Abstract
We developed a substitute for serum to produce fed-batch cultures of hybridoma cells in serum-free medium and confirmed that the cells could be successfully cultivated this way. Our substitute consisted of 12 components. The specific production rates of lactate and ammonia, which are harmful byproducts from the cells, were significantly reduced compared with a conventional serum-containing batch culture. This reduction led to a higher cell concentration and a longer production lifetime. As a result, the final concentration of monoclonal antibody was 400 mg/L, or five times greater than that in the conventional serum-containing batch culture. The developed substitute is expected to enable fed-batch cultivation in a serum-free condition.
Keywords: Serum-free culture, Fed-batch culture, Hybridoma cells, Ammonia, Lactate, Antibody production
Introduction
Fed-batch cultivation of mammalian cells has been routinely used for industrial production of biological compounds (Reuveny et al. 1985; Spier 1994; Liddell and Weeks 1995; Khoudi et al. 1999). In vitro culturing of hybridoma cells has been extensively studied for a high production rate of monoclonal antibody for several decades, and many efficient nutrient feeding strategies have been developed (Glacken 1987; Bibila and Robinson 1995; Distefano et al. 1996; Birch and Racher 2006). The most important strategy for doing fed-batch culture is to reduce the formation of byproducts, such as lactate and ammonia, and keep the nutrient concentration balanced and stable. Low accumulation of metabolites is critical to produce a high viable cell density and a long production lifetime, which can lead to high production of biological compounds. Cell growth over time, i.e. the integral of viable cells (IVC) is often used as an indicator for a cultivation producing monoclonal antibody. Lactate and ammonia are derived mainly from the cellular metabolism of glucose and glutamine, respectively. Their production can be partly decreased by reducing the glucose and glutamine concentrations in the medium. In recent studies, effective cultures of hybridoma and Chinese hamster ovary (CHO) cells have been developed through comprehensive research on nutrient metabolism (Bibila and Robinson 1995; Distefano et al. 1996; Birch and Racher 2006). The models in these studies incorporated not only major pathways of glucose and glutamine metabolism but also pathways of other amino acids into the stoichiometric model. As a result, highly efficient production of monoclonal antibody was achieved.
However, most of these studies have been done using serum-supplemented culture systems. Using serum is undesirable for the following reasons. Firstly, there have been a lot of problems related to virus infections transmitted by animal serum for production of biological compounds which can threaten safety of the products. Secondly, experimental data can be greatly affected by serum lots which have much influence on cell proliferation. Another point to consider is unknown factors in serum might affect the cellular metabolism and complicate optimizing nutrient feeding. Therefore, in this study, we have developed a manageable substitute for serum for fed-batch cultures of hybridoma cells and confirmed the cells could be successfully cultivated using our developed substitute.
Strategies for serum-free fed-batch cultivation
Mammalian cells require a complex nutrient environment for growth and survival in vitro. The typical cell culture medium is composed of carbon sources such as glucose, amino acids, vitamins, inorganic salts, buffers and various other components including serum. Some of the components, such as inorganic salts, are generally abundant, while others are often present in small amounts for cell growth. Some of the components are consumed in large amounts, but there must be others such as trace metals which are essential for biological functions even though they are consumed at a low level. A medium for batch cultivation must contain abundant nutrients at high concentrations whereas a medium for fed-batch cultivation are controlled to contain low concentration levels of glucose and amino acids according to a variety of stoichiometric models. Most of the fed-batch cultivations reported so far have been done by adding serum into the initial medium. However, to reduce unknown risks arising from serum when fed-batch cultivation is carried out, it would be very useful if there were a material of confirmed ingredients that could replace serum. Recently, some serum-free media for batch cultivation have been developed and released commercially. We tested some of these available serum-free media, and after confirming the proliferation of CRL-1606 and CHO cells in batch cultivation experimentally, which is not generally known, we chose IBL Media III (Immuno-Biological Laboratories, Takasaki, Japan) as a model for the development of a substitute for serum in fed-batch cultivation that has all the components needed for cell growth and survival by CRL-1606 and CHO cells.
For the first step, we analyzed all ingredients in IBL media III and categorized them into four groups: glucose and amino acids group, B vitamins group, inorganic salts group, and others. Moreover, the components in IBL Media III are divided logically into two categories which are modifiable and not modifiable for fed-batch cultivation.
Glucose and amino acids group
Glucose and amino acids are ingredients of IBL Media III. They are the most important factor for design of feeding media and should be added into media under a stoichiometric model, therefore, they were not considered as the components for a substitute for serum.
