Abstract
The Drosophila Schneider S2 (S2) Expression System enables expression of recombinant proteins constitutively, as well as inductively. This system can establish both transient and stable transformants with various selection markers. The generation of stable cell lines for increased expression or large scale expression of the desired protein is currently accomplished by cotransfection of both the expression and selection vectors. The selection vectors, pCoHYGRO and pCoBLAST, are commercially available using hygromycin-B and blasticidin S, respectively. Recently, we generated a plasmid, pCoPURO, for selection of transfected S2 cells using puromycin, which allows significant acceleration of the selection time. Although co-transfection of the selection marker with the plasmid for heterologous protein expression is functional in stable expression at short culture periods, the expression levels of stable transformants are continuously decreased during long culture times. To overcome this limitation, we generated pMT-PURO, a new plasmid that contains both the expression cassette and puromycin selection marker in a single plasmid. This system allows rapid selection and maintenance of the transformed S2 lines for extended culture periods.
Keywords: S2 cell, Puromycin, Selection, Single plasmid
Introduction
The Drosophila melanogaster Schneider 2 (S2) cell line was established in 1972 (Schneider 1972). These cells grow rapidly in culture at room temperature, without a need for CO2. Invitrogen (Carlsbad, CA) has established the Drosophila Expression System (DES) consisting of S2 cells and various plasmid vectors. This excellent system enables expression of recombinant proteins constitutively by the actin promoter (Ac5) (Chung and Keller 1990), as well as inductively by the metallothionein promoter (MT) (Bunch et al. 1988). Furthermore, the Drosophila homologue of the immunoglobulin binding chaperone protein secretion signal (BiP) has been introduced to the system to facilitate proper secretion of recombinant proteins from S2 cells, instead of secretion signals naive to the heterologous protein, which may not be recognized by S2 cells (Kirkpatrick et al. 1995). This system is used for both transient and transformants with various selection markers. However, there remain time-consuming stages in order to obtain good stable transformants for long culture periods. Some improvements in this aspect of S2 expression is the purpose of this review.
Selection of S2 cells with drug resistant marker genes
In the DES, the generation of stable cell lines for large scale expression of the desired protein is currently accomplished by cotransfection of both the expression and selection vectors. The vector “pCoHYGRO” was first established for hygromycin selection of cells that were cotranfected with the expression plasmid (van der Straten et al. 1989). In this case, hygromycin-B-phosphotransferase is expressed under the control of the copia promoter in S2 cells, that have been cotransfected with pCoHYGRO. This heterologous enzyme inactivates exogenous hygromycin-B, which would inhibit protein synthesis in S2 cells, and allows transformed cells to grow in the presence of hygromycin. However, the cells treated with hygromycin-B can still divide at the early phase until they can no longer survive. Therefore, it takes up to 4 weeks to obtain the transformants that are hygromycin-B-resistant.
Blasticidin S deaminase is a drug that inactivates an enzyme produced by Aspergillus terreus, which converts cytotoxic blasticidin S to its non-toxic deaminohydroxy derivative. Introduction of this enzyme in transfected cells has been used for transforming mammalian cells to Blasticidin S resistance (Kimura et al. 1994). Invitrogen has also generated the cotransfection vector “pCoBLAST”, which enables expression of blasticidin S deaminase in S2 cells under control of the copia promoter. Selection of S2 cells with blasticidin S can be accomplished around 2 weeks, which is faster than selection with hygromycin-B.
Puromycin is an aminonucleoside antibiotic produced by Streptomyces alboniger, which causes premature chain termination during translation in various cell types (de la Luna and Ortin 1992; Vara et al. 1985). Puromycin N-acetyltransferase inactivates cytotoxic puromycin by acetylating the amino position of its tyrosinyl moiety (Vara et al. 1985). In order to minimize selection times with transfected S2 cells, we recently generated cotransfection vector “pCoPURO”, which allows expression of puromycin N-acetyltransferase (Iwaki et al. 2003). This cotransfection vector is functional in S2 cells at the concentrations of puromycin between 2 and 10 μg/mL and eliminates non-transfected cells within 3 days (Iwaki et al. 2003).
