Abstract
CONTEXT: Lymphatic vessels are believed to be absent in the colon above the level of the mucularis mucosae. However, in our experience, lymphatic vessels are sometimes identifiable within the lamina propria in the setting of inflammation and neoplasia.
OBJECTIVE: We sought to assess the presence of lymphatics within the colonic lamina propria in neoplastic and inflammatory conditions using the lymphatic endothelium-specific immunohistochemical marker D2-40.
DESIGN: Representative sections of normal colon, inflamed colon, hyperplastic polyps, inflammatory polyps, adenomatous polyps, adenomatous polyps containing intramucosal carcinoma, and invasive colonic adenocarcinomas were subjected to immunohistochemical staining with D2-40. The presence of immunopositive lymphatic vessels was assessed. Lymphatic density within the lamina propria was calculated quantitatively, and the presence of inflammation was graded subjectively on a four-tiered scale (0-3).
RESULTS: Lymphatics were not identified within the lamina propria of normal colon. However, lymphatics were identified within the lamina propria in the majority of cases with neoplasia and/or inflammation. Additionally, there was a non-significant trend toward higher lymphatic vessel density in cases with increasing inflammation.
CONCLUSIONS: Lymphatic vessels are present within the lamina propria of colon in pathologic states, including cases of intramucosal carcinoma. This “aberrant” lymphangiogenesis is likely to be driven by inflammation and/or neoplasia.
Introduction
It has long been established that lymph node metastasis is an important prognostic factor in colorectal carcinoma, and this has lent relevance to the characterization of the lymphatic supply of the colon [1-4]. Although lymphatic spaces are generally abundant within the colonic mucosa, consensus states that lymphatic vessels are absent above the level of the muscularis mucosae. Most early attempts at assessment of lymphatics within the large bowel utilized standard hematoxylin and eosin (H&E) stained histologic sections and electron microscopy [5-7]. However, identification of lymphatics by conventional light microscopy and electron microscopy is not only quite challenging but also unreliable. Subsequent studies have utilized enzyme histochemical assays specific for 5’-nucleotide alkaline phosphatase in order to highlight lymphatics in colonic tissue [8-9]. Most studies using these methods have confirmed the hypothesis that lymphatics are absent in the lamina propria of colonic mucosa. Most recently, the lymphatic-specific monoclonal antibody D2-40 has been developed for immunohistochemical staining of lymphatic channels and has been used to study both lymphatic invasion and lymphatic vessel density in colon carcinoma [10-13]. In the only study of its kind thus far, Fogt et al. utilized D2-40 to identify lymphatics in normal colonic mucosa, adenomas, and invasive carcinomas. This group found that, while absent in normal colon, lymphatics were present within the lamina propria of in situ components of otherwise invasive carcinomas, as well as associated with early invasive epithelial nests in carcinoma [14]. While the latter finding is well established, the former finding suggests that lymphatic vessels may be present in colonic lamina propria in abnormal states other than frankly invasive carcinoma. However, this study showed no evidence of lymphatics within the lamina propria in adenomatous colonic tissue.
In the current American Joint Commission on Cancer (AJCC) staging system for colon carcinoma, both in situ and intramucosal (invasion into lamina propria) carcinomas are classified as tumor stage 0, while deeper invasion results in tumor stages of T1-T4. This is somewhat unique, as both in situ and invasive carcinomatous processes are considered equivalent for tumor staging purposes. The AJCC staging scheme is based in part on the very low clinical incidence of lymph node metastases seen in in situ and intramucosal carcinoma and on the fact that distinguishing in situ and intramucosal carcinoma can be difficult [15-16]. However, the system also is based on the historical concept that lymphatic vessels are absent from the colonic lamina propria. This may hold true for normal colon, but is clearly not the case for invasive carcinoma, and it may not be true for in situ and/or intramucosal carcinoma, either. In our experience, lymphatics are sometimes identifiable within the lamina propria of colon polyps and in cases of inflammatory bowel disease. Additionally, and most concerning, we have seen lymphatic tumor emboli in cases of intramucosal carcinoma. The purpose of this study is to assess for the presence of lymphatic vessels (LV) within the lamina propria of colon in a representative variety of neoplastic and inflamed states using the immunohistochemical marker D2-40.
Materials and Methods
Case selection
The database of the Department of Pathology at our institution was searched for cases of idiopathic inflammatory bowel disease (IBD, represented by both Crohn’s disease and ulcerative colitis), hyperplastic polyps, inflammatory polyps, adenomatous polyps, adenomatous polyps containing intramucosal carcinoma, and invasive colonic adenocarcinomas from 1995 to 2007. Biopsy specimens were rejected, and only resection and polypectomy specimens were utilized in order to maximize the stainable area of lamina propria in each case. Although this approach reduced the sample size of the study, it was deemed important in order to carefully assess the lamina propria both qualitatively and quantitatively for lymphatics. Future work likely would include biopsy specimens. Representative sections of normal colon were obtained from areas distant from the tumor in cases of colon carcinoma. All histologic material was reviewed by two pathologists (BK, DJ) to confirm the histologic diagnosis in each case. After careful review, cases were selected as follows: normal colonic tissue (n = 4), inflamed colonic tissue associated with inflammatory bowel disease (n = 4), hyperplastic polyps (n = 6), adenomatous polyps (n = 12), inflammatory polyps (n = 5), adenomatous polyps containing intramucosal carcinoma (n = 6), and invasive colonic adenocarcinomas (n = 5).
