arterial stiffness, pulse wave velocity
Increased arterial stiffness has emerged as an important risk factor for cardiovascular disease.1–3 Abnormal aortic stiffness adds to load on the heart and arteries and increases pulsatility of pressure and flow in central and peripheral arteries and the microvasculature. Increased pressure and flow pulsatility contributes to atherogenesis in large central arteries and is associated with increased incidence of ischemic events.4,5 Excessive pressure pulsatility also contributes to dysfunction and damage in the microcirculation,6 ultimately leading to end-organ damage. Interventions that reduce arterial stiffness offer an opportunity to minimize pulsatile damage and potentially reduce the incidence and severity of associated diseases in the heart, brain, kidneys, and other organs.
Previous studies have implicated the renin–angiotensin–aldosterone system (RAAS) in the pathogenesis of increased arterial stiffness. Polymorphisms in the genes for the angiotensin II type 1 receptor (AGTR1) and angiotensin-converting enzyme (ACE) are associated with elevated carotid–femoral pulse wave velocity (PWV).7 ACE inhibitors and angiotensin II type 1 receptor blockers (ARBs) have been shown to reduce aortic stiffness in relatively short-term (<12-month) intervention studies in patients with hypertension or heart failure.8–15 ACE inhibitors have also been shown to reduce ischemic events in high-risk groups,16–18 possibly because of their favorable effect on arterial properties and pulsatile hemodynamics. However, the large artery effects of long-term treatment with drugs that block the RAAS have not been assessed in relatively low-risk individuals with coronary disease and preserved ventricular function without a conventional indication for ACE inhibition or ARB therapy.
In addition, arterial stiffness is related to distending pressure, which often is reduced by interventions such as ACE inhibition or ARB. As a result, the question of whether therapy can produce a sustained reduction in measures of aortic wall stiffness, such as carotid–femoral PWV, independent of a concurrent reduction in mean arterial pressure (MAP), remains controversial. The Prevention of Events with Angiotensin Converting Enzyme inhibition (PEACE) Trial evaluated ACE inhibition with trandolapril as compared with placebo added to conventional therapy in patients with stable coronary disease and normal or near normal left ventricular function.19 The PEACE hemodynamic substudy measured pulsatile hemodynamics 2 to 7 years after initiation of therapy in a subset of the PEACE cohort to evaluate the effects of long-term ACE inhibition on large artery properties.
Methods
Study Design
The design of the PEACE Trial has been described previously.19,20 Patients were >50 years of age and had known coronary artery disease and a left ventricular ejection fraction >40%. Key exclusion criteria included an indication for an ACE inhibitor or ARB, unstable angina within 2 months or a coronary revascularization within the previous 3 months or planned revascularization, valvular heart disease, and elevated creatinine or potassium.19,20 In addition, to be eligible for the hemodynamic substudy, participants had to be enrolled at 1 of the 5 PEACE hemodynamic centers, actively taking assigned study medication, and willing to provide informed consent.
After a successful 2-week run-in phase, during which patients were instructed to take 2 mg of trandolapril per day, patients were randomly assigned to either continued trandolapril at 2 mg per day or matching placebo. At a visit 6 months after random assignment, patients who had tolerated the dose of 2 mg per day were advanced to the final dose of 4 mg of trandolapril per day or matching placebo.
The investigations performed in this study conform to the principles outlined in the Declaration of Helsinki. An institutional review board at each clinical center approved the study protocol, and each individual gave written informed consent before enrollment.
Hemodynamic Data Acquisition
Participants were studied in the supine position after ≈10 minutes of rest. Supine auscultatory blood pressures were obtained by using a computer-controlled device (Cardiovascular Engineering, Inc) that automatically inflated the cuff (Hokanson SC12, DE Hokanson, Inc) to a user preset maximum pressure and then precisely controlled deflation at 2 mm Hg/s. This device digitized and recorded the DC-coupled mean cuff pressure and an AC coupled, amplified oscillometric (pulsatile) cuff pressure, as well as the ECG and a cuff microphone channel throughout the cuff inflation and deflation sequence. Blood pressure was obtained 3 to 5 times at 2-minute intervals with a goal of obtaining 3 sequential readings that agreed to within 5 mm Hg for systolic and diastolic blood pressure. Arterial tonometry with ECG was obtained from the brachial, radial, femoral, and carotid arteries using a custom transducer. This transducer has a small sensor surface area and a frequency response that is flat from 0 to >1000 Hz. Next, echocardiographic images of the left ventricular outflow tract were obtained from a parasternal long axis view. This was followed by sequential acquisition of pulsed Doppler of the left ventricular outflow tract from an apical 5-chamber view followed by tonometry of the carotid artery. Finally, body surface measurements from suprasternal notch to brachial, radial, femoral, and carotid recording sites were obtained. All of the data were digitized during the primary acquisition, transferred to CD-ROM, and shipped to the core laboratory (Cardiovascular Engineering, Inc) for analysis.