B vitamins group
Vitamins are essential for cell growth in vitro. Many important enzymes and cofactors are synthesized from vitamins. Nine B vitamins are contained in IBL Media III. A brief description for each vitamin and its physiological functions in cells are as follows. Thiamine pyrophosphate is a coenzyme for several enzymes that catalyze the dehydrogenation of alpha-keto acids. Riboflavin is the central component of the cofactors flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN). Nicotinamide is incorporated into nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP) which function as coenzymes in a wide variety of enzymatic oxidation-reduction reactions essential for lipid metabolism, and glycogenolysis. Pantothenic acid is needed to form coenzyme-A (CoA), and is critical in the metabolism and synthesis of carbohydrates, proteins, and fats. Biotin is used in cell growth, the production of fatty acids, metabolism of fats, and amino acids. Folic acid is necessary for the production and maintenance of new cells. Cyanocobalamin is involved in cell metabolism, and especially affects DNA synthesis and regulation as well as fatty acid synthesis and energy production. Choline is one of the major donors for the one carbon metabolism. Inositol is needed for intracellular calcium concentration control, cell membrane potential maintenance and gene expression.
Thus, all of the B vitamins in the media are considered to be critical for cell survival and growth. Apoptosis at a late phase is a controversial issue for improving production of antibodies. The impact on apoptosis by depriving the media of the B vitamins group has been reported (Ishaque and Al-Rubeai 2002). Deletion of either pantothenic acid (D-Ca pantothenate), choline chloride, or riboflavin was responsible for the development of apoptosis. Media should not end up being deprived of B vitamins and we should keep more than limit concentrations by adding feeding media containing B vitamins. Most feeding media contain B vitamins whose composition is usually determined empirically (Eagle 1955). Regarding all these factors, as B vitamins should be added into the initial media and feeding media, we did not include them in the components of a substitute for serum.
The concentrations of vitamins in both the initial medium and the feeding medium were decided by following to Xie et al. work (1994), where the concentrations were decided so that they were not depleted under appropriate feeding control. In our study the vitamin concentrations were not analyzed due to the lack of a routine assay procedure. However, the amounts of vitamins added in fed-batch cultivations exceeded the requirements for cell growth. For example, in the experiment fed with the feeding media, the amount of vitamins added was estimated to be sufficient to attain a cell density of 3.8 × 106 cells mL−1, whereas the total cell density reached only 3.0 × 106 cells mL−1.
Inorganic salts group
Mammalian cells also consume significant amounts of metal ions and phosphorus in the form of phosphate. Most of the inorganic salts are present in excess in the basal medium, which is used in batch culture by mixing it with serum, to maintain a physiological osmolality. When an initial medium is designed, organic salts and their concentrations are determined by following the basal medium for targeted cells. Therefore these components should be added into the initial medium and they were not selected for the components of a substitute for serum.
Others
After we excluded glucose and amino acids, B vitamins and inorganic salts from IBL Media III, 12 components (Table 1) were left. A summary for each component and its physiological functions in cells follows below.
Table 1.
Components of substitutes for serum
| Component | Substitute (standard concentration) (mM) | Substitute (high concentration) (mM) |
|---|---|---|
| Insulin | 0.0017 | 0.017 |
| Transferrin | 0.00013 | 0.0013 |
| p-Aminobenzoic acid | 0.0015 | 0.015 |
| Pyridoxine hydrochloride | 0.011 | 0.11 |
| Sodium selenite | 0.000038 | 0.00038 |
| Sodium pyruvate | 0.75 | 7.5 |
| Hypoxanthine disodium | 0.0074 | 0.074 |
| Linoleic acid | 0.000073 | 0.00073 |
| Lipoic acid | 0.00025 | 0.0025 |
| Putrescine dihydrochloride | 0.00025 | 0.0025 |
| Thymidine | 0.00075 | 0.0075 |
| Glutathione | 0.00081 | 0.0081 |
Substitute (standard concentration) includes the selected components from IBL Media III as a substitute for serum. Substitute (high concentration) has a 10-fold increase on concentrations of the components of Substitute (standard concentration)
Insulin is a hormone that promotes glucose and amino acid uptake by the cell (Umpleby and Russell-Jones 1996). Transferrin is an iron transport protein that functions to transport iron into the cell (Yabe et al. 1987). Glutathione is also needed for the detoxification of methylglyoxal, a toxin produced as a by-product of metabolism (Abordo et al. 1999). Sodium selenite is a source of selenium found in many food supplements. Selenium is an enzyme cofactor that activates glutathione peroxidase, a player in the detoxification of oxygen radicals (Guérin and Gauthier 2003). p-Aminobenzoic acid is used in the formation of folic acid and the metabolism of protein (Whiteside-Carlson and Carlson 1949). Pyridoxine hydrochloride acts as a coenzyme in all transamination reactions, and in some decarboxylation and deamination reactions of amino acids (Yoshimura et al. 1996). Pyruvate is a key intersection in the network of metabolic pathways (De Meirleir 2002). Pyruvate is supplied in the form of sodium salt in the culture medium. Hypoxanthine is involved in DNA repair by preventing formation of deaminated adenine residues (Karran and Lindahl 1980). Hypoxanthine is added in the form of disodium salt in the culture medium. Linoleic acid leads to reinitiation of DNA synthesis and growth when added to quiescent cells (Holley and Kiernan 1974). The conjugate base of lipoic acid is lipoate which is mainly present at physiological conditions. Lipoate is able to scavenge reactive oxygen species and reduce other metabolites (Tirosh et al. 1999). Putrescine appears to be a growth factor necessary for cell division (Sjöholm 1993). Dihydrochloride salt is used for the source of putrescine in the culture medium. Thymidine is significant because of its involvement in the biosynthesis of DNA and in the preservation and transfer of genetic information (Sismour and Benner 2005).
