Abstract
From unfractionated embryonic mice liver cells, appreciable amount of spherical bodies containing nestin-positive cells were generated in the presence of neuronal growth factors. Following cultivation on poly-d-lysine/laminin-coated slips, approximately 70% of the cells expressed neuronal markers, and 16% had long processes. Functional analysis of these long-process-bearing cells with the whole-cell patch clamp method showed an inward current in response to glutamate, GABA, and serotonin as the neuronal characteristics. Furthermore, regenerating liver in adult mice also contained nestin-positive cells to the same extent as fetal liver. Regenerating liver could have potential as a source of neural cells for autologous transplantation.
Keywords: Liver, Nestin, Neural progenitor cell
Introduction
Brain has long been regarded as incapable of regeneration, however discovery of neural stem cells (NSC), the self renewing precursors of neurons and glia, generated intense interest aimed at developing transplantation strategies to promote neural recovery in the disease or injured nervous system (Reynolds and Weiss 1992; Bjorklund and Svendsen 1999). Although embryonic stem (ES) cells and fetal nervous tissue are considered to be candidates for transplantation source, they arise ethical and immune rejection problems. In addition, ES cells have the chance of teratoma incidence (Yanai et al. 1995). Therefore, autologous tissue would be ideal source for transplantation.
One possible source of autologous tissue is the adult brain. It has been shown that NSC can be isolated, cultured, and propagated from adult mammalian brain tissue (Gage 2000; Arsenijevic et al. 2001). However, for clinical applications this source is fairly inaccessible and the generation of NSC from adult brain has variable efficiency and reproducibility.
On the other hand, bone marrow, skin and muscle have been investigated as alternative source of neural stem cells (Toma et al. 2001; Romero-Ramos et al. 2002; Sanchez-Ramos et al. 2002). While transplanted into brain in vivo or cultured in suitable condition for neuronal condition in vitro, they express neural markers. Those experiments may suggest that tissue-specific adult stem cells have significant plasticity and the potential to differentiate into cells of other tissues. The phenomena are called transdifferentiation. That procedure may make it possible to obtain autologous neurons from non-neuronal cells. Since it is unclear how many kinds of cells that are able to transdifferentiate into neural derivatives are distributed in whole body, here we sought another cell population for the liver. The reason why we focused on the liver is that the liver itself possess strong regenerating activity like bone marrow, skin and mesenchymal tissue in the muscle (Toma et al. 2001; Romero-Ramos et al. 2002; Sanchez-Ramos et al. 2002).
Transdifferentiation strategy also has problems. In many experiments functional analysis was not performed, moreover it is uncertain whether the tissue specific stem cell transdifferentiates or whether a subpopulation persists that has the appropriate neural differentiation ability. Therefore we attempted to evaluate obtained neuron-like cells by patch clamp method and considered the mechanism as to transdifferentiation based on our results and reports concerning about liver development.
Material and methods
Tissue dissociation and formation of spheres
C57BL/6 fetal mouse livers were removed at ED13 and single cell suspensions were prepared by gentle pipetting in DMEM/F12 (Invitrogen Corp., Carlsbad, CA). Isolated cells were passed through a nylon mesh filter (pore size, 35 μm) and centrifuged at 200 g for 5 min. The cellular pellet was washed twice (200 g, for 5 min) with DMEM/F12. It was then resuspended in DMEM/F12 supplemented with epidermal growth factor (EGF) (20 ng/ml, Sigma, St. Louis, MO), basic fibroblast growth factor (bFGF) (40 ng/ml, WAKO, Tokyo, Japan), N2 supplement (10 μl/ml, Invitrogen Corp.), penicillin G (100 U/ml), streptomycin (100 μg/ml) and Amphotericin B (250 ng/ml) at a cell density of 1 × 106 cells/ml. The cells were cultured in poly-HEMA (poly 2-hydroxyethyl methacrylate) (Sigma)-coated dishes for one week. The culture medium was changed twice a week. Aggregation of cells into spheres was checked regularly under a microscope.
Neuronal induction
After 1 week, the spheres prepared as above were collected and centrifuged, and then plated on poly-d-lysine/laminin-coated slips (BD Biosciences) in DMEM/F12 containing 10% FBS, 1 μM retinoic acid (RA) (Sigma), 1 ng/ml brain-derived neurotrophic factor (BDNF) (Invitrogen Corp.), 100 ng/ml nerve growth factor (NGF) (Chemicon International Inc., Temecula, CA) and 50 ng/ml neurotrophin-3 (NT-3) (WAKO) for 7–14 days.
