Abstract
Effect of [6]-gingerol, a major pungent component in ginger, on the proliferation of a rat ascites hepatoma AH109A cells was investigated by measuring [3H]thymidine incorporation into acid-insoluble fraction of the cultured cells and that on the invasion by co-culturing the hepatoma cells with rat mesentery-derived mesothelial cells. [6]-Gingerol inhibited both the proliferation and invasion of hepatoma cells in a dose-dependent manner at concentrations of 6.25–200 μM (proliferation) and 50–200 μM (invasion). [6]-Gingerol accumulated cells in S phase and elongated doubling time of hepatoma cells, and increased the rate of apoptosis. Hepatoma cells previously cultured with hypoxanthine (HX) and xanthine oxidase (XO) or with hydrogen peroxide showed increased invasive activities. [6]-Gingerol suppressed the reactive oxygen species-potentiated invasive capacity by simultaneously treating AH109A cells with [6]-gingerol, HX and XO or with [6]-gingerol and hydrogen peroxide. Furthermore, [6]-gingerol reduced the intracellular peroxide levels in AH109A cells. These results suggest that the suppression of hepatoma cell proliferation by [6]-gingerol may be due to cell cycle arrest and apoptosis induction. They also suggest that the anti-oxidative property of [6]-gingerol may be involved in its anti-invasive activity of hepatoma cells.
Keywords: Anti-oxidative property, Apoptosis, Cell cycle, [6]-Gingerol, Hepatoma cells, Hydrogen peroxide, Invasion, Proliferation, Reactive oxygen species
Introduction
[6]-Gingerol, a major pungent component in ginger has been shown to possess various pharmacological activities such as anti-oxidative and anti-inflammatory activities (Surh 2002; Wang et al. 2003). The latter is due to the inhibitory effect of [6]-gingerol on the expression of cyclooxygenase 2, a key enzyme in the prostaglandin biosynthesis and recognized as a molecular target for anti-inflammatory and cancer chemopreventive agents (Kim et al. 2004). [6]-Gingerol has been reported to induce apoptosis in HL60 cells (Lee and Surh 1998) and inhibit metastasis of B16 melanoma cells (Suzuki et al. 1997). The invasion is particularly complicated process and key step in the cancer metastatic cascade (Liotta et al. 1988). During the past 20 years, primary liver cancer, 95% of which is hepatocellular carcinoma, has ranked third in men and fifth in women as a cause of death from malignant neoplasm in Japan (Kiyosawa et al. 2004). AH109A cells, a rat ascites hepatoma line, grow rapidly both in vitro and in vivo, and they form solid tumors when subcutaneously inoculated into rats. Using AH109A cells, we have demonstrated that some food factors such as carotenoids (Kozuki et al. 2000), catechins (Zhang et al. 2000), resveratrol (Kozuki et al. 2001), ascorbic acid (Hirakawa et al. 2005), lignans (Miura D et al. 2007) suppress the proliferation and/or invasion of the hepatoma cells. Furthermore, we have confirmed an involvement of reactive oxygen species (ROS) in hepatoma cell invasion: both the exogenous and endogenous ROS induce the invasion of AH109A cells (Kozuki et al. 2000; Miura Y et al. 2003a). The present study was attempted to investigate the effect of [6]-gingerol on the proliferation and invasion of AH109A cells in culture and its modes of actions.
Materials and methods
Materials
[6]-Gingerol was purchased from Nakalai Tesque, Inc., Kyoto, Japan. It was dissolved in ethanol and the solution was added to the medium at a final ethanol concentration of 0.2%. Control medium contained 0.2% ethanol alone. All other reagents were of the best grade commercially available.
Culture of AH109A hepatoma cells
The experiments in this article were conducted in accordance with guidelines established by the Animal Care and Use Committee of Tokyo Noko University. Male Donryu rats (4 weeks of age) were purchased from NRC Haruna (Gunma, Japan). AH109A cells were generously provided by the Cell Resource Center for Biomedical Research, Tohoku University, Sendai, Japan. AH109A cells were maintained in peritoneal cavity of male Donryu rats, and isolated from accumulated ascites and then cultured in Eagle’s minimum essential medium (MEM) (Nissui Pharmaceutical Co., Tokyo, Japan) containing 10% calf serum (CS, Equitech-Bio, Inc., Kerrville, TX, USA) (10% CS/MEM). These cells were cultured for at least 2 weeks after isolation to eliminate contaminated macrophages and neutrophils, and used for the assays described below.