Immunohistochemical staining
In each case, appropriate 4-µm–thick sections from formalin-fixed paraffin-embedded tissue were immunostained for D2-40 (dilution 1:200, Dako, Carpinteria, California) and CD34 (dilution 1:4, BioGenex, San Ramon, California) using the indirect immunoperoxidase method without antigen retrieval. Immunostaining was accomplished on a Ventana Autostainer (Ventana, Tucson, Arizona) using Envision+ Detection (Dako, Carpinteria, California). Negative controls were established with nonspecific immunoglobulin-G as the primary antibody. CD34 staining was included to qualitatively assess the differential presence of vascular and lymphatic channels within the mucosa and identify possible neovascularization in the presence of inflammation and neoplasia.
Interpretation of immunostaining
Lymphatic vessels were identified by strong D2-40 staining and the formation of slit-like or rounded luminal spaces. After low-power scanning to identify areas of greatest qualitative density, a formal lymphatic vessel count was performed within three high-power (40X) fields in each case. Similar to previous studies, the lymphatic vessel density (LVD) is reported as the mean value of those three counts [13,17]. Only lymphatic vessels with convincing morphology and positive staining for D2-40, as agreed by two investigators (BK, DJ) were counted. D2-40-positive lymphatics in and around lymphoid follicles or near muscle fibers were not included. If immunopositive lymphatics were not readily identified on initial examination, a scrupulous search of the entire lamina propria was made to thoroughly rule out their presence before assignment to the negative category. In cases of discrepancy, the slides were reviewed at a two-headed microscope. Each identifiable D2-40-positive vessel was identified and discussed relative to the inclusion criteria stated above, and formal counts were repeated until consensus was achieved. Vascular spaces were identified based on strong CD34 staining and the formation of clearly defined luminal spaces.
Interpretation of inflammation
In each case, the degree of mucosal inflammation was graded using a four-tiered system. The overall density of inflammation was assessed irrespective of inflammatory cell type. Grade 0 was defined as scattered inflammatory cells consistent with those seen in normal colon. Grade 2 was defined as mildly increased inflammatory cells just above baseline for normal colon. Grade 3 was defined as a significant increase in inflammatory cells within the lamina propria, well above baseline for normal colon. Grade 4 was defined as a florid inflammatory infiltrate with ulceration and/or significant architectural disorganization (Figure 1). The assigned levels for each case were agreed upon by two investigators (BK, DJ), who were blinded to the immunostaining characteristics of each case. In cases of discrepancy, the slides were reviewed at a two-headed microscope. Both reviewers explained their grade assignment relative to the inclusion criteria stated above, and after discussion of possible reasons for discordance, consensus was achieved.
Figure 1.
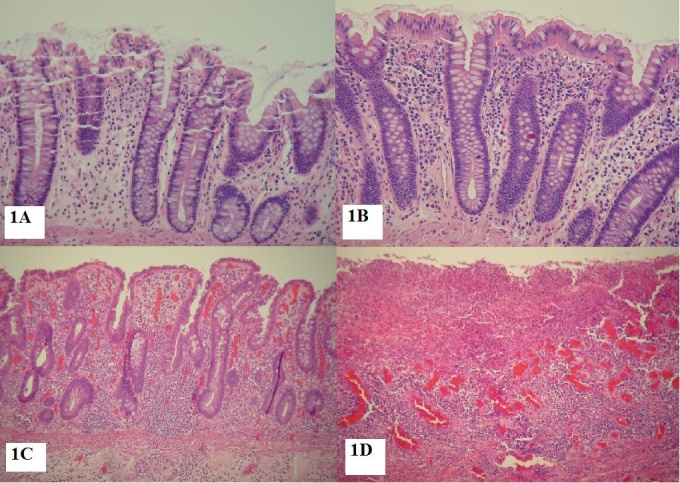
Inflammatory grading: Histologic examples of different inflammatory grades: A. Grade 0 (H&E 20X) B. Grade 1 (H&E 20X) C. Grade 3 (H&E 10X) D. Grade 4 (H&E 10X).
