Abstract
This paper reports for the first time, the Primary cell culture of hepatopancreas from edible crab Scylla serrata using crab saline, L-15 (Leibovitz), 1 × L-15 + crab saline, 2 × L-15 + crab saline, 3 × L-15 and citrate buffer without any serum. We could isolate and maintain E (Embryonalzellen), F (Fibrenzellen), B (Blasenzellen), R (Restzellen) and G (Granular cells). Upon seeding the hepatopancreatic E, F, B, and R cells showed different survival pattern over time than granular cells. A modified L-15 (3×) medium supported the best survival of hepatopancreatic E, F B, and R cells in in-vitro culture. However granular cells could be maintained for 184 days with L-15 (1×) + crab saline. Fetal bovine serum was not effective additive and hampered cell viability in present study.
Keywords: Hepatopancreas, Scylla serrata, Primary cultures, E (Embryonalzellen), F (Fibrenzellen), B (Blasenzellen), R (Restzellen) and G (Granular cells)
Introduction
Crustacean cell culture has gained momentum due to viral diseases affecting commercially important species. Hence, cell culture techniques were developed: (a) to assist understanding the mechanism of host pathogenesis interaction Chen et al. 1995, (b) to produce large amount of viral material for their characterization (c) to improve tools for diagnosis and cure of diseases. Attempts have been made to establish several cell culture systems of shrimps (Al-Mohanna and Nott 1987; Chen et al. 1986, 1995; Hsu et al. 1995; Ke et al. 1990; Mulford and Austin 1998; Mulford et al. 2001; Nadala et al. 1993; Toullec et al. 1996; Uma et al. 2002) and other crustaceans (Peponnet and Quiol 1971).
Mud crab fishery has become commercially important in many parts of the world. In India mud crab, Scylla serrata has gained economic importance since early eighties with the commencement of live crab export to south East Asian countries. Though crab fishery is growing in India, it is blighted by disease outbreaks resulting from environmental and pathogenic factors. This has resulted in growing a need to develop cell culture techniques for understanding the pathogenicity. Earlier studies on crab were related to cytotoxicology and histology (Al-Mohanna and Nott 1985, 1987). Ballard et al. (1993) attempted integumentary cuticular epithelial cell culture of crab. However, there is hardly any report on development of primary cell cultures of commercially important crab species. Therefore, it was decided to develop a primary cell culture of hepatopancreas an organ responsible for detoxification of xenobiotics of Scylla serrata.
Materials
Animals
Live Scylla serrata (13.83 ± 0.60 g) were obtained from a local supplier and were maintained in aerated seawater (Salinity 29‰, temperature 28 °C). Prior to the experiment all crabs were immersed in 5% sodium hypochlorite.
Cell culture material
Sterile disposable 35 mm tissue culture petridishes were used. Leibovitz L-15 medium (1×, 2× and 3×), crab saline, L-15 + crab Saline (1:1), 2 × L-15 + crab saline (1:1), 3 × L-15 + crab saline, citrate buffer and artificial seawater were used as culture media for comparison of their effectiveness. Leibovitz L-15 medium with l-glutamine powder was used. The media as powder were reconstituted with sterile double distilled water and filtered (0.22 μm porosity filters) artificial sea-water (salinity 29‰). The media used were serum free. Crab saline was constituted with NaCl (440 mM), KCl (11.3 mM), CaCl2 (13.3 mM), MgCl2 (26 mM), Na2SO4 (23 mM), HEPES (10 mM). The citrate buffer was constituted with sodium citrate (27 mM), NaCl (440 mM), KCl (3 mM), NaH2PO4 (5 mM), Na2SO4 (5.6 mM), EDTA (10 mM), Poly vinyl pyrrolidone (0.25 mM) and artificial seawater was constituted with NaCl (480 mM), KCl (10 mM), MgSO4·7H2O (30 mM), MgCl2·6H2O (20 mM), and HEPES (5 mM). All media were filtered through 0.22 μm pore size cellulose membrane prior to use. Antibiotics were routinely used and included penicillin, streptomycin (Himedia, India A001 diluted to 10 × 4 IU/mL–10 × 4 μg/mL), ampicillin or gentamycin (300 μg/mL) and flagyl (10 μg/mL). The osmolarity was adjusted with crab saline and citrate buffer having poly vinyl pyrrolidone (15 mM). The pH was adjusted to 7.4.
