Abstract
A recombinant plasmid harboring heterologous genes coding human ribonuclease/angiogenin inhibitor (RAI) was expressed in stably transformed Drosophila melanogaster Schneider 2 (S2) cells. Stably transformed polyclonal cell populations expressing RAI were isolated after 4 weeks of selection with hygromycin B. Recombinant RAI with a molecular weight of 50 kDa was detected in the intracellular (cell) and extracellular (medium) fractions of S2 cells. Recombinant RAI was purified from the extracellular fraction using a two-step purification scheme comprised of Ni-NTA and ion-exchange chromatography. Purified RAI migrated on SDS-PAGE as a single band in the elution fraction containing 300 mM NaCl. The ribonuclease inhibitor activity of purified RAI was measured using yeast tRNA and RNase A. Purified RAI exhibited an activity of ∼8 U μg−1 for the inhibition of RNA degradation by RNase A. Cultivation of stably transformed S2 cells using HyQ®SFX-insect MP medium increased cell growth by 79% and approximately doubled the production of recombinant RAI.
Keywords: Drosophila melanogaster S2 cells, Ribonuclease/angiogenin inhibitor, RAI, Purification, Ribonuclease inhibitor activity
Introduction
Human placental ribonuclease/angiogenin inhibitor (RAI) is a cytoplasmic ribonuclease inhibitor that occurs in several mammalian tissues (Lee and Vallee 1994). RAI (50 kDa) purified from the human placenta has been shown to control intracellular RNases by binding bovine pancreatic RNase A, and functioning as a non-competitive inhibitor (Blackburn 1979). In addition, RAI has a role in abolishing the ribonucleolytic and angiogenic activities of angiogenin (Shapiro and Vallee 1987). Angiogenin is an angiogenic factor that is involved in angiogenesis, the process of blood vessel formation in which new vessels develop from existing vessels. Since tumor growth is dependent on angiogenesis (Kerbel 1997), studies of RAI might be important to develop anti-angiogenic therapy in cancer. RAI expression or purification has been reported from human erythrocytes and an Escherichia coli expression system (Frank and Vallee 1989; Moenner et al. 1998; Klink et al. 2001). However, expression of recombinant RAI by stably transformed insect cells has not yet been examined. Compared with a bacterial expression system insect cells are generally better for the production of eukaryotic recombinant proteins requiring post-translational modification. Advantages of the insect cell, Drosophila melanogaster Schneider 2 (S2), used in this study include high-level and low cost production of eukaryotic proteins (Schneider 1972), high density growth without CO2 supplementation in a serum-free medium, stable gene insertion into chromosomal DNA (Johansen et al. 1989), easy secretion of protein products into the medium, and no interaction of endogenous Drosophila proteins with mammalian proteins (Courey and Tjian 1988). In this report, we describe stable expression of the cDNA for human RAI in D. melanogaster S2 cells and purification of the recombinant RAI. We also investigate the in vitro activity of recombinant RAI derived from stably transformed S2 cells.
Materials and methods
Cell limes, plasmids, and enzymes
Drosophila melanogaster S2 cells were grown at 27 °C in T-25 culture flasks (Nunc, Roskilde, Denmark) in M3 (Shields and Sang M3) Insect Medium (Sigma, St. Louis, MO, USA) containing 10% IMS (Insect Medium Supplement from Sigma). The 3.6 kb pMT/BiP/V5-His plasmid (Invitrogen, Carlsbad, CA, USA) contained a metallothionein promoter, a BiP signal sequence, a V5 epitope tag, and a polyhistidine region. The selection plasmid pCoHygro (Invitrogen), which contained the bacterial hygromycin B phosphotransferase gene under control of the constitutive Drosophila Copia 5′-LTR promoter, was used for stable transformation. The pLBA/RAI (ATCC 85539) plasmid contained the cDNA for human RAI. E. coli JM109 was used as the primary host for constructing and propagating plasmids. E. coli cells were routinely grown with agitation at 37 °C in LB medium (1% tryptone, 0.5% yeast extract, and 0.5% NaCl at pH 7.3) containing 50 μg ml−1 of ampicillin. We used DNA restriction enzymes from Promega, Madison, WI, USA, or Takara, Shiga, Japan according to manufacturer instructions.