Data Analysis
Tonometry waveforms were signal averaged using the electrocardiographic QRS as the fiducial point. Average systolic and diastolic cuff pressures were used to calibrate peak and trough of the signal-averaged brachial waveform. Mean brachial pressure (obtained by integration of the calibrated brachial waveform) and diastolic pressure were then used to calibrate carotid, radial, and femoral waveforms.21 All of the blood pressure recordings were overread by the core laboratory. Carotid–brachial, carotid–radial, and carotid–femoral PWVs; aortic compliance; characteristic impedance; and total arterial compliance were calculated as described previously.12,22 Briefly, characteristic impedance was estimated in the time domain as the early change in pressure divided by the corresponding change in flow before return of the reflected wave. Values obtained by using a time domain approach to estimate characteristic impedance are highly correlated with frequency domain techniques, with R=0.948 to 0.994, depending on the averaging criteria used for the frequency domain estimate.22 The foot of the carotid pressure waveform was first aligned with the foot of the aortic flow waveform. Total arterial compliance was estimated by using the diastolic area method applied to the last two thirds of diastole.23 Aortic compliance was computed from the inverse of the product of characteristic impedance and carotid–femoral PWV. PWVs were computed from foot-to-foot time delays obtained by tonometry and body surface measurements corrected for parallel transmission.22 Augmentation index, a measure of the relative contribution of wave reflection to central pulse pressure, was assessed from the calibrated carotid pressure waveform.22 As reported previously, reproducibility of measures of central aortic stiffness using our protocol in a multicenter setting is high, with intraclass correlation coefficients for repeated measures of characteristic impedance of 0.93 to 0.95.22
Statistical Analysis
Baseline characteristics for the entire hemodynamic substudy sample were tabulated and compared with the characteristics of the full PEACE cohort. Baseline characteristics of the hemodynamic sample were then tabulated and compared according to treatment group. Differences in baseline characteristics between the trandolapril and placebo groups were tested using an F test for continuous variables and χ2 for categorical variables. Subsequent models adjusted for baseline characteristics that differed with a P<0.15.
Distributions for several key arterial stiffness measures (aortic compliance, carotid–femoral PWV, characteristic impedance, and total arterial compliance) were skewed and were therefore log transformed to normalize variance. We used general linear models to test for treatment differences between hemodynamic variables. Treatment MAP and significant baseline variables were then entered into a general linear model as additional independent variables predicting arterial stiffness measures along with treatment group. Models were assessed separately with treatment MAP included as a continuous variable and as a categorical variable according to quintiles of MAP. Clinical site was assessed as a random effect using maximum likelihood estimation and was not found to be a significant source of variability for aortic compliance. Therefore, analyses were not adjusted for clinical site. Power calculations were based on duplicate measurements conducted in a pilot evaluation of 8 PEACE Study patients before random assignment into the parent trial. We assumed that aortic compliance mean and SD would be 0.51±0.20×10−5 cm4/dyne. For the sample size estimate, we further assume a 2-tailed type 1 error of 0.05. A sample size of 150 patients per group provided 80% power to detect a 14% difference between the placebo and ACE inhibitor groups. This sample size also provided 90% power to detect a 16% difference in aortic compliance between the 2 groups. This sample size was sufficient to detect comparable or smaller differences in PWV, characteristic impedance, and augmentation index, because these measurements generally have a smaller relative variance than aortic compliance. A 2-sided P<0.05 was considered to indicate statistical significance.
Results
Baseline characteristics of the hemodynamic substudy sample according to treatment assignment are presented in Table 1. Patients randomly assigned to trandolapril were older, had higher body mass index, and had a lower ejection fraction; they were more likely treated with β-blockers and less likely treated with aspirin. The median duration of treatment from random assignment to hemodynamic substudy was 52 months, with a minimum duration of 25 months and a maximum duration of 80 months. Treatment duration did not differ between treatment groups (P=0.98). At the time of the hemodynamic study, 84% of the participants in the active treatment group were taking the target 4 mg dose of trandolapril.
Table 1. Baseline Characteristics of the Substudy Sample According to Treatment Group.