Procedures
Development of primary cell cultures
The animals were anaesthetized for 2 min in a jar containing ether anesthesia, dipped briefly in 10% (w/v) sodium hypochlorite to inactivate extraneous microorganisms and rinsed for 10 min in 2% (w/v) potassium permanganate followed by a rapid immersion in crab saline containing penicillin-streptomycin (10 × 4 IU/mL–10 μg/mL), ampicillin (10 μg/mL) and flagyl (10 μg/mL). Then, the animals were rinsed thrice with sterile artificial seawater followed by rinse in 70% ethanol prepared with artificial seawater. The animals were dissected open to expose the thoracic region having hepatopancreas symmetrically arranged as two half-organs flanking the stomach. The hepatopancreas was separated with the use of sterile scalpel and forceps and transferred to a sterile centrifuge tube containing sterile crab saline. The tissue was rinsed thrice in sterile crab saline, and then was cut aseptically into 1 mm3 fragments and transferred to 15 mL sterile centrifuge tube containing sterile crab saline, 1 × L-15, 2 × L15, 3 × L15, crab saline, 1 × L-15 + crab saline, 2 × L-15 + crab saline, 3 × L-15, 3 × L-15 + crab saline, citrate buffer, L-15 + sea water and artificial sea water + 1% glucose (pH 7.4) (Table 1). Small fragments were used for explant culture. These fragments were placed in 35 mm sterile petridishes containing different culture media. In addition, fragments of hepatopancreas were triturated by several passages through 10 mL sterile pipette to obtain dissociated cell suspension. Previously mentioned media were used for trituration. The suspension was then filtered through a 100 μm nylon mesh and spun for 3 min at 150g. The resulting pellet had stratification from bottom to top: white cell fraction, yellow cell fraction. The two cell fractions were carefully aspirated separately into centrifuge tubes using pasteur pipette and washed twice with culture media. Each cell fraction was cultured separately in the optimized media and assessed for cell viability by trypan blue dye exclusion technique.
Table 1.
The percentage yield of viable cells obtained using distinct dissociating media
| Medium | Working solution | % Viable cells obtained after dissociation |
|---|---|---|
| 1 | 1 × Leibovitz’s | 33.75 ± 3.34 |
| 2 | 2 × Leibovitz’s | 42.0 ± 2.4 |
| 3 | 3 × Leibovitz’s | 72.0 ± 3.53 |
| 4 | Crab saline | 40.25 ± 2.94 |
| 5 | 1 × Leibovitz’s + Crab saline (1:1) | 49.25 ± 4.81 |
| 6 | 2 × Leibovitz’s + Crab saline (1:1) | 44.75 ± 13.27 |
| 7 | 3 × Leibovitz’s + Crab saline (1:1) | 76.0 ± 3.50 |
| 8 | Citrate buffer | 92.5 ± 2.08 |
| 9 | Leibovitz’s + Seawater | 62.0 ± 3.0 |
| 10 | ASW | 41.0 ± 1.4 |
Note: The percent viable cells means the number of viable cells per 100 cell count after mechanical dissociation of cells from the parental tissue
The hepatopancreatic tissue fragments’ trituration using different media and their combinations (Table 1) were studied. Cell viability was assessed by resuspending an aliquot of 0.1 mL of cell suspension in 0.2 mL of 0.4% trypan blue dye and determined, using a Neubauer haemocytometer, the proportion of living (cell excluding dye) and non living (cell containing dye) cells.
Two techniques were evaluated for seeding. In one, small tissue fragments well moistened with diluted hemolymph were placed aseptically in sterile petridishes and allowed to attach for 10–15 min before the addition of media. After ensuring attachment of fragments, 2 mL of serum free platting media were added. With the second technique, aliquots of 1 mL of the separated cell pellets (yellow, white cell fractions) at density of ~3 × 103 cells/mL were seeded into culture petridishes containing an additional 2.0 mL of desired culture media. Inoculated cells were mixed by gently swirling the petridishes. All the cultures were incubated at 20–24 °C, media were refreshed on day 2 and there after at 2-day intervals. Cultured cells were observed daily with an Olympus inverted microscope at magnification of 1,000× and 200×.
Attempts to sub culture the hepatopancreatic cells were carried out after 7 days. For procuring cells for subculture, a rubber-scraper was used to remove the cells attached to the bottom of parent dishes. These cells were transferred to new tissue culture dishes with fresh medium. Representative cells were stained with Giemsa stain (+). The stained preparations were used for determining size and shape with Image analyzer (Nikon Eclipse TS100, TI-SM Japan).
