Abstract
Healthy 90-day-old ostrich chicks were used in the present study. The ultrastructure and melatonin 1a receptor (MT1) distribution in the ovaries of ostrich chicks was observed by transmission electron microscope and light microscope. The results showed that the ostrich chick ovary contained primordial follicles, primary follicles and secondary follicles, but no mature follicles. There are some unique ultrastructural characteristics observed in the secondary follicle, such as the cortical granule, which was located in cytoplasm beside the nucleus and appeared first in the oocyte. The zona radiata appeared in the secondary follicle, and there was an obvious vitelline membrane. There were intraovarian rete, connecting rete, and extraovarian rete in the ovaries of ostrich chicks. This is the first study that provides immunohistochemical evidence for the localization of the melatonin MT1 in the ostrich chick ovary. The germinal epithelium, follicular cell layer of every grade of follicle, cytoplasm of the oocyte and interstitial cells all expressed MT1. The expression of positive immunoreactivity materials was the strongest in the follicular cell layer of the primordial follicle and germinal epithelium, was weaker in the follicular cell layer of the primary follicle and secondary follicle, and was weakest in the oocytes of all grades of follicle. In addition, the extraovarian rete displayed strong positive expression of MT1, while there was no positive expression in the intraovarian rete or connecting rete. The positive expression of MT1 immunoreactivity in the ovary was very strong, implying that the ovary is an important organ for synthesizing MT1.
Keywords: Melatonin 1a receptor, Ostrich chicks, Ovary, Ultrastructure
Introduction
Compared with other birds, there are some specific differences in the reproduction and physiology of ostriches, such as their low fecundity. The female ostrich achieves sexual maturity at 2–2.5 years of age, and the duration of its brood time is 90 days. These observations suggest that there are likely some special characteristics of the gonad of the ostrich. In the current literature, there are several reports on the microstructure and ultrastructure of gonads in birds (Guraya 1976; Rothwell and Solomon 1977; Perry et al. 1978; Yoshimura et al. 1993), but there is little known about the gonads of ostrich chicks. There have been reports on the morphology of ovarian follicles (Madekurozwa and Kimaro 2006a) and ultrastructure of the follicular wall (Madekurozwa and Kimaro 2006b) in sexually immature ostriches (aged 12–14 months), but there are no reports on the histologic structure of the ovary of ostrich chicks. Therefore, the aim of the present study is to delineate the gross anatomy, microstructure and ultrastructure of the gonads of ostrich chicks, and in doing so, explore the underlying structural basis for the differences observed in the reproductive physiology of the ostrich.
Melatonin was first separated from pituitary gland of the hog in 1956 (Lee and Lerner 1956). It is secreted by the pineal body of birds and mammals (Ralph 1975), Melatonin is an important hormonal signal regulated by illumination, which is transferred to the hypothalamic–pituitary–gonadal axis to modulate reproductive function. Melatonin may have an additive function in regulating the reproductive system, with multi-site pathways of action (Pang et al. 1998). The regulation of the physiological function of target cells by melatonin occurs not only through the melatonin receptor-mediated G protein signal transduction pathway, but also through the signaling pathway activated by the MT1 cytosolic receptor or nuclear receptor. Studies in chickens have shown that melatonin functions as an antioxidant to improve the glutathione peroxidase activity and enhance immunity (Pablos et al. 1995). The melatonin can reduce the weight of the gonad and its accessory organs, so it can delay the sexual maturity of the immaturity animals (Shacoori et al. 1996). Ayre et al. (1992) pointed out that the melatonin binding site in the chicken ovary is located in the follicular cell layer. Up to now, there has been no report of an immunohistochemical study of MT1 in the ovary of birds. The brood time of the ostrich is 3 months long, which is different from other birds. Studying the location of MT1 in the ovary may provide the theory evidence for the unique reproductive and physiological functions of ostrich chicks.
Materials and methods
Animals
A total of 8 female ostrich chicks, aged 90 days and weighing 11–15 kg, were used in the present study. The birds originated from commercial farms, and were killed by the administration of an overdose of sodium pentobarbitone.
Transmission electron microscopy (TEM)
Ovary tissue sample was fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer. Thereafter, the ovarian tissues were fixed in osmium tetroxide, dehydrated and embedded in epoxy resin. Ultra-thin sections of the samples were stained with lead citrate and uranyl acetate and subsequently analyzed with a Holland FEI TecnaiG212 transmission electron microscope.