After considering the above information, the remaining 12 components seemed to allow cells to survive, so we chose them as the components for the substitute to be developed, though some of the components might be non-essential to cells. In addition to that, we had to consider the special features of the fed-batch cultivation to determine the concentration of the components for the substitute. It has been reported that fed-batch cultivation produces several times more maximum viable cells than batch cultivation does and it also needs a longer cultivation time than batch cultivation does, which means that the effective components in the initial medium might degrade and they might be present in too-small amounts at the late phase of fed-batch cultivation. Though we did not analyze the degradation of initial media in this study due to the lack of a routine assay procedure, for instance, insulin, which is one of the remaining 12 components, is reported to degrade with increasing incubation time at 37 °C in isolated swine adipocytes (Etherton et al. 1984). In Etherton et al.’s report, insulin decreased by half in 2 h. After we considered all these factors, we prepared two substitutes for serum, which we called Substitute (standard concentration) and Substitute (high concentration); their compositions are shown in Table 1. Substitute (standard concentration) was prepared from the selected 12 components from IBL Media III. Substitute (high concentration) had 10-fold higher concentrations of the components of Substitute (standard concentration). This was done based on the fact that IBL Media III is designed for batch cultivation and it is known that fed-batch cultivation has a 10-fold larger IVC than batch cultivation.
Materials and methods
Cell line and culture medium
The host cell line in this experiment was a mouse–mouse hybridoma, CRL-1606, producing anti-human fibronectin monoclonal antibody. We purchased the cell line from American Type Culture Collection (ATCC; Manassas, VA).
In the serum-containing batch culture we used Iscove’s Modified Dulbecco’s Medium (IMDM; Sigma-Aldrich, St. Louis, MO) and 5% of fetal bovine serum (BioSource International, Inc., Camarillo, CA). In the serum-free batch culture we used IBL Media III medium (Immuno-Biological Laboratories, Takasaki, Japan).
In the serum-free fed-batch cultivation we prepared the culture medium according to the method of Xie and Wang (1994). The culture medium consisted of an initial medium and a feeding medium. The initial medium was based on IMDM, which is often used in combination with serum for the conventional cultivation of hybridoma. The concentration of vitamins in the initial medium was similar to that in IMDM. However, to reduce harmful ammonia formation, the levels of amino acids were kept lower than in IMDM. In this fed-batch culture we prepared the initial medium just using the 12 components shown in Table 1 instead of serum. For the source of the inorganic salts, we used IBL Media III. The feeding medium contained multiple nutrients with a stoichiometrically balanced composition. These nutrients were controlled simultaneously through feeding by controlling one of them. The feeding medium had the same composition as the medium of Xie and Wang did. All ingredients of the medium we used are shown in Table 2.
Table 2.