Statistical analysis
Overall statistical significance of LVD variation between tissue types was calculated by one-way analysis of variance (ANOVA) methods using Microsoft Excel software (Microsoft Corporation, Redmond, Washington). Once overall statistical significance was demonstrated by ANOVA calculations, multiple comparison statistics were applied via pair-wise applications of the least significant difference (LSD) calculation using Analyse-it software (Analyse-it, Leeds, UK). Similarly, overall statistical significance of LVD vs. inflammatory grade was calculated by one-way ANOVA using Microsoft Excel software.
Results
Using the lymphatic marker D2-40, lymphatic vessels were not identified in the lamina propria of normal colon, consistent with previous reports. The lymphatic vessels in normal mucosa were localized to the submucosa and in the immediate vicinity of the muscularis mucosae (Figure 2). However, lymphatics were present within the lamina propria in a significant percentage of all pathologic cases studied (Table 1). Seventy-five percent of cases of IBD showed positive staining (Figure 3). Among benign polyps, 80 percent of inflammatory polyps and 66 percent of hyperplastic polyps showed positivity (Figure 4). Among neoplastic polyps, 75 percent of adenomas and 67 percent of polyps with intramucosal carcinoma revealed D2-40-positive lymphatic channels within the lamina propria (Figure 4). Invasive carcinomas showed frequent lymphatics within the tumor stroma, particularly within fibrous stromal bands running between tumor islands (Figure 5). However, in immediately adjacent non-neoplastic colon, there were no identifiable lymphatics within the lamina propria. The highest mean lamina propria LVD of 3.4 was seen in inflammatory polyps (Table 1). Inflamed colon (IBD) had the next highest mean LVD at 2.3, followed closely by adenomas at 2.2. Interestingly, polyps with intramucosal carcinoma had a relatively low LVD of 1.5. One-way ANOVA statistics showed an overall statistically significant difference in LVD between groups, i.e., by tissue type (p < 0.005). Pair-wise comparison of LVD by tissue type revealed a statistically significant difference in LVD between adenoma vs. normal colon, adenoma vs. adenocarcinoma, intramucosal carcinoma vs. carcinoma, inflamed polyps vs. normal colon, inflamed colon vs. adenocarcinoma, normal colon vs. adenocarcinoma, and adenocarcinoma vs. hyperplastic polyps at the 95 percent confidence interval (Table 2).
Figure 2.
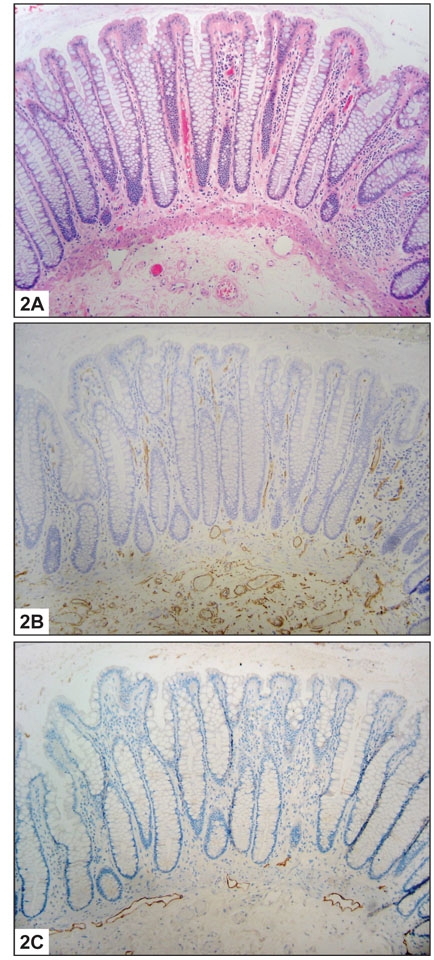
Normal Colon: A. Histologic section showing normal colonic mucosa (H&E 10X). B. Histologic section showing normal colonic mucosa immunolabelled for CD34. Blood vessels are present at all levels of the mucosa (CD34 10X). C. Histologic section showing normal colonic mucosa immunolabelled for D2-40. Lymphatic vessels are limited to the submucosa and immediately adjacent to the muscularis mucosae (D2-40 10X).
Figure 3.
