Abstract
Drosophila research has been and continues to be an essential tool for many aspects of biological scientific research and has provided insight into numerous genetic, biochemical, and behavioral processes. As well, due to the remarkable conservation of gene function between Drosophila and humans, and the easy ability to manipulate these genes in a whole organism, Drosophila research has proven critical for studying human disease and the physiological response to chemical reagents. Methotrexate, a widely prescribed pharmaceutical which inhibits dihydrofolate reductase and therefore folate metabolism, is known to cause teratogenic effects in human fetuses. Recently, there has been resurgence in the use of methotrexate for inflammatory diseases and ectopic or unwanted pregnancies thus, increasing the need to fully understand the cytotoxicity of this pharmaceutical. Concerns have been raised over the ethics of studying teratogenic drugs like methotrexate in mammalian systems and thus, we have proposed a Drosophila model. We have shown that exposure of female Drosophila to methotrexate results in progeny with developmental abnormalities. We have also shown that methotrexate exposure changes the abundance of many fundamental cellular transcripts. Expression of a dihydrofolate reductase with a reduced affinity for methotrexate can not only prevent much of the abnormal transcript profile but the teratogenesis seen after drug treatment. In the future, such studies may generate useful tools for mammalian antifolate “rescue” therapies.
Keywords: Dihydrofolate reductase, Drosophila, homolog, Methotrexate, Resistance, Teratogenesis, Transgenics
Introduction
In a few short years we will celebrate the 100th anniversary of T.H. Morgan’s discovery of the role of chromosomes in heredity and the coveted “white-eyed” fly. Since these initial discoveries, continuing research using Drosophila has uncovered many guiding genetic principles such as: chromosomes almost invariably contain genes, genes are mostly arranged linearly, chromosomes are often the basis of sex determination, and genetic linkage. As well, Drosophila research has revealed the harmful genetic consequence of ionizing radiation, pioneered DNA cloning and library screening, chromosome walking techniques, and genome-wide mutational screens. Such discoveries using Drosophila have been, and continue to be, critical for every aspect of biological scientific research.
Perhaps one other attribute that makes Drosophila such an enticing research model is the remarkable conservation of gene function shared with mammalian homologs. Examples of conserved gene function are genes involved in the Notch signaling pathway, homeobox genes for developmental pattern formation, cyclins for control of cell cycle progression, and genes involved in folate metabolism including the dihydrofolate reducase gene (Dhfr) which is homologous to human DHFR. In addition, there are a vast number of scientific tools available to Drosophila researchers that cannot be easily applied to a mammalian system such as the ethical manipulation of these genes to understand developmental expression pattern, loss-of-function phenotypes, and over expression phenotypes. This, in turn, has lead to the discovery and experimentation of Drosophila homologs to many human diseases including alpha-synuclein involved in Parkinson’s disease (Feany and Bender 2000), the tumor suppressor p53 (Jin et al. 2000), and an insulin receptor homolog (Fernandez et al. 1995). It is not always feasible to investigate these genes and the proteins they encode in humans, however; they can be easily manipulated in a Drosophila system and applied to our understanding of human disease.
Conservation of gene function in Drosophila and mammals not only begins at the gene and protein level but extends to downstream and divergent processes comprising developmental regulation, circadian rhythm, neurodegeneration, and the physiological response to stress, including chemical reagents. Antifolates, such as methotrexate (MTX), inhibit the activity of DHFR in the folate pathway, and are examples of therapeutic pharmaceuticals that can produce, as an unfortunate side effect, the same toxic and teratogenic effects in Drosophila as they do in humans (Adam et al. 2003; Affleck et al. 2006b). Due to the continued and even increased prescription of MTX for human disease, it is essential that the pathway of events following this treatment be fully understood. This is crucial in the case of teratogenicity. Thus, the focus of this review is the utility of a Drosophila model for the study of toxicity and teratogenicity following antifolate administration.
Dihydrofolate reductase from Drosophila and mammals
Folate, an important B vitamin, can be synthesized by plants and bacteria, conversely, mammals and insects must acquire folate through their diet (Dadd 1973). Insufficient folate in the diet of human adults can lead to anemia and accumulation of homocysteine, which has been linked to heart disease (Weikert et al. 2005) and cancer (Fairfield and Fletcher 2002). In pregnant mothers, deficiencies in folic acid are a well known cause of neural tube defects and anencephaly (Oakley et al. 2004). Thus, it is not surprising folic acid is supplemented in certain grains (Malinow et al. 1998) and additional folic acid is prescribed during and post pregnancy (Fernandez-Ballart and Murphy 2001).