Morphologic analysis
The remaining tissue samples were then fixed in Bouin’s fluid for 12 h. After fixation, tissues underwent routine processing for histological analysis and were embedded in paraffin wax. Some sections were also stained with hematoxylin-eosin for morphological observations, and other sections were stained for immunohistochemical analysis.
Immunohistochemical analysis
Sections 4 μm in thickness were deparaffinized, and endogenous peroxidase activity was blocked by immersion in 3% (v/v) hydrogen peroxide solution for 20 min. Thereafter, the slides were microwaved at 750 W twice for 10 min each time. The sections were then incubated for 12 h at 4 °C with specific rabbit anti-chicken affinity-purified polyclonal antibody against MT1 (Boster, China) at dilutions of 1:100, and then were incubated for 20 min with a biotinylated secondary antibody (Boster, China). Thereafter, the slides were incubated for 20 min with the streptavidin peroxidase component of the SABC staining kit (Boster, China). Slides were then rinsed in PBS and bound antibody was visualized after the addition of a 3,3′-diaminobenzidine tetrachloride solution (Boster, China). Negative controls for immunostaining were created by substituting the primary antibody with normal mouse serum. The positive staining of immunohistochemical test presented brown.
Quantification
The relative intensity of the immunoreactivity was scored semiquantitatively as follows: lack of immunoreactivity (−), weak immunoreactivity (+), moderate immunoreactivity (++), strong immunoreactivity (+++).
Protein isolation and western blot analysis
Ovary tissue samples from ostrich chicks were centrifuged 10 min at 3000 g in icecold lysis buffer [17 mM Tris–HCl pH 7.3 containing 0.144 M NH4Cl (Sigma)] to eliminate lymphocytes that express melatonin receptors (Pozo et al. 1997). Tissues were homogenized using a Potter–Elvehjem homogenizer (20 strokes, on ice). Total proteins were extracted from the ovary tissue using the cell lysis buffer for western (1%Triton X-100, 1%deoxycholate, 0.1% SDS) according to manufacturer’s instructions (Beyotime, China). Protein concentration of each sample was assayed by the BCA protein Assay Reagent according to manufacturer’s instructions (Beyotime, China).
Proteins samples were heated at 95C for 5 min in 2× sodium dodecyl sulphate (SDS) gel-loading buffer [100 mM Tris–HCl pH 6.8, 200 mM β-mercaptoethanol, 20% glycerol, 4% SDS and 0.2%bromophenol blue (Sigma)]. Proteins were fractionated by SDS–polyacrylamide gel electrophoresis (SDS–PAGE) on 12% separating gels with 4.4% stacking gels. Proteins were then transferred to nitrocellulose membranes (Amersham Biosciences). Blots were blocked in blocking buffer [20 mM Tris–HCl (pH 7.4) containing 150 mM NaCl, 0.02% (v/v) Tween 20 (TBST; tris-buffered saline with Tween-20) and 5% non-fat dry milk (Sigma)]. Western blot analysis was carried out using a 1:200 dilution of specific rabbit anti-chicken affinity-purified polyclonal antibody against MT1 (Boster, China) in blocking buffer for 2 h at 37 °C. After TBST washing procedure, the blots were incubated with 1:2000 goat anti-rabbit horse-radish peroxidaselabelled specific antibodies (Boster, China) in TBST for 1 h at 37 °C.
The immunoreactive bands were developed after using the 3,3′-diaminobenzidine tetrachloride solution (Boster, China).
Results
Anatomy of the ovary
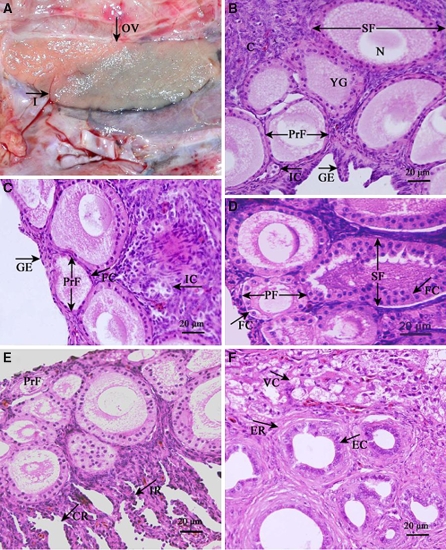
As with other kinds of birds, only the left side generative organ of ostrich developed. The left ovary is near the spine at the back of the inner margin rib and is attached to the left flank by mesovarium. The center of the ovary’s dorsal surface tightly connects with the left adrenal gland. Shaped as a long oval, the ovary, which is dark pink, has a notch in the surface and no mature ovarian follicles (Fig. 1a).