| Variable | Trandolapril | Placebo |
|---|---|---|
| N | 152 | 148 |
| Age, y | 64.2±7.9* | 62.9±7.7 |
| Women (% of patients) | 16 (11) | 13 (9) |
| Height, cm | 173±9 | 173±8 |
| Weight, kg | 86±16* | 83±14 |
| Body mass index, kg/m2 | 29±4* | 28±4 |
| Systolic pressure, mm Hg | 133±16 | 133±17 |
| Diastolic pressure, mm Hg | 78±9 | 80±10 |
| Mean pressure, mm Hg | 97±10 | 97±11 |
| Heart rate, min−1 | 59±10 | 61±11 |
| Serum creatinine, mg/dL | 1.05±0.23 | 1.04±0.21 |
| LV ejection fraction, % | 57.1±8.1* | 58.7±8.4 |
| Medical history, N (%) | ||
| Diabetes | 16 (11) | 10 (7) |
| Documented MI | 104 (68) | 93 (63) |
| Angina pectoris | 86 (57) | 91 (62) |
| PTCA or CABG | 111 (73) | 117 (79) |
| Hypertension | 54 (36) | 50 (34) |
| Stroke | 8 (5) | 4 (3) |
| Current smoker | 22 (15) | 23 (16) |
| Medications, N (%) | ||
| Calcium channel blocker | 44 (29) | 44 (30) |
| β blocker | 99 (65)* | 82 (55) |
| Aspirin/antiplatelet | 133 (88)* | 138 (93) |
| Lipid-lowering drug | 114 (75) | 106 (72) |
The following medications were being taken by <10% of patients with no differences between groups: antiarrhythmic, anticoagulant, digitalis, diuretics, and insulin. LV indicates left ventricular; MI, myocardial infarction; PTCA, percutaneous coronary angioplasty; CABG, aortocoronary bypass graft surgery.
P<0.15 for trandolapril vs placebo.
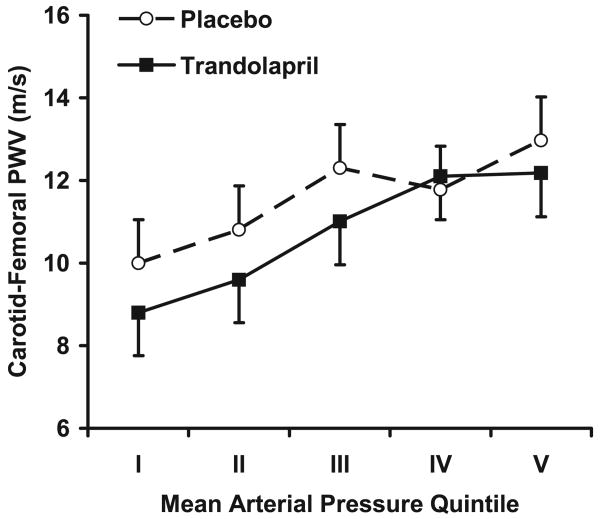
The primary hemodynamic variables are presented in Table 2. Patients randomly assigned to trandolapril versus placebo had lower MAP and carotid–femoral PWV at the time of the follow-up hemodynamic examination. In unadjusted analyses, there were no differences in aortic compliance, augmentation index, characteristic impedance, or total arterial compliance (Table 2). In models that adjusted for baseline differences between treatment groups, aortic compliance was higher in the trandolapril group and carotid–femoral PWV was lower, whereas augmentation index, characteristic impedance, and total arterial compliance still did not differ by treatment group (Table 2). In the aortic compliance model, baseline covariates increased the model R2 from 0% to 28%. In the PWV model, R2 increased from 2% to 29%. MAP was also added to models as a continuous variable. For aortic compliance, the model R2 increased to 50%, and the treatment effect was no longer significant (P=0.4). For carotid–femoral PWV, the model R2 increased to 45%; however, the treatment effect remained significant (P=0.002). Values for carotid–femoral PWV, adjusted for differences in baseline characteristics and grouped according to quintiles of MAP, are presented in the Figure.
Table 2. On-Treatment Pulsatile Hemodynamic Variables According to Treatment Group.
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| Variable | Trandolapril, Mean
(95% CI) |
Placebo, Mean
(95% CI) |
P | Trandolapril, Mean
(95% CI) |
Placebo, Mean
(95% CI) |
P |
| Mean pressure, mm Hg | 93 (91 to 95) | 96 (95 to 98) | <0.001 | 93 (91 to 95) | 97 (94 to 99) | 0.004 |
| Brachial pulse pressure, mm Hg | 63 (61 to 66) | 65 (62 to 68) | 0.40 | 61 (57 to 64) | 63 (60 to 67) | 0.15 |
| Carotid pulse pressure, mm Hg | 64 (61 to 67) | 65 (62 to 68) | 0.77 | 61 (57 to 64) | 62 (58 to 66) | 0.41 |
| Carotid to femoral PWV, m/s | 10.4 (10.0 to 10.9) | 11.3 (10.7 to 11.8) | 0.02 | 10.5 (10.0 to 11.1) | 11.9 (11.2 to 12.6) | <0.001 |
| Aortic compliance, 10−5 cm4/dyne | 0.49 (0.45 to 0.54) | 0.46 (0.41 to 0.51) | 0.38 | 0.50 (0.45 to 0.57) | 0.44 (0.38 to 0.49) | 0.02 |
| Augmentation index, % | 14.7 (12.7 to 16.7) | 15.8 (13.6 to 18.0) | 0.46 | 11.9 (9.1 to 14.7) | 12.7 (9.7 to 15.6) | 0.61 |
| Characteristic impedance, dyne×s/cm5 | 199 (188 to 211) | 199 (186 to 213) | 0.96 | 191 (176 to 207) | 197 (181 to 215) | 0.46 |
| Total arterial compliance, mL/mm Hg | 1.66 (1.57 to 1.75) | 1.66 (1.55 to 1.77) | 0.98 | 1.65 (1.51 to 1.79) | 1.64 (1.50 to 1.79) | 0.95 |
Adjusted models include: age, body mass index, ejection fraction, and β-blocker and aspirin usage. Values for aortic compliance, carotid–femoral PWV, characteristic impedance, and total arterial compliance represent geometric means.