DHFR is an essential housekeeping enzyme involved in the conversion of folate to its active form. In most organisms, DHFR catalyzes the reduction of folate to dihydrofolate (DHF) followed by a second reduction of DHF to tetrahydrofolate (THF). THF, the key product of DHFR activity, is used as a cofactor for the transfer of one-carbon units and THF-dependant reactions are essential for the biosynthesis of thymidylate, purines, and homeostatic levels of glycine, serine, and homocysteine (Kompis et al. 2005). THF is also used by methionine synthase to produce methionine from homocysteine, which in turn, is modified by methionine adenosyltransferase to produce S-adenosylmethionine (SAM) (McKay et al. 2004). SAM is the substrate used for methylation reactions catalyzed by DNA methyltransferases and plays a role in essential epigenetic mechanisms involved in correct genomic expression during embryogenesis. Given the crucial role of DHFR and folate in many biological processes and development, it is apparent why the inhibition of DHFR activity or a reduction in folate levels can produce teratogenic effects.
The physical properties of Drosophila DHFR are similar to that of mammalian DHFRs, with approximately the same molecular weights, two optimal pH values, and similar kinetic values for cofactors and substrates (Table 1). DHFRs have been purified from a variety of organisms and models based on crystal coordinates have been used to determine the molecular mechanisms involved in DHFR catalysis (Hammes 2002). Although Drosophila DHFR has not been crystallized, the insect and human enzyme share 17/24 of the residues involved in cofactor and substrate binding, allowing the judicious use of the mammalian coordinates for structural studies. Overall Drosophila DHFR shares a 49% identity to mammalian DHFRs (Hao et al. 1994) and like the mammalian enzyme, is inhibited by aminopterin and MTX but uninhibited by the bacterial DHFR inhibitor, trimethoprim and the plasmodium DHFR inhibitor, pyrimethamine (Affleck et al. 2006a). Although MTX is a tight-binding competitive inhibitor of both Drosophila and mammalian DHFRs, the Kd for the Drosophila DHFR is 860 pM, a value 10 to 1000 times higher than mammalian DHFRs (Rancourt and Walker 1990). It is presumed that the few non-conserved active site residues may contribute to this higher Kd.
Table 1.
Comparison of human and Drosophila DHFRs
| Molecular weight (kD) | Optimum pH | Km NADPH (μM) | Km DHF (μM) | |
|---|---|---|---|---|
| D. melanogaster | 22a | 4.7, 8.5a | 5.2a | 0.3a |
| H. sapiens | 20b | 4.5, 8.0c | 7.1c | 1.0c |
Despite the similarity in mammalian and Drosophila DHFRs, the genes that encode this enzyme and regulation of transcription are distinct. This is not surprising however, when considering the difference in genome organization of mammals and Drosophila (von Sternberg et al. 1992). The Drosophila genome is quite compact as exemplified by the 1 kb Dhfr gene with a lone 50 bp intron and a single TATA sequence for transcription initiation (Hao et al. 1994). In contrast, the mammalian genes are more complex. The human DHFR gene spans 30 kb with a total of 5 introns ranging from 362 to 12,000 bp and it is controlled by both a major bidirectional promoter with a Sp1 consensus sequence and a minor bidirectional promoter with several Sp1-binding sites (Chen et al. 1984). The higher complexity of the mammalian DHFR argues that a simpler insect model may be valuable in understanding the genomic responses to MTX.
Antifolate inhibition of dihydrofolate reductase
Any chemical that interferes with folic acid metabolism is termed an antifolate. Folate and MTX both have a pterin ring, aminobenzoic acid, and a minimum and maximum of 1 and 6 glutamates, respectively. The fundamental difference between folate and most antifolates designed for mammalian therapy is a substitution of the hydroxyl at the C4-position of the pterin ring for an amino group. MTX, in addition to the C4-amino group, has a methyl group at N10. These changes to the structure are sufficient to make MTX a potent inhibitor of DHFR, and thus a valuable pharmaceutical (McGuire 2003). Indeed, MTX is used for the treatment of a wide variety of cancers (Huennekens 1994) as well as for the treatment of ectopic pregnancy (Fernandez et al. 1998), inflammatory skin disease (Goujon et al. 2006), Crohn’s disease (Sun and Das 2005), rheumatoid arthritis (Nakazawa et al. 2001), and systemic lupus (Wise et al. 1996). However, MTX is a well known teratogen and therefore must be prescribed with caution to women of reproductive age (Lloyd et al. 1999; Lewden et al. 2004). Embryonic lethality has been observed in developing embryos of many mammalian systems, including rat (Vinson and Hales 2002), mouse (Darab et al. 1987), rabbit (DeSesso and Goeringer 1991) and cat (Khera 1976).
