Abstract
An animal protein-free medium was developed for Drosophila melanogaster S2 (S2AcGPV2) cells genetically modified to produce the rabies virus G glycoprotein (GPV). IPL-41, used as a basal medium, was supplemented with yeastolate, carbohydrates, amino acids and lipids aiming initially to reduce and further to eliminate the need of fetal bovine serum. The S2AcGPV2 cells were fully capable of growing in serum-free supplemented IPL-41 medium containing 6 g L−1 yeastolate ultrafiltrate, 10 g L−1 glucose, 3.5 g L−1 glutamine, 0.5 g L−1 fructose, 2 g L−1 lactose, 0.6 g L−1 tyrosine, 1.48 g L−1 methionine and 1% (v/v) lipid emulsion, reaching 19 × 106 cells mL−1. Maximum specific growth rate and cell productivity were 0.025 h−1 and 0.57 × 105 cells mL−1 h−1, respectively. Glucose and lactose were consumed during cell culture, but not fructose. Lactate concentration generally decreased during cell culture, while ammonium concentration reached 167 mg L−1, however, without noticeable deleterious effects on cell growth. GPV concentration values achieved were, however, modest in the proposed medium formulation.
Keywords: GPV protein, IPL-41 medium, Metabolism and S2 cells
Introduction
Animal cell medium formulation, supplementation and optimization to achieve high cell densities have been the subject of intense research in the last years. Specifically for insect cells, basal media such as IPL-41, TC100, Grace and TNM-FH supplemented with serum have been widely used (Bachmann et al. 2004; Kioukia et al. 1995; Batista et al. 2005; Donaldson and Schuler 1998). However, medium formulations free of animal protein are desirable for cell growth, due to limitations associated to serum and other animal-derived compounds supplementation. Serum replacement can reduce costs, lot-to-lot variability in medium formulation and also diminish protein load, facilitating the downstream processing steps of recombinant proteins. In addition, when components of animal origin are added to the culture medium, there is a potential risk of adventitious agents transfer, such as viruses and prions. Therefore, according to guidelines from the Food and Drug Administration (FDA, USA) and also from the European Pharmacopoeia (Chu and Robinson 2001), animal-derived components should be avoided in the formulation of culture media used in the production of biopharmaceuticals.
The elimination of animal-derived proteins is possible for different expression systems types, such as for transfected Drosophilamelanogaster S2 cells. The Drosophila expression system has several advantages that make it adequate for protein expression at laboratory and industrial scales. It is a non-lytic insect expression system that uses simple plasmid vectors and fast growing cells which do not require CO2 incubation for proven high-level protein production.
Drosophila melanogaster cells are often maintained in Schneider’s Drosophila medium supplemented with 10% fetal bovine serum, but this cell line can be also cultivated in formulations such as Shields and Sang M3 Insect Medium containing from 2% to 10% FBS (Mosher and Crews 1999; Nybakken et al. 2005) and in Drosophila Serum-Free medium (Southon et al. 2004). The last medium composition, however, similarly to that of Sf900II medium (a culture medium frequently employed for insect cell culture), is not directly available in the literature.
When designing a new formulation, it is usual to begin by adding individual components to a basal medium and monitoring the behavior of cells maintained in such formulation (Zimmerman et al. 2000). Therefore, the first step in the formulation of a serum-free medium is choosing an appropriate basal medium and the set of supplements to be used. The basal medium should provide salts, trace elements, carbohydrates, vitamins, and amino acids. Specific cellular metabolic characteristics should be taken into account when selecting basal medium supplements. For instance, many cells, especially non-transformed cells, require a source of fatty acids. If there is no supply of exogenous lipids, some cells are able to synthesize the required lipid materials from glucose but not all cells in culture will grow in the absence of lipids (Hewlett 1991). Therefore, lipids are often considered as supplements to basal media not enriched with serum, serving also to disperse hydrophobic components.
The literature presents a number of studies in which IPL-41 medium is employed as a basal medium for insect cells. However, supplements such as growth factors, trace metals, shear protecting agents and lipids should be provided. An effective low cost medium formulation based on IPL-41 supplemented with 6 g L−1 of yeastolate ultrafiltrate (autolytic digested yeasts), 1% (v/v) of lipid mixture containing Pluronic PF68 and 4 g L−1 of Hy Soy (a soybean hydrolysate) is reported in the literature (Donaldson and Shuler 1998). This formulation supported BTI-Tn5B1-4 growth of up to 6 × 106 cells mL−1. Another formulation (developed for Spodoptera frugiperda cells) based on IPL-41 medium resulted in 5.5 × 106 cell mL−1 (Maiorella et al. 1988). For Drosophila Schneider 2 cells, IPL-41 supplemented with 1% Pluronic PF-68, 1% lipid concentrate, and 2% yeastolate ultrafiltrate was successfully used, resulting in up to 2 × 107 cells mL−1 actively producing virus-like particles (Bachmann et al. 2004). More recently, Galesi et al. (2007) have shown that in TC100 basal medium containing 10 g L−1 glucose and 3.5 g L−1 glutamine, supplemented with 1% lipid emulsion, 0.1% Pluronic F68 and 3 g L−1 yeastolate, it was possible to attain up to 10.7 × 106Drosophila S2 cells mL−1 in culture. The use of HySoy as an alternative to yeastolate was not as effective, though.
Therefore, supplements such as yeastolate ultrafiltrate and lipids are frequently employed for IPL-41 enrichment. Mixtures of lipids are frequently added to improve cell growth (Maiorella et al. 1988), providing cholesterol, an important constituent of biological membranes which most insect cells cannot synthesize (Mitsuhashi 1989), essential fatty acids, α-tocopherol as an antioxidant and free-radical scavenger, and the shear protectant Pluronic F-68. Fatty acids, in free-state or bound to carriers like albumin or lipoproteins, act as precursors for prostaglandin synthesis, representing an alternative energy source and being major components of the cell membrane (Hewlett 1991). The lipid metabolism of dipteran cells (as Drosophila cells) seems to the rather peculiar: the lack of lipids decrease the cloning efficiency of cells (Echalier 1997).