Carotid–femoral PWV plotted separately by treatment group according to quintile of on-treatment MAP. Treatment with trandolapril was associated with a downward shift in the relation between mean pressure and carotid–femoral PWV (P=0.003), indicating a pressure-independent reduction in aortic stiffness. A test for an interaction between treatment group and quintile of MAP was not significant (P=0.45). MAP minimum and maximum values (mm Hg) in the groups were as follows: I (68.5 to 86.0), II (86.1 to 91.0), III (91.1 to 96.0), IV (96.1 to 102.5), and V (102.6 to 133.0).
Because augmentation index may be affected by heart rate, height, and weight, we ran models with and without adjustment for MAP that also adjusted for heart rate and replaced body mass index with height and weight. Augmentation index still did not differ between treatment groups in either of these models (P>0.25).
Effect Modification
Effect modification was assessed by adding an interaction term for treatment group and each of several key variables to the model for carotid–femoral PWV. The model also included terms for baseline characteristics and MAP. The effect of treatment group on carotid–femoral PWV was not modified by age (P=0.51), on-treatment MAP quintile (P=0.45), or treatment duration (P=0.09).
Discussion
This study evaluated pulsatile hemodynamics after long-term ACE inhibition with trandolapril in a sample of the PEACE cohort, which is a middle-aged-to-elderly cohort with known coronary artery disease and normal or near normal left ventricular ejection fraction. Accounting for baseline differences, patients randomly assigned to trandolapril had higher aortic compliance and lower carotid–femoral PWV. The difference in aortic compliance was not significant after adjusting for a small but significant reduction in MAP in the trandolapril group. However, the difference in carotid–femoral PWV persisted after adjusting for differences in MAP, suggesting that long-term ACE inhibition with trandolapril reduced aortic stiffness beyond what would be expected from the passive effects of a reduction in distending pressure alone.
There are a number of plausible mechanisms for a favorable effect of ACE inhibition on carotid–femoral PWV. Activation of the RAAS promotes myocyte hypertrophy and extracellular fibrosis and upregulates enzymes involved in the production of reactive oxygen species.24,25 The resulting oxidative stress impairs NO availability and endothelial function and may, therefore, increase functional arterial stiffness.26,27 Increased myocyte mass or tone and fibrosis in the arterial wall contribute to stiffness and would be expected to increase carotid–femoral PWV. In addition, variants in RAAS genes have been related to carotid–femoral PWV in humans, making inhibition of this pathway an attractive option for reducing arterial stiffness.7,28–33 Consistent with this hypothesis, a previous 12-week study in middle-aged hypertensive subjects demonstrated a reduction in carotid–femoral PWV with the ACE inhibitor enalapril, although a component of the reduction in PWV in that study may have been attributable to a reduction in MAP.12 A study in patients with peripheral vascular disease demonstrated a reduction in carotid–femoral PWV with a nonsignificant change in MAP after 24 weeks of ramipril therapy.34 These previous short-term studies, together with the present long-term data, suggest a prompt and sustained effect of ACE inhibition on carotid–femoral PWV, even in relatively low-risk individuals, such as the PEACE population.
Additional measures of aortic stiffness (characteristic impedance), wave reflection (augmentation index), and global arterial properties (brachial and carotid pulse pressure and total arterial compliance) were not affected by long-term ACE inhibition in our study. The apparently discrepant effects of treatment on various measures of arterial function underscore the diversity of factors that influence this family of related but distinct hemodynamic variables. Carotid–femoral PWV is a measure of the spatially averaged properties of the descending thoracic and abdominal aorta, iliac, and femoral arteries and is primarily affected by changes in the stiffness or thickness of the arterial wall. In contrast, characteristic impedance is a measure of the properties of the proximal aortic root and is highly sensitive to changes in aortic diameter.35 Augmentation index is a measure of relative wave reflection that is affected by a number of modifiable and nonmodifiable factors, including age, sex, height, weight, heart rate, peripheral resistance, and the degree of impedance mismatch between aorta and muscular arteries.36 Aortic compliance is derived from characteristic impedance and carotid–femoral PWV and is, therefore, dependent on proximal and distal aortic properties, with a modest contribution from the iliac arteries as well. Total arterial compliance is a complex average of arterial properties throughout the body, from proximal aorta to resistance vessels, and, therefore, has limited specificity for regional change in arterial properties. Our finding of a reduction in carotid–femoral PWV suggests that long-term ACE inhibition with trandolapril primarily affected the mid-to-distal aorta, probably via a reduction in aortic wall stiffness rather than a change in aortic diameter. A concomitant reduction in aortic diameter, as reported previously after 6 months of treatment with trandolapril,10 may have offset the reduction in wall stiffness, leading to our observed lack of change in characteristic impedance and pulse pressure despite evidence for a reduction in aortic wall stiffness.