Composition of culture media in the experiments
| Batch cultivation with serum (IMDM + 5% FBS) (mM) | Batch cultivation with Substitute I (IBL Media III) (mM) | Fed-batch cultivation with Substitute | ||
|---|---|---|---|---|
| Initial medium (mM) | Feeding medium (mM) | |||
| Serum or substitute for serum | 5% FBS | Substitute (standard concentration) | Substitute (standard or high concentration) | – |
| Glucose and amino acids | ||||
| Glucose | 0.75 | 17.8 | 2.0 | 115.4 |
| Alanine | – | 0.17 | – | – |
| Arginine | 0.1 | 0.74 | 0.1 | 7.4 |
| Asparagine | 0.5 | 0.22 | 0.5 | 3.08 |
| Aspartic acid | 1.0 | 0.18 | 1.0 | 8.64 |
| Cystine | 0.1 | 0.15 | – | – |
| Cysteine | – | 0.15 | 0.1 | 3.49 |
| Glutamic acid | 0.5 | 0.31 | 0.5 | 5.01 |
| Glutamine | 0.2 | 2.8 | 0.2 | 41.9 |
| Glycine | 1.0 | 0.26 | 1.0 | 5.41 |
| Histidine | 0.1 | 0.048 | 0.1 | 2.69 |
| Hydroxyproline | – | 0.038 | 0.038 | – |
| Isoleucine | 0.2 | 0.5 | 0.2 | 5.27 |
| Leucine | 0.2 | 0.52 | 0.2 | 10.1 |
| Lysine | 0.2 | 0.5 | 0.2 | 8.47 |
| Methionine | 0.1 | 0.13 | 0.1 | 2.71 |
| Phenylalanine | 0.1 | 0.23 | 0.1 | 3.98 |
| Proline | 0.2 | 0.29 | 0.2 | 2.0 |
| Serine | 0.5 | 0.3 | 0.5 | 3.19 |
| Threonine | 0.2 | 0.47 | 0.2 | 7.15 |
| Tryptophan | 0.1 | 0.047 | 0.1 | 1.37 |
| Tyrosine | 0.2 | 0.27 | 0.2 | 3.23 |
| Valine | 0.2 | 0.47 | 0.2 | 7.51 |
| B vitamine | ||||
| D-Biotin | 0.00005 | 0.002 | 0.00005 | 0.051 |
| Choline chlorede | 0.029 | 0.044 | 0.029 | 2.54 |
| Folic acid | 0.009 | 0.0057 | 0.009 | 0.09 |
| Myo-inositol | 0.04 | 0.093 | 0.04 | 1.35 |
| Niacinamide | 0.033 | 0.018 | 0.033 | 0.37 |
| Pyridoxal | 0.02 | 0.011 | 0.02 | 0.51 |
| Riboflavin | 0.001 | 0.0008 | 0.001 | 0.033 |
| Thiamine | 0.012 | 0.0068 | 0.012 | 0.084 |
| D-Ca pantothenate | 0.017 | 0.0045 | 0.017 | 0.11 |
| Vitamin B12 | 0.00005 | 0.00026 | 0.00001 | 0.007 |
| Inorganic salts | ||||
| NaHCO3 | 60.0 | 27.4 | 27.4 | – |
| HEPES | – | 18.75 | 18.75 | – |
| Ca(NO3)2 | – | 0.15 | 0.15 | – |
| KCl | 5.0 | 4.3 | 4.3 | – |
| MgSO4 | 2.0 | 0.5 | 0.5 | – |
| NaCl | 60.0 | 97.0 | 97.0 | – |
| Na2HPO4 | 3.0 | 1.66 | 1.66 | – |
| KNO3 | – | 0.00038 | 0.00038 | – |
| NaH2PO4 | – | 0.45 | 0.45 | – |
| CaSO4 | – | 0.0000026 | 0.0000026 | – |
| FeSO4 | 0.1 | 0.0013 | 0.0013 | – |
| ZnSO4 | – | 0.00074 | 0.00074 | – |
| CaCl2 | 2.0 | 0.81 | 0.81 | – |
| MgCl2 | – | 0.00015 | 0.00015 | – |
Culture conditions
The experiment was performed in a 1-L bioreactor (Able Co., Tokyo, Japan) with a volume of 800 mL of culture medium and an initial cell density of 1 × 108 cells L−1 at a stirring rate of 30 rpm at 37 °C. Dissolved oxygen was monitored and controlled at 60% of saturated air by adjusting the inlet gas composition. The inlet gas, composed of oxygen, carbon dioxide, and nitrogen, was passed through a porous (10 μm) SUS316 sparger immersed in the culture medium at a gas flow rate of 1 mL min−1. pH was controlled at 7.2 by the addition of 0.5 M NaOH. We aseptically took 5 mL of samples two or three times a day to measure the density and viability of the cells as well as the concentrations of glutamine, glucose, ammonia and lactate. After centrifugation at 800g for 10 min, supernatant was then stored at −20 °C for later analysis of monoclonal antibody concentration. In the fed-batch culture, feeding medium was added to maintain a relatively constant nutritional environment. Volume of feeding medium was decided by prediction of cell growth using the following equation when we took samples every 12 h. As the concentration of components of feeding medium was very high, the volume of feeding medium was very small, (less than 40 mL against 800 mL of media in the reactor at one time.
 |
Xie and Wang controlled the concentration of nutrients at a low level without depletion of nutrients through their cultivation and we did a fed-batch cultivation applying the same feeding strategy as for Xie and Wang. So we thought the concentration of medium components in supernatant was less than that in batch cultivation and not depleted. We adopted glutamine as an indicator for feeding control. The glutamine concentration ranged from 0.3 to 1.8 mM for the cultivations.