Yeastolate, on the other hand, is a source of amino acids (Ikonomou et al. 2003) as well as of polysaccharides and vitamins (Echalier 1997). The presence of a potent cell culture enhancer factor in yeastolate low molecular weigh fractions is reported in the literature (Mendonça et al. 2007). Similarly to fetal bovine serum, yeastolate has undefined components in its formulation. However, most of the ultrafiltrate yeastolates commercially available presents standardized production processes, consisting of highly controlled steps which basically involve yeast culture, cell enzymatic digestion, ultrafiltration and microfiltration stages. Such highly standardized production protocols result also in a standardized product with much lower lot-to-lot variability than fetal bovine serum.
In addition to yeastolate, other hydrolysates can be employed as FBS substitutes in insect cell culture, such as Primatone RL (an enzymatic digest of animal tissue), NZ-Soy (a soy peptone) and Hy-Soy. However, the most widely used hydrolysate in insect cell culture is yeastolate (Donaldson and Shuler 1998; Ikonomou et al. 2001; Marteijn et al. 2003), due to the superior results observed with its use.
Other supplements can also increase insect cell growth. Fructose, for instance, can provide cell growth when glucose is exhausted in the culture medium (Mendonça et al. 1999), but with lower maximum specific growth rates. Further, amino acids as methionine and tyrosine can increase the length of the stationary phase for Sf9 cells cultivated in TNM-FH medium, prolonging recombinant protein production (Mendonça et al. 1999).
Rabies is a serious public health problem and a cause of mortality in many regions of the world. The annual number of deaths worldwide caused by rabies is estimated in 50,000 (Rupprecht et al. 1995). The first rabies vaccine successfully employed in humans was developed by Louis Pasteur and colleagues in 1885 (Pérez and Paolazzi 1997). Since then, several vaccine types have been developed.
Rabies virus subunit vaccines use viral surface antigens to evoke an immune response. Subunit vaccines consist of small proteins or peptide portions from pathogenic viruses, which can be also produced by genetically engineered cells. The G glycoprotein from rabies virus (GPV) is a transmembrane protein that forms the viral envelope and induces the production of antibodies that neutralize the virus attack. It can be formulated as a subunit vaccine and is effectively produced by recombinant S2 cells (Yokomizo et al. 2007), which present approximately associated growth and GPV production kinetics. Therefore, optimization of cell growth would also potentially imply in optimization of GPV production.
In the present report it is demonstrated that media formulation free of animal proteins based on IPL-41 medium supplemented with the carbohydrates glucose, fructose and lactose, the amino acids glutamine, tyrosine and methionine, with lipid emulsion and yeastolate ultrafiltrate can provide high transfected S2 cell concentrations, assuring potential to achieve the safety requirements of recombinant proteins produced through this expression system.
Materials and methods
Cell line, media and supplements
Wild (Invitrogen) and transfected (Yokomizo et al. 2007) Drosophila melanogaster S2 cells with pAc 5.1/V5-His A (S2AcGPV2 cells) were used in this study, as well as Sf9 cells (CRL 1711, from ATCC). For S2AcGPV2 cell construction, the plasmid pGPV/PV with the sequence of interest, the c-DNA fragment encoding the glycoprotein surface antigen, GPV, GT1 subtype of rabies virus (obtained from Dr. Yves Jacob, Pasteur Institute, France), and the vector pAc 5.1/V5-His A (Invitrogen, Carlsbad, CA, USA) were utilized. The GPV cDNA, (1.6-Kbp) was inserted into pAc5.1/V5HisA under the control of the Drosophila actin promoter, generating pAcGPV. For cell selection, the selection vector pCoHygro (Invitrogen, Carlsbad, CA, USA) carrying genes coding for hygromicin-inactivating enzymes was utilized. Escherichia coli DH5alfa was used as the primary host for the construction and propagation of plasmids. S2 cells were transfected with pAcGPV and pCoHygro vectors using Cellfectin (Invitrogen, Carlsbad, CA, USA). For the selection of cell populations with higher GPV expression, confluent transfected cultures were submitted to subculture in 96 well plates at a density of 105 cells per well and cultured subsequently in 24, 12 and 6 well plates. Cultures with 106 cells mL−1 of each subculture were performed in 25 cm2 T-flasks (Nunc), in 5 mL of SF900II (GIBCO BRL) medium with 200 μg mL−1 of hygromicin (19).
The cells were cultured in Sf900II (Gibco BRL), in TC100 (Cultilab, Brazil) supplemented with 10% FBS (Cultilab, Brazil) and in IPL-41 (Gibco BRL) supplemented with glutamine, fructose, lactose, methionine and tyrosine (Sigma Chemical Co.), and also with FBS, glucose, yeastolate ultrafiltrate and a chemically defined lipid emulsion concentrate (Gibco BRL). Sf900 II was employed as a control medium due to its capacity to effectively support S2 cells in culture after cell thawing, while the Drosophila SFM medium commonly employed for these cells was not.
Inoculum preparation
Frozen cells, originally adapted to TC100 medium with 10% FBS, were thawed and propagated in the tested media (Sf900 II, TC100 with 10% FBS or supplemented IPL-41). After thawing, around 1 mL of cell suspension was transferred to 4 mL of the medium to be tested. Cells were cultivated in T-flasks (Nunc) for 72 h at 28 °C. After that, the cells were centrifuged at 3000 rpm for 5 min and the pellet was resuspended in fresh medium. For the experiments performed in schott flasks, the cells were cultivated in a 100 mL schott flask with 20 mL of culture medium for 72 h at 28 °C. For the experiments performed in spinners, the cells were previously adapted to each tested medium and cultivated in 60 mL culture medium in 100 mL Bellco spinner flasks, also at 72 h and 28 °C. After this period, the cells were centrifuged at 3000 rpm for 5 min, the pellet was resuspended in fresh medium and transferred to new spinners for furthers experiments.