In contrast to our findings, several short-term studies found a reduction in augmentation index with ACE inhibition, particularly in hypertensive patients, although augmentation was not normalized in these studies.8,37 Short-term alterations in augmentation index after administration of an ACE inhibitor are predominantly related to a fall in peripheral resistance and shortening of the systolic ejection period.38 Shortening of the systolic ejection period is partially attributable to a reflex increase in heart rate after acute ACE inhibition; although after 12 weeks of ACE inhibition, the systolic ejection period was reduced in the absence of a change in heart rate.12 In contrast to these short-term studies, a 6-month study demonstrated a significant reduction of distal aortic and carotid distensibility and aortic PWV after trandolapril treatment in hypertensive patients.10 In addition, in a 12-month study that compared ACE inhibition with perindopril (plus indapamide) versus β-blockade with atenolol in a hypertensive sample, heart rate and carotid augmentation index were unchanged, and carotid–femoral PWV was reduced in the ACE inhibitor group, similar to our findings.11 However, MAP was substantially reduced in both of these previous studies involving hypertensive patients, suggesting that the reduction in PWV was potentially attributable to the reduction in MAP alone. In the present study with a median follow-up of >4 years, heart rate, systolic ejection period, and augmentation index did not differ between treatment groups. Thus, attenuation of acute or subacute changes in heart rate, systolic ejection period, or peripheral resistance after long-term ACE inhibition may have contributed to the lack of a change in augmentation index in the present study.
Many of the hemodynamic effects of ACE inhibition, including effects on arterial structure and function, are enhanced in the presence of RAAS activation, such as occurs with heart failure, sodium restriction, or concomitant administration of diuretics or other natriuretic agents.39 The low prevalences of heart failure and diuretic usage at baseline in our study prevented us from analyzing relations between these conditions and effectiveness of ACE inhibition. The combination of a low probability that the RAAS was activated in these stable elderly patients together with the long duration of treatment may have attenuated changes in global hemodynamic variables, such as cardiac output, peripheral resistance, total arterial compliance, and augmentation index. Importantly, however, ACE inhibition with trandolapril had a favorable effect on the arterial wall that persisted for the full duration of our long-term study.
Increased carotid–femoral PWV is a risk factor for adverse cardiovascular events, including mortality, heart attack, stroke, and heart failure.1–3,40,41 If increased PWV represents a causal factor in the pathophysiology of these adverse outcomes, a significant reduction in carotid–femoral PWV would be expected to reduce the incidence of these clinical end points. PEACE failed to show a statistically significant reduction in many of these events, although there was a significant reduction in heart failure–related events and a trend toward a reduction in strokes in the trandolapril group. Importantly, the modest reduction in carotid–femoral PWV observed in our study (0.9 m/s) would be expected to reduce cardiovascular mortality by only 4% to 5% in a relatively low risk sample,1,3 which is consistent with the statistically nonsignificant 7% reduction in the composite end point of cardiovascular deaths, nonfatal myocardial infarction, or stroke observed in the main PEACE Trial.
A number of limitations of our study need to be considered. The hemodynamic substudy had a lower percentage of women, largely because 1 center was a Veteran's Administration hospital that had enrolled only men. Patients enrolled in the study were known to be compliant with randomized therapy and are, therefore, a nonrandom subset of the full PEACE cohort that may not be representative of the full sample. There were fewer diabetic and hypertensive patients and less frequent usage of several classes of medication in the substudy as compared with the main trial, suggesting that the substudy sample was somewhat healthier than the full sample. In addition, baseline evaluations were not performed on the study participants. Therefore, longitudinal change during trandolapril therapy was inferred from a cross-sectional analysis performed at the end of the treatment period. To offset the lack of baseline evaluations, our sample size was powered to detect clinically relevant differences in key hemodynamic variables between treatment groups. In addition, it is important to note that we cannot differentiate a reduction in stiffness in the treatment group from an ongoing age-related increase in stiffness in the placebo group that was attenuated in the treatment group. We suspect that both factors contributed to the observed difference in stiffness.