Inside the cell, MTX competes with folate and DHF for the active site of DHFR and is transported and modified by the same cellular factors. Reduced folate carrier (RFC), folate receptor (FR), and low pH transporters are used for cellular uptake of folate and MTX in mammals (Brzezinska et al. 2000). Once inside the cell both folate and MTX are polyglutamated by folylpolyglutamate synthase (FPGS) (Gorlick et al. 1999). Organic anion transporter (OAT), multidrug resistance protein (MRP) and bidirectional RFC are responsible for efflux. Although these proteins have not been formally characterized in Drosophila, in all cases, putative genes have been described in FlyBase (http://flybase.bio.indiana.edu/; Crosby et al. 2007) by sequence homology. Hence, it is assumed that transport and glutamate modification of folate and MTX in Drosophila are comparable processes to that of mammals.
Once in the cell, the affinity of DHFR for MTX is much higher than for either folic acid or DHF (Appleman et al. 1998) so that the enzyme-bound MTX leads to partial or complete depletion of reduced folate levels and in turn, the inhibition of processes involving folate derivatives. DNA synthesis is compromised when levels of 5,10-CH2-THF are reduced as the thymidylate synthase cycle requires this THF derivative to donate a methyl group to dUMP for synthesis of de novo dTMP. It is this DNA inhibition that is thought to cause most of MTX’s cytotoxicity (McGuire 2003). Purine synthesis by glycinamide ribonucleotide and 5-aminoimidazole-4-carboxamide ribonucleotide is also affected by a deficiency of reduced folates, further disrupting DNA synthesis as well as RNA synthesis. Homeostatic levels of certain amino acids are affected as well as S-adenosylmethionine, which is essential for correct gene expression (McKay et al. 2004).
MTX resistance and expression of Drosophila DHFR in mammalian cells
In humans one of the consequences of using MTX therapy for various diseases, including certain cancers, is that the treated somatic cells can mutate such that cell division is no longer inhibited at that concentration. The dosage can be increased, with the possible consequence that an increased level of resistance results. In human cells this resistance is due to decreasing drug influx, increasing drug efflux, decreased polyglutamation, amplification of DHFR, mutations producing an altered DHFR, or a combination of these mechanisms. The conservation of DHFR function and the catalytic amino acid residues in Drosophila and mammalian cells (Hao et al. 1994) is also reflected in mutations recovered after MTX selection in both systems. “Hot spot” residues in mammalian cells include mutations at L22 and L31 that alter DHFR structure to decrease the binding affinity of MTX within the active site (Dicker et al. 1989; Meisel et al. 2003; Cody et al. 2005). These residues correspond to L22 and L30 in Drosophila DHFR, and substitution of residues at these positions also provide MTX resistance to mammalian cells after transfection (Affleck et al. 2006a). Drosophila DHFR with a L22R substitution allowed mammalian cells to continue to divide in concentrations of MTX that were 200-fold higher than the levels conferred by transfection of wild-type Drosophila DHFR and 2-fold higher than L22R murine DHFR (Simonsen and Levinson 1983). Significantly, this is the highest level of MTX resistance observed by a single amino acid substitution in a mammalian cell line. Such experiments offer the promise that highly resistant DHFR mutations could confer myeloprotection during chemotherapy treatment. Drosophila DHFR with a L30Q mutation, originally obtained from MTX-selected Drosophila cell line, also provided protection from MTX cytotoxicity allowing cell division even at a concentration of 2 μM MTX (Affleck et al. 2006a). This demonstrates that Drosophila DHFR is not only fully functional in mammalian cells but that mutations analogous to mammalian “hot spot” residues in Drosophila DHFR can similarly provide MTX resistance.
It is curious that a common mechanism of acquisition of a resistant phenotype in mammalian cells is by gene amplification yet in Drosophila, resistance has only been observed to occur by mutation to Dhfr producing a MTX-resistant DHFR. It is unclear if differences in gene structure and/or genome organization between Drosophila and mammals may play a role in the observed difference in the preferred method of acquired drug resistance. To investigate if gene structure is involved, Drosophila Dhfr cDNA was transfected into Chinese hamster ovary (CHO) cells with no endogenous DHFR and together with wild-type CHO cells were selected for MTX resistance over 19 months. DrosophilaDhfr amplification appears to have been a mode of acquired resistance in at least some of the CHO cells transfected with Drosophila Dhfr (unpublished observations). This observation and the similar effect of MTX on mammalian and Drosophila cells in culture implies that the observed difference in acquired resistance between these two eukaryotic cells cannot be attributed to differences in cell physiology or in DHFR gene structure. Thus, it is likely the difference in overall genome organization explains the lack of Dhfr amplification in Drosophila cells.