Evaluation of medium supplements on cell survival, growth and function
This study consisted basically of two steps, the first being the analysis of cell behavior in supplemented IPL-41 containing low amounts of FBS and the second being the evaluation of cell performance in supplemented IPL-41 free of FBS. All experiments were carried out in 100 mL schott flasks (with 20 mL of medium each) inoculated with 7.5 × 105 viable cells mL−1, incubated at 100 rpm in a rotary shaker at 28 °C.
Despite the cells were originally adapted to TC100 supplemented with FBS, this medium could not be used for the intended work because it contains not only serum proteins but also tryptose phosphate obtained from animal sources. Therefore, the animal protein-free IPL-41 formulation was selected as the basal medium and the results were compared to those achieved with TC100 medium containing 10% FBS (minimal culture medium) and Sf900II medium (rich serum-free medium). Culture media optimization studies were performed employing the factorial design approach (which is based on the analysis of the statistical effects of different independent variables on response variables) and the surface diagram methodology.
In the first step, the effects of low FBS percentage (1–3% v/v) and of different yeastolate ultrafiltrate concentrations (4–8 g L−1) on cell behavior were determined employing IPL-41 medium supplemented with glucose (10 g L−1), glutamine (3.5 g L−1), fructose (3.75 g L−1), lactose (0.75 g L−1), methionine (1.4 g L−1), tyrosine (1.4 g L−1) and lipid emulsion (3% v/v). The range of concentrations selected for these supplements is in agreement with regular insect cell culture practice. The analysis of the effects of FBS and yeastolate ultrafiltrate was performed through a 22 factorial experimental design expanded in star configuration, in a total of 13 experiments, as indicated in Table 1.
Table 1.
22 Complete factorial design experiment for yeastolate and FBS supplements, including three replicates of the central point plus a set of tests in star configuration
| Medium formulation | Independent variables | Dependent variables | ||||
|---|---|---|---|---|---|---|
| Yeastolate ultrafiltrate (g L−1) | FBS (%) | Maximum cell concentration (cells mL−1) | Viability (%) | μmax (h−1) | Maximum cell productivity (cells mL−1 h−1) | |
| 1A | 4 | 1 | 25 × 105 | 97 | 0.0106 | 0.17 × 105 |
| 2A | 8 | 1 | 90 × 105 | 99 | 0.0155 | 0.18 × 105 |
| 3A | 4 | 3 | 37 × 105 | 99 | 0.0114 | 0.24 × 105 |
| 4A | 8 | 3 | 100 × 105 | 99 | 0.0169 | 0.23 × 105 |
| 5A | 3.2 | 2 | 18 × 105 | 99 | 0.0062 | 0.13 × 105 |
| 6A | 8.8 | 2 | 79 × 105 | n.d | n.d. | n.d. |
| 7A | 6 | 0.59 | 110 × 105 | 99 | 0.0220 | 0.18 × 105 |
| 8A | 6 | 3.4 | 110 × 105 | 99 | 0.0144 | 0.16 × 105 |
| 9A | 6 | 2 | 91 × 105 | 99 | 0.0244 | 0.42 × 105 |
| 10A | 6 | 2 | 80 × 105 | 99 | 0.0238 | 0.38 × 105 |
| 11A | 6 | 2 | 95 × 105 | 99 | 0.0153 | 0.35 × 105 |
| TC100 + 10%FBS | n.a. | 10 | 35 × 105 | 99 | 0.0134 | 0.18 × 105 |
| Sf900 II | n.a. | n.a. | 91 × 105 | 99 | 0.0201 | 0.58 × 105 |
Formulations 5A–8A correspond to the star points, and 9A to 11A, to the central points; n.a.: Not added; n.d.: Not determined
In the second step of the study, the effects of fructose (1–3 g L−1), lactose (1–3 g L−1), methionine (2.6–2.9 g L−1), tyrosine (0.46–0.76 g L−1) and lipid emulsion (1–3%) were analyzed employing a 25−2 fractional factorial design, in a total of 13 experiments detailed in Table 2. In this set of tests, FBS was eliminated and all media formulations were also supplemented with glucose, glutamine and yeastolate to give final concentrations equal to 10, 3.5 and 6 g L−1, respectively. Following, the concentration of lipid emulsion was fixed at 1% and further studies were performed employing a 24 factorial design, depicted in Table 3, reducing fructose and methionine concentration ranges to 0.5–1 and 1.48–1.88 g L−1, respectively. Simultaneously, lactose and tyrosine concentration ranges were increased to 2–4 and 0.6–1.1 g L−1, respectively.
Table 2.
25−2 Factorial experimental design for supplement screening with three replicates at the central point
| Medium formulation | Independent variables | Dependent variables | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Fructose (g L−1) | Lactose (g L−1) | MET (g L−1) | TYR (g L−1) | Lipids (%) | Max. cell concentration (cells mL−1) | Viability (%) | μmax (h−1) | Max. cell Productivity (cells mL−1 h−1) | |
| 1B | 1 | 1 | 2.6 | 0.46 | 3 | 0 | 0 | n.d. | n.d. |
| 2B | 3 | 1 | 2.6 | 0.76 | 1 | 97 × 105 | 97 | 0.029 | 0.57 × 105 |
| 3B | 1 | 3 | 2.6 | 0.76 | 1 | 88 × 105 | 99 | 0.017 | 0.30 × 105 |
| 4B | 3 | 3 | 2.6 | 0.46 | 3 | 0 | 0 | n.d. | n.d. |
| 5B | 1 | 1 | 2.9 | 0.76 | 3 | 0 | 0 | n.d. | n.d. |
| 6B | 3 | 1 | 2.9 | 0.46 | 1 | 60 × 105 | 97 | 0.015 | 0.22 × 105 |
| 7B | 1 | 3 | 2.9 | 0.46 | 1 | 120 × 105 | 97 | 0.018 | 0.40 × 105 |
| 8B | 3 | 3 | 2.9 | 0.76 | 3 | 0 | n.d. | 0 | n.d. |
| 9B | 2 | 2 | 2.75 | 0.61 | 2 | 80 × 105 | 91 | 0.015 | 0.19 × 105 |
| 10B | 2 | 2 | 2.75 | 0.61 | 2 | 78 × 105 | 93 | 0.021 | 0.15 × 105 |
| 11B | 2 | 2 | 2.75 | 0.61 | 2 | 79 × 105 | 77 | 0.014 | 0.16 × 105 |
| TC100 + 10% FBS | n.a. | n.a. | n.a. | n.a. | n.a. | 55 × 105 | 99 | 0.015 | 0.40 × 105 |
| Sf900 II | n.a. | n.a. | n.a. | n.a. | n.a. | 91 × 105 | 99 | 0.020 | 0.58 × 105 |
Formulations 9B to 11B correspond to the central points; MET: Methionine; TYR: Tyrosine; n.a.: Not added; n.d.: Not determined
Table 3.