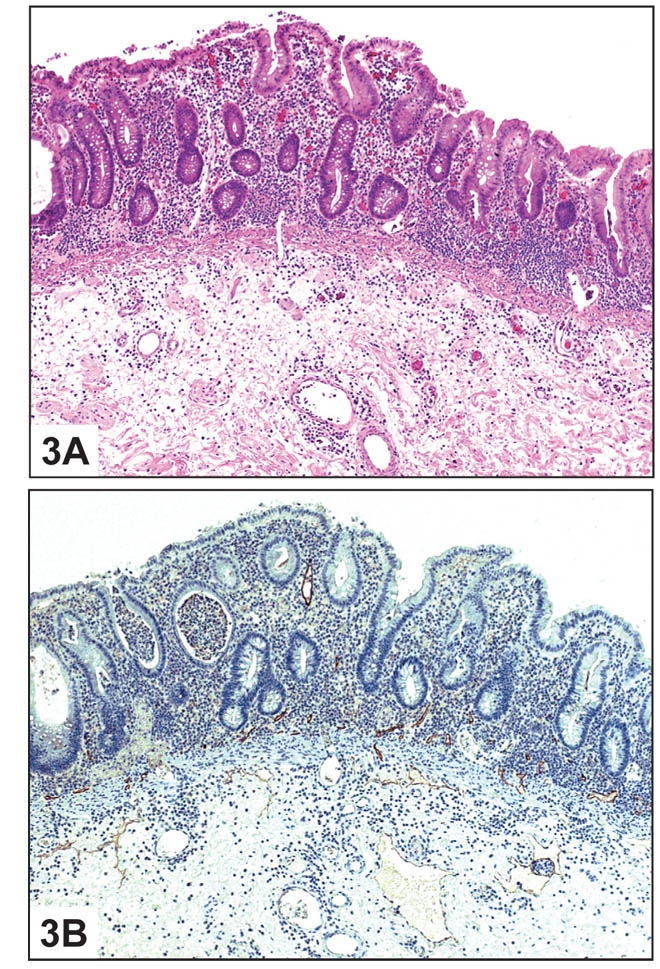
Inflammatory Bowel Disease: A. Histologic section of colon with inflammatory bowel disease (H&E 10X).B. Immunostain showing D2-40-positive lymphatic vessels within the lamina propria (D2-40 10X).
Figure 4.
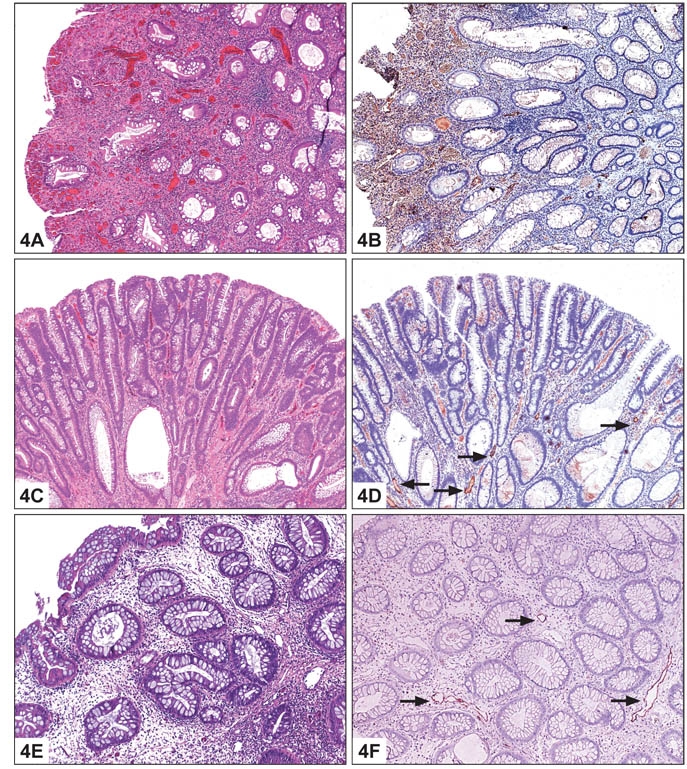
Colonic Polyps: A. Histologic section of inflammatory polyp (H&E 10X). B. Immunostain showing D2-40-positive lymphatic vessels within the lamina propria (D2-40 10X). C. Histologic section of adenomatous polyp (H&E 20X). D. Immunostain showing D2-40-positive lymphatic vessels within the lamina propria (D2-40 20X). E. Histologic section of hyperplastic polyp (H&E 10X). F. Immunostain showing D2-40-positive lymphatic vessels within the lamina propria (D2-40 10X).
Figure 5.
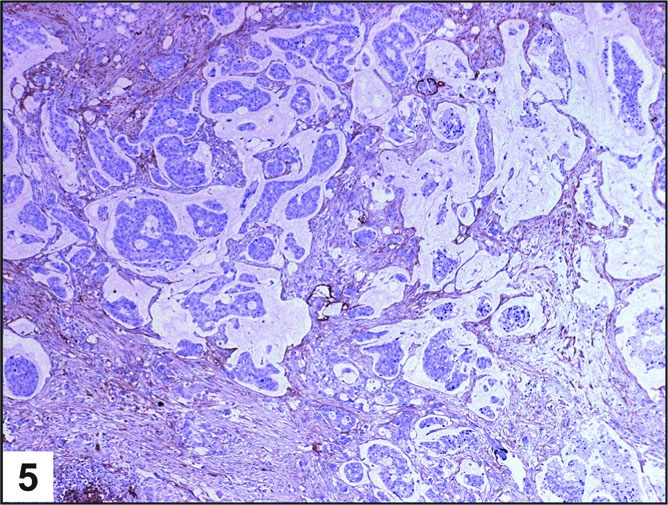
Invasive Carcinoma: Histologic section showing D2-40-positive lymphatic vessels in a case of invasive colonic carcinoma (D2-40 10X).