Limitation of co-transfection methods of selection markers in S2 cells
Although co-transfection of the selection marker with expression plasmids is functional process for generating stable expressing S2 lines in short culture periods, the expression levels by stable transformants are continuously decreased during long culture periods. This results from heterogeneous populations of S2 cells generated by cotransfection, in which some cells are present that contain the selection marker without the gene of interest in the chromosome. These non-productive drug-resistant cells grow faster than productive cells which also contain the gene of interest. Therefore, the former cells overgrow in culture and the productivity of cells also expressing recombinant proteins decreases during long culture periods.
Cloning of single cells is the theoretical answer in order to avoid this phenomenon. However, the processes for this type of cloning are complicated and time-consuming.
Single plasmid transfection
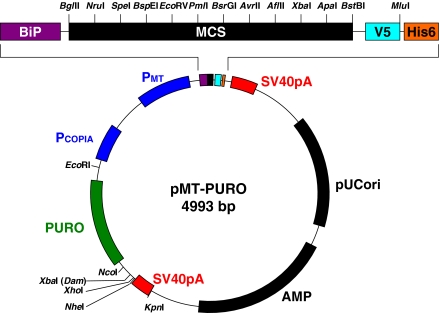
In order to avoid generating nonproductive puromycin-resistant cells, we generated pMT-PURO, which contains the heterologous protein expression cassette driven by MT promoter, along with the puromycin selection marker driven by the copia promoter, all in a single plasmid (Fig. 1). For assembling this vector, the original vector “pMT/BiP/V5-HisA” from Invitrogen was digested by NarI, which is located upstream of the MT promoter. The puromycin-resistant cassette, as well as the copia promoter and SV40 pA signal sequences, were inserted into the site in a head-to-head proportion to avoid promoter interference. New multiple cloning sites (MCS) were substituted for the original sites after the integration of the puromycin-resistant cassette.
Fig. 1.
Map of pMT-PURO. PMT, metallothionein promoter; BiP, BiP signal sequence; MCS, multiple cloning site; V5, V5 peptide tag; His6, 6× histidine; SV40 pA, SV40 late polyadenylation signal; PCOPIA, copia promoter; Amp, ampicillin resistant gene; pUCori, pUC origin; PURO, puromycin N-acetyl-transferase (pac). There are two XbaI site in this vector although one of them is Dam methylated
The advantage of this strategy is to avoid generating nonproductive puromycin-resistant cells. However, this vector would normally integrate a 1:1 ratio of the gene of interest to the puromycin-resistant marker into the chromosome. Therefore, the puromycin-resistant cells might contain relatively lower amounts of the gene of interest, compared to the resistant cells generated by a cotransfection procedure.
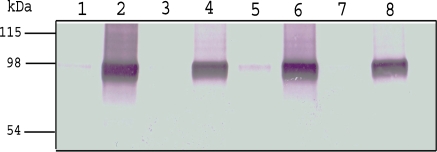
Therefore, we compared the results obtained with the cotransfection procedure using the pCoPURO and a heterologous expression vector for human plasminogen (hPlg) production, with those obtained using the single expression/selection vector, pMT-PURO, in S2 cells. Plg was indeed expressed using both methods, and no significant differences were observed in both methods. Moreover, we also constructed pMT-HYGRO (data not shown) and compared the results obtained with the cotransfection and single expression/selection for Hygromycin selection. No obvious differences were noted either (Fig. 2) although the establishment of stable transformants by hygromycin selection took longer periods (3 weeks) than puromycin selection (3 days).
Fig. 2.
Western-blot for recombinant S2 cells expression media. Lanes 1, 3, 5, and 7 are samples taken before induction. Lanes 2, 4, 6, and 8 are samples removed 96 h post-induction. Lanes1 and 2: cells transfected with pMT-PURO-hPlg. Lanes 3 and 4: cells transfected with pMT-HYGRO-hPlg. Lanes 5 and 6: cells co-transfected with pMT-hPlg and pCoPURO. Lanes 7 and 8: cells co-transfected with pMT-hPlg and pCoHYGRO
Furthermore, the cell cultures obtained with the pMT-PURO/hPlg single vector transfection were stable for more than 20 passages. In addition, long term storage of these cells (−80 °C) was possible using the Invitrogen freezing protocol for S2 cells up to 3 months. Utilization of cells as old as 20 passages, or thawed cells, showed no significant decrease in the protein expression level.