24 Factorial experimental design for fructose, lactose, tyrosine and methionine supplements with three replicates at the central point
| Medium | Independent variables | Dependent variables | ||||||
|---|---|---|---|---|---|---|---|---|
| Fructose (g L−1) | Lactose (g L−1) | TYR (g L−1) | MET (g L−1) | Max. cell concentration (cells mL−1) | Viability (%) | μmax (h−1) | Max. cell Productivity (cells mL−1 h−1) | |
| 1C | 0.50 | 2 | 0.60 | 1.48 | 310 × 105 | 99 | 0.009 | 0.41 × 105 |
| 2C | 1.00 | 2 | 0.60 | 1.48 | 420 × 105 | 99 | 0.018 | 0.83 × 105 |
| 3C | 0.50 | 4 | 0.60 | 1.48 | 370 × 105 | 99 | 0.019 | 1.11 × 105 |
| 4C | 1.00 | 4 | 0.60 | 1.48 | 240 × 105 | 99 | 0.020 | 0.55 × 105 |
| 5C | 0.50 | 2 | 1.10 | 1.48 | 270 × 105 | 99 | 0.011 | 0.40 × 105 |
| 6C | 1.00 | 2 | 1.10 | 1.48 | 330 × 105 | 99 | 0.012 | 0.50 × 105 |
| 7C | 0.50 | 4 | 1.10 | 1.48 | 270 × 105 | 98 | 0.020 | 0.71 × 105 |
| 8C | 1.00 | 4 | 1.10 | 1.48 | 260 × 105 | 99 | 0.029 | 1.19 × 105 |
| 9C | 0.50 | 2 | 0.60 | 1.88 | 350 × 105 | 98 | 0.015 | 0.44 × 105 |
| 10C | 1.00 | 2 | 0.60 | 1.88 | 290 × 105 | 99 | 0.036 | 0.76 × 105 |
| 11C | 0.50 | 4 | 0.60 | 1.88 | 260 × 105 | 99 | 0.016 | 0.44 × 105 |
| 12C | 1.00 | 4 | 0.60 | 1.88 | 250 × 105 | 99 | 0.031 | 0.75 × 105 |
| 13C | 0.50 | 2 | 1.10 | 1.88 | 280 × 105 | 99 | 0.036 | 0.96 × 105 |
| 14C | 1.00 | 2 | 1.10 | 1.88 | 300 × 105 | 99 | 0.040 | 0.79 × 105 |
| 15C | 0.50 | 4 | 1.10 | 1.88 | 240 × 105 | 99 | 0.024 | 1.04 × 105 |
| 16C | 1.00 | 4 | 1.10 | 1.88 | 290 × 105 | 99 | 0.039 | 1.14 × 105 |
| 17C | 0.75 | 3 | 0.86 | 1.68 | 300 × 105 | 99 | 0.034 | 0.93 × 105 |
| 18C | 0.75 | 3 | 0.86 | 1.68 | 310 × 105 | 98 | 0.020 | 0.72 × 105 |
| 19C | 0.75 | 3 | 0.86 | 1.68 | 290 × 105 | 99 | 0.026 | 0.78 × 105 |
| Sf900 II | n.a. | n.a. | n.a. | n.a. | 160 × 105 | 98 | 0.024 | 0.76 × 105 |
Formulations 17C to 19C correspond to the central points; MET: Methionine; TYR: Tyrosine; n.a.: Not added
The responses analyzed in all experiments were cell viability, maximum viable cell concentration, maximum specific growth rate (μmax) and cell productivity. When required, the statistical effects of the independent variables on the response variables were calculated using the software Statistica.
The maximum specific growth rate was calculated through the following equation:
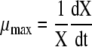 |
where X is the viable cell concentration and t is the time.
The maximum cell productivity was determined during the exponential growth phase using the following equation (Wang et al. 1979):
 |
where Xf and Xo refer to final and initial viable cell concentration, respectively, μmax is the maximum specific growth rate, and tlag is the length of the lag phase.
The length of the lag phase was estimated as the period beginning at the inoculation time and ending when exponential growth was initially observed.
Cell culture in spinners
In the last step of the study, cell behavior in the selected optimum medium (IPL-41 supplemented with 0.5 g L−1 fructose, 10 g L−1 glucose, 2 g L−1 lactose, 1.48 g L−1 methionine, 0.6 g L−1 tyrosine, 3.5 g L−1 glutamine, 1% lipids and 6 g L−1 yeastolate) and in Sf900II medium was compared, employing 100 mL spinner flasks (with working volumes of 60 mL) at 28 °C and 100 rpm inoculated with 7.5 × 105 viable cells mL−1. In these experiments, cell viability, maximum viable cell concentration, maximum specific growth rate and cell productivity, as well as media osmolality and the concentrations of carbohydrates, amino acids, lactate, ammonium and GPV were monitored.
Analytical methods
Cell concentration and viability were determined by optical microscopy (Olympus, model CK2) with trypan blue. Glucose, fructose and lactose concentrations were measured by HPLC using a Bio-Rad HPX-87H organic acid column. Amino acid concentrations in the culture supernatants were analyzed by the Pico-tag system, using a reverse-phase HPLC column (Waters). Lactate concentration was determined by using the 2700 YSI Biochemical Analyser (Yellow Spring Instruments). Ammonium content was determined through a 95-12 Orion probe analyzer coupled to a SA720 Procyon potenciometer. Media osmolalities were analyzed employing an Osmette A Precision System Inc osmometer. The G glycoprotein was measured using the rabies glycoprotein enzyme immunoassay from the Pasteur Institute (France), essentially as indicated by the manufacturer after cell recovery by centrifugation at 3,000 rpm for 5 min, ressuspension in phosphate-saline buffer and lysis by sonication (Vibra-Cell (Sonics and Materials).