MTX-induced developmental effects
Although targeting of DHFR by antifolates is undisputedly the major contributor of toxicity and teratogenicity, other potential targets, either directly or indirectly, may contribute to the observed harmful effects of these drugs, and these can be best examined using expression profiles in response to treatment. Control and MTX-exposed Drosophila Schneider’s (S3) cell lines were compared using microarrays. Remarkably, a large number of changes were observed; perturbed transcripts levels associated with cell cycle regulation, metabolism, signaling, transport, and defense response were apparent and subsequently confirmed using quantitative real-time RT-PCR (Table 2) (Affleck et al. 2006b). Similarly, Drosophila ovarian tissues showed a substantial number of overlapping, altered transcript levels in response to MTX. Studies with mammalian cells also showed that a large number of transcripts were affected with a majority of these transcripts unrelated to folate biosynthesis. Although the response of some of the transcripts identified is a predictable response to a toxin, the role of many of the perturbed transcripts is still not clear. These observations underscore the difficulty in understanding cytotoxity. Although as mentioned, earlier studies implicated impaired DNA synthesis in MTX toxicity, technological advances now show that a myriad of transcriptional changes actually occur.
Table 2.
Drosophila transcript abundance as assessed by qRT-PCR in response to MTX in a cell line (S3), ovaries from control fly lines (Canton S and w1118) and ovaries from a transgenic, MTX-resistant fly line expressing L30Q DHFR (pINDY5L30Qa)
| Gene symbol | Function | Drosophila S3 cellsa | Canton S ovariesb | w1118 ovariesc | pINDY5L30Qa ovariesc |
|---|---|---|---|---|---|
| Est21C | Transcription | 0.7 ± 0.1 | 3.6 ± 0.2 | 28.7 ± 0.7 | 0.0 ± 0.0 |
| Obp99a | Transport | 2.4 ± 0.0 | 4.2 ± 0.0 | 13.1 ± 0.5 | −0.3 ± 0.2 |
| Ance | Metamorphosis | 1.3 ± 0.3 | 3.1 ± 0.1 | 4.5 ± 1.7 | −0.1 ± 0.1 |
| – | Protease inhibition | 0.9 ± 0.2 | 3.0 ± 0.2 | 3.9 ± 0.6 | −0.3 ± 0.1 |
| Zen | Transcription | −3.5 ± 0.1 | 2.8 ± 0.3 | 1.5 ± 0.9 | −0.4 ± 0.2 |
| GstE9 | Defense response | 0.7 ± 0.2 | 4.0 ± 0.3 | 0.6 ± 1.0 | 0.0 ± 0.0 |
| Fst | Response to cold | −0.5 ± 0.5 | 3.8 ± 0.2 | 0.2 ± 1.0 | 0.3 ± 0.1 |
| CycE | Cell cycle | −0.1 ± 0.3 | −2.2 ± 0.3 | −9.6 ± 1.1 | −0.4 ± 0.3 |
| Mcm6 | Chorion gene amplification | −4.0 ± 0.3 | −3.5 ± 0.0 | −8.8 ± 0.5 | −2.8 ± 0.4 |
| Slbp | Cell cycle | −5.4 ± 0.2 | −2.4 ± 0.1 | −8.3 ± 1.2 | 0.2 ± 0.2 |
| stai | Signaling | −2.8 ± 0.3 | −9.8 ± 0.2 | −7.8 ± 0.4 | 0.1 ± 0.2 |
| cdc2c | Cell cycle | −0.6 ± 0.1 | −2.7 ± 0.1 | −7.2 ± 0.3 | 0.1 ± 0.0 |
| Mdr65 | Transport | −3.4 ± 0.2 | −4.3 ± 1.8 | −6.5 ± 1.1 | 0.3 ± 0.1 |
| loki | Cell cycle | −2.0 ± 0.2 | −10.8 ± 0.3 | −4.3 ± 0.9 | −2.0 ± 1.0 |
It is important to note that microarray analyses in mammals include tissue taken directly from laboratory animals (Huang et al. 2004; Ganter et al. 2005), tissue biopsies (Takata et al. 2005), or cell lines (Brachat et al. 2002). Unfortunately these studies are limited due to the inevitable sacrifice of large numbers of mammalian subjects or by the availability of tissues and impractability of many experimental manipulations. With the demonstration of the similar response in expression arrays in Drosophila however, limits to sample availability and experimental design are virtually eliminated, and there is not the ethical dilemma of deliberately exposing mammalian fetuses to this powerful teratogen.