Perspectives
Increased aortic stiffness is associated with excess risk for various adverse cardiovascular disease end points, including mortality, myocardial infarction, stroke, and heart failure. In addition, a number of conditions not recognized previously as having a vascular etiology, including retinal disease and many forms of dementia, have recently been related to abnormal aortic stiffness. Increasing awareness of the adverse effects of aortic stiffening has stimulated interest in defining interventions specifically targeted toward reducing aortic stiffness. Several lines of evidence suggest that the RAAS may be involved in vascular fibrosis and stiffening, and a number of relatively short-term studies have suggested that ACE inhibition or ARB may reduce arterial stiffness. The present study has demonstrated that long-term treatment with the ACE inhibitor trandolapril was associated with a greater reduction in carotid–femoral PWV than would be expected from passive changes because of a reduction in MAP alone, indicating that long-term ACE inhibition has a direct favorable effect on aortic stiffness. Thus, contrary to popular belief, aortic stiffening does appear to be reversible. Favorable effects of ACE inhibition on arterial stiffness likely contribute to the favorable clinical effects of ACE inhibition, particularly in higher-risk patient groups, such as those with hypertension or heart failure.
Acknowledgments
We gratefully acknowledge the efforts of the PEACE investigators, research coordinators, and committee members. A list of these individuals has been published previously.19
Sources of Funding: K.A.J. and M.M.R. are supported in part by National Institutes of Health/National Heart, Lung, and Blood Institute grant N01HC065149 and a supplement from Knoll Pharmaceuticals and Abbott Laboratories, which also provided the study medication. The PEACE hemodynamic substudy was funded by an unrestricted grant from Knoll Pharmaceuticals to Brigham and Women's Hospital.
Footnotes
Disclosures: Brigham and Women's Hospital has been awarded patents regarding the use of inhibition of the renin–angiotensin system in selected survivors of myocardial infarction; M.A.P. and Dr Eugene Braunwald are among the coinventors. The licensing agreement with Abbott and Novartis is not linked to sales. G.F.M. is owner of Cardiovascular Engineering, Inc., a company that designs and manufactures devices that measure vascular stiffness. The company uses these devices in clinical trials that evaluate the effects of diseases and interventions on vascular stiffness. G.F.M. has reported receiving consulting and speaking fees from OMRON Healthcare, Inc., and consulting fees from Inverness Medical Innovations Inc. J.M.O.A. and M.A.P. have reported receiving grants, honoraria, and consulting fees from various pharmaceutical companies. The remaining authors report no conflicts.
References
- 1.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 2.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 3.Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 4.Domanski MJ, Davis BR, Pfeffer MA, Kastantin M, Mitchell GF. Isolated systolic hypertension: prognostic information provided by pulse pressure. Hypertension. 1999;34:375–380. doi: 10.1161/01.hyp.34.3.375. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell GF, Moye LA, Braunwald E, Rouleau JL, Bernstein V, Geltman EM, Flaker GC, Pfeffer MA. Sphygmomanometrically determined pulse pressure is a powerful independent predictor of recurrent events after myocardial infarction in patients with impaired left ventricular function. SAVE investigators. Survival and Ventricular Enlargement. Circulation. 1997;96:4254–4260. doi: 10.1161/01.cir.96.12.4254. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell GF, Vita JA, Larson MG, Parise H, Keyes MJ, Warner E, Vasan RS, Levy D, Benjamin EJ. Cross-sectional relations of peripheral micro-vascular function, cardiovascular disease risk factors, and aortic stiffness: the Framingham Heart Study. Circulation. 2005;112:3722–3728. doi: 10.1161/CIRCULATIONAHA.105.551168. [DOI] [PubMed] [Google Scholar]
- 7.Benetos A, Topouchian J, Ricard S, Gautier S, Bonnardeaux A, Asmar R, Poirier O, Soubrier F, Safar M, Cambien F. Influence of angiotensin II type 1 receptor polymorphism on aortic stiffness in never-treated hypertensive patients. Hypertension. 1995;26:44–47. doi: 10.1161/01.hyp.26.1.44. [DOI] [PubMed] [Google Scholar]
- 8.Ting CT, Chen CH, Chang MS, Yin FC. Short- and long-term effects of antihypertensive drugs on arterial reflections, compliance, and impedance. Hypertension. 1995;26:524–530. doi: 10.1161/01.hyp.26.3.524. [DOI] [PubMed] [Google Scholar]