Analytical methods
The concentration of viable cells and viability were automatically determined using the Vi-CELLTM automated cell viability analyzer (Beckman Coulter, Fullerton, CA). The concentration of the main components of the culture medium such as glucose, glutamine, glutamate, ammonia and lactate was determined using a Nova Bioprofile 100 Plus Analyzer (Nova Biomedical Corp., Waltham, MA). The enzyme-linked immunosorption assay (ELISA) was employed to determine the antibody titer. Cells were centrifuged for 10 min at 800g with a centrifuge (Kokusan, Tokyo, Japan) prior to the antibody assay. Kappa mouse IgG1 (Sigma-Aldrich Corp., St. Louis, MO) was used as a standard. First, 100 mL antigen solution (antibody to mouse IgG1; Sigma) were placed in a 96-well microtiter plate and incubated for 1 h. After rinsing with 3 × 100 mL washing buffer (1:10 diluted Block Ace solution plus 0.05% Tween20; Snow Brand Milk Products Co., Ltd., Tokyo, Japan), the wells were blocked with 1:4 diluted Block Ace solution at 37 °C for 1 h. Samples were added to the wells, and antibody was able to bind to them by incubating the wells at 37 °C for 1 h. The wells were then washed three times with washing buffer. Bound antibodies were detected with alkaline phosphatase conjugate antibody (Vector Laboratories, Inc., Burlingame, CA). Incubation and washing steps were as described above. Signals were developed with p-nitrophenyl phosphate (PNPP) substrate (Vector Laboratories, Inc., Burlingame, CA), and the absorbance at 405 nm was measured.
Results and discussion
A fed-batch example of CRL-1606 cell line is given here to demonstrate the fed-batch capacity and potential of using our developed substitute for serum. We conducted fed-batch and batch cultivations in 1-L bioreactors under appropriate conditions. Fed batch cultivations were conducted with initial medium containing Substitute (standard concentration) or Substitute (high concentration) instead of serum and feeding medium which was designed by Xie and Wang (1994). In batch cultivations, Iscove’s Modified Dulbecco’s medium (IMDM) containing 5% fetal bovine serum was used as a conventional cultivation, while IBL Media III was used in order to compare with serum-free fed-batch cultivation. All ingredients of the medium used in our study are shown in Table 2.
Cell growth
We confirmed that CRL-1606 cells could survive and grow in a serum-free fed-batch cultivation just by adding Substitute (standard concentration) or Substitute (high concentration) instead of serum. As shown in Fig. 1 these cells began to increase after a lag phase and decreased after about 490 h. But significant differences were seen between Substitute (standard concentration) and Substitute (high concentration) regarding the proliferation. In the case of using Substitute (high concentration) viable cells increased continuously until the end of the culture and reached 3 × 109 cells L−1 while in the case of using Substitute (standard concentration) viable cells increased until 140 h and then viable cell density was maintained at a constant value of 1 × 109 cells L−1. A fed-batch cultivation with Substitute (standard concentration) had early entry into the stationary phase and the stationary phase was long. We attribute that to the following. In Bordetella pertussis, transcript abundance levels are varied in response to nutrient depletion and then they enter into the stationary phase in order to survive longer (Nakamura et al. 2006). So depletion of some nutrients of Substitute (standard concentration) might lead the cells to enter the stationary phase. The values of proliferation and viable cell density in fed-batch cultivation were higher than those of batch cultivation. IVC of fed-batch cultivation using Substitute (high concentration) and Substitute (standard concentration) were 0.54 and 0.32 × 1012 cells h L−1, respectively. These values were much larger than those of serum-containing and serum-free batch cultures, which were 0.1 and 0.02 × 1012 cells h L−1, respectively. In conclusion, effective fed-batch cultivations could be carried out by applying Substitute (standard concentration) and Substitute (high concentration).
Fig. 1.
A growth profile of CRL-1606 cells in fed-batch cultures using designed substitutes and conventional batch cultures. Medium in batch cultivations with serum and with Substitute (standard concentration) were Iscove’s Modified Dulbecco’s medium (IMDM) containing 5% fetal bovine serum and IBL Media III, respectively. Fed-batch cultivations with Substitute (standard concentration) and Substitute (high concentration) were done with IMDM based initial medium containing Substitute (standard concentration) or Substitute (high concentration) instead of serum and feeding medium. The feeding medium was designed by Xie and Wang (1994)
Production of lactate and ammonia
Figure 2 shows production of byproducts, ammonia and lactate, correlated with IVC. Production of ammonia increased concomitant with IVC (Fig. 2A). The specific formation rate of ammonia (the slope of the line) varied depending on the stage of a culture but significant differences were not seen between fed-batch cultivations with Substitute (high concentration) and Substitute (standard concentration) nor between batch cultivation with serum and batch cultivation with Substitute (standard concentration). The slope of the line for fed-batch cultivations was lower than that of batch cultivations at every IVC value. This means that the difference in slope (ammonia production rate) is not due to the difference in ingredients between Substitute (high concentration) and Substitute (standard concentration) and serum, but due to culture methods (fed-batch or batch).
Fig. 2.