Results and discussion
Effects of yeastolate on cell viability, growth and productivity in low serum medium
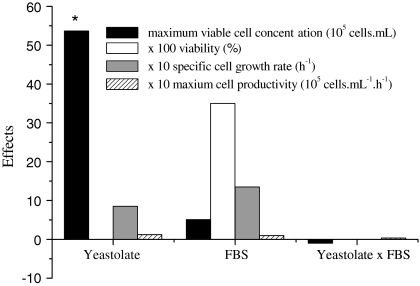
Most of the available literature on culture of Drosophila S2 cells mention the use of either expensive serum-free media or formulations containing around 10% of FBS. Aiming to initially reduce FBS requirements from these cells by substituting this component by yeastolate, a 22 experimental design was performed. The influence of yeastolate ultrafiltrate concentration (4–8 g L−1) and FBS percentage (1–3% v/v) on maximum viable cell concentration, viability, maximum specific growth rate and cell productivity was evaluated. The results achieved are indicated in Table 1, while the calculated statistical effects are shown in Fig. 1.
Fig. 1.
Statistical effects obtained for the 22 factorial experimental design on maximum viable cell concentration (at the stationary phase), viability, maximum specific cell growth rate and cell productivity at the exponential phase. * Indicates that the variable is statistically significant at 95% confidence
Maximum viable cell concentration in the stationary phase varied from 1.8 to 11 × 106 cells mL−1. Several of the proposed medium formulations resulted in higher maximum viable cell densities when compared to Sf900 II and, mostly, to TC100 supplemented with serum. Except for cells cultured in formulation 1A, cell viability was equal or superior to 97% in all formulations. Statistically significant effects were observed only for maximum viable cell concentration (according to non linear multiple regression and analysis of variance, the calculated F value was 32.5, being, therefore, more than 7 times higher than the listed value, equal to 4.46). As shown in Fig. 1, increases on yeastolate concentration augment viable cell concentration more than the level verified for serum addition. On the other hand, since FBS contains a large number of different growth-promoting activities in a physiologically balanced blend, supplementation with this component increased cell viability, however, at a non statistically significant level.
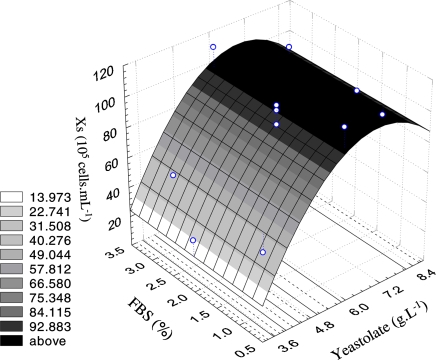
Since only yeastolate presented a statistically significant effect on maximum cell concentration, a mathematical model was generated to describe this response variable behavior as a function of yeastolate concentration. The coded model was used to generate the correspondent response surface, as shown in Fig. 2.
Fig. 2.
Response surface of the maximum viable cell concentration at the stationary phase as a function of yeastolate ultrafiltrate concentration and FBS percentage
The response surface indicated that a yeastolate ultrafiltrate concentration of 6 g L−1 is the optimal condition to achieve maximum viable cell concentration. This result can be compared to that obtained for Sf9 cells in serum supplemented IPL-41 medium. When yeastolate ultrafiltrate was used at a range from 4 to 8 g L−1, high cell density was achieved (Drews et al. 1995). According to Fig. 2, FBS percentage in the range from 1% to 3% practically did not affect cell growth. Therefore, this complex supplement was not employed in the next performed experiments.
Table 1 shows also the maximum specific growth rate (μmax) and cell productivity for the 22 factorial design. It is observed that μmax varied from 0.0062 h−1 to 0.0244 h−1, while cell productivity ranged from 0.13 × 105 to 0.42 × 105 cells mL−1 h−1 in all IPL-41 based formulations. These results clearly show that high specific growth rates can be obtained when employing yeastolate ultrafiltrate at 6 g L−1. Interestingly, on the medium formulation 6A, the cells did not present the classic growth behavior; cell concentration oscillated in this particular experiment, therefore not allowing the calculation of the kinetic parameters.
Effects of fructose, lactose, methionine, tyrosine and lipids on cell viability, growth and productivity in serum-free medium formulations
Aiming to further increase maximum specific cell growth rate, concentration and productivity, a 25−2 fractional design was performed totally eliminating FBS and varying the concentrations of fructose (1–3 g L−1), lactose (1–3 g L−1), methionine (2.6–2.9 g L−1), tyrosine (0.46–0.76 g L−1) and lipid emulsion (1–3%), while maintaining constant the initial amounts of yeastolate (6 g L−1), glucose (10 g L−1) and glutamine (3.5 g L−1). The results are presented in Table 2.
Interestingly, when the highest concentration of lipid emulsion (3%) was employed, in almost all situations the cells did not survive, despite the fact that in previous experiments this concentration was employed. Possibly, at this time cells were not effectively protected from eventual deleterious effects caused by the lipid emulsion because of total FBS absence in this set of experiments. Hydrophobic components from the lipid emulsion could have formerly associated to FBS components, not being therefore directly exposed to the cells, which would be hence protected from them. For this reason, high concentration of lipid emulsion in serum-free IPL-41 medium might reduce cell growth. In the remaining experiments, cell concentration varied from 6 × 106 to 12 × 106 viable cells mL−1, being always larger than the value achieved in the TC100 medium supplemented with FBS control.