Partial hepatectomy
A 70% partial hepatectomy under anesthesia with pentobarbital was performed on 8 week-oldC57BL/6 male mice using a technique described by Higgins and Anderson (Higgins 1931). Mice were sacrificed 3 days after partial hepatectomy.
Immunocyto- and histochemistry
Each antigen was examined in at least three independent experiments. Fetal mouse livers for histological analysis were fixed in 4% para-formaldehyde in 0.1 M phosphate buffer (4% PFA) for 1 h at 4°C. After hepatectomy, the regenerating livers were perfused with 4% PFA. The livers were then frozen and sectioned into 10 μm-thick slices using a cryostat. Cultured cells were also fixed in cold 4% PFA for 20 min. After washing with PBS, specimens were incubated in 3% BSA for 30 min at room temperature, and then covered with the primary antibody solution containing 1% BSA at 4°C overnight. On the following day, they were washed in PBS and incubated for 1 h at room temperature with secondary antibody. Sources and concentrations of the primary antibodies were as follows: mouse anti-nestin, monoclonal (1:600;Chemicon), rabbit anti-MAP-2, polyclonal (1:200; Chemicon), mouse anti-β-tubulin type III, monoclonal (1:200; Chemicon), rabbit anti-neurofilament-H, polyclonal (1:200; Chemicon), rabbit anti-tyrosine hydroxylase (TH), polyclonal (1:200), rabbit anti-glutamic acid decarboxylase (GAD), polyclonal (1:2000; Sigma), rabbit anti-choline acetyltransferase (ChAT), polyclonal (1:500; Chemicon), rabbit anti-GFAP, polyclonal (DAKO, Carpinteria, CA, USA), mouse anti-O4, monoclonal (1:40 Chemicon), rabbit anti-albumin, polyclonal (1:600). The second antibodies were Alexa 488- or Cy3-coupled anti-mouse and anti-rabbit antibodies (1:200 Invitrogen Corp., Chemicon).
Cellular nuclei were counterstained with Hoechst 33258 (Sigma). Staining was visualized under a fluorescence microscope (Zeiss, Oberkochen, Germany) or a confocal laser scanning microscope (Nikon, Tokyo, Japan). All of data were analyzed using a contemporary statistical package (SPSS 12.0 J, Chicago, IL).
Patch clamp experiments
The cells were voltage-clamped at a −50 mV holding potential at room temperature using a whole-cell clamp configuration. The instruments used for electrophysiology were as follows: an Axopatch 200-A patch clamp amplifier, a Digidata-1200 data acquisition system and pCLAMP 6.02 software from Axon Instruments Inc. (Foster City, USA). The headstage of the amplifier was fitted to an MHW-3 hydraulic manipulator manufactured by Narishige Inc., (Tokyo, Japan), and the cells were visualized with an Olympus IMT-2 invert microscope (Olympus, Tokyo, Japan). The software was run on an IBM compatible personal computer with a 90 MHz Pentium processor. The patch electrodes (OD = 1.5 mm, thin wall, Garner Co., Lincoln, NE, USA) were pulled with a PP-83 puller and polished by an MF-83 microforge (Narishige Inc.). The resistance of the patch electrodes was 8–10 MΩ. The solutions used were as follows: the extracellular solution contained 10 mM HEPES, 140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 10 mM glucose, pH 7.34, and the intracellular pipette solution contained 10 mM HEPES, 110 mM KCl, 15 mM NaCl, 0.1 mM CaCl2, 2 mM MgCl2, 1 mM EGTA, 2 mM ATP-disodium, pH 7.25. In order to block the K-current, modified solutions were used: the extracellular solution contained 10 mM HEPES, 110 mM NaCl, 5 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 30 mM tetraethyl-ammonium chloride (TEA-Cl), 1 mM 4-aminopyridine, 10 mM glucose, pH 7.34, and the intracellular pipette solution contained 10 mM HEPES, 110 mM CsCl, 15 mM NaCl, 0.1 mM CaCl2, 2 mM MgCl2, 1 mM EGTA, 2 mM ATP-disodium, pH 7.25. Neurotransmitters (10 mM glutamate, 1.25 mM serotonin, 10 mM dopamine, 10 mM acetylcholine, 10 mM GABA) were added directly to the extracellular bath solution. Recording was started simultaneously with the drug applications. The voltage-gated potassium current was measured using step command pulses starting from −90 mV to +40 mV in 10 mV steps.