When all types of specimens were combined, a vague trend toward increased LVD with increasing degree of inflammation was seen (Table 3). Cases with grade 0 inflammation had an average LVD of 0.6, while those with grade 1 inflammation increased to 2.1. This general trend continued, but tapered, in the more severely inflamed cases, with grade 2 and 3 specimens showing average LVDs of 3.3 and 3 respectively. Application of ANOVA statistics showed an overall statistically significant difference in inflammatory grade between groups, i.e., by tissue type (p < 0.0001). However, calculations failed to demonstrate a statistically significant difference in LVD based on inflammatory grade (p = 0.07).
Immunohistochemical staining for CD34 revealed the presence of blood vessels within the lamina propria in both normal and all study cases. This finding confirms the presumed presence of vascular channels within all levels of the mucosa that must be distinguished from lymphatic spaces. The subjective density of lamina propria blood vessels was relatively uniform across sample types.
Discussion
It has been well established that lymph node metastasis is an important prognostic factor in colorectal carcinoma, and for this reason, the study of colonic lymphangiogenesis is pertinent [1-4]. However, the events initiating and regulating lymphatic vascular invasion and subsequent metastasis are not well understood. At a basic level, lymphatic invasion is a starting requirement for lymph node metastasis, and tumor cells with greater exposure to lymphatic vasculature have been shown in certain tumors to have higher rates of lymphatic metastasis [17-18]. Perhaps not surprisingly, it has been shown that increased lymphatic vessel density is associated with poor outcome in a number of tumors, including squamous cell carcinoma of the head and neck, thyroid carcinoma, breast carcinoma, and melanoma [19-23]. Recent work has shown a similar association of high lymphatic microvessel density with increased rates of lymph node metastasis and poor outcome in colorectal carcinoma [9,12,24]. Additional work on cases of superficially invasive colorectal carcinoma limited to the submucosa has shown a similar trend toward greater incidence of lymph node metastasis in cases with high lymphatic vessel density [13].
Based on conventional light and electron microscopy, it has been shown that lymphatic vessels are absent within the colonic lamina propria. However, we have shown there are circumstances wherein induction of lymphangiogenesis occurs, including neoplasia and inflammation. In this study, normal colon showed no evidence of lymphatic channels within the lamina propria by immunohistochemical staining for D2-40, in keeping with what has been proposed by other authors. However, the majority of cases with pathologic findings, whether neoplastic or inflammatory, did show the presence of lymphatics above the level of the muscularis mucosae.
Inflammation has been shown to be associated with the presence of lymphatics in the colonic mucosa under specific circumstances. In a small series, Kaiserling et al. utilized D2-40 staining to show that lymphatic vessels can be present in the colonic mucosa in cases of long-standing ulcerative colitis where there is widening of the muscularis mucosae, penetration of the mucosa by muscle fibers, filiform changes in the mucosa, or hyperplasia of mucosa-associated lymphoid tissue [25]. More recently, Kaneko et al. revealed that lymphatic vessel density was proportional to the degree of associated inflammation in cases of invasive colonic adenocarcinoma penetrating into submucosa [13]. It is likely that inflammatory mediators induce lymphangiogenesis in these settings. Our results suggest that increasing inflammation may be associated with higher LVD in colonic lamina propria. Overall, mean LVD was highest in cases with an inflammatory component (inflamed colon in cases of IBD and inflammatory polyps). In particular, when all specimens were combined, the mean LVD increased with inflammatory grade. When broken down by each category, hyperplastic polyps and adenomas with intramucosal carcinoma showed a vague trend toward increased mean LVD with increased inflammation. However, the number of cases in each category was too small to draw any strong conclusions. When all categories were combined, this trend was more robust. Unfortunately, statistical significance was not achieved in comparisons of LVD with inflammatory grade, likely due to small sample size. While this preliminary study shows the presence of lymphatics in lamina propria and suggests a relationship between inflammation and LVD, further studies are needed to see if this correlation is statistically significant.
Taken together, these findings suggest that inflammatory mediators may indeed play a significant role in the regulation of lymphatic proliferation in the colonic lamina propria. Several interesting questions are raised. The first is whether newly formed lymphatics in the lamina propria regress once an inflammatory stimulus is removed. Further investigation using serial biopsy specimens eventually may answer this. Another question involves the sequence of lymphangiogenesis. In our specimens, lymphatics were unambiguously seen at all levels of the lamina propria. However, there was a subjective zonal preference for lymphatics to be more prevalent near the muscularis mucosae rather than in the superficial lamina propria. It remains to be answered whether newly formed lymphatic vessels migrate across the muscularis mucosae from areas of normal lymphatics or whether they arise de novo within the lamina propria driven by inflammation. Finally, our study did not categorize the type of inflammation, but rather focused on overall degree of inflammatory infiltrate regardless of subtype. It is unclear whether the drive for lymphangiogenesis varies depending on the acuity or chronicity of inflammation; this is another question that may be answered through further investigation.