In the experiments in which cell growth was indeed observed, cell viability reached high levels, varying from 77% to 99%, demonstrating clearly the importance of adequate nutrient supply. The medium formulation 2B presented the highest maximum specific growth rate, 0.029 h−1, and cell productivity varied from 0.15 to 0.57 × 105 cells mL−1 h−1. In the control cultures, performed in serum-supplemented TC100 and in Sf900 II media, μmax values were, respectively, 0.015 h−1 and 0.020 h−1, while cell productivities were 0.40 × 105 cells mL−1 h−1 and 0.58 × 105 cells mL−1 h−1 in TC100 supplemented with FBS and Sf900 II, respectively.
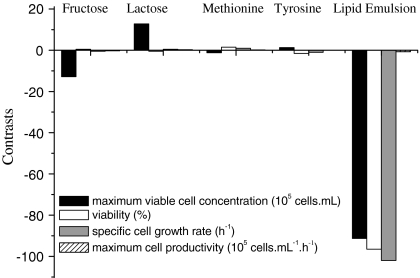
Figure 3 shows the statistical contrasts of the supplements employed in the fractional design on cell behavior. Increments in fructose concentration and lipid emulsion percentage decreased maximum cell concentration. On the other hand, increments on lactose concentration provided stimulatory effects on maximum viable cell concentration. Viability was strongly affected only by the lipid emulsion percentage. In addition, high lipid emulsion percentage (above 2%) did not increase cell growth.
Fig. 3.
Statistical contrasts obtained for the 25−2 fractional experimental design on maximum cell concentration, viability, maximum cell specific growth rate and cell productivity
Since the previous experiments indicated that high lipid concentration affected all the dependent variables, in the next set of experiments lipid concentration was fixed at 1% v/v. The statistical effects of the tested carbohydrates and amino acids was then further evaluated employing a 24 factorial design, in which IPL-41 medium was also supplemented with yeastolate (6 g L−1), glucose (10 g L−1), glutamine (3.5 g L−1) and lipid emulsion (1% v/v). Table 3 presents the achieved results.
In the IPL-41 based formulations, maximum viable cell concentration varied from 24 × 106 to 42 × 106cells mL−1, while cell viability varied from 98 to 99%. The maximum specific specific growth rates varied from 0.009 to 0.040 h−1 and cell productivity varied from 0.4 × 105 to 1.19 × 105 cells mL−1 h−1. In the control assay performed in Sf900 II medium, μmax was 0.024 h−1 and cell productivity was 0.76 × 105 cells mL−1 h−1.
In fact, in this experiment, all formulations resulted in the increase of cell concentration, when compared to Sf900 II, according to the Table 3. The Tukey test was performed for the formulations containing the lowest and highest supplement concentration levels of the 24 factorial design (formulations 1C and 16C, respectively), showing that no significant differences could be observed on maximum viable cell concentration in both media. Therefore, in the next set of experiments, the medium formulation corresponding to the lower level was employed, consisting of IPL-41 supplemented with 10 g L−1 glucose, 3.5 g L−1 glutamine, 6 g L−1 yeastolate, 0.5 g L−1 fructose, 2 g L−1 lactose, 0.6 g L−1 tyrosine, 1.48 g L−1 methionine and 1% lipid emulsion. Since this formulation provided high cell growth, satisfactory potential for recombinant protein production was expected.
Behavior of S2AcGPV2 cells cultured in the selected medium formulation in spinner flasks
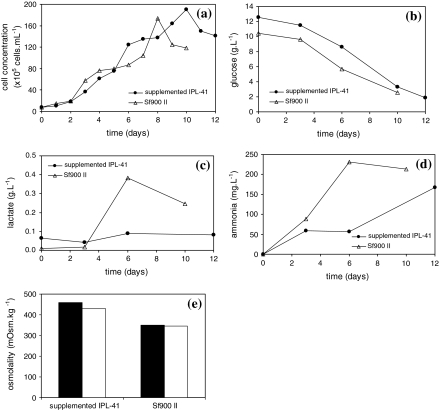
After twelve subculture steps both in SF900 II and in supplemented IPL-41 containing 10 g L−1glucose, 3.5 g L−1glutamine, 6 g L−1 yeastolate, 0.5 g L−1 fructose, 2 g L−1 lactose, 0.6 g L−1 tyrosine, 1.48 g L−1 methionine and 1% lipid emulsion, to ensure cell adaptation mostly to the formulated medium, the maximum viable cell concentration on spinner flasks was lower than that achieved with the same medium prepared according to the 24 factorial design studies on schott flasks. Figure 4a shows that viable cell concentration in supplemented IPL-41 medium reached 19 × 106 cells mL−1, while in Sf900 II medium cell concentration reached 17.4 × 106 cells mL−1. Glucose was continuously depleted in IPL-41 supplemented and Sf900 II media during cell culture, as shown in Fig. 4b. Fructose was not consumed during cell culture in supplemented IPL-41 medium, while lactose was moderately consumed (data not shown). The presence of fructose or lactose in Sf900 II medium was not detected.
Fig. 4.
Kinetic behavior of S2AcGPV2 cells in the selected medium formulation, IPL-41 supplemented with 10 g L−1 glucose, 3.5 g L−1 glutamine, 6 g L−1 yeastolate, 0.5 g L−1 fructose, 2 g L−1 lactose, 0.6 g L−1 tyrosine, 1.48 g L−1 methionine, 1% lipid emulsion, and SF900 II. Solid symbols refer to supplemented IPL-41 and open symbols, to Sf900 II medium. (a) Viable cell concentration; (b) glucose concentration; (c) lactate concentration; (d) ammonia concentration and (e) medium (■) initial osmolatity and (□) final osmolality. Cells were inoculated at the final concentration of 7.5 × 105 cells mL−1 and cultivated at 28 °C
The concentration of the main product from glucose metabolism, lactic acid, practically did not vary in the supplemented IPL-41 formulation, but in Sf900 II medium, lactate accumulated during the whole culture period, as shown in Fig. 4c, similarly as previously reported by other groups for Sf9 cells (Mendonça et al. 1999; Palomares et al. 2004). Probably, the increase in lactate concentration, while glucose was still present, was associated to oxygen limitation. Sf-9 cells, for instance, do not produce lactate under non-limiting oxygen conditions even in media with high initial glucose content (Ikonomou et al. 2003). Ammonia concentration reached values around 167mg L−1 in IPL-41 supplemented medium and 230m g L−1 in Sf900 II medium (Fig. 4d), suggesting that inhibitory levels were not reached. Insect cells are not as sensitive to the presence of ammonia as mammalian cells (Ikonomou et al. 2003).