Fig. 1.
Microstructural characteristics of the ostrich chick ovary. (a) The ovary (OV) is a long oval slice, dark pink in color, with a notch in the surface and no mature ovarian follicles. (b) The cortex (C) of the ovary in ostrich chicks and the shape of the germinal epithelium (GE) are cubic or prismatic; the yolk granules (YG) in the oocyte of the primordial follicles (PrF) and secondary follicle (SF). (c) The follicular cell layer (FC) of the primordial follicles (PrF) and interstitial cells (IC) of the ostrich chick ovary. (d) The follicular cell layer (FC) in the primary follicle (PF) and secondary follicle (SF) of the ostrich chick ovary. (e) Intraovarian rete (IR) and connecting rete (CR) of the ostrich chick ovary. (f) Epithelial cell (EC) of the extraovarian rete (ER) and vacuolated cell (VC) of the ostrich chick ovary
Primordial follicles
Primordial follicles distribute to the surface of the subcapsular cortex and composed of an oocyte surrounded by a single layer of flat follicular cells (Fig. 1c). The oocyte, 61.88 ± 7.04 μm in diameter, is a simple structure that contains yolk granules.
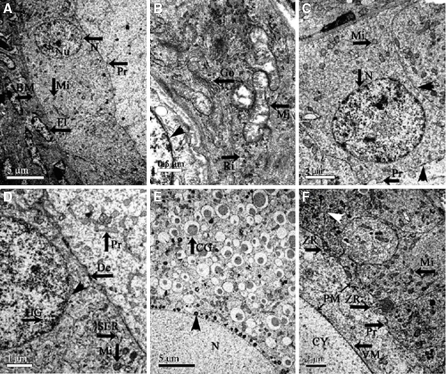
Under the electron microscope, the follicular cells that surround the oocyte are flat-shaped (Fig. 2a) and have one or two nucleoli each. The cytoplasmic organelles include many bacilliform mitochondria, ribosomes and Golgi complexes (Fig. 2b). The inner layer of the follicular membrane is located outside the basal lamina, and is composed of fibroblasts (Fig. 2a) that are lined by electron-dense substances in the karyotheca inner wall.
Fig. 2.
Ultramicrostructural characteristics of the ostrich chick ovary. (a) The part of the oocyte, follicular cells and basal membrane in the primordial follicle of the ostrich chicks, showing the nucleus (N), nucleolus (Nu) and cell process (Pr) of the granulosa cell; basal lamina (BM); and fibroblast (FI). (b) The cytoplasm of the follicular cells in the primordial follicle of ostrich chicks, showing the mitochondria (Mi), Golgi complex (Go), free ribosome (Ri); the nuclear pore is clear (△). (c) The part of the oocyte, cubic follicular cells in the primary follicle and basal lamina, showing that there is a desmosome (△) connecting the follicular cells and the oocytes; the digitations of follicular cells (Pr). (d) The part of the oocyte and follicular cells in primary follicle, showing the desmosome between the follicular cells and oocytes, the heterochromatin granules (HG), intranucleus of the follicular cells and nuclear membrane (△). (e) The oocyte nuclei and cytoplasm of the secondary follicle, showing the trachychromatic granules clinging to the nucleus (△), and cortical granules in cytoplasm of the oocyte (CG). (f) The cytoplasm of the oocyte, vitelline membrane (VM), perivitelline membrane (PM), zona radiate (ZR), and multilayer follicular cells (△) in the secondary follicle
Primary follicle
Primary follicles distributed in the periphery and middle of the ovarian cortex. The volume of the primary follicle is larger than that of the primordial follicle, as is the oocyte, which has a diameter of 80.94 ± 4.81 μm. The primary follicle has a single cubical follicular cell layer (Fig. 1d). The nucleus is located at the free end of the follicular cell and it increases in diameter and becomes round in the primary follicle (Fig. 2c). The karyotheca is distinct, and the granules of heterochromatin cling to the inner wall of the nucleus (Fig. 2d). The cytoplasm of the follicular cells contains a great number of mitochondria, most of which are round (Fig. 2c). The oolemma changes throughout the maturation process of the oocyte, with continued elaboration of the microvilli, which begin to have connections with the projections from the surface of the follicular cells. There are desmosomes located between the projections of follicular cells and the microvilli of the oocytes (Fig. 2c).