Preparations of cell for photograph
The cultured cells were harvested with a scraper and sedimented by centrifugation at 150g for 3 min. The pellet was suspended in sea water containing 2% glutaraldehyde with periodic agitation for 90 min at room temperature. The fixed cell were centrifuged at 150g for 1 min and then dehydrated in a series of ethanol grade (15–100%). Digital images were acquired on an Olympus BX41 microscope using an Olympus DP12 Digital Microscope camera.
Genomic deoxyribonucleic acid analysis by polymerase chain reaction
The genomic DNA was isolated from cultured cells and parental tissue using DNA extraction solution (Genie, Bangalore India—CAT #FC-46). The isolated DNA was stored at 4 °C until required. Two specific oligonucleotide primers, UBC457 (58-CGACGCCCTG-38), and YNZ22 (58-CTCTGGGTGTCGTGC-38) were designed based on (Klinbunga et al. 2000) reference. These primers are specific for Scylla serrata. The amplification reaction was carried out using 50 μL reaction volume containing 25 μL PCR master mix (Fermentas, India—Cat. No. K0171), 21 μL nuclear free water, 2 μL of each primer, 2 μL of sample DNA, RAPD—PCR was performed in a Hybaid thermocycler for 35 cycles consisting of two denaturation steps at 94 °C for 5 min and 94 °C for 30 s, annealing at 36 °C (or 50 °C for YNZ22) for 60 s, and extension at 72 °C for 60 s. The final extension was carried out at the same temperature for 10 min. The resulting products were electrophoretically analyzed through 1.6% agarose gels, stained with ethidium bromide, and visualized using a UV transilluminator. Molecular weight marker, DNA ladder, was from Fermentas (Cat. No. SM1553).
Results
A cell isolation procedure for Scylla serrata hepatopancreas was developed based on mechanical dissociation and centrifugation method which produced viable cell population. Table 1 shows the observed cell recovery/yields during tissue preparation using different media and their combinations. Maximum cell recovery/yield were obtained with citrate buffer (92.5 ± 2.08). We therefore, selected citrate buffer as dissociating media in mechanical dissociation (trituration) technique.
Ten trials were carried out in which pentuplicate tissue samples were examined for their potency and efficiency to grow in vitro (Table 2). The dissociated cells of hepatopancreas and explants were examined everyday using 1 × L-15, 2 × L15 and 3 × L-15, Crab saline, 1 × L-15 + Crab saline, 2 × L-15 + crab saline, 3 × L-15 + Crab saline, citrate buffer and L-15 + seawater. Overall good viability of cells was observed in the tested media and their combinations. Comparison of strengths (1×, 2× and 3×) of L-15 medium showed that 3 × L-15 was better and even its combination (1:1) with crab saline gave best results. EDTA: citrate buffer fortified with PVP was also equally effective. The dissociated hepatopancreas showed presence of E (Embryonalzellen), F (Fibrenzellen), B (Blasenzellen), R (Restzellen) and G (Granular) cells (Fig. 1a–d). For obtaining these cells in isolation we attempted trituration of hepatopancreas of premoult, moulting and postmoult crabs.
Table 2.