Drosophila, similar to mammals, have a pair of ovaries, and although the fertilized egg does not implant in the Drosophila uterus, mothers provide their offspring with essential factors for development during oogenesis through follicle and nurse cells until the progeny are sufficiently developed in the larval stage to obtain their own nutrition (King 1970). Therefore, not surprisingly, maternal MTX exposure in female flies produces developmental abnormalities, including abnormal tufts of bristles, appendage curvature, and eye and wing deformities in some surviving progeny (Affleck et al. 2006b). As well, flies exposed to increasing concentrations of MTX show a dose- and time-dependant reduction in egg production reflecting pre-embryonic lethality (Affleck et al. 2006b). After 2 days of exposure to either 4.4, 11, 22, or 44 μM MTX, egg production was reduced to 61%, 61%, 35%, and 22%, respectively, when compared to untreated female egg production. Egg production on day 3 was reduced to less than 25% of untreated controls at all MTX concentrations. By day 4 no oviposition was observed by females exposed to MTX, thereby emphasizing that DHFR inhibition is also the major contributor to teratogenicity in Drosophila and strengthening the attractiveness of a Drosophila model for the understanding of antifolate-induced toxicity and teratogenicity.
Rescue of MTX-induced teratogenesis by MTX-resistant DHFRs
MTX can cause irreversible damage during fetal development; however, the value of MTX as a therapeutic agent is indispensable. Therefore, endeavors to “rescue” mammalian fetuses from the teratogenic effects of antifolate therapy have been attempted. In rabbits, a structural analog of THF, leucovorin, has been shown to lessen the teratogenic effects caused by MTX (DeSesso and Goeringer 1991). In addition to folate analogs, expression of “drug-resistant” DHFRs have been used to protect against teratogenicity. L22R DHFR has been constitutively expressed in transgenic murine embryos and placental tissue and results in the amelioration of the teratogenic effects of MTX (Sutton et al. 1998). Mice receiving a bone marrow transplant of marrow expressing either L22R or F31S provided chemoprotection from lethal and sublethal toxic effects of MTX, respectively (May et al. 1995).
Since Drosophila DHFRs with either a L22R or L30Q mutation permitted mammalian cells to divide in concentrations of MTX 200-fold and 2-fold higher, respectively, than control cells expressing wild-type Drosophila DHFR, fly lines ubiquitously expressing these mutations under the control of a UAS-GAL4 system were created (Affleck and Walker 2007). Unexpectedly, despite the observed high tolerance of the mammalian cells expressing L22R DHFR, expression of this mutant DHFR in flies did not provide protection to developing embryos when compared to control flies (either non-transgenic flies or transgenic flies expressing an extra copy of wild-type Drosophila DHFR) (Affleck and Walker 2007). Although the L22R enzyme does not appreciably bind MTX, it also does not reduce DHF at a rate sufficient to protect fertility (Affleck et al. 2006a). These experiments emphasize the importance of using whole organisms to test observations made in vitro and ex vivo.
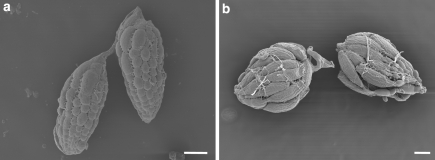
Conversely, transgenic flies expressing L30Q DHFR continued to produce viable offspring at a concentration 10-fold higher (1 μM MTX) than control lines. Transgenic L30Q DHFR flies produced stage 9–14 follicles (Fig. 1) and oviposited for a 21 day period even when exposed to 1 μM MTX. In contrast, at this MTX concentration, follicle maturation in ovaries of all other fly lines was not observed after day 4 (Fig. 1) and oviposition ceased post-day 4. When the L30Q transgene expression was driven solely in ovaries using specific promoters, a similar result was observed, suggesting the resistant enzyme must be expressed in ovarian tissue to protect the developing oocytes. All fly lines ceased oviposition after 4 days when exposed to 10 μM MTX. Interestingly, if MTX exposure was terminated after 13 days, L30Q transgenic flies recovered (regaining the ability to produce stage 9–14 follicles and oviposit), whereas, all other fly lines did not. These results have important implications for the “rescue” of mammalian fertility after MTX therapy at high dosages.
Fig. 1.
Scanning electron micrographs of ovaries from (a) control w1118 females and (b) transgenic females expressing L30Q DHFR after 4 days on medium with MTX. Ovaries from w1118 females only show immature follicles at stages 1–8. Ovaries from females expressing L30Q DHFR show follicles at all stages of development (1–14)
The generation of MTX-resistant, transgenic Drosophila provides unique experimental subjects to examine not only the morphological effects of teratogenesis but also the recovery of transcript homeostasis. As previously mentioned, MTX perturbs transcript levels involved in cell cycle, transport, signaling, transcription, and defense. A subset of these perturbed transcripts were analyzed from the ovaries of MTX-resistant fly lines and compared to control flies. The outcome was that a majority (12/14) of the transcripts appeared to be rescued (transcript abundance was similar to untreated controls) in the transgenic flies (Table 2) (Affleck and Walker 2007). Although two transcripts were still not at control levels, these messages were less affected than those from non-transgenic control females exposed to the same concentration of MTX.