Results
We cultivated fetal liver cells (106/ml) from embryonic day 13 (ED13) mice according to the method used for isolation and propagation of neurospheres from brain. (Weiss et al. 1996) After a few days, many cells (approximately 90%) died, but viable cellular spheroids had begun to form. These cells continued to proliferate and the size of the spheres increased until 14 days after the initial culturing (Fig. 1a). However, after 14 days, the rate of growth gradually diminished and the cells finally ceased dividing.
Fig. 1.
Generation and characterization of spheres. Phase micrographs of floating spheres of cells (arrows) isolated from ED13 mouse liver and cultured for 1 week (a). The spheres displayed strong expression of albumin (b). Approximately 10% of cells in the spheres stained for nestin (green) (blue: nuclei counter-stained with Hoechst 33258) (c). Scales in the subsequent figures are in 1 μm increments
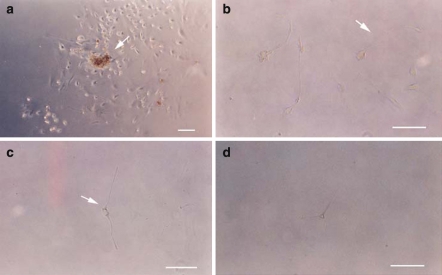
Although most of the cells within the spheres at day 7 of cultivation expressed albumin (Fig. 1b), a marker for hepatocytes or hepatoblasts, 11.4 ± 3.3% of these cells expressed nestin (Fig. 1c) which is expressed on neuronal progenitor cells. Therefore, some of the cells from fetal liver might have a capacity for neuronal differentiation in response to culture conditions for neuronal progenitor cells as in the case of skin, muscle and gut (Toma et al. 2001; Romero-Ramos et al. 2002; Schafer et al. 2003) We attempted to differentiate the day 7 spheres by plating them on poly-d-lysine/laminin-coated slips in DMEM/F12 containing 10% FBS and growth factors (EGF, bFGF, RA, BDNF, NGF and NT-3). The spheres attached to the slips, then some cells began to migrate away from the primary site of attachment and started to lengthen and develop processes (Fig. 2a). The differentiated cells displayed various morphologies including those of monopolar, bipolar and multipolar neuron-like cells (16.0 ± 10.5%) (Fig. 2b–d). After in vitro differentiation, we analyzed the cells for neuronal and glial markers. On day 7 of differentiation, the neuron-like cells expressed β-tubulin III (64.3 ± 13.9%), a marker for newly born neurons, microtubule-associated proteins-2 (MAP-2) (71.3 ± 18.2%) and neurofilament-H (NF-H) (89.5 ± 6.8%) all of which are markers for mature neurons (Fig. 3a–c). In contrast, the cells were negative for GFAP and O4 that are known as glial markers. The cells expressed GAD (62.4 ± 12.6%), a marker for GABAergic neurons, which are not found in the peripheral nerve system (Fig. 3d). However, ChAT-positive cells or TH-positive cells were not detected.
Fig. 2.
Morphology of cells derived from fetal liver after 7 days of neural induction. The sphere (arrow) is attached to the slip and many of its cells have migrated away from primary sites of attachment (a). After neuronal induction, some cells showed monopolar (arrow) (b), bipolar (arrow) (c), and multipolar (d) morphology. Scales in the subsequent figures are in 1 μm increments
Fig. 3.