A standard transfection protocol using pMT-PURO/with a heterologous expression plasmid
Seed 6 × 105 S2 cells in each well of a 24-well plate in 600 μL ExCell 420 medium (SAFC Biosciences, Kansas City, MO), containing 10% fetal bovine serum (FBS), and 1× antibiotic/antimycotic solution (Mediatech, Herndon, VA). This is designated as complete media (CM).
Incubate cells for 16 h at 25–28 °C.
Prepare the following transfection reagents:
Solution A:
| 2 M CaCl2 | 7.2 μL |
| pMT-PURO based expression vector | 4 μg |
| Tissue culture sterile water | Bring to a final volume of 50 μL |
Solution B:
| 2× HBS | 50 μL |
We employ the calcium phosphate transfection kit from Invitrogen. Plasmid DNA is purified using Qiaprep spin miniprep kit (QIAGEN, Valencia, CA). Both the quantity and quality of plasmid DNA obtained with this miniprep kit suffices for sequence analysis and transfection.
Add 50 μL Solution A to 50 μL Solution B with continuously mixing.
Incubate the mixture at 25–28 °C for 40 min.
Pour the mixture into the S2 cells.
Incubate the cells with the calcium phosphate-DNA precipitate for 24 h at 25–28 °C.
Remove the calcium phosphate-DNA precipitate by centrifugation at 100g for 5 min and then discard the supernatant.
Resuspend cells in 600 μL CM and replate into the same well.
Incubate the cells for 48 h at 25–28 °C.
Centrifuge the cells at 100g for 5 min and discard the supernatant.
Resuspend the cells in 600 μL CM containing 10 μg/mL puromycin (RPI, Mount Prospect, IL).
Incubate the cells for 72 h at 25–28 °C.
Centrifuge the cells at 100g for 5 min and discard the supernatant.
Resuspend the cells in 1,500 μL CM containing 10 μg/mL puromycin (RPI, Mount Prospect, IL) and seed the cells into each well of a 12-well plate.
Incubate the cells for 72 h at 25–28 °C.
Centrifuge the cells, resuspend in 3 mL plain ExCell 420 media (incomplete media), and then seed onto each well of a 6-well plate.
Expand the cells using incomplete media.
Conclusion
In this study, it was shown that a protein as large as hPlg (ca., 90 kDa) can be efficiently expressed at high levels (ca., 2–6 mg/L) in S2 cells. Therefore, the fusion of the selection vector with the expression vector does not impose an obvious upper limit, at least up to this molecular weight, of the recombinant protein for efficient expression. Moreover, the increased stability of the cell lines will allow long term utilization of the same cell culture for protein expression. We have expressed with pMT-PURO several human and murine proteins related coagulation and fibrinolysis, e.g., plasminogen (Plg), urokinase-type plasminogen (uPA) activator, coagulation factor XII (FXII), high molecular weight kininogen (HMWK), and prekallikrein (PK) along with large number of variants of these proteins (Table 1).
Table 1.
Recombinant hemostasis-related proteins with this system
| Gene | M.W. |
|---|---|
| Plg | 90,000 |
| uPA | 53,000 |
| FXII | 80,000 |
| PK | 86,000 |
| HMWK | 110,000 |
One advantage of expressing hemostasis genes in this system is that the majority of them are too large to express in Escherichia coli-based systems. While mammalian expression systems are also extensively used, most mammalian cells express proteinases that activate or degrade hemostasis-related proteins, thus making purification of intact recombinant proteins difficult (Nilsen and Castellino 1999). Although we expressed hemostasis-related genes successfully with the Drosophila system, certain limitations need to be addressed. It is believed that insect cells possess most of the protein processing capabilities that occur in mammalian cells. Therefore, it is likely that a recombinant protein expressed in insect cells may contain the normal activity (Osterrieder et al. 1994). However, recombinant proteins expressed in S2 cells typically contain lower molecular masses than the native proteins (Altmann et al. 1999). In our case, the molecular mass of recombinant Plg expressed in S2 cells is slightly smaller than that of native Plg purified from plasma. This discrepancy is due to different glycan structures between mammalian and insects cells (Kost et al. 2005). The different glycan structures may affect some functional properties obtained with use of the S2 cell system (Chang et al. 2005).