- 9.Topouchian J, Brisac AM, Pannier B, Vicaut E, Safar M, Asmar R. Assessment of the acute arterial effects of converting enzyme inhibition in essential hypertension: a double-blind, comparative and crossover study. J Hum Hypertens. 1998;12:181–187. doi: 10.1038/sj.jhh.1000581. [DOI] [PubMed] [Google Scholar]
- 10.Topouchian J, Asmar R, Sayegh F, Rudnicki A, Benetos A, Bacri AM, Safar ME. Changes in arterial structure and function under trandolapril-verapamil combination in hypertension. Stroke. 1999;30:1056–1064. doi: 10.1161/01.str.30.5.1056. [DOI] [PubMed] [Google Scholar]
- 11.Asmar RG, London GM, O'Rourke ME, Safar ME. Improvement in blood pressure, arterial stiffness and wave reflections with a very-low-dose perindopril/indapamide combination in hypertensive patient: a comparison with atenolol. Hypertension. 2001;38:922–926. doi: 10.1161/hy1001.095774. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell GF, Izzo JL, Jr, Lacourciere Y, Ouellet JP, Neutel J, Qian C, Kerwin LJ, Block AJ, Pfeffer MA. Omapatrilat reduces pulse pressure and proximal aortic stiffness in patients with systolic hypertension: results of the conduit hemodynamics of omapatrilat international research study. Circulation. 2002;105:2955–2961. doi: 10.1161/01.cir.0000020500.77568.3c. [DOI] [PubMed] [Google Scholar]
- 13.Mahmud A, Feely J. Reduction in arterial stiffness with angiotensin II antagonist is comparable with and additive to ACE inhibition. Am J Hypertens. 2002;15:321–325. doi: 10.1016/s0895-7061(01)02313-5. [DOI] [PubMed] [Google Scholar]
- 14.Lacourciere Y, Beliveau R, Conter HS, Burgess ED, Lepage S, Pesant Y, Spence JD, Asmar R, Carriere S, Plante GE. Effects of perindopril on elastic and structural properties of large arteries in essential hypertension. Can J Cardiol. 2004;20:795–799. [PubMed] [Google Scholar]
- 15.Mitchell GF, Arnold JM, Dunlap ME, O'Brien TX, Marchiori G, Warner E, Granger CB, Desai SS, Pfeffer MA. Pulsatile hemodynamic effects of candesartan in patients with chronic heart failure: the CHARM Program. Eur J Heart Fail. 2006;8:191–197. doi: 10.1016/j.ejheart.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Rutherford JD, Pfeffer MA, Moye LA, Davis BR, Flaker GC, Kowey PR, Lamas GA, Miller HS, Packer M, Rouleau JL. Effects of captopril on ischemic events after myocardial infarction. Results of the Survival and Ventricular Enlargement trial. SAVE Investigators. Circulation. 1994;90:1731–1738. doi: 10.1161/01.cir.90.4.1731. [DOI] [PubMed] [Google Scholar]
- 17.Yusuf S, Pepine CJ, Garces C, Pouleur H, Salem D, Kostis J, Benedict C, Rousseau M, Bourassa M, Pitt B. Effect of enalapril on myocardial infarction and unstable angina in patients with low ejection fractions. Lancet. 1992;340:1173–1178. doi: 10.1016/0140-6736(92)92889-n. [DOI] [PubMed] [Google Scholar]
- 18.The SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions The SOLVD Investigators. N Engl J Med. 1992;327:685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 19.Braunwald E, Domanski MJ, Fowler SE, Geller NL, Gersh BJ, Hsia J, Pfeffer MA, Rice MM, Rosenberg YD, Rouleau JL. Angiotensin-converting-enzyme inhibition in stable coronary artery disease. N Engl J Med. 2004;351:2058–2068. doi: 10.1056/NEJMoa042739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfeffer MA, Domanski M, Rosenberg Y, Verter J, Geller N, Albert P, Hsia J, Braunwald E. Prevention of events with angiotensin-converting enzyme inhibition (the PEACE study design). Prevention of Events with Angiotensin-Converting Enzyme Inhibition. Am J Cardiol. 1998;82:25H–30H. doi: 10.1016/s0002-9149(98)00488-3. [DOI] [PubMed] [Google Scholar]
- 21.Kelly R, Fitchett D. Noninvasive determination of aortic input impedance and external left ventricular power output: a validation and repeatability study of a new technique. J Am Coll Cardiol. 1992;20:952–963. doi: 10.1016/0735-1097(92)90198-v. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell GF, Tardif JC, Arnold JM, Marchiori G, O'Brien TX, Dunlap ME, Pfeffer MA. Pulsatile hemodynamics in congestive heart failure. Hypertension. 2001;38:1433–1439. doi: 10.1161/hy1201.098298. [DOI] [PubMed] [Google Scholar]
- 23.Liu Z, Brin KP, Yin FC. Estimation of total arterial compliance: an improved method and evaluation of current methods. Am J Physiol. 1986;251:H588–H600. doi: 10.1152/ajpheart.1986.251.3.H588. [DOI] [PubMed] [Google Scholar]