In vitro proliferation and invasion assays
Effect of [6]-gingerol on the proliferation was examined by measuring the incorporation of [methyl-3H]thymidine (20 Ci/mmol, Perkin Elmer Life and Analytical Sciences, Inc., Boston, MA, USA) into acid-insoluble fraction of cells, as described previously (Yagasaki et al. 1992). Briefly, 1.0 × 104 AH109A cells were seeded into a 48-well plate and exposed to [6]-gingerol for 24 h in 400 μL of 10% CS/MEM. The proliferation of AH109A cells was evaluated by measuring the incorporation for last 4 h of [methyl-3H]thymidine (0.15 μCi/well) into the DNA fraction. Effect of [6]-gingerol on the invasion was examined by the co-culture system (Akedo et al. 1986) with slight modifications as described previously (Kozuki et al. 2000). Briefly, mesothelial cells (M-cells) were isolated from mesentery of male Donryu rats (6–8 weeks of age). After digestion by trypsin, 1.3–2.0 × 105 M-cells were plated in a 60 mm culture dish with 2 mm grids (Corning Incorporated, Corning, NY, USA), and cultured for 5–7 days to attain a confluent state in 10% CS/MEM. Then, AH109A cells (2.4 × 105 cells per dish) were applied on the monolayer of M-cells in 10% CS/MEM with [6]-gingerol for 24 h. Invaded cells and colonies underneath M-cells were counted with a phase-contrast microscope. The invasive activity of AH109A cells was expressed as the number of invaded cells and colonies/cm2.
Flowcytometric analyses of cell cycle phases and Annexin-V-FITC staining of apoptotic cells
For cell cycle analysis, 2.5 × 105 cells of AH109A per well were seeded in a 6-well plate in the medium containing 0, 25, 50, 100, and 200 μM [6]-gingerol and cultured for 24 h. Cells were collected and washed twice with sterilized, Ca2+- and M2+-free, phosphate-buffered saline [PBS(−)]. Thereafter, 500 μL of propidium iodide (PI) solution containing 1 mg of PI (Sigma Chemical Co., St. Louis, MO, USA) in 20 mL of 1% Triton X-100 (Sigma), and 0.1% of sodium citrate (Wako Pure Chemical Industries Ltd., Osaka, Japan) was added and cells were incubated for 30 min on ice. Cells at different cell cycle phases were then analyzed with a flow cytometer (EPICS ELITE EPS, Beckman-Coulter, Hialeah, FL, USA) as previously described (Zhang et al. 2000).
The effect of [6]-gingerol on apoptosis in AH109A cells was assessed using ANNEXIN-V FITC kit (IMMUNOTECH, Marseille, France) according to the manufacturer’s instructions. Phosphatidylserine (PS), which can specifically bind to Annexin-V, is one of the phospholipids in the cell membrane and exists predominantly in inner leaflet of the cell membrane of normal cells. When apoptosis occurs, PS in the cell membrane immediately appears on the outer leaflet of the cell membrane. The cells with PS on their surface can thus be thought to be early apoptotic cells. Briefly, 5 × 105 cells of AH109A per well were seeded in a 6-well plate and cultured in the medium containing 0, 100, and 200 μM [6]-gingerol for 3 h. At the end of culture, cells were labeled with Annexin-V-FITC and analyzed with a flow cytometer as described previously (Miura Y et al. 2004).
Pretreatment of AH109A cells with hypoxanthine and xanthine oxidase or hydrogen peroxide
AH109A cells were cultured for 4 h in the absence or presence of 20 μM [6]-gingerol with or without a ROS-generating system, i.e., 4 μg/mL hypoxanthine (HX, Sigma, St. Louis, MO) with 7 × 10−4 U/mL xanthine oxidase (XO, Sigma) (Shinkai et al. 1986; Tanaka et al. 1997) or 25 μM hydrogen peroxide (H2O2, Wako Pure Chemical Industries). AH109A cells were then washed once with 10% CS/MEM and seeded on the M-cell monolayer in 10% CS/MEM without [6]-gingerol and ROS. After cultured for 24 h, invaded cells and colonies underneath M-cells were counted with a phase-contrast microscope as described above.
Flowcytometric analysis of intracellular peroxide in AH109A cells
Intracellular peroxide levels in AH109A cells were assessed by flow cytometric analysis using a fluorometric probe (2′, 7′-dichlorofluorescin diacetate; DCFH-DA, Molecular Probes, Eugene, OR) (Bass et al. 1983) with a flow cytometer as described previously (Miura Y et al. 2003b).
Statistical analysis
Data were expressed as means ± SEM. Multiple comparison was performed by one-way analysis of variance (ANOVA) followed by Tukey–Kramer multiple comparisons test, and p < 0.05 was considered statistically significant.
Results
Effect of [6]-gingerol on the proliferation and invasion of AH109A cells
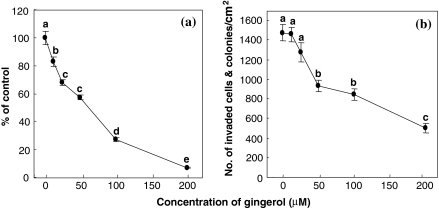
The effect of [6]-gingerol on the proliferation and invasion of AH109A cells is shown in Fig. 1. [6]-Gingerol dose-dependently and significantly inhibited the AH109A proliferation at concentrations 12.5–200 μM (Fig. 1a). Likewise, [6]-gingerol commenced to significantly suppress the AH109A invasion at a concentration of 50 μM, and thereafter maintained the inhibitory effect up to 200 μM (Fig. 1b). The inhibitory action of [6]-gingerol appeared to be stronger on the proliferation than on the invasion.