Neoplasia also may be associated with the presence of lymphatics in colonic mucosa. In addition to inflammation-related findings, Kaiserling et al. found an association between dysplastic epithelial changes and quantitative increases in lymphatics in cases of UC with epithelial dysplasia [25]. Kuroyama et al. utilized enzyme histochemical assays to identify lymphatics in cases of colon carcinoma and showed that intratumoral lymphatic vessels were present in 91 percent of carcinomas. Lymphatic density was significantly higher within the tumor than in the surrounding submucosa in these cases [9]. Fogt et al. reported lymphatic vessels both adjacent to nests of invasive tumor and in the lamina propria surrounding regions of in situ carcinoma using D2-40 [14]. These findings imply an association between epithelial neoplasia and lymphangiogenesis in the colon. Our results show that neoplastic conditions (adenoma and intramucosal carcinoma) are associated with the presence of lymphatic vessels within colonic lamina propria. The mean LVD for adenomas was 2.2. Interestingly, however, the mean LVD for adenomas with intramucosal carcinoma was only 1.5, which on an intuitive level seems paradoxical. This may be reflective of the small sample size in this category (an issue which may arise in any study assessing intramucosal carcinoma in the colon). Within the invasive carcinoma group, the mean LVD within and around tumor was 4.8. Overall, there was a statistically significant difference in LVD between groups. Additionally, pair-wise comparison showed significant differences in LVD between adenoma vs. normal colon, intramucosal carcinoma vs. invasive adenocarcinoma, invasive carcinoma vs. normal colon, invasive adenocarcinoma vs. inflamed colon, and invasive carcinoma vs. hyperplastic polyp. While the comparisons involving invasive carcinoma are not unexpected, the significant differences between adenoma and normal colon and between intramucosal carcinoma and invasive carcinoma are novel and supportive of our overall observations. It is of interest that non-neoplastic colon immediately adjacent to invasive carcinoma did not show any increase or aberrant localization of lymphatic channels in our cases, contrary to what was seen in the study by Fogt et al. [14]. If mediators of lymphangiogenesis are at work within the tumor, then it appears they may have predominantly localized or paracrine effects within invasive carcinomas.
One final issue related to lymphatics in lamina propria that warrants brief discussion regards colon cancer staging. While the potential incidence of lymph node metastasis in intramucosal carcinoma is extremely low, there are cases in which metastasis has been suggested [7]. If access to lymphatic vessels is available to superficial lesions within the lamina propria, then the difference between intramucosal and in situ carcinoma becomes more significant. The invasive lesion, however superficial, indeed may have metastatic potential due to the presence of nearby lymphatics. Based on clinical outcome data, there would be no compelling reason to modify the current AJCC staging system. However, with a more precise definition of intramucosal carcinoma, prospective follow-up may indeed reveal a higher rate of lymph node metastases in patients with intramucosal carcinomas than in those with purely in situ lesions.
In conclusion, lymphatics are identifiable within the lamina propria of the colon in various abnormal conditions, including pre-neoplastic, neoplastic, and inflammatory states. The driving force behind lymphangiogenesis may be stimulus from inflammation, surrounding neoplasia, or a combination of both. While the necessarily small sample size of this study prevented robust statistical analysis, future work including biopsy specimens may provide further insight into the lymphatic vasculature of the colon in pathologic states.
Table 1. Results of immunohistochemical staining for D2-40 and CD34, as well as mean lymphatic density by tissue type. A statistically significant difference between LVD among different tissue types was identified (p < 0.005).
| Tissue | D2-40 positivity | %positive D2-40 | Mean lymphatic density** | CD34 positivity | %positive CD34 |
| Normal colon | 0/4 | 0% | 0 | 4/4 | 100% |
| Inflamed colon | 3/4 | 75% | 2.3 | 4/4 | 100% |
| Hyperplastic polyps | 4/6 | 66% | 2 | 6/6 | 100% |
| Inflammatory polyps | 4/5 | 80% | 3.4 | 5/5 | 100% |
| Adenomatous polyps | 9/12 | 75% | 2.2 | 12/12 | 100% |
| Adenomatous polyps containing intramucosal carcinoma | 4/6 | 67% | 1.5 | 6/6 | 100% |
| Invasive adenocarcinoma | 5/5* | 100%* | 4.8 | 5/5 | 100% |
| Total | 29/42 | 69% | 2.3 | 42/42 | 100% |
*positivity within and around tumor islands
**there was discrepancy in LVD calculation in one case of invasive adenocarcinoma which was resolved by consensus
Table 2. Results of pair-wise LSD multiple comparison statistic.