- 24.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 25.Morishita R, Gibbons GH, Ellison KE, Lee W, Zhang L, Yu H, Kaneda Y, Ogihara T, Dzau VJ. Evidence for direct local effect of angiotensin in vascular hypertrophy In vivo gene transfer of angiotensin converting enzyme. J Clin Invest. 1994;94:978–984. doi: 10.1172/JCI117464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinlay S, Creager MA, Fukumoto M, Hikita H, Fang JC, Selwyn AP, Ganz P. Endothelium-derived nitric oxide regulates arterial elasticity in human arteries in vivo. Hypertension. 2001;38:1049–1053. doi: 10.1161/hy1101.095329. [DOI] [PubMed] [Google Scholar]
- 27.Ramsey MW, Goodfellow J, Jones CJ, Luddington LA, Lewis MJ, Henderson AH. Endothelial control of arterial distensibility is impaired in chronic heart failure. Circulation. 1995;92:3212–3219. doi: 10.1161/01.cir.92.11.3212. [DOI] [PubMed] [Google Scholar]
- 28.Balkestein EJ, Staessen JA, Wang JG, van der Heijden-Spek JJ, Van Bortel LM, Barlassina C, Bianchi G, Brand E, Herrmann SM, Struijker-Boudier HA. Carotid and femoral artery stiffness in relation to three candidate genes in a white population. Hypertension. 2001;38:1190–1197. doi: 10.1161/hy1101.095992. [DOI] [PubMed] [Google Scholar]
- 29.Benetos A, Gautier S, Ricard S, Topouchian J, Asmar R, Poirier O, Larosa E, Guize L, Safar M, Soubrier F, Cambien F. Influence of angiotensin-converting enzyme and angiotensin II type 1 receptor gene polymorphisms on aortic stiffness in normotensive and hypertensive patients. Circulation. 1996;94:698–703. doi: 10.1161/01.cir.94.4.698. [DOI] [PubMed] [Google Scholar]
- 30.Hosoi M, Nishizawa Y, Kogawa K, Kawagishi T, Konishi T, Maekawa K, Emoto M, Fukumoto S, Shioi A, Shoji T, Inaba M, Okuno Y, Morii H. Angiotensin-converting enzyme gene polymorphism is associated with carotid arterial wall thickness in non-insulin-dependent diabetic patients. Circulation. 1996;94:704–707. doi: 10.1161/01.cir.94.4.704. [DOI] [PubMed] [Google Scholar]
- 31.Mattace-Raso FU, van der Cammen TJ, Sayed-Tabatabaei FA, van Popele NM, Asmar R, Schalekamp MA, Hofman A, van Duijn CM, Witteman JC. Angiotensin-converting enzyme gene polymorphism and common carotid stiffness. The Rotterdam study. Atherosclerosis. 2004;174:121–126. doi: 10.1016/j.atherosclerosis.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 32.Safar ME, Cattan V, Lacolley P, Nzietchueng R, Labat C, Lajemi M, De Luca N, Benetos A. Aldosterone synthase gene polymorphism, stroke volume and age-related changes in aortic pulse wave velocity in subjects with hypertension. J Hypertens. 2005;23:1159–1166. doi: 10.1097/01.hjh.0000170378.08214.13. [DOI] [PubMed] [Google Scholar]
- 33.Safar ME, Lajemi M, Rudnichi A, Asmar R, Benetos A. Angiotensin-converting enzyme D/I gene polymorphism and age-related changes in pulse pressure in subjects with hypertension. Arterioscler Thromb Vasc Biol. 2004;24:782–786. doi: 10.1161/01.ATV.0000119354.41615.33. [DOI] [PubMed] [Google Scholar]
- 34.Ahimastos AA, Natoli AK, Lawler A, Blombery PA, Kingwell BA. Ramipril reduces large-artery stiffness in peripheral arterial disease and promotes elastogenic remodeling in cell culture. Hypertension. 2005;45:1194–1199. doi: 10.1161/01.HYP.0000168945.44069.aa. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell GF, Lacourciere Y, Ouellet JP, Izzo JL, Jr, Neutel J, Kerwin LJ, Block AJ, Pfeffer MA. Determinants of elevated pulse pressure in middle-aged and older subjects with uncomplicated systolic hypertension: the role of proximal aortic diameter and the aortic pressure-flow relationship. Circulation. 2003;108:1592–1598. doi: 10.1161/01.CIR.0000093435.04334.1F. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 37.Ting CT, Yang TM, Chen JW, Chang MS, Yin FC. Arterial hemodynamics in human hypertension. Effects of angiotensin converting enzyme inhibition. Hypertension. 1993;22:839–846. doi: 10.1161/01.hyp.22.6.839. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell GF, Lacourciere Y, Arnold JM, Dunlap ME, Conlin PR, Izzo JL., Jr Changes in aortic stiffness and augmentation index after acute converting enzyme or vasopeptidase inhibition. Hypertension. 2005;46:1111–1117. doi: 10.1161/01.HYP.0000186331.47557.ae. [DOI] [PubMed] [Google Scholar]
- 39.Safar ME, Van Bortel LM, Struijker-Boudier HA. Resistance and conduit arteries following converting enzyme inhibition in hypertension. J Vasc Res. 1997;34:67–81. doi: 10.1159/000159204. [DOI] [PubMed] [Google Scholar]
- 40.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 41.Meaume S, Benetos A, Henry OF, Rudnichi A, Safar ME. Aortic pulse wave velocity predicts cardiovascular mortality in subjects >70 years of age. Arterioscler Thromb Vasc Biol. 2001;21:2046–2050. doi: 10.1161/hq1201.100226. [DOI] [PubMed] [Google Scholar]