Comparison of each culture medium on primary culture of hepatopancreas of Scylla serrata
| Culture No. | Medium | Osmolarity (mOsm/kg) | E | F | B | R | G | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | A | B | A | B | A | B | A | B | |||
| 1 | Leibovitz’s | 485 | +++ | 2–3 | ++ | 2–3 | + | 1–2 | ++ | 2–3 | +++ | 10–15 |
| 2 | 2 × Leibovitz’s | 740 | +++ | 2–3 | ++ | 2–3 | + | 1–2 | ++ | 2–3 | +++ | 15–20 |
| 3 | 3 × Leibovitz’s | 950 | +++ | 10–15 | ++ | 10 | + | 4–5 | ++ | 30 | +++ | 153 days |
| 4 | Crab saline | 860 | +++ | 2–3 | ++ | 2–3 | + | 1–2 | ++ | 2–3 | +++ | 10–15 |
| 5 | Leibovitz’s + Crab saline(1:1) | 880 | +++ | 2–3 | ++ | 2–3 | + | 1–2 | ++ | 2–3 | +++ | 184 days |
| 6 | 2 × Leibovitz’s + Crab saline(1:1) | 800 | +++ | 2–3 | ++ | 2–3 | + | 1–2 | ++ | 2–3 | +++ | 10–15 |
| 7 | 3 × Leibovitz’s + Crab saline | 840 | +++ | 2–3 | ++ | 2–3 | + | 1–2 | ++ | 2–3 | +++ | 15–20 |
| 8 | EDTA Citrate buffer | 860 | +++ | 5–10 | ++ | 10 | + | 3–5 | ++ | 30 | +++ | 180 days |
| 9 | Leibovitz’s +Seawater | 800 | +++ | 4–6 | ++ | 5–7 | + | 4–5 | ++ | 10–20 | +++ | 30–35 |
E, Embryonalzellen cell; F, Fibrenzellen cell; B, Blasenzellen cell; R, Restzellen cell; G, Granular cells; A, attachment of cells; B, maximum day of survival; +, approximately 30–50% of cells adhere on the culture petridish; ++, approximately 60–80% of cells adhere on the culture petridish; +++, majority of cells adheres on the culture petridish
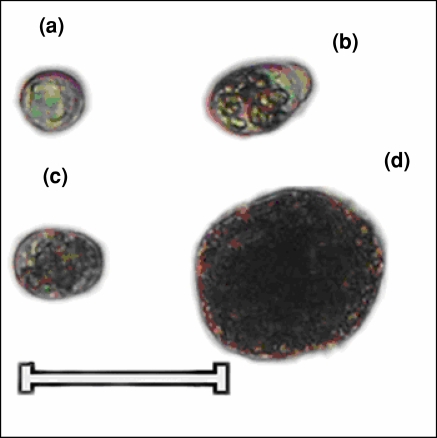
Fig. 1.
Different cell types present in hepatopancreatic tissue of Scylla serrata. (a) Embryonalzellen cells. (b) Fibrenzellen cells. (c) Restzellen cells. (d) Blasenzellen cells. Scale bar 60 μm
The hepatopancreatic cell suspension of crab on centrifugation at 150g produced two-layered pellet. The bottom layer was white and was formed exclusively of granular cells, while the upper yellow fraction included E, F, B, and R cells. During the course of experiment it was noted that the moulting cycle had significant effect on cell type composition.
Figure 2 shows the changes occurring in the cellular composition of E, F, B, R cells of hepatopancreas during the moulting cycle. It is observed that population of E, F, B and R cells varied during moulting cycle but during premoult, moult and post moult stages the population density of R cells was highest while that of E cells was lowest, it did not show any significant changes in its density during moulting cycle. F and B cells exhibited rise in population density during postmoult stage.
Fig. 2.
Composition of cell types in three different molting stages of Scylla serrata
Primary culture
Isolated cells from hepatopancreatic tissue of Scylla serrata were obtained by mechanical dissociation technique. Centrifugation of the cell suspension yielded two layered cell fraction, which was inoculated separately. The upper yellow fraction included mixed population of E, F, B, R cells (Fig. 3), while the bottom white fraction included small granular cells. Upon seeding the hepatopancreatic mixed cell population showed different survival pattern over time than the granular cell. Initial viability of the mixed cell population was >90% and were maintained constant for 24 h in the culture. After 5 days B cells began to disappear from the culture while, F cells survived for 10 days, E cells for 15 days and R cells for 30 days. However, granular cells could survive for 184 days with regular media change. F, B and R cells did show periodic proliferation and were loosely attached to the substratum but E and granular cells showed firm attachment to the substratum. E cells in the culture occasionally showed mitotic division and were alive for less than two weeks. Table 2 reveals that 1 × Leibovitz’s + crab saline supplemented with 1% glucose at an osmolarity of 880 mOsm/kg was the most effective media for granular cell survival. However for mixed cell population best results were obtained in 3 × Leibovitz’s supplement with glucose + Nacl 5 g/L at 950 mOsm/kg and EDTA: citrate buffer fortified with PVP at 860 mOsm/kg.
Fig. 3.
Primary cell culture of hepatopancreatic cells (Embryonalzellen cells, Fibrenzellen cells, Blasenzellen cells, Restzellen) of Scylla serrata. (fixed cells in 2% glutaraldehyde)
Fetal bovine serum, a standard supplement in many cell culture systems, was not effective additive and hampered cell viability in this study. Hepatopancreatic cells cultured in the media without supplementation with fetal bovine serum retained much higher viability. Other supplements, such as heamolymph, sterile filtrate from homogenized hepatopancreatic tissue, were not effective for cell viability.