Secondary follicle
Secondary follicles located in the middle of the cortex. At this stage the follicle is bigger, as is the oocyte, whose diameter increases to 136.88 ± 31.64 μm. The structure of the ooplasm is approximately the same as described above, but it now contains a greater number of yolk granules (Fig. 1b). The follicular cell layer of the secondary follicle differentiates to a multi-layer structure; the boundary between the follicular cells is distinct. There are many highly electron-dense materials clinging to the nuclear membrane (Fig. 2e), which is round and smooth. The cortical granules spread in the cytoplasm near the nucleus (Fig. 2e). The yolk membrane of the secondary follicle is obvious, and the protrusions from the yolk membrane run to the follicular cell layer, which forms a zona radiata. There are many layers of follicular cells. The cell nuclei of the follicular cells appear to be round and situated near the oocyte (Fig. 2f). The cellular organs in the cytoplasm of the secondary follicle are much more plentiful as compared to the primary ovarian follicle.
Rete ovarii
From the reconstruction of the rete ovarii of the ostrich chicks, it becomes apparent that this structure is composed of a continuous association of tubes and cell cords that extend from the ovary into the periovarian tissue.
The intraovarian rete (Fig. 1e) is constructed of numerous tubules that consist of many small cell bundles. The connecting rete (Fig. 1e) has tissue that joins the extraovarian rete with the intraovarian rete and leads to the follicle by the basal membrane. The irregular tubular structure in the medulla can be seen at the hilum of the ovary, which is called the extraovarian rete of the ovary (Fig. 1f). Its epidermis is composed of simple cubical cells, with strong basophilia and a high nuclear/cytoplasmic ratio (N/P ratio). The cell nuclei are large and round, and the nucleoli are obvious. The periphery is surrounded by the spindle cells of connective tissue.
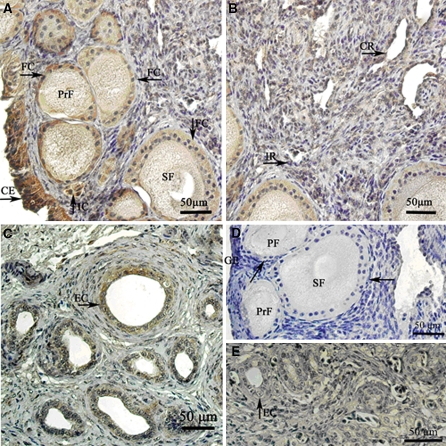
Immunohistochemical localization of MT1 in ovary of the ostrich chicks
Positive MT1 immunoreactive in the ovary of ostrich chicks was intensively present in the germinal epithelial cells, follicular cells in every follicle phase, cytoplasm of oocytes and interstitial cells (Fig. 3a). The cell membranes and cytoplasm both expressed immunoreactivity for MT1. The follicular cells of the primordial follicle and germinal epithelium exhibited the strongest immunoreactivity (Table 1). The immunoreactivity in the follicular cells of the primary follicle and secondary follicle was moderate, and that in the oocyte of every follicle grade was weak. In addition, the interstitial cells and the extraovarian rete showed stronger immunoreactivity (Fig. 3c). Immunoreactivity was absent in the intraovarian rete and connecting rete (Fig. 3b, Table 2). No positive staining was detected in the negative control sections (Fig. 3d, e).
Fig. 3.
MT1 expression in the ostrich chick ovary. (a) The cells with positive MT1 immunoreactivity in the cortex of the ostrich chick ovary. The expression of positive products in the germinal epithelium (GE), follicular cell layer (FC) of every grade of follicle, and the interstitial cell (IC). (b) There were no positive MT1 immunoreactivity cells in the intraovarian rete (IR) and connecting rete (CR). (c) The positive MT1 immunoreactivity cells in the extraovarian rete. The expression of positive products in the epithelial cell was much stronger. (d) The negative expression of MT1 immunoreactivity in the cortex of ostrich chick ovary. (e) The negative expression of MT1 immunoreactivity in the extraovarian rete
Table 1.