Taken together, these studies show that Drosophila is indeed an excellent model to investigate drug toxicity, teratogenesis, alterations to transcript abundance, and rescue of antifolate-induced toxicity.
The future of Drosophila in drug research
Drosophila is an attractive organism due to the conserved gene function between the fly and humans, the short generation time, fully sequenced genome, and the availability of a wide range of scientific tools and manipulations. Currently, there are many other examples of genetic, biochemical, and behavioral research that are conducted using the invertebrate Drosophila. Here we have presented one case for the use of Drosophila to study the developmental and cytotoxic effects of a common human pharmaceutical, thereby preventing the sacrifice of mammalian animals and embryos. These studies have laid the ground work for both understanding the effects of other harmful pharmaceuticals and for future testing of potential harmful drugs in a non-vertebrate, whole organism system. For these reasons and many more, the use of Drosophila in research will continue to grow and provide the scientific community with valuable knowledge for at least the next 100 years.
Acknowledgements
Natural Sciences and Engineering Research Council of Canada (NSERC) is acknowledged for scholarship and grant support to the authors.
Abbreviations
- CHO
Chinese hamster ovary
- DHF
dihydrofolate
- DHFR
dihydrofolate reductase
- MTX
methotrexate
- THF
tetrahydrofolate
- SAM
S-adenosylmethionine
- UAS
upstream activating sequence
References
- Adam MP, Manning MA, Beck AE, Kwan A, Enns GM, Clericuzio C, Hoyme HE (2003) Methotrexate/misoprostol embryopathy: report of four cases resulting from failed medical abortion. Am J Med Genet A 123:72–78 [DOI] [PubMed]
- Affleck JG, Al-Batayneh KM, Neumann K, Cole SP, Walker VK (2006) Drosophila dihydrofolate reductase mutations confer resistance to mammalian cells. Eur J Pharmacol 529:71–78 [DOI] [PubMed]
- Affleck JG, Neumann K, Wong L, Walker VK (2006) The effects of methotrexate on Drosophila development, female fecundity, and gene expression. Toxicol Sci 89:495–503 [DOI] [PubMed]
- Affleck JG, Walker VK (2007) Transgenic rescue of methotrexate-induced teratogenicity in Drosophila melanogaster. Toxicol Sci 99:522–531 [DOI] [PubMed]
- Appleman JR, Prendergast N, Delcamp TJ, Freisheim JH, Blakley RL (1988) Kinetics of the formation and isomerization of methotrexate complexes of recombinant human dihydrofolate reductase. J Biol Chem 263:10304–10413 [PubMed]
- Brachat A, Pierrat B, Xynos A, Brecht K, Simonen M, Brungger A, Heim J (2002) A microarray-based, integrated approach to identify novel regulators of cancer drug response and apoptosis. Oncogene 21:8361–8371 [DOI] [PubMed]
- Brzezinska A, Winska P, Balinska M (2000) Cellular aspects of folate and antifolate membrane transport. Acta Biochim Pol 47:735–749 [PubMed]
- Chen MJ, Shimada T, Moulton AD, Cline A, Humphries RK, Maizel J, Nienhuis AW (1984) The functional human dihydrofolate reductase gene. J Biol Chem 259:3933–3943 [PubMed]
- Cody V, Luft JR, Pangborn W (2005) Understanding the role of Leu22 variants in methotrexate resistance: comparison of wild-type and Leu22Arg variant mouse and human dihydrofolate reductase ternary crystal complexes with methotrexate and NADPH. Acta Crystallogr D Biol Crystallogr 61:147–155 [DOI] [PubMed]
- Crosby MA, Goodman JL, Strelets VB, Zhang P, Gelbart WM, FlyBase Consortium (2007) FlyBase: genomes by the dozen. Nucleic Acids Res 35:D486–D491 [DOI] [PMC free article] [PubMed]
- Dadd RH (1973) Insect nutrition: current developments and metabolic implications. Annu Rev Entomol 18:381–420 [DOI] [PubMed]
- Darab DJ, Minkoff R, Sciote J, Sulik KK (1987) Pathogenesis of median facial clefts in mice treated with methotrexate. Teratology 36:77–86 [DOI] [PubMed]