Explant culture
Only granular and very few E cells were found to migrate from explants. Upon migration they were found to attach to the substratum. The E cells upon subculture gradually began to disappear from the culture. In contrast granular cells survived, and could be maintained up to 6 months. Rest of the cells did not migrate and explant tissue deteriorated after 7–8 days. For survival and growth of migrated cells, removal of explant tissue within 4–5 days followed by their subculturing was essential. The cell yield from explant cultures were inferior to the yields obtained by mechanical dissociation. We therefore decided the dissociation technique as the easier and quicker method to initiate the primary cell culture.
Morphology
E cells
These were 23–28 μm diameter, round cells with nucleoplasm to cytoplasm ratio more than one (Fig. 1a). They did not show lipid or glycogen reserves when stained with Sudan III or Para-aldehyde Schiff (PAS).
F cells
These were columnar 30–50 μm diameter cells with a central or on few occasions, a basal nucleus. The cytoplasm showed few vacuoles (Fig. 1b).
B cells
These were 50–71 μm diameter cells having 30–50 μm vacuole occupying about 80–90% of cytoplasm and on a few occasions multiple vacuoles were seen near the optical surface (Fig. 1d).
R cells
These were 21–42 μm diameter and had multivacuaolated cytoplasm (Fig. 1c). The cytoplasm was Sudan black B/III and PAS positive.
Granular cells
These were ~1–5 μm diameter spheroid cells showing tendency to attach to the substratum (Fig. 4).
Fig. 4.
Primary cell culture of hepatopancreatic granular cells (fixed cells in 2% glutaraldehyde)
Figure 5 depicts RAPD patterns of PCR amplified DNA fragments of cultured and parental tissue of Scylla serrata, using primers UBC457 and YNZ22. The result shows similarity in, sizes and number of RAPD bands between cultured cells and parental tissue, confirming the progeny belongs to parental tissue (Table 3, Fig. 5).
Fig. 5.
RAPD patterns of Scylla serrata using primers UBC457 and YNZ22, Lane M: DNA marker; Lane 1—parental DNA amplified using primer UBC457, Lane 2—cultured cell DNA amplified using primer UBC457, Lane 3—parental DNA amplified using primer YNZ22, Lane 4—Cultured cell DNA amplified using primer UBC457. Arrowheads indicate RAPD bands describe in Table 3
Table 3.
Sequences of oligonucleotide primers, sizes and number of RAPD Bands using Primers UBC457, and YNZ22
| Primer | Sequence | Parental DNA | Cultured cell DNA |
|---|---|---|---|
| Size range and (number of bands) | |||
| UBC457 | CGACGCCCTG | 500–1,000 (3) | 500–1000 (3) |
| YNZ22 | CTCTGGGTGTCGTGC | 1,400–2,000 (2) | 1,400–2,000 (2) |
Discussion
This is the first report of the development of primary cell culture from economically important crab, Scylla serrata. As such the results are comparable with studies on primary culture of hepatopancreas from Penaeous monodon (Uma et al. 2002), Penaeus orientalis (Ke et al. 1990), Palaemontes argentinus (Sousa et al. 2005), Marscupenaeus japonicus (Zilli et al. 2003). However with Scylla serrata the cell cultures were achieved at 20–24 °C. In agreement with previous work (Chen et al. 1986; Nadala et al. 1993) on crustaceans, the efficiency of L-15 medium for promoting growth and cell survival of invertebrate tissue such as hepatopancreas is demonstrated by the present work. However, our study demonstrates the suitability of 3 × L-15 and EDTA: citrate buffer fortified with PVP for growth and survival of hepatopancreatic cells. The use of EDTA-citrate buffer with PMSF pH 7.1 for maintenance of hepatopancreatic cells was demonstrated by Zilli et al. (2003) but our work demonstrates the use of modified citrate buffer where in PVP could be used for adjusting osmolarity to 860 mOsm/L at alkaline pH (7.4). Yet in this study usefulness of crustacean saline buffer with 3 × L-15 is also demonstrated. But seawater with L-15 was relatively less effective. It is possible that seawater with L-15 has poor buffering capacity. Though there are reports of better growth of invertebrate cells at pH range of 7.0–7.4 (Rosenthal and Diamant 1990; Toullec et al. 1996) and 7.6–8.1 (Hsu et al. 1995) the present work indicates that for hepatopancreatic cells of crab pH 7.6 is suitable.