Comparison of by-products production in fed-batch culture using designed substitutes and conventional batch cultures. Medium in batch cultivations with serum and with Substitute (standard concentration) were Iscove’s Modified Dulbecco’s medium (IMDM) containing 5% fetal bovine serum and IBL Media III, respectively. Fed-batch cultivations with Substitute (standard concentration) and Substitute (high concentration) were done with IMDM based initial medium containing Substitute (standard concentration) or Substitute (high concentration) instead of serum and feeding medium. The feeding medium was designed by Xie and Wang (1994)
The specific formation rate of lactate (the slope of the line) varied depending on the stage of a culture (Fig. 2B). Significant differences of specific formation rate of lactate were not seen between cultivations. But interestingly, production of lactate was changed to consumption in the case of fed-batch cultivation with Substitute (high concentration). Though glucose is the most preferred source for glycolysis, after cell growth approached an apparent stationary phase, the cellular metabolism seems to have shifted from glucose to lactate consumption. Lactate can also be utilized together with glucose as carbon and energy sources with an approximate molar exchange of one glucose molecule = two lactate molecules when glucose is limiting. Therefore both lactate and glucose can be considered as carbon and energy sources and the cumulative consumption of glucose and lactate combined QGL exhibits a linear relationship relative to the IVC in CHO cells. QGL is defined as follows:
 |
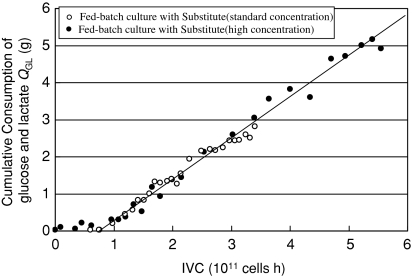
where G represents glucose concentration and L represents lactate concentration. Though L0 − Lt is less than 0 at the early stage of cultivation, lactate can be produced and stocked as an energy source (Tsao et al. 2005). Therefore we investigated the relationship between cumulative consumption of glucose and lactate combined and IVC in our fed-batch cultivation and found the linear relationship between them as shown in Fig. 3. Judging from this result, consumption of lactate is probably due to utilization of lactate as carbon and energy sources, though the mechanism is unclear still.
Fig. 3.
Correlation between cumulative consumption of glucose and lactate combined (QGL) plotted and integral of viable cells (IVC). QGL = (G0 + L0) − (Gt + Lt), where G represents glucose concentration and L represents lactate concentration. Fed-batch cultivations with Substitute (standard concentration) and Substitute (high concentration) were done with IMDM based initial medium containing Substitute (standard concentration) or Substitute (high concentration) instead of serum and feeding medium. The feeding medium was designed by Xie and Wang (1994)
This feature is very useful for producing antibodies because lactate is known to inhibit antibody production (Glacken 1987). In fed-batch cultivation with Substitute (high concentration) lactate concentration was cut to 3.1 mM. So the improvement of the antibody production rate at the late stage in fed-batch culture is thought to be due to reduction of lactate concentration.
Production of antibody
The concentrations of antibody increased concomitant with the integrated value of viable density over time as shown in Fig. 4. All of the production rates of antibody exhibited a linear relationship relative to the IVC below 3.4 × 1012 cells h L−1 The production rate of antibody with Substitute (standard concentration) was almost the same as that of Substitute (high concentration), but the production rate of antibody with serum was a little higher than that of substitutes. This means serum is a little more effective than our substitutes. Above the IVC value of 3.4 × 1012 cells h L−1, the antibody production rate increased dramatically. This is thought to be due to decrease of lactate concentration, as we stated. The enhancement of antibody production by the fed-batch cultivation was due to the increase of ICV. The final concentration of monoclonal antibody of the serum-free fed-batch culture was 400 mg L−1, or five times greater than that in the serum-containing batch culture, which is the conventional method.
Fig. 4.
Comparison of antibody concentrations in fed-batch cultures using designed substitutes and conventional batch cultures plotted against integral of viable cells (IVC). Medium in batch cultivations with serum and with Substitute (standard concentration) were Iscove’s Modified Dulbecco’s medium (IMDM) containing 5% fetal bovine serum and IBL Media III, respectively. Fed-batch cultivations with Substitute (standard concentration) and Substitute (high concentration) were done with IMDM based initial medium containing Substitute (standard concentration) or Substitute (high concentration) instead of serum and feeding medium. The feeding medium was designed by Xie and Wang (1994)
Comparison of culture performance
We prepared two substitutes for serum, Substitute (standard concentration) and Substitute (high concentration) and compared their performances. As stated above, Substitute (high concentration) had better performance in fed-batch cultivation than Substitute (standard concentration), therefore we would like to propose Substitute (high concentration) as a substitute for serum. Culture performances among batch and fed-batch experiments are summarized in Table 3.
Table 3.