Although there are possible limitations and/or concerns in the S2 system, this single plasmid transfection system is easily handled and gives reliable and reproductive results for recombinant protein synthesis. The vectors “pCoPURO” and “pMT-PURO” are now available from Addgene (http://www.addgene.org/pgvec1) and Riken Bio resource center (http://www.brc.riken.jp/lab/dna/).
Acknowledgments
We thank Mr. Liang Zhong for inserting the puromycin cassette into pMT/BiP/V5-HisA, Ms. Mariana Figuera Losada for expressing human plasminogen and its variants, Ms. Diana Cruz-Topete for expressing human and murine plasminogen and their variants, and Dr. Qihua Fu for expressing human and murine urokinase-type plasminogen activator and their variants with this system. We also thank many laboratories who used pCoPURO and provided to us important feedback. This work was supported in part by grants HL13423 and HL19982 (to FJC).
References
- Altmann F, Staudacher E, Wilson IB, Marz L (1999) Insect cells as hosts for the expression of recombinant glycoproteins. Glycoconj J 16:109–123 [DOI] [PubMed]
- Bunch TA, Grinblat Y, Goldstein LS (1988) Characterization and use of the Drosophila metallothionein promoter in cultured Drosophila melanogaster cells. Nucleic Acids Res 16:1043–1061 [DOI] [PMC free article] [PubMed]
- Chang KH, Yang JM, Chun HO, Chung IS (2005) Enhanced activity of recombinant beta-secretase from Drosophila melanogaster S2 cells transformed with cDNAs encoding human beta1,4-galactosyltransferase and galbeta1,4-GlcNAc alpha2,6-sialyltransferase. J Biotechnol 116:359–367 [DOI] [PubMed]
- Chung YT, Keller EB (1990) Positive and negative regulatory elements mediating transcription from the Drosophila melanogaster actin 5C distal promoter. Mol Cell Biol 10:6172–6180 [DOI] [PMC free article] [PubMed]
- de la Luna S, Ortin J (1992) pac gene as efficient dominant marker and reporter gene in mammalian cells. Methods Enzymol 216:376–385 [DOI] [PubMed]
- Iwaki T, Figuera M, Ploplis VA, Castellino FJ (2003) Rapid selection of Drosophila S2 cells with the puromycin resistance gene. Biotechniques 35:482–484, 486 [DOI] [PubMed]
- Kimura M, Takatsuki A, Yamaguchi I (1994) Blasticidin S deaminase gene from Aspergillus terreus (BSD): a new drug resistance gene for transfection of mammalian cells. Biochim Biophys Acta 1219:653–659 [DOI] [PubMed]
- Kirkpatrick RB, Ganguly S, Angelichio M, Griego S, Shatzman A, Silverman C, Rosenberg M (1995) Heavy chain dimers as well as complete antibodies are efficiently formed and secreted from Drosophila via a BiP-mediated pathway. J Biol Chem 270:19800–19805 [DOI] [PubMed]
- Kost TA, Condreay JP, Jarvis DL (2005) Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat Biotechnol 23:567–575 [DOI] [PMC free article] [PubMed]
- Nilsen SL, Castellino FJ (1999) Expression of human plasminogen in Drosophila Schneider S2 cells. Protein Expr Purif 16:136–143 [DOI] [PubMed]
- Osterrieder N, Wagner R, Pfeffer M, Kaaden OR (1994) Expression of equine herpesvirus type 1 glycoprotein gp14 in Escherichia coli and in insect cells: a comparative study on protein processing and humoral immune responses. J Gen Virol 75(Pt 8):2041–2046 [DOI] [PubMed]
- Schneider I (1972) Cell lines derived from late embryonic stages of Drosophila melanogaster. J Embryol Exp Morphol 27:353–365 [PubMed]
- van der Straten A, Johansen H, Rosenberg M, Sweet R (1989) Introduction and constitutive expression of gene products in cultured Drosophila cells using hygromycin-B selection. Curr Methods Mol Cell Biol 1:1–8
- Vara J, Perez-Gonzalez JA, Jimenez A (1985) Biosynthesis of puromycin by Streptomyces alboniger: characterization of puromycin N-acetyltransferase. Biochemistry 24:8074–8081 [DOI] [PubMed]