Distribution of immunoreactivity for MT1 in the ovarian follicles of the ostrich chicks
| Follicle type | Follicular region | MT1 | Negative controls |
|---|---|---|---|
| Primordial | Follicular cell layer | +++ | − |
| Cytoplasm of oocyte | + | − | |
| Primary | Follicular cell layer | ++ | − |
| Cytoplasm of oocyte | + | − | |
| Secondary | Follicular cell layer | ++ | − |
| Cytoplasm of oocyte | + | − |
Intensities of immunostaining: −, absent; +, weak; ++, moderate; +++, strong
Table 2.
Immunohistochemical localization of MT1 in ovary (excluding ovarian follicles) of the ostrich chicks
| Location | MT1 | Negative controls |
|---|---|---|
| Germinal epithelium | +++ | − |
| Interstitial cells | ++ | − |
| Intraovarian rete | − | − |
| Connecting rete | − | − |
| Extraovarian rete | ++ | − |
Intensities of immunostaining: −, absent; +, weak; ++, moderate; +++, strong
Expression of MT1 protein in the ostrich chick ovary
Western blot analysis was used to determine MT1 protein expression in the ovary of ostrich chicks. Optimal antibody signal was initially determined by separating increasing concentrations of total proteins by SDS–PAGE and immunoblotting using specific rabbit purified polyclonal anti-MT1 antibodies. Densitometric analysis of the immunoblots showed that the signal was proportional to the amount of protein applied to gel within a range of 20–150 μg (correlation coefficients ≤ 0.98) for the antibodies. We carried the study with 60 μg of total protein that gave the optimal antibody signal, The results revealed a single band of 37 kDa for ostrich chicks ovary tissue proteins for MT1 (Fig. 4).
Fig. 4.
Expression of MT1 protein in ostrich chicks ovary tissues. Western blots were hybridized with specific, anti-MT1 antibodies. low molecular protein marker; ostrich chicks ovary MT1 representative results from the experiments are shown
Discussion
The oocyte of the primordial follicle doesn’t usually contain cortical granules. Just after the follicle begins to grow, the cortical granule starts to form (Kim et al. 1996). The cortical granule of 90-day-old ostrich chicks first appears near the nucleus of the secondary oocyte, as observed by electron microscopy. The morphological structure, location of the cortical granule and its change in composition are closely related to oocyte maturation and fertilization (Hyttel et al. 1989). In the later development of the oocyte, the cortical granule increases significantly in size and moves slowly to the cortical area. When the oocyte matures, the cortical granule becomes situated under the plasmalemma; this is considered one of the typical characteristics of oocyte maturation (Oterino et al. 2001).
The present study has shown that the zona radiata grow slowly in the primordial follicle and primary follicle, but in the secondary follicle the long cytoplasmic processes form the zona radiata. This phenomenon demonstrates that after the yolk precursor enters the ooplasm, it gradually changes from having restricted motion to being active. At this moment, the oocyte actively receives nutrients transported from the follicular cell layer for vigorous anabolic metabolism (Tan et al. 1992). Meanwhile, the distribution of the zona radiata is disproportionate, which is in concordance with Madekurozwa’s report. The present study shows that the existence of the perivitelline layer in the ovarian follicles of the ostrich chicks is the same as that of other birds. The amorphous material forming the perivitelline layer has been shown to be vitellogenin (Ito et al.2003). Thus, the existence of the perivitelline layer in the sexually immature ostrich suggests that the rate of vitellogenin transfer from the follicular cell layer into the oocyte is not low at this stage of development. This conclusion is completely different from that proposed in the study by Madkurozwa and Kimaro (2006b).
In the nineteenth century, anatomists first recognized the morphological resemblance between the rete ovarii and the rete testis during the embryonic development of the mammalian gonad. The tubular networks in these structures arose from the mesonephros, formed by a single layer of cubical and cylindrical epithelial cells, and connected to the Wolffian duct (Byskov and Lintern-Moore 1973). Some researches (Byskov et al. 1997; FrÖjdman et al. 1995; Glickman et al. 2005; Mcnatty et al. 1995; Nikitin 1985) focused on the conversion and morphologic changes of the mesonephros cells to demonstrate that the follicular cells of mammals arise through the differentiation of the rete ovarii from the mesonephridium. They presumed that the rete ovarii plays a part in the formation of the granular layer. In addition, some reports have shown that the rete ovarii is involved in the formation of the follicle and accompanies its growth (Byskov et al. 1997). This research, for the first time, reported that the rete ovarii exists in ostrich chicks. The endothelial cells of the intraovarian rete and connecting rete of 90-day-old ostrich chicks had weak basophilia, but the endothelial cells of the extraovarian rete had pronounced basophilia. This was different from Jimpy mice, in which the endothelial cells of the connecting rete had much stronger basophilia (Byskov et al. 1997). Up till now, there have not been any reports on the developmental features and functions of avian rete ovarii. Further research must be performed to demonstrate whether the avian rete ovarii play an important role in the formation of follicles before sexual maturity, as they do in mammals.