Construction of expression plasmids
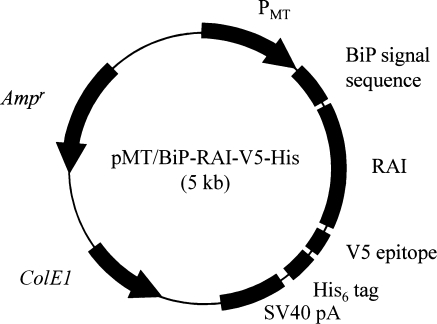
Human RAI cDNA was amplified from pLBA/RAI by PCR using oligonucleotide primers containing BglII or XhoI restriction enzyme sites. PCR was performed using a Thermal Cycler (PE Biosystems, Foster City, CA, USA) using PCR Mix (Takara) in a volume of 50 μl. Amplified RAI cDNA was then inserted into the T/A cloning vector, pGEM-T (Promega) to yield pGEM-T-RAI, and the construct was verified by DNA sequencing. pMT/BiP/RAI-V5-His was constructed by inserting the BglII-XhoI fragment of pGEM-T-RAI between the BglII and XhoI sites of pMT/BiP/V5-His (Fig. 1). The proper orientation and reading frame of the insertions in pMT/BiP/RAI-V5-His were confirmed by both restriction enzyme mapping and DNA sequencing.
Fig. 1.
Schematic representation of the expression plasmid pMT/BiP/RAI-V5-His
Stable transformation
Exponentially growing S2 cells were co-transfected with the pMT/BiP/RAI-V5-His and pCoHygro plasmids (1:1) using the lipofectin method. To make the transfection medium, plasmid DNA and the lipofectin reagent (Invitrogen) were diluted separately in IMS-free M3 medium, and then mixed in a 1:5 ratio. The transfection medium was incubated at room temperature for 15 min and transferred to six-well plates pre-seeded 2 h earlier with S2 cells in IMS-free M3 medium. After a 24-h incubation, the medium was changed to remove the lipofectin reagent, and the cells were incubated for five additional days without drug selection in M3 medium containing 10% IMS. The cells were then centrifuged and resuspended in selective M3 medium containing 10% IMS and 300 μg ml−1 hygromycin B (Invitrogen). The selective medium was replaced every 5 days, and stably transformed polyclonal cell populations were isolated after 4 weeks of selection using hygromycin B, which was maintained continuously in the medium after the selection procedure.
Cell culture and analysis of gene expression
Stably transformed S2 cells expressing RAI were grown at 27 °C in T-25 flasks in 5 ml of M3 medium containing 10% IMS and 300 μg ml−1 hygromycin B. Stably transformed S2 cells were cultured in multiple T-25 flasks to analyze cell growth and RAI expression. RAI expression was induced by the addition of 0.5 mM CuSO4 after the start of the run. After centrifuging the cultures at 3,000 rpm for 5 min, the supernatants were used to identify extracellular recombinant proteins. The cell fraction was incubated with rocking for 1 h in lysis buffer [50 mM Tris–HCl (pH 8.0), 150 mM NaCl, 0.02% sodium azide, 100 μg ml−1 PMSF, 1 μg ml−1 aprotinin, and 1% Triton X-100]. The cell extracts were centrifuged at 14,000 rpm for 15 min to remove cell debris, and the supernatant was used to identify intracellular recombinant proteins. Protein concentrations were determined using a Bradford protein assay kit (Bio-Rad, Hercules, CA, USA). RAI expression was determined by densitometric scanning after SDS-PAGE and Western blot analysis.
Purification of recombinant RAI
Polyhistidine-tagged RAI was purified using Ni-NTA affinity chromatography (Qiagen, Valencia, CA, USA) followed by ion-exchange chromatography using a Vivapure spin column (Vivascience, Germany), according to manufacturer instructions. Stably transformed S2 cells were cultured in a medium containing 0.5 mM CuSO4. After 5 days of incubation, medium fractions were collected by centrifugation, filtrated through a 0.2 μm syringe filter, dialyzed for 2 days with dialysis buffer [20 mM Tris–HCl (pH 7.9), 500 mM NaCl], then used in Ni-NTA affinity chromatography. Ni-NTA resin was equilibrated with binding buffer containing 10 mM imidazole and poured into a column. The dialyzed medium fraction was then slowly applied to the column containing the equilibrated resin. Weakly bound proteins were washed from the resin using the binding buffer, and contaminating proteins still bound to the resin were removed by increasing the imidazole concentration to 30 mM. Proteins bound to the resin were eluted in the buffer containing high concentrations of imidazole (from 50 to 500 mM). Fractions containing recombinant RAI were dialyzed with 20 mM Tris–HCl (pH 8.0) buffer for 3 days then applied to the Vivapure spin column packed with the equilibrated anion-exchanger diethylamine. Unbound proteins were washed from the resin using 20 mM Tris–HCl (pH 8.0) buffer. The recombinant RAI was finally eluted in the 20 mM Tris–HCl (pH 8.0) buffer containing different concentrations of NaCl (from 100 to 500 mM). Fractions containing recombinant RAI were dialyzed in phosphate buffered saline (pH 7.4) and analyzed by SDS-PAGE and Western blot analysis. All steps were carried out at 4 °C.