Comparison of culture performances
| Serum-free fed-batch Substitute (high concentration) | Serum-free fed-batch Substitute (standard concentration) | Serum-free batch | Serum-containing batch | |
|---|---|---|---|---|
| Culture span (h) | 537 | 547 | 117 | 132 |
| Maximum viable cell density (109 cells L−1) | 3.0 | 1.0 | 0.36 | 1.7 |
| Integral of viable cells (1012 cells h L−1) | 0.54 | 0.32 | 0.02 | 0.10 |
| Monoclonal antibody concentration (mg L−1) | 400 | 200 | 9 | 80 |
| Average specific antibody production rate (10−9 mg cell−1 h−1) | 0.74 | 0.62 | 0.45 | 0.80 |
| Final ammonia concentration (mM) | 8.0 | 8.2 | 3.5 | 3.8 |
| Final lactate concentration (mM) | 3.1 | 15.8 | 1.6 | 31.0 |
| Average specific formation rate of ammonia (10−9 mmole cell−1 h−1) | 0.014 | 0.025 | 0.18 | 0.038 |
| Average specific formation rate of lactate (10−9 mmole cell−1 h−1) | 0.006 | 0.049 | 0.080 | 0.31 |
In the case of fed-batch cultivation with Substitute (high concentration) for serum, both maximum viable cell density and culture time were extended significantly, compared to serum-free and serum-containing batch cultures. The value of IVC reached 0.54 × 1012 cells h L−1, or more than five times that of conventional batch culture using serum in mammalian cells. As a result, we could get large production of antibody. From the viewpoint of productivity per day, the value in this fed-batch was 19 mg L−1 day−1. It was 1.5-fold higher than the productivity per day for conventional batch culture, though the fed-batch culture needed a longer culture time than the batch culture. Higher concentration of antibody is considered to be better for manufacturing production because low concentration of antibody leads to a bigger loss during purification (Fahrner et al. 1999). There was no marked difference for average specific antibody production rate between fed-batch and conventional batch cultures, though Substitute (high concentration) was thought to be less effective than serum as stated above. It was because the amount of lactate, an inhibitor for producing antibody was dramatically reduced at the late stage of fed-batch cultivation.
Average specific formation rate of ammonia was 0.014 × 10−9 mmole cell−1 h−1, a decrease of 60% from batch cultivation. Average specific formation rate of lactate was 0.006 × 10−9 mmole cell−1 h−1, a decrease of 98% from batch cultivation. Thus, the rates of average specific formation of ammonia and lactate were significantly reduced by keeping glucose, glutamine and other amino acids at a low level through feeding control of concentration based on a stoichiometric model. Thus, the Substitute (high concentration) we developed was effective for maintaining the features of a fed-batch cultivation. We consider serum substitute to have a potential for application to other cell lines as we could culture CHO cells successfully in the batch cultivation using Substitute (high concentration) and to satisfy various user needs for fed-batch cultivation.
Conclusion
A substitute for serum for fed-batch cultivation has been developed and it has been confirmed to effectively cut the amounts of ammonia and lactate formed as byproducts and to enhance monoclonal antibody production in the serum-free fed-batch culture of CRL-1606 cells. Judging from the proliferation of CHO cells in a batch cultivation using the substitute, we expect that our developed substitute will be suitable for fed-batch culture application to other cell lines (e.g. CHO cells) and it is expected to facilitate serum-free fed-batch culturing.
Nomenclature
- ΔVF
Volume of feeding medium fed to the reactor since the nth sample was taken, L
- β
Total stoichiometric coefficient of glucose, amino acids, and vitamins, nmole cell−1
- Nt
Total cell number in the reactor at culture time t, number of cells
- Nt,n
Total cells in the reactor when the nth sample was taken, number of cells
- Ct
Total concentration of glucose, amino acids, and vitamins in the supplemental medium, mM
References
- Abordo EA, Minhas HS, Thornalley PJ (1999) Accumulation of alpha-oxoaldehydes during oxidative stress: a role in cytotoxicity. Biochem Pharmacol 58(4):641–648 [DOI] [PubMed]
- Bibila TA, Robinson DK (1995) In pursuit of the optimal fed-batch process for monoclonal antibody production. Biotechnological Prog 11:1–13 [DOI] [PubMed]
- Birch JR, Racher AJ (2006) Antibody production. Adv Drug Deliv Rev 58(5–6):671–685 [DOI] [PubMed]