The maximum specific growth rate reached in supplemented IPL-41 was 0.025 h−1 and cell productivity was 0.57 × 105 cells mL−1 h−1. The maximum specific growth rate in Sf900II medium reached 0.012 h−1, while cell productivity was 0.32 × 105 cells mL−1 h−1.
Concerning amino acids analysis, methionine and tyrosine were not consumed (data not shown). Also, glutamine limitation was not apparently observed (data not shown). Differences can be observed in amino acid metabolism of dipterous cells (such as transfected S2 cells) and lepdopiterous cells (e.g. Sf9 cells). Sf9 cells in TNM-FH medium intensively consume methionine and tyrosine (Mendonça et al. 1999). However, Galesi et al. (2007) verified that glutamine was rapidly consumed during exponential phase by S2 cells maintained in TC100 medium supplemented with 10 g L−1 glucose, 3.5 g L−1 glutamine, from 1 to 3 g L−1 yeastolate, from 1 to 3% FBS and 0.1% PF-68.
The osmolalities did not vary during cell culture, being although higher for the proposed formulation, around 460mOsm. kg−1 and 346mOsm.kg−1 for supplemented IPL-41 and Sf900 II media, respectively (Fig. 4e). As discussed by Olejnik et al. (2003), the release of additional energy and precursors as a result of increased metabolism by osmotically stressed cultures can affect recombinant protein synthesis. Oh et al. (1995) reported that hybridomas subjected to high osmotic pressure increased the production level of total RNA, of which 80% was ribosomal RNA. Higher ribosomal RNA content can result in the increase of protein translation rates, reflecting in higher total protein concentration.
The highest GPV concentrations in these media were 1.19 and 3.42 ng mL−1 for the proposed formulation and Sf900II, respectively. These values are lower than those reported by Yokomizo et al. (2007). Different factors may have contributed to the low protein production observed, such as the fact that only about 30% of the cells in the total population employed in that work were actively producing the target protein, with a heterogeneous distribution of the recombinant protein, as verified by immunofluorescence (Yokomizo et al. 2007). The same cell population was employed in the present work and selection of less productive subpopulations can have occurred during the adaptation process, which involved 12 subculture steps.
Also, the G glycoprotein from rabies virus is a relatively complex trimeric, membrane-anchored protein (Sissoëff et al. 2005), and the expression of membrane proteins is not a simple process (Grisshammer 2006). In the case of transmembrane proteins, folding occurs in three different environments: the endoplasmic reticulum (ER) lumen, the ER membrane and the cytosol. For glycoproteins such as the rabies virus one, it seems that the ectodomain, the transmembrane domain and the cytoplasmic domain constitute independent folding domains, and membrane anchor type on protein ectodomain, as well as other different factors, such as pH (Gaudin et al. 1993), can affect protein trimerization and folding, inducing conformational changes. These alterations could affect the biological activity of this protein, and its capacity to interact with the ELISA kit antibody. Besides, interference in protein detection associated with protein recovery from the cells may also have occurred, since no protease inhibitors were added to the extraction medium or even to the culture media itself.
However, since kinetic studies performed by Yokomizo et al. (2007) showed that recombinant S2 cells can produce up to 300 ng mL-1 of GPV, other bioprocess variables should be investigated to improve the target protein production.
The performance of wild and recombinant S2 cells as well as of Sf9 cells was preliminarily evaluated in the formulated medium already including slight modifications based on the achieved results for the recombinant S2 cells. IPL-41 medium was supplemented with 6 g L−1 yeastolate ultrafiltrate, 10 g L−1 glucose, 5 g L−1 glutamine, 2 g L−1 lactose, 1% lipid emulsion and 0.05% PF-68, but not with fructose, tyrosine and methionine, since the recombinant S2 cells did not effectively consumed them. Glutamine concentration, on the other hand, was increased to 5 g L−1, since it showed to be a limiting substrate to Sf9 cells. The cells were fully capable of growing in the modified IPL-41 medium, reaching 3.3 × 106 cells mL−1 (Sf9 cells), 26 × 106cells mL−1 (wild S2 cells) and 19 × 106 cells mL−1 (recombinant S2 cells), being the maximum recombinant S2 cell concentration identical to the value achieved in the previous formulation.
In conclusion, this work provided a formulation for an animal protein-free medium effective for culturing S2AcGPV2 cells. IPL-41 basal medium supplying salts and trace elements, as well as vitamins and others amino acids, and supplemented with yeastolate ultrafiltrate (final concentration 6 g L−1), glucose (10 g L−1) and glutamine (3.5 g L−1) is recommended for culturing Drosophila melanogaster S2 cells. Furthermore, the serum-free medium required other carbohydrates (fructose, 0.5 g L−1and lactose, 2 g L−1), amino acids (methionine 1.48 g L−1 and tyrosine, 0.6 g L−1) and lipids (1% v/v), providing high cell densities (19 × 106 viable cells mL−1), and adequate specific growth rate (0.025 h−1) and cell productivity (0.57 × 105 cells mL−1 h−1). The supplemented IPL-41 medium can support higher cell growth than that observed for the commercial serum-free Sf900 II medium (17.4 × 106 cells mL−1), however, protein production in the tested expression system can be further improved.
Acknowledgments
The authors acknowledge Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brasília, Brazil), Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP grant no. 02/09482-3, São Paulo, Brazil) and Instituto Butantan (São Paulo, Brazil) for the financial support. The authors wish to acknowledge Prof Dr. Cláudio Alberto Torres Suazo (Federal University of São Carlos, Brazil) and team for amino acids analysis. We are also grateful to Prof Dr. Andreas Karoly Gombert and Saul Nitsche Rocha from University of São Paulo for sugars measurements.