Enzymatic activity assay of recombinant RAI
Ribonuclease inhibitory activities of recombinant RAI were determined using the end point assay procedure of Moenner et al. (1998) with minor modification. Briefly, yeast tRNA (30 μg; GibcoBRL) was incubated alone or with RNase A (2 μg, 0.2 U; Amresco, Solon, OH, USA) in an assay mixture containing a TE buffer [10 mM Tris–HCl (pH 8.0), 1 mM EDTA] in a total volume of 1 ml. After the addition of either recombinant RAI or commercial ribonuclease inhibitor (RI, Invitrogen), the reaction mixtures were incubated for 15 min. RNA concentrations were quantified by spectrophotometric analysis at OD260. One unit of ribonuclease inhibitor is the amount required to inhibit the activity of 5 ng of ribonuclease A by 50%.
Western blot analysis
Protein samples were separated by electrophoresis on 12% polyacrylamide-SDS gel (Laemmli 1970), and then visualized by silver staining (Sambrook et al. 1989), or subjected to Western blot analysis. The electrophoresed proteins on the gel were transferred to a nitrocellulose membrane (Amersham-Pharmacia Biotech, Piscataway, NJ, USA), pre-incubated for 2 h at room temperature with a blocking solution (3% skim milk in TBS), incubated with mouse anti-V5 (1:1,000 dilution in blocking solution, Invitrogen), and probed with alkaline phosphatase-conjugated goat anti-mouse IgG antibody (1:1,000 dilution in blocking solution, Sigma). The membranes were washed and BCIP/NBT solution (Amresco) was added. The reaction was quenched with distilled water.
Results and discussion
Recombinant RAI expression
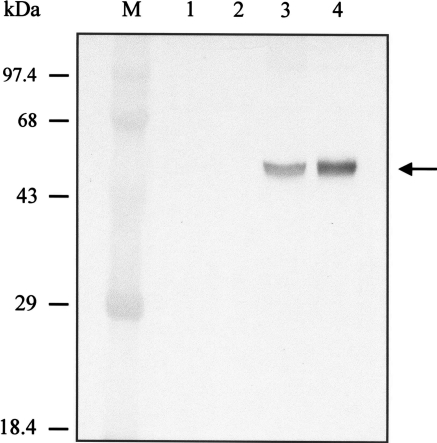
We examined the expression of RAI in stably transformed S2 cells by Western blot analysis. At 5 days post-induction with 0.5 mM CuSO4 the cell density was 1.2 × 107 cells ml−1. Shown in Fig. 2, the recombinant RAI protein of ∼50 kDa was detected in transformed S2 cells carrying pMT/BiP/RAI-V5-His by Western blot analysis with mouse anti-V5 antibodies (Invitrogen). The molecular size was approximated from the predicted molecular weight of RAI containing the C-terminal tag of V5 and His6. Recombinant RAI was present in the intracellular and extracellular (medium) fractions of transfected S2 cells. Densitometric scanning showed that the secreted RAI (i.e., RAI in the medium) accounted for ∼70% of the total RAI production. The total of recombinant RAI expression was ∼1.4 μg (107 cells)−1, as estimated by the intensity of Western blot in comparison with known standards. Recombinant RAI was not detected in either the cellular or medium fractions of non-transfected S2 cells.
Fig. 2.