- DeSesso JM, Goeringer GC (1991) Amelioration by leucovorin of methotrexate developmental toxicity in rabbits. Teratology 43:201–215 [DOI] [PubMed]
- Dicker AP, Volkenandt M, Bertino JR (1989) Detection of a single base mutation in the human dihydrofolate reductase gene from a methotrexate-resistant cell line using the polymerase chain reaction. Cancer Commun 1:7–12 [DOI] [PubMed]
- Fairfield KM, Fletcher RH (2002) Vitamins for chronic disease prevention in adults: scientific review. J Amer Med Assoc 287:3116–3126 [DOI] [PubMed]
- Feany MB, Bender WW (2000) A Drosophila model of Parkinson’s disease. Nature 404:394–398 [DOI] [PubMed]
- Fernandez H, Yves VSC, Pauthier S, Audibert F, Frydman R (1998) Randomized trial of conservative laparoscopic treatment and methotrexate administration in ectopic pregnancy and subsequent fertility. Human Repro 13:3239–3243 [DOI] [PubMed]
- Fernandez R, Tabarini D, Azpiazu N, Frasch M, Schlessinger J (1995) The Drosophila insulin receptor homolog: a gene essential for embryonic development encodes two receptor isoforms with different signaling potential. EMBO J 14:3373–3384 [DOI] [PMC free article] [PubMed]
- Fernandez-Ballart J, Murphy MM (2001) Preventive nutritional supplementation through the reproductive life cycle. Public Health Nutr 4:1363–1366 [DOI] [PubMed]
- Ganter B, Tugendreich S, Pearson CI, Ayanoglu E, Baumhueter S, Bostian KA, Brady L, Browne LJ, Calvin JT, Day GJ, Breckenridge N, Dunlea S, Eynon BP, Furness LM, Ferng J, Fielden MR, Fujimoto SY, Gong L, Hu C, Idury R, Judo MS, Kolaja KL, Lee MD, McSorley C, Minor JM, Nair RV, Natsoulis G, Nguyen P, Nicholson SM, Pham H, Roter AH, Sun D, Tan S, Thode S, Tolley AM, Vladimirova A, Yang J, Zhou Z, Jarnagin K (2005) Development of a large-scale chemogenomics database to improve drug candidate selection and to understand mechanisms of chemical toxicity and action. J Biotechnol 119:219–244 [DOI] [PubMed]
- Gorlick R, Cole P, Banerjee D, Longo G, Li WW, Hochhauser D, Bertino JR (1999) Mechanisms of methotrexate resistance in acute leukemia. Decreased transport and polyglutamylation. Adv Exp Med Biol 457:543–550 [DOI] [PubMed]
- Goujon C, Berard F, Dahel K, Guillot I, Hennino A, Nosbaum A, Saad N, Nicolas JF (2006) Methotrexate for treatment of adult atopic dermatitis. Eur J Dermatol 16:155–158 [PubMed]
- Hammes GG (2002) Multiple conformational changes in enzymes catalysis. Biochemistry 41:8221–8228 [DOI] [PubMed]
- Hao H, Tyshenko MG, Walker VK (1994) Dihydrofolate reductase of Drosophila. Cloning and expression of a gene with a rare transcript. J Biol Chem 269:15179–15185 [PubMed]
- Huang Q, Jin X, Gaillard ET, Knight BL, Pack FD, Stoltz JH, Jayadev S, Blanchard KT (2004) Gene expression profiling reveals multiple toxicity endpoints by hepatotoxicants. Mutat Res 549:147–167 [DOI] [PubMed]
- Huennekens FM (1994) The methotrexate story: a paradigm for development of cancer chemotherapeutic agents. Adv Enzyme Regul 34:397–419 [DOI] [PubMed]
- Jarabak J, Bachur NR (1971) A soluble dihydrofolate reductase from human placenta: purification and properties. Arch Biochem Biophys 142:417–425 [DOI] [PubMed]
- Jin S, Martinek S, Joo WS, Wortman JR, Mirkovic N, Sali A, Yandell MD, Pavletich NP, Young MW, Levine AJ (2000) Identification and characterization of a p53 homologue in Drosophila melanogaster. PNAS 97:7301–7306 [DOI] [PMC free article] [PubMed]
- Khera KS (1976) Teratogenicity studies with methotrexate, aminopterin, and acetylsalicylic acid in domestic cats. Teratology 14:21–27 [DOI] [PubMed]
- King RC (1970) Ovarian development in Drosophila melanogaster. Academic Press, New York
- Kompis IM, Islam K, Then RL (2005) DNA and RNA synthesis: antifolates. Chem Rev 105:593–620 [DOI] [PubMed]
- Lewden B, Vial T, Elefant E, Nelva A, Carlier P, Descotes J (2004) Low dose methotrexate in the first trimester of pregnancy: results of a French collaborative study. J Rheumatol 31:2360–2365 [PubMed]