| Tissue | 95% CI | Significant |
| Adenoma vs. IMC | -1.0 to 2.3 | No |
| Adenoma vs. IP | -3.0 to 1.6 | No |
| Adenoma vs. IC | -2.0 to 1.9 | No |
| Adenoma vs. NC | 0.2 to 4.1 | Yes |
| Adenoma vs. HP | -1.5 to 1.8 | No |
| IMC vs. IP | -3.9 to 0.1 | No |
| IMC vs. IC | -2.9 to 1.4 | No |
| IMC vs. NC | -0.7 to 3.7 | No |
| IMC vs. CA | -5.3 to -1.3 | Yes |
| IMC vs. HP | -2.4 to 1.4 | No |
| IP vs. IC | -1.1 to 3.4 | No |
| IP vs. NC | 1.1 to 5.7 | Yes |
| IP vs. CA | -3.5 to 0.7 | No |
| IP vs. HP | -0.6 to 3.4 | No |
| IC vs. NC | -0.1 to 4.6 | No |
| IC vs. CA | -4.8 to -0.3 | Yes |
| IC vs. HP | -1.9 to 2.4 | No |
| NC vs. CA | -4.2 to 0.2 | Yes |
| NC vs. HP | -4.2 to 0.2 | No |
| CA vs. HP | 0.8 to 4.8 | Yes |
CI- confidence interval, IMC-intramucosal carcinoma, IP-inflamed polyp, IC-inflamed colon, NC-normal colon, HP-hyperplastic polyp, CA-adenocarcinoma
Table 3. Average lymphatic density (LVD) and inflammatory grade.
| Tissue | Grade 0 Inflammation | Grade 1 Inflammation* | Grade 2 Inflammation | Grade 3 Inflammation | |
| Normal colon (n = 4) | Number | 4 | 0 | 0 | 0 |
| Avg. LVD | 0 | - | - | - | |
| Inflamed colon (n = 4) | Number | 0 | 0 | 0 | 4 |
| Avg. LVD | - | - | - | 2.3 | |
| Hyperplastic polyps (n = 6) | Number | 0 | 4 | 2 | 0 |
| Avg. LVD | - | 1.5 | 3 | - | |
| Inflammatory polyps (n = 5) | Number | 0 | 0 | 0 | 5 |
| Avg. LVD | - | - | - | 3.4 | |
| Adenomatous polyps (n = 12) | Number | 1 | 11 | 0 | 0 |
| Avg. LVD | 3 | 2.1 | - | - | |
| Adenomatous polyps containing intramucosal carcinoma (n = 6) | Number | 0 | 4 | 2 | 0 |
| Avg. LVD | - | 0.75 | 3 | - | |
| Invasive adeno-carcinoma (n = 5) | Number | 0 | 2 | 2 | 1 |
| Avg. LVD | - | 6 | 4 | 4 | |
| Total | Avg. LVD | 0.6 | 2.1 | 3.3 | 3 |
* there was discrepancy in inflammatory grade assignment in five cases, each of which was resolved by consensus
Acknowledgments
We wish to thank Mary Helie, Chuck Frederick, and Marcy Verde for their essential role in the immunohistochemical staining of our study cases. The authors declare no conflict of interest associated with this manuscript.
Abbreviations
- AJCC
American Joint Commission on Cancer
- LV
lymphatic vessels
- IBD
idiopathic inflammatory bowel disease
- LVD
lymphatic vessel density
- ANOVA
analysis of variance
- LSD
least significant difference
References
- Fielding L, Phillips R, Fry J, et al. Prediction of outcome after curative resection for large bowel cancer. Lancet. 1986;2:904–907. doi: 10.1016/s0140-6736(86)90422-8. [DOI] [PubMed] [Google Scholar]
- Chapuis P, Dent O, Fisher R, et al. A multivariate analysis of clinical and pathological variables in prognosis after resection of large bowel cancer. Br J Surg. 1985;72:698–702. doi: 10.1002/bjs.1800720909. [DOI] [PubMed] [Google Scholar]
- Dukes C, Bussey H. The spread of rectal cancer and its effect on prognosis. Br J Cancer. 1958;12:309–320. doi: 10.1038/bjc.1958.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newland R, Chapius P, Pheils M, et al. The relationship of survival to staging and grading of colorectal carcinoma: a prospective study of 503 cases. Cancer. 1981;47:1424–1429. doi: 10.1002/1097-0142(19810315)47:6<1424::aid-cncr2820470630>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Dobbins W. The intestinal mucosal lymphatic in man. A light and electron microscopic study. Gastroenterol. 1966;51(6):994–1003. [PubMed] [Google Scholar]
- Azzali G. Three-dimensional and ultrastructural aspects of the lymphatic vascularization of the vermiform appendix. J Submicrosc Cytol Pathol. 1998;30(4):545–553. [PubMed] [Google Scholar]
- Fenoglio C, Kaye G, Lane N. Distribution of human colonic lymphatics in normal, hyperplastic and adenomatous tissue. Its relationship to metastasis from small carcinomas in pedunculated adenomas, with two case reports. Gastroenterol. 1973;64(1):51–66. [PubMed] [Google Scholar]