- De Meirleir L (2002) Defects of pyruvate metabolism and the Krebs cycle. J Child Neurol 17(Suppl 3):3S26–3S33 (discussion 3S33–3S34) [PubMed]
- Distefano DJ, Mark GE, Robinson DK (1996) Feeding of nutrients delays apoptotic death in fed-batch cultures of recombinant NS0 myeloma cells. Biotechnol Lett 18:1067–1072 [DOI]
- Eagle H (1955) Nutrition needs of mammalian cells in tissue culture. Science 122:501–504 [DOI] [PubMed]
- Etherton TD, Chung CS, Wiggins JP (1984) Receptor-dependent and independent degradation of insulin by isolated swine adipocytes at 37°C. J Anim Sci 59:366–375 [DOI] [PubMed]
- Fahrner RL, Whitney DH, Vanderlaan M, Blank GS (1999) Performance comparison of protein A affinity-chromatography sorbents for purifying recombinant monoclonal antibodies. Biotechnol Appl Biochem 30(2):121–128 [PubMed]
- Glacken MW (1987) Development of mathematical descriptions of mammalian cell culture kinetics for the optimization of fed-batch bioreactors. Ph.D. thesis, MIT, Cambridge, MA, USA
- Guérin PJ, Gauthier ER (2003) Induction of cellular necrosis by the glutathione peroxidase mimetic ebselen. J Cell Biochem 89(1):203–211 [DOI] [PubMed]
- Holley RW, Kiernan JA (1974) Control of the initiation of DNA synthesis in 3T3 cells: low-molecular weight nutrients. Proc Natl Acad Sci USA 71(8):2942–2945 [DOI] [PMC free article] [PubMed]
- Ishaque A, Al-Rubeai M (2002) Role of vitamins in determining apoptosis and extent of suppression by bcl–2 during hybridoma cell culture. Apoptosis 7(3):231–239 [DOI] [PubMed]
- Karran P, Lindahl T (1980) Hypoxanthine in deoxyribonucleic acid: generation by heat-induced hydrolysis of adenine residues and release in free form by a deoxyribonucleic acid glycosylase from calf thymus. Biochemistry 19:6005–6011 [DOI] [PubMed]
- Khoudi H, Laberge S, Ferullo JM, Bazin R, Darveau A, Castonguay Y, Allard G, Lemieux R, Vézina LP (1999) Production of a diagnostic monoclonal antibody in perennial Alfalfa plants. Biotechnol Bioeng 64:135–143 [DOI] [PubMed]
- Liddell E, Weeks I (1995) Anitibody technology. Bios Scientific Publishers, UK, pp 65–130
- Nakamura MM, Liew SY, Cummings CA, Brinig MM, Dieterich C, Relman DA (2006) Growth phase- and nutrient limitation-associated transcript abundance regulation in Bordetella pertussis. Infect Immun. 74(10):5537–5548 [DOI] [PMC free article] [PubMed]
- Reuveny S, Velez D, Riske F, MacMillan JD, Miller L (1985) Production of monoclonal antibodies in culture. Dev Biol Stand 60:185–197 [PubMed]
- Sismour AM, Benner SA (2005) The use of thymidine analogs to improve the replication of an extra DNA base pair: a synthetic biological system. Nucleic Acids Res 33(17):5640–5646 (Print 2005) [DOI] [PMC free article] [PubMed]
- Sjöholm A (1993) Role of polyamines in the regulation of proliferation and hormone production by insulin-secreting cells. Am J Physiol 264(3 Pt 1):C501–C518 [DOI] [PubMed]
- Spier RE (1994) Animal cell biotechnology in the 1990 s, from models to morals. In: Spier RE, Griffiths JB (eds) Animal cell biotechnology. Cambridge, UK, pp 1–43
- Tirosh O, Sen CK, Roy S, Kobayashi MS, Packer L (1999) Neuroprotective effects of alpha-lipoic acid and its positively charged amide analogue. Free Radic Biol Med 26(11–12):1418–1426 [DOI] [PubMed]
- Tsao YS, Cardoso AG, Condon RG, Voloch M, Lio P, Lagos JC, Kearns BG, Liu Z (2005) Monitoring Chinese hamster ovary cell culture by the analysis of glucose and lactate metabolism. J Biotechnol 118(3):316–327 [DOI] [PubMed]
- Umpleby AM, Russell-Jones DL (1996) The hormonal control of protein metabolism. Baillieres Clin Endocrinol Metab 10(4):551–570 [DOI] [PubMed]
- Whiteside-Carlson V, Carlson WW (1949) Studies on the effect of para-aminobenzoic acid, folic acid, and sulfanilamide on dextran synthesis by leuconostoc. J Bacteriol 58(2):143–149 [PMC free article] [PubMed]
- Xie L, Wang DI (1994) Applications of improved stoichiometric model in medium design and fed-batch cultivation of animal cells in bioreactor. Cytotechnology 15(1–3):17–29 [DOI] [PubMed]
- Yabe N, Kato M, Matsuya Y, Yamane I, Iizuka M, Takayoshi H, Suzuki K (1987) Role of iron chelators in growth-promoting effect on mouse hybridoma cells in a chemically defined medium. In vitro Cell Dev Biol 23(12):815–820 [DOI] [PubMed]
- Yoshimura T, Jhee KH, Soda K (1996) Stereospecificity for the hydrogen transfer and molecular evolution of pyridoxal enzymes. Biosci Biotechnol Biochem 60(2):181–187 [DOI] [PubMed]