- Lloyd ME, Carr M, McElhatton P, Hall GM, Hughes RA (1999) The effects of methotrexate on pregnancy, fertility and lactation. Q J Med 92:551–563 [DOI] [PubMed]
- Malinow MR, Duell PB, Hess DL, Anderson PH, Kruger WD, Phillipson BE, Gluckman RA, Block PC, Upson BM (1998) Reduction of plasma homocyst(e)ine levels by breakfast cereal fortified with folic acid in patients with coronary heart disease. N Engl J Med 338:1009–1015 [DOI] [PubMed]
- May C, Gunther R, McIvor RS (1995) Protection of mice from lethal doses of methotrexate by transplantation with transgenic marrow expressing drug-resistant dihydrofolate reductase activity. Blood 86:2439–2348 [PubMed]
- McGuire JJ (2003) Anticancer antifolates: Current status and future directions. Curr Pharm Design 9:2593–2613 [DOI] [PubMed]
- McKay JA, Williams EA, Mathers JC (2004) Folate and DNA methylation during in utero development and aging. Biochem Soc Trans 32:1006–1007 [DOI] [PubMed]
- Meisel R, Bardenheuer W, Strehblow C, Sorg UR, Elmaagacli A, Seeber S, Flasshove M, Moritz T (2003) Efficient protection from methotrexate toxicity and selection of transduced human hematopoietic cells following gene transfer of dihydrofolate reductase mutants. Exp Hematol 31:1215–1222 [DOI] [PubMed]
- Nakazawa F, Matsuno H, Yudoh K, Katayama R, Sawai T, Uzuki M, Kimura T (2001) Methotrexate inhibits rheumatoid synovitis by inducing apoptosis. J Rheumatol 28:1800–1808 [PubMed]
- Oakley GP Jr., Bell KN, Weber MB (2004) Recommendations for accelerating global action to prevent folic acid-preventable birth defects and other folate-deficiency diseases: meeting of experts on preventing folic acid-preventable neural tube defects. Birth Defects Res A Clin Mol Teratol 70:835–837 [DOI] [PubMed]
- Rancourt SL, Walker VK (1990) Kinetic characterization of dihydrofolate reductase from Drosophila melanogaster. Biochem Cell Biol 68:1075–1082 [DOI] [PubMed]
- Simonsen CC, Levinson AD (1983) Isolation and expression of an altered mouse dihydrofolate reductase cDNA. PNAS 80:2495–2499 [DOI] [PMC free article] [PubMed]
- Srimatkandada S, Medina WD, Cashmore AR, Whyte W, Engel D, Moroson BA, Franco CT, Dube SK, Bertino JR (1983) Amplification and organization of dihydrofolate reductase genes in a human leukemic cell line, K-562, resistant to methotrexate. Biochemistry 22:5774–5781 [DOI] [PubMed]
- Sun JH, Das KM (2005) Low-dose oral methotrexate for maintaining Crohn’s disease remission: where we stand. J Clin Gastrointerol 39:751–756 [DOI] [PubMed]
- Sutton C, McIvor RS, Vagt M, Doggett B, Kapur RP (1998) Methotrexate-resistant form of dihydrofolate reductase protects transgenic murine embryos from teratogenic effects of methotrexate. Pediatr Dev Pathol 1:503–512 [DOI] [PubMed]
- Takata R, Katagiri T, Kanehira M, Tsunoda T, Shuin T, Miki T, Namiki M, Kohri K, Matsushita Y, Fujioka T, Nakamura Y (2005) Predicting response to methotrexate, vinblastine, doxorubicin, and cisplatin neoadjuvant chemotherapy for bladder cancers through genome-wide gene expression profiling. Clin Cancer Res 11:2625–2636 [DOI] [PubMed]
- Vinson RK, Hales BF (2002) Expression and activity of the DNA repair enzyme uracil DNA glycosylase during organogenesis in the rat conceptus and following methotrexate exposure in vitro. Biochem Pharmacol 64:711–721 [DOI] [PubMed]
- von Sternberg RM, Novick GE, Gao GP, Herrera RJ (1992) Genome canalization: the coevolution of transposable and interspersed repetitive elements with single copy DNA. Genetica 86:215–246 [DOI] [PubMed]
- Weikert C, Hoffmann K, Dierkes J, Zyriax BC, Klipstein-Grobusch K, Schulze MB, Jung R, Windler E, Boeing H (2005) A homocysteine metabolism-related dietary pattern and the risk of coronary heart disease in two independent German study populations. J Nutr 135:1981–1988 [DOI] [PubMed]
- Wise CM, Vuyyuru S, Roberts WN (1996) Methotrexate in nonrenal lupus and undifferentiated connective tissue disease – a review of 36 patients. J Rheumatol 23:1005–1010 [PubMed]