Western blot analysis of non-transfected and stably transformed S2 cells. The expression of recombinant RAI from cellular and medium fractions was detected with Western blot analysis using anti-V5 antibody. M molecular weight marker, 1 cellular fraction of non-transfected cells, 2 medium fraction of non-transfected cells, 3 cellular fraction of stably transformed S2 cells, and 4 medium fraction of stably transformed S2 cells. The arrow indicates the recombinant RAI protein
Purification of recombinant RAI
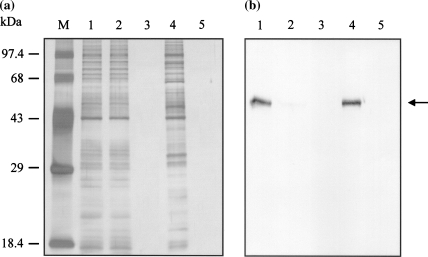
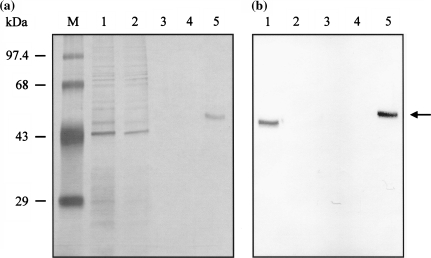
Recombinant RAI protein in the extracellular fraction of stably transformed S2 cells was purified by Ni-NTA affinity chromatography followed by ion-exchange chromatography. The purity of the protein was analyzed using SDS-PAGE and silver staining. Western blot analysis further confirmed the identity of the purified protein. Ni-NTA affinity chromatography showed that most of recombinant RAI protein (polyhistidine-tagged RAI, RAI-V5-His6) was eluted in the wash buffer containing a low concentration of imidazole (60 mM), and the presence of recombinant RAI was confirmed by Western blot analysis (Fig. 3b). This fraction contained a number of non-specific proteins (Fig. 3a). Many polyhistidine-tagged recombinant proteins were successfully purified from the extracellular fractions of stably transformed S2 cells by simple one-step Ni-NTA affinity chromatography (Chang et al. 2002; Jeon et al. 2003). However, this procedure was not suitable for the purification of recombinant RAI. Binding activity between the recombinant RAI protein and the Ni-NTA resin was probably too weak for use in affinity purification. Since successful purification of RAI has been reported using ion-exchange chromatography (Nadano et al. 1994), we used two-step purification. The first step was purification of recombinant RAI protein by Ni-NTA affinity chromatography followed by the second step of ion-exchange chromatography using a Vivapure spin column containing the anion-exchanger diethylamine. Recombinant RAI protein was detected in the eluent fraction containing 300 mM of NaCl without visible contaminating proteins on silver nitrate-stained SDS-PAGE gel (Fig. 4).
Fig. 3.
SDS-PAGE (a) and Western blot analytical results (b) for Ni-NTA affinity chromatography purification of His-tagged recombinant RAI protein from the medium fraction of a stably transformed S2 cell culture. M indicates the molecular weight marker. Lane 1 represents the medium fraction of stably transformed S2 cells loaded onto the Ni-NTA affinity column. Lane 2 represents the flow-through fraction of the affinity column. Lanes 3, 4, and 5 represent eluent fractions containing 30, 60, and 100 of mM imidazole, respectively. The arrow indicates the recombinant RAI protein
Fig. 4.
SDS-PAGE (a) and Western blot analytical results (b) for the purification of recombinant RAI by ion-exchange chromatography from the eluent fraction (containing 60 mM imidazole) that was obtained in Ni-NTA affinity chromatography. M indicates the molecular weight marker. Lane 1 represents the elute fraction from the affinity chromatography column. Lane 2 represents the flow-through fraction from the anion-exchanger column. Lanes 3, 4, and 5 represent the washing and eluent fractions containing 100 and 300 mM of NaCl, respectively. The arrow indicates the recombinant RAI protein
Ribonuclease inhibitory activity assay of purified RAI
Since RAI is known to suppress RNA degradation by ribonuclease in vitro (Lee et al. 1989; Lee and Vallee 1994), we investigated the biological activity of the purified recombinant RAI on the degradation of RNA by RNase A. Four enzymatic reactions were performed for 15 min at room temperature. The first reaction used only yeast tRNA (30 μg ml−1), the second used yeast tRNA (30 μg ml−1) and RNase A (0.2 U), the third used the second condition plus purified RAI (10 μg), and the fourth used the second condition plus commercial RI (80 U). In the second reaction, RNase A increased the ΔOD260/min (×10−3) value from 0.37 to 8.63 due to degradation of RNA. In the third reaction, the addition of 10 μg of purified RAI suppressed RNase A activity by 61%, as shown in the ΔOD260/min data (Table 1). The suppression level of the purified RAI at 10 μg (the third reaction) was almost equal to the level using commercial RI at 80 U (fourth reaction of Table 1), indicating that 1 μg of purified RAI was equivalent to 8 U of commercial RAI.