Footnotes
Â. M. Moraes and C. A. Pereira are recipient of CNPq fellowships.
References
- Bachmann AS, Corpuz G, Hareld WP et al (2004) A simple method for the rapid purification of copia virus-like particles from Drosophila Schneider 2 cells. J Virol Methods 115:159–165 [DOI] [PubMed]
- Batista FRX, Pereira CA, Mendonça RZ et al (2005) Enhancement of Sf9 cells and baculovirus production employing Graces’s medium supplemented with milk whey ultrafiltrate. Cytotechnology 49:1–9 [DOI] [PMC free article] [PubMed]
- Chu L, Robinson DK (2001) Industrial choices for protein production by large-scale cell culture. Curr Opin Biotechnol 12:180–187 [DOI] [PubMed]
- Donaldson MS, Shuler ML (1998) Low-cost serum free medium for the BTI-Tn5B1-4 insect cell line. Biotechnol Prog 14:573–579 [DOI] [PubMed]
- Drews M, Paalme T, Vilu R (1995) The growth and nutrient utilisation of insect cell line Spodoptera frugiperda Sf9 in batch and continuous culture. J Biotechnol 40:187–198 [DOI]
- Echalier G (1997) Composition of the body fluid of Drosophila and the design of culture media for Drosophila cells. In: Drosophila cells in culture. Academic Press, San Diego, pp 1–67
- Galesi ALL, Pereira CA, Moraes AM (2007) Culture of transgenic Drosophila melanogaster Schneider 2 cells in serum-free media based on TC100 basal medium. Biotechnol J (in press). doi:10.1002/biot.200700048 [DOI] [PubMed]
- Gaudin Y, Ruigrok RWH, Knossow M et al (1993). Low-pH Conformational changes of rabies virus glycoprotein and their role in membrane fusion. J Virol 67:1365–1372 [DOI] [PMC free article] [PubMed]
- Grisshammer R (2006) Understanding recombinant expression of membrane proteins. Curr Opin Biotechnol 17:337–340 [DOI] [PubMed]
- Hewlett G (1991) Strategies for optimising serum-free media. Cytotechnology 5:3–14 [DOI] [PubMed]
- Ikonomou L, Bastin G, Schneider YJ et al (2001) Design of an efficient medium for insect cell growth and recombinant protein production. In vitro Cell Dev Biol Anim 37:549–559 [DOI] [PubMed]
- Ikonomou L, Schneider YJ, Agathos SN (2003) Insect cell culture for industrial production of recombinant proteins. Appl Microbiol Biot 62:1–20 [DOI] [PubMed]
- Kioukia N, Nienow AW, Emery AN et al (1995) Physiological and environmental factors affecting the growth of insect cells and infection with baculovirus. J Biotecnol 38:243–251 [DOI] [PubMed]
- Maiorella B, Inlow D, Shauger A et al (1988) Large-scale insect cell culture for recombinant protein production. Bio/Technol 6:1406–1410 [DOI]
- Marteijn RL, Jurrius O, Dhont J et al (2003) Optimisation of a feed medium for fed-batch culture of insect cells using a genetic algorithm. Biotechnol Bioeng 81:269–278 [DOI] [PubMed]
- Mendonça RZ, Palomares LA, Ramírez OT (1999) An insight into insect cell metabolism through selective nutrient manipulation. JBiotech 72:61–75 [DOI]
- Mendonça RZ, Oliveira EC, Pereira CA, Lebrum I (2007) Effect of bioactive peptides isolated from yeastolate, lactalbumin and NZ case in the insect cell growth. Bioprocess Biosyst Eng 30:157–164 [DOI] [PubMed]
- Mitsuhashi J (1989) In: Mitsuhashi J (ed) Invertebrate cell system applications. CRC Press, pp 3–20
- Mosher JT, Crews ST (1999) Effecten reagent yields high trasnfection efficiencies with Drosophila melanogaster S2 cells. Qiagen News 4:7–9
- Nybakken K, Vokes SA, Lin TY et al (2005) Genome-wide RNA interference screen in Drosophila melanogaster cells for new components of the Hh signaling pathway. Nat Genet 37:1323–1332 [DOI] [PMC free article] [PubMed]
- Oh SKW, Cha FKF et al (1995) Intracellular responses of productivity hybridomas subjected to high osmotic pressure. Biotechnol Bioeng 46:525–535 [DOI] [PubMed]
- Palomares LA, López S, Ramírez OT (2004) Utilization of oxygen uptake rate to assess the role of glucose and glutamine in the metabolism of insect cell cultures. Biochem Eng J 19:87–93 [DOI]
- Pérez O, Paolazzi CC (1997) Production methods for rabies vaccine. J Ind Microbiol Biot 18:340–347 [DOI] [PubMed]
- Rupprecht CE, Smith JS, Fekadu M et al (1995) The ascension of wildlife rabies: a cause for public health concern or intervention? Emerg Infec Dis 1:101–114 [DOI] [PMC free article] [PubMed]
- Sissoëff L, Mousli M, England P et al (2005) Stable trimerization of recombinant rabies virus glycoprotein ectodomain is required for interaction with the p75NTR receptor. J Gen Virol 86:2543–2552 [DOI] [PubMed]
- Southon A, Burke R, Norgate M et al (2004) Copper homoeostasis in Drosophila melonogaster S2 cells. Biochem J 383:303–309 [DOI] [PMC free article] [PubMed]
- Wang DI, Cooney CL, Demain AL et al (1979) Fermentation and enzyme technology. Wiley, New York
- Yokomizo AY, Jorge SAC, Astray RM et al (2007) Rabies virus glycoprotein expression in Drosophila S2 cells. I. Functional recombinant protein in stable co-transfected cell line. Biotechnol J 2:102–109 [DOI] [PubMed]
- Zimmerman AM, Vierck JL, O’ Really BA et al (2000) Formulation of defined medium to maintain cell health and viability in vitro. Methods Cell Sci 22:43–49 [DOI] [PubMed]