Though the necessity of FBS or FCS at concentrations of 5–20% (v/v) as an ingredient in crustacean cell culture medium has been emphasized by some researchers (Chen and Wen 1999; Mulford and Austin 1998; Mulford et al. 2001; Leigh and Jan 1999; Tong and Miao 1996; Uma et al. 2002), Luedeman (1990) and Frerichs (1996) suggested that serum could be toxic for crustacean cells. Our study demonstrates that serum degrades the viability of hepatopancreatic cells of Scylla serrata.
The general epithelial cytology of the hepatopancreas in Scylla serrata is consistent with that observed in a few Penaedon species (Al-Mohanna and Nott 1985; Icely and Nott 1992; Johnston et al. 1998; Sousa et al. 2005). The present study clearly demonstrates that the mechanically dissociated cells remain viable and centrifugation of cell suspensions results in forming a stratified pellet.
The variation in population density observed for E, F, B and R cells in yellow fraction of the pellet could be a result of physiological demand. In the present study R cells show inverse relationship with F and B cells i.e. the number of F and B cells decrease as the number of R cells increase. As R cells are involved in storage of calcium, phosphate etc. (Zilli et al. 2003) the increase in their number indicate elevation in storage activities and under such conditions the F and B cells are not required in large number as they are responsible for synthesis and secretion of enzymes required for digestion. Both the storage and digestion activities may not occur simultaneously. After enzymatic digestion, the end products of digestion need to be taken up. Hence subsequent to digestion F and B cells number drops paving way for rise in number of R cells required for storage during moulting cycle.
The cell attachment observed in this study is in disagreement with that reported for P. vannamei by Toullec et al. (1996) as they reported negligible attachment of hepatopancreatic cells. Our study demonstrates poor attachment of B cells to the substratum in comparison to rest of the cells in primary culture.
Owens and Smith (1999) reported 48 h survival of hepatopancreatic explant culture in double strength L-15 medium with 20% FBS. However our study shows that only E and granular cells migrate from explant with tendency to attach to the substratum and to proliferation slowly in 3 × L-15 and citrate buffer. Upon subculture granular cells are capable of surviving for six months. The explant survives for 7–8 days.
In conclusion it is apparent that different types of hepatopancreatic cells of Scylla serrata could be separated, cultured/maintained and they did not proliferate much individually probably owing to the lack of growth factors or mitogenic agents in our culture media. However, this requires further investigation to develop ideal medium for culturing each type of hepatopancreatic cell of Scylla serrata in vitro. Certainly this study has demonstrated the viability of primary cell cultures of Scylla serrata which should have value in associated toxicological and pathogenicity studies as well as studies directed to isolation of viruses affecting the crab population.
Acknowledgement
The authors acknowledge the receipts of financial assistances from Department of Biotechnology, New Delhi under the project number BT/PR8029/AAQ/03/287/2006.
References
- Al-Mohanna SY, Nott JA (1985) Mitotic E-and secretory F-cells in the hepatopancreas of the shrimp Penaeus Semisulcatus (Crustacea: Decapoda). J mar Biol ASS UK 65:901–910
- Al-Mohanna SY, Nott JA (1987) R-cells and the digestive cycle in Penaeus semisulcatus (Crustacean: Decapoda). Mar Biol (Berl) 95:129–137. doi:10.1007/BF00447494 [DOI]
- Ballard TA, Roer RD, Dillaman RM (1993) Long-term culture of integumental explants from premolt blue crabs, Callinectes sapidus. J Tissue Cult Methods 15:11–14. doi:10.1007/BF02387283 [DOI]
- Chen S-N, Chi S-C, Kou G-H, Liao IC (1986) Cell cultures from tissues of Grass Prawn, Penaeus monodon. Fish Pathol 21:161–166