- Jia Y, Li Z, He Y, et al. Expression of vascular endothelial growth factor-C and the relationship between lymphangiogenesis and lymphatic metastasis in colorectal carcinoma. World J Gastroenterol. 2004;10(22):3261–3263. doi: 10.3748/wjg.v10.i22.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroyama S, Kobayashi N, Ohbu M, et al. Enzyme histochemical analysis of lymphatic vessels in colon carcinoma: occurrence of lymphangiogenesis within the tumor. Hepatogastroenterology. 2005;52(64):1057–1061. [PubMed] [Google Scholar]
- Breiteneder-Geleff S, Soleiman A, Kowalski H, et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154:385–394. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walgenbach-Bruenagel G, Tolba R, Varnai A, et al. Detection of lymphatic invasion in early stage primary colorectal cancer with the monoclonal antibody D2-40. Eur Surg Res. 2006;38(5):438–444. doi: 10.1159/000095086. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Nakayama Y, Inoue Y, et al. Lymphatic microvessel density is an independent prognostic factor in colorectal cancer. Dis Colon Rectum. 2007;50(3):308–314. doi: 10.1007/s10350-006-0792-y. [DOI] [PubMed] [Google Scholar]
- Kaneko I, Tanaka S, Oka S, et al. Lymphatic vessel density at the site of deepest penetration as a predictor of lymph node metastasis in submucosal colorectal cancer. Dis Colon Rectum. 2006;50:13–21. doi: 10.1007/s10350-006-0745-5. [DOI] [PubMed] [Google Scholar]
- Fogt F, Zimmerman R, Ross H, et al. Identification of lymphatic vessels in malignant, adenomatous, and colonic mucosa using the novel immunostain D2-40. Oncol Rep. 2004;11(1):47–50. doi: 10.3892/or.11.1.47. [DOI] [PubMed] [Google Scholar]
- Kodaira S, Teramoto T, Ono S, et al. Lymph node metastases from carcinomas developing in pedunculated and semipedunculated colorectal adenomas. Aust N Z J Surg. 1981;15(5):429–433. doi: 10.1111/j.1445-2197.1981.tb05977.x. [DOI] [PubMed] [Google Scholar]
- Tada S, Yao T, Iida M, et al. A clinicopathologic study of small flat colorectal carcinoma. Cancer. 1994;74(9):2430–2435. doi: 10.1002/1097-0142(19941101)74:9<2430::aid-cncr2820740907>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Weidner N, Semple J, Welch W, et al. Tumor angiogenesis and metastasis-correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- Weidner N, Folkman J, Pozza F, et al. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst. 1992;84:1875–1887. doi: 10.1093/jnci/84.24.1875. [DOI] [PubMed] [Google Scholar]
- Maula S, Luukkaa M, Grenman R, et al. Intratumoral lymphatics are essential for the metastatic spread and prognosis in squamous cell carcinomas of the head and neck region. Cancer Res. 2003;63:1920–1926. [PubMed] [Google Scholar]
- Kayzas P, Gellef S, Batistatou A, et al. Evidence for lymphangiogenesis and its prognostic implications in head and neck squamous cell carcinoma. J Pathol. 2005;206:170–177. doi: 10.1002/path.1776. [DOI] [PubMed] [Google Scholar]
- Straume O, Jackson D, Akslen L, et al. Independent prognostic impact of lymphatic vessel density and presence of low-grade lymphangiogenesis in cutaneous melanoma. Clin Cancer Res. 2003;9:250–256. [PubMed] [Google Scholar]
- Nakamura Y, Yasuoka H, Tsujimoto M, et al. Lymph vessel density correlates with nodal status, VEGF-C expression, and prognosis in breast cancer. Cancer Res Treat. 2005;91:125–132. doi: 10.1007/s10549-004-5783-x. [DOI] [PubMed] [Google Scholar]
- Dadras S, Paul Y, Bertoncini J, et al. Tumor lymphangiogenesis: a novel prognostic indicator for cutaneous melanoma metastasis and survival. Am J Pathol. 2003;162:1951–1960. doi: 10.1016/S0002-9440(10)64328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad R, Kordunsky L, Liu Y, et al. Lymphatic microvessel density as prognostic marker in colorectal cancer. Mod Pathol. 2006;19:1317–1323. doi: 10.1038/modpathol.3800651. [DOI] [PubMed] [Google Scholar]
- Kaiseling E, Krober S, Geleff S. Lymphatic vessels in the colonic mucosa in ulcerative colitis. Lymphology. 2003;36(2):52–61. [PubMed] [Google Scholar]