Phenotype of cells derived from fetal liver after 7 days of neural induction. Cells with neural morphology were immunoreactive with β-III tubulin (a), MAP-2 (b), neurofilament-H (c) and GAD(d) (green) (blue: nuclei counter-stained with Hoechst 33258). Scales in the subsequent figures are in 1 μm increments
We analyzed the functional activity of the neuron-like cells from fetal liver by whole-cell patch clamp recording. On day 14 after neuronal induction, the resting membrane potential was −26 ± 9.7 mV in the fusiform neuron-like cells. The ionic current was then measured by the patch clamp method under voltage clamp conditions. With an increase in the voltage, an outward current was detected (87% of the cells), which disappeared under treatment with K+-channel blockers (TEA-Cl and 4-amynopyridine), indicating the presence of a voltage-dependent K+-current in the neuron-like cells (Fig. 4c, d). The ion current contained no tetrodotoxin-sensitive component (TTX: 1 μM in the extracellular solution). No action potential could be triggered under current clamp conditions (up to 10 pA injected current). We also analyzed the ability of the cells to respond to neurotransmitters. An inward current was elicited in the neuron-like cells in response to glutamate (75% of cells), GABA (65% of cells) and serotonin (100% of cells) (Fig. 4f, h, j) but not to dopamine and acetylcholine (not shown). The control cells from the fetal liver were cultured in Williams’ medium with 10% FBS which are culture conditions used for hepatocytes. These control cells with no neuronal induction failed to respond to GABA, glutamate and serotonin (Fig. 4e, g, i). Nevertheless, approximately 50% of these cells showed the presence of a voltage-dependent K+-current. These results suggest that neuron-like cells may be capable of neuronal cell function.
Fig. 4.
Functional analysis of neuron-like cells. Cells cultured in hepatocyte medium (a) and cells cultured in neuronal medium (b) for 14 days were used for whole-cell patch clamp recording. The contour of the patch clamp electrode can be seen on the right side of the photo. Cells cultured in neuronal medium had a voltage-gated potassium current (c) that was inhibited by K-channel blockers (d) and responded to glutamate (f), GABA (h) and serotonin (j) but not to dopamine and acetylcholine. Cells cultured in hepatocyte medium did not exhibit any response to glutamate (e), GABA (g) or serotonin (i). Scales in the subsequent figures are in 1 μm increments
The immunostaining of ED13 mouse liver revealed that numerous albumin-positive cells thought to be hepatoblasts occupied the fetal liver (Fig. 5a). However, nestin-positive cells extending long processes were scattered among foci of nestin-negative hepatoblasts and haematopoietic cells (Fig. 5b). These nestin-positive cells could be the cells with neuronal differentiation potential. Similar nestin-positive cells with extended long processes were also observed in the tissue of regenerating adult liver on day 3 (Fig. 5d). In contrast, essentially no positive cells were observed in normal adult liver (Fig. 5c).
Fig. 5.
Nestin-positive cells in ED13 mouse liver. Double immunostaining showed that abundant nestin-positive cells (b) (green) extending long processes were scattered among the albumin-positive cells (a) (red). Similar nestin-positive cells with extended long processes were observed in the tissue of regenerating liver on day 3(d), although essentially no positive cells were observed in normal liver (c). Scales in the subsequent figures are in 1 μm increments
Discussion
So far many experiments related to transdifferentiation into neurons from non-CNS tissue in vitro have been reported (Toma et al. 2001; Sanchez-Ramos 2002; Romero-Ramos et al. 2002; Schafer et al. 2003). The source of cells was mainly focused on bone marrow cells, in addition, the cells from—skin, muscle, gut, peripheral nerve were also used (Toma et al. 2001; Sanchez-Ramos 2002; Romero-Ramos et al. 2002; Schafer et al. 2003; Morrison et al. 1999). In some experiments of those, the key to transdifferentiation into neurons is the isolation of nestin positive cells by neurosphere method that is originally identified for neuronal precursor cells (Toma et al. 2001; Kabos et al. 2002; Schafer et al. 2003). We isolated the spheres containing nestin-positive cells from fetal liver by neurosphere method and induced them to neurons. Some cells differentiated from the spheres expressed neuronal markers. Moreover, functional analysis showed the presence of voltage-dependent potassium channels and inward current in response to glutamate, GABA and serotonin. These results suggested we generated functional neurons from the fetal liver. They could be used for neuronal regeneration as autologous sourse for transplantation.
In the liver, the existence of nestin-positive cells has been already reported, and they are known as stellate cells or Ito cells (Niki et al. 1999). We showed that abundant stellate cells expressing nestin are present in fetal liver. Stellate cells are non-parenchymal cell and are thought to serve as liver support and repair after liver insults such as resection or carbon tetra-chlorine administration (Sato et al. 2003; Cassiman et al. 2002). Recent studies suggested the possibility that they are originated from neural crest, because they express N-CAM, synaptophisine, neurotrophin receptors, and GFAP that are neuronal and glial markers during the process of liver repair (Nakatani et al. 1996; Cassiman et al. 1999; Cassiman et al. 2002; Sato et al. 2003).