- Chen S-N, Shih H-H, Kou G-H (1995) Primary cell cultures from tissues of penaeid shrimps and their susceptibilities to monodon-type baculovirus (MBV). Rep Fish Dis Res 16:1–14
- Chen SN, Wen CM (1999) Establishment of cell lines derived from oyster, Crassostrea gigas Thunberg and hard clam, Meretrix lusoria Roding. Methods Cell Sci 21:183–192. doi:10.1023/A:1009829807954 [DOI] [PubMed]
- Frerichs GN (1996) In vitro culture of embryonic cells from the freshwater prawn Macrobrachium rosenbergii. Aquaculture 143:227–232. doi:10.1016/0044-8486(96)01281-1 [DOI]
- Hsu Y-L, Yang Y-H, Chen Y-C, Tung M-C, Wu J-L, Engelking MH et al (1995) Development of an in vitro subculture system for prawn tissues. Asian Fish Soc Spec Pub 10:161–170
- Icely JD, Nott JA (1992) Digestion and absorption: digestive system and associated organs. In: Harrison FW, Humes AG (eds) Microscopic anatomy of invertebrates, vol 10. Decapod crustacea, Wiley-Liss, New York, pp 147–201
- Johnston DJ, Alexander CG, Yellowhees D (1998) Epithelial cytology and function in the digestive gland of Thenus orientalis (Decapoda, scyllaridae). J Crust Biol 18(12):271–278. doi:10.2307/1549320 [DOI]
- Ke H, Liping W, Yumei D, Shuji Z (1990) Studies on cell culture from the hepato-pancreas of the oriental shrimp, Penaeus orientalis Kishinouye. Asian Fish Sci 3:299–307
- Klinbunga S, Boonyapakdee A, Pratoomchat B (2000) Genetic diversity and species-diagnostic markers of mud crabs (Genus Scylla) in Eastern Thailand determined by RAPD analysis. Mar Biotechnol 2:180–187 [DOI] [PubMed]
- Luedeman RA (1990) Development of an in vitro primary cell cultures from the penaeid shrimp Penaeus stylirostris and Penaeus vannamei and evaluation of a potential application. Masters Thesis. The University of Arizona, Tucson, AZ
- Mulford AL, Austin B (1998) Development of primary cell cultures from Nephrops norvegicus. Methods Cell Sci 19:269–275. doi:10.1023/A:1009787223797 [DOI] [PubMed]
- Mulford AL, Lyng F, Mothersill C, Austin B (2001) Development and characterization of primary cell cultures from the hematopoietic tissues of the Dublin Bay prawn, Nephrops norvegicus. Methods Cell Sci 22:265–275. doi:10.1023/A:1017971618398 [DOI] [PubMed]
- Nadala EC, Lu Y, Loh PC (1993) Primary culture of lymphoid, nerve and ovary cells from Penaeus stylirostris and Penaeus vannamei. In Vitro Cell Dev Biol Anim 29A:620–622. doi:10.1007/BF02634546 [DOI] [PubMed]
- Leigh Owens, Jan Smith (1999) Early attempts at production of prawn cell lines. Methods Cell Sci 21:207–211. doi:10.1023/A:1009806606562 [DOI] [PubMed]
- Peponnet F, Quiol JM (1971) Cell cultures of crustacea Arachnida and merostomacea. In: Vago C (ed) Invertebrate tissue culture. vol I pp 341–359
- Rosenthal J, Diamant A (1990) In vitro primary cell culture from penaeus semisulcatus. In: perkins FO, Cheng TC (eds) Pathology in Marine Science. Academic Press, San Diego, pp 7–13
- Sousa LG, Elena I, Cuartas , Petriella AM (2005) Fine structural analysis of the epithelial cells in the hepatopancreas of Palaemonetes argentinus (Crustacea, Decapoda, Caridea) in intermoult. Biocell 29(1):25–31 [PubMed]
- Tong S-L, Miao H-Z (1996) Attempts to initiate cell cultures from Penaeus chinensis tissues. Aquaculture 147:151–157. doi:10.1016/S0044-8486(96)01386-5 [DOI]
- Toullec J-Y, Crozat Y, Patrois J, Porcheron P (1996) Development of primary cell cultures from the penaeid shrimps Penaeus vannamei and P. indicus. J Crust Biol 16:643–649. doi:10.2307/1549183 [DOI]
- Uma A, Prabhakar TG, Koteeswaran A, Ravikumar G (2002) Establishment of primary cell culture from hepatopancreas of Penaeus monodon for the study of whitespot syndrome virus (WSSV). Asian Fish Sci 15:365–370
- Zilli L, Schiavone R, Scordella G, Zonno V, Verri T, Storelli C et al (2003) Changes in cell type composition and enzymatic activities in the hepatopancreas of Marsupenaeus japonicus during the moulting cycle. J Comp Physiol [B] 173:355–363. doi:10.1007/s00360-003-0348-6 [DOI] [PubMed]