Table 1.
Ribonuclease inhibitor assay of purified recombinant RAI
| Reaction | ΔOD260/min (×10−3) |
|---|---|
| Yeast tRNA (30 μg) | 0.37 (±0.02) |
| Yeast tRNA (30 μg) + RNase A (0.2 U) | 8.63 (±0.24) |
| Yeast tRNA (30 μg) + RNase A (0.2 U) + Purified RAI (10 μg) | 3.37 (±0.16) |
| Yeast tRNA (30 μg) + RNase A (0.2 U) + Commercial RI (80 U) | 3.43 (±0.22) |
All data represent the average of triplicate analyses. One unit of ribonuclease inhibitor is the amount required to inhibit the activity of 5 ng of ribonuclease A by 50%
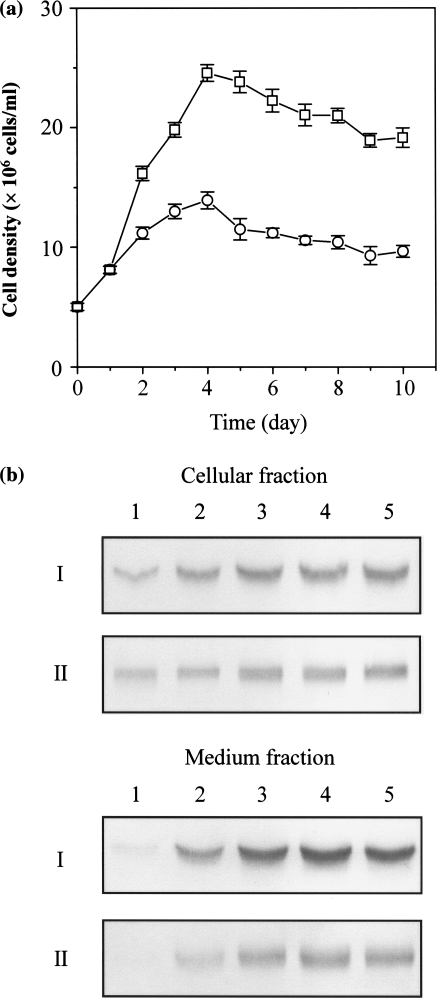
Effect of HyQ®SFX-Insect MP medium on cell growth and recombinant RAI production
HyQ®SFX-insect MP medium (Hyclone) is a protein-free cell culture medium developed through metabolic pathway design. Use of HyQ®SFX-insect MP medium increased growth and recombinant RAI production from transformed S2 cells pre-adapted for 2 weeks with the same medium. Cells were cultured for 10 days in HyQ®SFX-insect MP medium without IMS, or in M3 medium containing 10% IMS at the same initial cell density (5 × 106 cells ml−1). Recombinant RAI expression was induced by adding 0.5 mM CuSO4 after the start of the run. The maximum cell density after 4 days of incubation in HyQ®SFX-insect MP medium (2.5 × 107 cells ml−1) was 79% higher than the density (1.4 × 107 cells ml−1) on M3 medium containing 10% IMS (Fig. 5a). Recombinant RAI production after 5 days of incubation from HyQ®SFX-insect MP medium was ∼2 times higher compared with M3 medium, as measured by the densitometry of the blot (Fig. 5b). The expression patterns of RAI in the cellular and medium fractions were almost the same in both media (Fig. 5b), indicating that HyQ®SFX-insect MP medium can be effectively used for production of recombinant proteins in stably transformed S2 cells. Our experimental results for transformed S2 cells were similar to results reported by Barnett (1998) regarding superior growth and production of recombinant proteins in the insect cells Sf9, Sf21, and High Five™. Our preliminary analyses showed that supplementation with IMS (at the level of 1–10%) in HyQ®SFX-insect MP medium did not have any positive effect on cell growth and recombinant RAI production, as measured after 5 days of incubation (data not shown).
Fig. 5.