Neural crest cells are derived from the dorsal aspect of the neural tube and migrate to the whole body (Bronner-Fraser 1993). They finally differentiate into various sorts of cells such as sensory and sympathetic neurons, Schwann cells, mesenchymal cells, adrenal chromaffin cells, melanocytes, endocrine cells, smooth muscle, skeletal muscle, and osteocytes in different locations (Le Douarin and Ziller 1993; Dupin et al. 2001). Transplantation and culture studies suggest the fate of neural crest cells can be determined by the environment (Dupin et al. 2001; Smith et al. 1977; Dulac and Le Douarin 1991; Bronner-Fraser 1993). It’s intriguing that they are capable of differentiating into the cells which compose three kinds of germ layers, but still uncertain about all of the mechanism.
Morrison et al. (1999) isolated neural crest stem cells from fetal sciatic nerve by flow cytometry, and directed them to neuronal and glial cells in vitro. Schafer et al. (2003) formed neurospheres including nestin-positive cells from enteric nervous system in fetal mice, and showed neuronal and glial differentiation in vitro. Those reports indicate that neural crest stem cells are still present in non-CNS tissues after they migrated to outside of CNS. Although noggin, retinoic acid, and neurotrophin have been used for the induction from bone marrow stromal cells to neurons (Sanchez-Ramos et al. 2000; Kohyama et al. 2001). They also play an important role during the development of neural linkage in neural crest cells. (Kohyama et al. 2001; Pinco et al. 1993; Henion and Weston 1994; Dupin and Le Douarin 1995; McMahon et al. 1998) From those view points, our result might suggest that neural crest stem cells migrated to fetal liver or dedifferentiated stellate progenitor cells are isolated by neurosphere method and that they are able to be directed to neuronal linkage. Since neural crest cells migrate to bone marrow, muscle and skin (Hainfellner et al. 2001), the referred stem cells to transdifferentiation into neurons are indistinguishable from neural crest stem cells.
In order to provide the neurons from non-CNS tissues as autologous sourse for regeneration of CNS, it is indispensable to detect the origin of stem cells and to clarify the mechanism of development and differentiation in the stem cells so that we can harvest the stem cells efficiently and direct them to target cells correctly and functionally. Our results may contribute to find a clue to the question.
Finally, we have revealed experimentally the presence of neural progenitor cells, which derived probably from the embryonic neural crest, in the fetal liver. The liver is one of the easy organs possessing a high regenerating ability in the body. Our results, therefore, infer that the liver may be a useful tissue as well as the myeloid tissue to obtain neural progenitor cells for neural repair medicine after neural injuries or diseases in connection with the stellate cells.
References
- Arsenijevic Y, Villemure JG, Brunet JF et al (2001) Isolation of multipotent neural precursors residing in the cortex of the adult human brain. Exp Neurol 170:48–62 [DOI] [PubMed]
- Bjorklund A, Svendsen C (1999) Stem cells. Breaking the brain–blood barrier. Nature 397:569–570 [DOI] [PubMed]
- Bronner-Fraser M (1993) Neural crest cell migration in the developing embryo. Trends Cell Biol 3:392–397 [DOI] [PubMed]
- Cassiman D, van Pelt J, De Vos R et al (1999) Synaptophysin: a novel marker for human and rat hepatic stellate cells. Am J Pathol 155:1831–1839 [DOI] [PMC free article] [PubMed]
- Cassiman D, Libbrecht L, Desmet V et al (2002) Hepatic stellate cell/myofibroblast subpopulations in fibrotic human and rat livers. J Hepatol 36:200–209 [DOI] [PubMed]
- Dulac C, Le Douarin NM (1991) Phenotypic plasticity of Schwann cells and enteric glial cells in response to the microenvironment. Proc Natl Acad Sci USA 88:6358–6362 [DOI] [PMC free article] [PubMed]