We studied the expression and distribution of MR in ovary of ostrich chicks by means of western blotting and immunohistochemistry for the first time. The results showed that positive cells located in germinal epithelium, extraovarian rete, follicular cells of the primordial follicle, primary follicle and oocytes in every follicle grade, and the staining intensity was strong. The presence of melatonin receptors in the ovary, mammary gland, testis, epididymis indicate that the direct melatonin actions on different levels of the reproductive system (Pang et al. 1998; Pozo et al. 1997). It indicates that there should be a part of melatonin effect on the ovary of ostrich chicks directly, besides the indirect effect through the hypothalamic–pituitary–gonadal axis. Previous studies have reported that many tissues of birds have a very strong [125I]-melatonin binding site, so birds are more suitable subjects than mammals for research on the melatonin receptor (Dubocovich 1988). The melatonin binding site is located in the spleen of chicken, pigeon, quail and duck, and in the duck it is also found in the thymus and bursa of the fabricius (Pang et al. 1993). In ostrich chicks, four parts of ovary have positive MT1-immunoreactive cells which were melatonin binding sites. However, the only kind of melatonin binding site in the chicken ovary is follicular cells (Ayre et al. 1992). Melatonin may be involved in the sexual maturation, ovulation or menopause of women (Boczek-Leszczy and Juszczak 2007). Ostrich’s sex-maturity period was the longest in birds and melatonin play the inhibitory action on the development of gonad. So, we presume that melatonin has a direct effect on the ovary of ostrich chicks which result to an inhibition of its development, but the mechanism of action need further study.
In extraovarian rete of ostrich chicks, we can also find MT1-immunoreactive positive cells with strong staining intensity, from which we supposed that the extraovarian rete may have the function of regulating the development of the gonad, especially on the follicular cells.
Acknowledgments
The authors would like to thank Dr. Liu Huazhen for her help in preparation of the manuscript. This study was supported by the National Natural Science Fund Project of China No. 30471249 and No. 39970547.
Contributor Information
Yan Wang, Phone: +86-27-87286970, FAX: +86-27-87280408, Email: wy6696@yahoo.com.cn.
Ke-Mei Peng, Phone: +86-27-87286970, FAX: +86-27-87280408, Email: wangyan1658@webmail.hzau.edu.cn, Email: kmpeng@sohu.com.
References
- Ayre EA, Yuan H, Pang SF (1992) The identification of 125I-labelled iodomelatonin-binding sites in the testes and ovaries of the chicken (Gallus domesticus). J Endocrinol 133:5–11 [DOI] [PubMed]
- Boczek-Leszczy KE, Juszczak M (2007) The influence of melatonin on human reproduction. Pol Merkur Lekarski 23:128–130 [PubMed]
- Byskov AG, Lintern-Moore S (1973) Follicle formation in the immature mouse ovary: the role of the rete ovarii. J Anat 116:207–217 [PMC free article] [PubMed]
- Byskov AG, Skakkebaek NE, Stafanger G, Peters H (1997) Influence of Ovarian surface epithelium and reteovanii on follicle formation. J Anat 123:77–86 [PMC free article] [PubMed]
- Dubocovich ML (1988) Pharmacology and function of melatonin receptors. FASEB J 2:2765–2773 [DOI] [PubMed]
- FrÖjdman K, Ekblom P, Sorokin L, Yaqi A, Pelliniemi LJ (1995) Differential distribution of laminin chains in the development and sex differentiation of mouse internal genitalia. Int J Dev Biol 39:335–344 [PubMed]
- Glickman SE, Short RV, Renfree MB (2005) Sexual differentiation in three unconventional mammals: spotted hyenas, elephants and tammar wallabies. Horm Behav 48:403–417 [DOI] [PubMed]