Time-course changes of cell growth and recombinant RAI expression in T-flask cultures of stably transformed S2 cells. (a) Cell densities obtained in M3 (open circle) and HyQ®SFX-insect MP medium (open square) containing 0.5 mM CuSO4 are plotted against the incubation time. The error bars indicate the standard deviation of the mean for three independent runs. (b) Recombinant RAI expression in the cellular and medium fractions was confirmed by Western blot analysis. I and II indicate HyQ®SFX-insect MP and M3 medium, respectively. Lanes 1–5 represent the incubation times of 1, 3, 4, 5, and 6 days, respectively
This is the first report on efficient expression of recombinant RAI in a stably transformed S2 cell system. Purified recombinant RAI protein from S2 cells is biologically active, as evidenced by its inhibitory activity on RNA degradation due to RNase A. HyQ®SFX-insect MP medium is superior M3 medium for the cell growth and recombinant RAI production by stably transformed S2 cells.
Acknowledgment
This work was supported by a Korea Research Foundation Grant (KRF-2004-041-F00019).
References
- Barnett BB (1998) Insect cell culture technology: research & production development, Hyclone. Art To Sci 17:1–7
- Blackburn P (1979) Ribonuclease inhibitor from human placenta: rapid purification and assay. J Biol Chem 254:12484–12487 [PubMed]
- Chang KH, Park JH, Lee YH, Kim JH, Chun HO, Kim JH, Chung IS (2002) Dimethylsulfoxide and sodium butyrate enhance the production of recombinant cyclooxygenase 2 in stably transformed Drosophila melanogaster S2 cells. Biotechnol Lett 24:1353–1359 [DOI]
- Courey AJ, Tjian R (1988) Analysis of Sp1 in vivo reveals multiple transcriptional domains, including a novel glutamine-rich activation motif. Cell 55:887–898 [DOI] [PubMed]
- Frank S, Vallee BL (1989) Expression of human placental ribonuclease inhibitor in Escherichia coli. Biochem Biophys Res Commun 160:115–120 [DOI] [PubMed]
- Jeon HK, Chang KH, Kim KI, Chung IS (2003) Functional expression of recombinant tumstatin in stably transformed Drosophila melanogaster S2 cells. Biotechnol Lett 25:185–189 [DOI] [PubMed]
- Johansen H, Van Der Straten A, Sweet R, Otto E, Maroni G, Rosenberg M (1989) Regulated expression at high copy number allows production of a growth-inhibitory oncogene product in Drosophila Schneider cells. Genes Dev 3:882–889 [DOI] [PubMed]
- Kerbel RS (1997) A cancer therapy to resistance. Nature 390:335–336 [DOI] [PubMed]
- Klink TA, Vicentini AM, Hofsteenge J, Raines RT (2001) High-level soluble production and characterization of porcine ribonuclease inhibitor. Protein Expr Purif 22:174–179 [DOI] [PubMed]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of head of bacteriophage. Nature 227:680–685 [DOI] [PubMed]
- Lee FS, Shapiro R, Vallee BL (1989) Tight-binding inhibition of angiogenin and ribonuclease A by placental ribonuclease inhibitor. Biochemistry 28:225–230 [DOI] [PubMed]
- Lee FS, Vallee BL (1994) Structure and action of mammalian ribonuclease (angiogenin) inhibitor. Prog Nucleic Acid Res Mol Biol 44:1–30 [DOI] [PubMed]
- Moenner M, Vosoghi M, Ryazantsev S, Glitz DG (1998) Ribonuclease inhibitor protein of human erythrocytes: characterization, loss of activity in response to oxidative stress, and association with Heinz bodies. Blood Cells Mol Dis 24:149–164 [DOI] [PubMed]
- Nadano D, Yasuda T, Takeshita H, Uchide K, Kishi K (1994) Purification and characterization of human brain ribonuclease inhibitor. Arch Biochem Biophys 312:421–428 [DOI] [PubMed]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory mannual. Cold Spring Harbour Laboratory Press, New York
- Schneider I (1972) Cell lines derived from late embryonic stages of Drosophila melanogaster. J Embryol Exp Morphol 27:353–365 [PubMed]
- Shapiro R, Vallee BL (1987) Human placental ribonuclease inhibitor abolishes both angiogenic and ribonucleic activities of angiogenin. Proc Natl Aca Sci 84:2238–2241 [DOI] [PMC free article] [PubMed]