- Dupin E, Le Douarin NM (1995) Retinoic acid promotes the differentiation of adrenergic cells and melanocytes in quail neural crest cultures. Dev Biol 168:529–548 [DOI] [PubMed]
- Dupin E, Real C, Ledouarin N (2001) The neural crest stem cells: control of neural crest cell fate and plasticity by endothelin-3. An Acad Bras Cienc 73:533–545 [DOI] [PubMed]
- Gage FH (2000) Mammalian neural stem cells. Science 287:1433–1438 [DOI] [PubMed]
- Hainfellner JA, Voigtlander T, Strobel T et al (2001) Fibroblasts can express glial fibrillary acidic protein (GFAP) in vivo. J Neuropathol Exp Neurol 60:449–461 [DOI] [PubMed]
- Henion PD, Weston JA (1994) Retinoic acid selectively promotes the survival and proliferation of neurogenic precursors in cultured neural crest cell populations. Dev Biol 161:243–250 [DOI] [PubMed]
- Higgins GM (1931) Experimental pathology of the liver: 1. Restoration of liver of white rat following surgical removal. Arch Pathol Lab Med 12:186–202
- Kabos P, Ehtesham M, Kabosova A et al (2002) Generation of neural progenitor cells from whole adult bone marrow. Exp Neurol 178:288–293 [DOI] [PubMed]
- Kohyama J, Abe H, Shimazaki T et al (2001) Brain from bone: efficient “meta-differentiation” of marrow stroma-derived mature osteoblasts to neurons with Noggin or a demethylating agent. Differentiation 68:235–244 [DOI] [PubMed]
- Le Douarin NM, Ziller C (1993) Plasticity in neural crest cell differentiation. Curr Opin Cell Biol 5:1036–1043 [DOI] [PubMed]
- McMahon JA, Takada S, Zimmerman LB et al (1998) Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev 12:1438–1452 [DOI] [PMC free article] [PubMed]
- Morrison SJ, White PM, Zock C et al (1999) Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell 96:737–749 [DOI] [PubMed]
- Nakatani K, Seki S, Kawada N et al (1996) Expression of neural cell adhesion molecule (N-CAM) in perisinusoidal stellate cells of the human liver. Cell Tissue Res 283:159–165 [DOI] [PubMed]
- Niki T, Pekny M, Hellemans K et al (1999) Class VI intermediate filament protein nestin is induced during activation of rat hepatic stellate cells. Hepatology 29:520–527 [DOI] [PubMed]
- Pinco O, Carmeli C, Rosenthal A et al (1993) Neurotrophin-3 affects proliferation and differentiation of distinct neural crest cells and is present in the early neural tube of avian embryos. J Neurobiol 24:1626–1641 [DOI] [PubMed]
- Reynolds BA, Weiss S (1992) Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255:1707–1710 [DOI] [PubMed]
- Romero-Ramos M, Vourc’h P, Young HE et al (2002) Neuronal differentiation of stem cells isolated from adult muscle. J Neurosci Res 69:894–907 [DOI] [PubMed]
- Sanchez-Ramos JR (2002) Neural cells derived from adult bone marrow and umbilical cord blood. J Neurosci Res 69:880–893 [DOI] [PubMed]
- Sanchez-Ramos J, Song S, Cardozo-Pelaez F et al (2000) Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol 164:247–256 [DOI] [PubMed]
- Sato M, Suzuki S, Senoo H (2003) Hepatic stellate cells: unique characteristics in cell biology and phenotype. Cell Struct Funct 28:105–112 [DOI] [PubMed]
- Schafer KH, Hagl CI, Rauch U (2003) Differentiation of neurospheres from the enteric nervous system. Pediatr Surg Int 19:340–344 [DOI] [PubMed]
- Smith J, Cochard P, Le Douarin NM (1977) Development of choline acetyltransferase and cholinesterase activities in enteric ganglia derives from presumptive adrenergic and cholinergic levels of the neural crest. Cell Differ 6:199–216 [DOI] [PubMed]
- Toma JG, Akhavan M, Fernandes KJ et al (2001) Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol 3:778–784 [DOI] [PubMed]
- Weiss S, Dunne C, Hewson J et al (1996) Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci 16:7599–7609 [DOI] [PMC free article] [PubMed]
- Yanai J, Doetchman T, Laufer N et al (1995) Embryonic cultures but not embryos transplanted to the mouse’s brain grow rapidly without immunosuppression. Int J Neurosci 81:21–26 [DOI] [PubMed]