- Guraya SS (1976) Correlative cytological and histochemical studies on the avian oogenesis. Z Mikrosk Anat Forsch 90:91–150 [PubMed]
- Hyttel P, Greve T, Callesen H (1989) Ultrastructural aspects of oocyte maturation and fertilization in cattle. J Reprod Fertil. Supplement 38:35–47 [PubMed]
- Ito Y, Kihara M, Nakamura E, Yonezawa S, Yoshizaki N (2003) Vitellogenin transport and yolk formation in the quail ovary. Zool Sci 20:717–726 [DOI] [PubMed]
- Kim NH, Funahashi H, Abeydeera LR, Moon SJ, Prather RS, Day BN (1996) Effects of oviductal fluid on sperm penetration and cortical granule exocytosis during fertilization of pig oocytes in vitro. J Reprod Fertil 107:79–86 [DOI] [PubMed]
- Lee TH, Lerner AB (1956) Isolation of melanocyte-stimulating hormone from hog pituitary gland. J Biol Chem 221:943–959 [PubMed]
- Madekurozwa MC, Kimaro WH (2006a) A morphological and immunohistochemical study of healthy and atretic follicles in the ovary of the sexually immature Ostrich (Struthio camelus). Anat Histol Embryol 35:253–258 [DOI] [PubMed]
- Madekurozwa MC, Kimaro WH (2006b) Ultrastructural features of the follicular wall in developing follicles of the sexually immature ostrich (Struthio camelus). Onderstepoort J Vet Res 73:199–205 [DOI] [PubMed]
- Mcnatty KP, Smith P, Hudson NL, Heath DA, Tisdall DJ, O WS, Braw-Tal R (1995) Development of the sheep ovary during fetal and early neonatal life and the effect of fecundity genes. J Reprod Fertil Supplement 49:123–35 [PubMed]
- Nikitin AI (1985) Problems in the early differentiation of mammalian gonads (embryonic histogenesis and mechanisms of regulation). Arkh Anat Gistol Embriol 89:5–17 [PubMed]
- Oterino J, Sanchez TG, Zelarayán L, Valz-Gianinet JN, Bühler MI (2001) Cortical granule exocytosis in Bufo arenarum oocytes matured in vitro. Zygote 9:251–259 [DOI] [PubMed]
- Pablos MI, Chuang J, Reiter RJ, Ortiz GG, Daniels WM, Sewerynek E, Melchiorri D, Poeggeler B (1995) Time course of the melatonin-induced increase in glutathione peroxidase activity in chick tissues. Biol Signals 4:325–330 [DOI] [PubMed]
- Pang CS, Brown GM, Tang PL, Pang SF (1993) G-protein linked melatonin binding sites in the chicken lung. Neurosci Lett 162:17–20 [DOI] [PubMed]
- Pang SF, Li L, Ayre EA, Pang CS, Lee PP, Xu RK, Chow PH, Yu ZH, Shiu SY (1998) Neuroendocrinology of melatonin in reproduction: recent developments. J Chem Neuroanat 14:157–166 [DOI] [PubMed]
- Perry MM, Gilbert AB, Evans AJ (1978) Electron microscope observations on the ovarian follicle of the domestic fowl during the rapid growth phase. J Anat 125:481–497 [PMC free article] [PubMed]
- Pozo D, Delgado M, Fernandez-Santos JM, Calvo JR, Gomariz RP, Martin-Lacave I, Ortiz GG, Guerrero JM (1997) Expression of the Mel1a-melatonin receptor mRNA in T and B subsets of lymphocytes from rat thymus and spleen. FASEB J 11:466–473 [DOI] [PubMed]
- Ralph CL (1975) The pineal gland and geographical distribution of animals. Int J Biometeorol 19:289–303 [DOI] [PubMed]
- Rothwell B, Solomon SE (1977) The ultrastructure of the follicle wall of the domestic fowl during the phase of rapid growth. Br Poult Sci 18:605–610 [DOI] [PubMed]
- Shacoori V, Saïag B, Lemay V, Girre A, Rault B (1996) Effects of melatonin in vivo upon luteinizing hormone and prolactin releases induced by opiate receptor antagonists in adult male rats. J Endocrinol Invest 19:76–82 [DOI] [PubMed]
- Tan JH, Sun QY, Yang ZM, Qin PC (1992) Ultrastructural studies on the goat oogenesis. Acta Anatomica Sinica 23:106–110
- Yoshimura Y, Okamoto T, Tamura T (1993) Ultrastructural changes of oocyte and follicular wall during oocyte maturation in the Japanese quail (Coturnix coturnix japonica). J Reprod Fertil 97:189–196 [DOI] [PubMed]