Fig. 1.
Effect of [6]-gingerol on the proliferation (a) and invasion (b) of AH109A cells. [6]-Gingerol was dissolved in ethanol. The [6]-gingerol solution was added to the culture medium at a final ethanol concentration of 0.2%. The proliferative activity of AH109A cells (a) was determined by the incorporation of [methyl-3H]thymidine and the invasive activity (b) by invasion assay as described in “Materials and methods” section. Data are the means ± SEM of six wells (a: proliferation) and 10 areas (b: invasion). Values not sharing a common letter are significantly different at p < 0.05 by Tukey–Kramer multiple comparisons test
Accumulation in S phase and induction of apoptosis in [6]-gingerol-treated AH109A cells
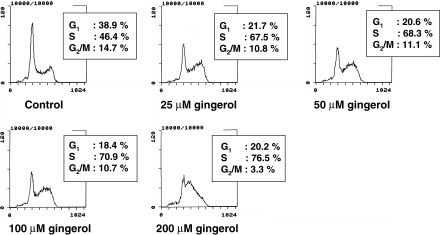
To elucidate the mechanisms of [6]-gingerol action, its effect on cell cycle was examined by flow cytometry (Fig. 2). [6]-Gingerol treatment led to dose-dependent increases in the fractions of AH109A cells in the S phase: the fraction of S phase increased from 46% at 0 μM to 77% at 200 μM.
Fig. 2.
Effect of [6]-gingerol on cell cycle of AH109A cells by propidium iodide stained flow cytometric analysis. AH109A cells were treated with [6]-gingerol at 0, 25, 50, 100 and 200 μM, and were processed for flow cytometric analysis. 10,000 events were acquired. Data are from a representative experiment repeated three times with similar results
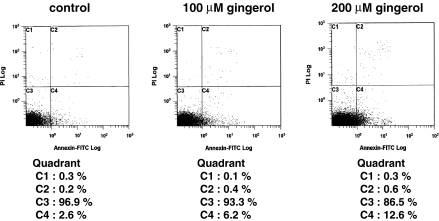
The induction of apoptosis in AH109A cells was studied by using Annexin-V-FITC labeling. Analysis of the cell population showed four distinct sets of cells (Fig. 3). Annexin-V-negative and PI-negative cells (lower left quadrant) designated as viable cells. Annexin-V-positive and PI-negative cells (lower right quadrant) designated as early apoptotic cells, Annexin-V-positive and PI-positive cells (upper right quadrant) designated as late apoptosis, and Annexin-V-negative and PI-positive cells (upper left quadrant) designated as necrotic cells. In this study, AH109A cells were treated with 100 and 200 μM [6]-gingerol for 3 h. As depicted in Fig. 3, [6]-gingerol led to the dose-dependent appearance of PS on the outer leaflet of the plasma membrane as shown by increased portions of Annexin-V-positive and PI-negative cells (lower right quadrant).
Fig. 3.
Induction of apoptosis by [6]-gingerol in AH109A cells. Hepatoma cells were treated with [6]-gingerol at 0, 100 and 200 μM for 3 h, and stained with Annexin-V-FITC as described in “Materials and methods” section. Data are from a representative experiment repeated three times with similar results
Effect of [6]-gingerol on the invasion of AH109A cells pre-treated with hypoxanthine and xanthine oxidase or hydrogen peroxide
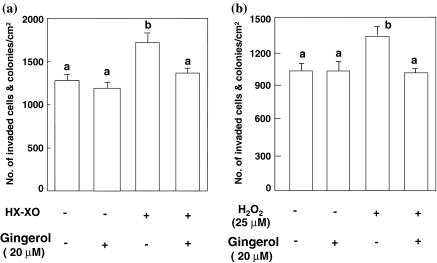
To examine whether or not [6]-gingerol would inhibit the invasion of tumor cells by its anti-oxidative activity, the invasion assay was performed with AH109A cells pre-cultured in ROS-containing medium. As shown in Fig. 4a, the invasive activity of AH109A cells pre-cultured in the HX–XO system, which generates ROS, was significantly higher than that of AH109A cells with no treatment. [6]-Gingerol inhibited the ROS-potentiated invasive activity of the pre-cultured cells when added to the medium at a concentration of 20 μM on pre-culturing the hepatoma cells with HX and XO. Likewise, the invasive activity of AH109A pre-treated with hydrogen peroxide (H2O2) was significantly elevated, and this rise was canceled by adding [6]-gingerol (20 μM) to the medium containing 25 μM hydrogen peroxide (Fig. 4b). In our preliminary experiment, 20 μM of [6]-gingerol exposure for 4 h to AH109A cells exerted no influence on the following AH109A proliferation during 24 h (data not shown). Thus, the concentration of 20 μM was adopted in these experiments to avoid an influence of the [6]-gingerol-mediated proliferation inhibition on the AH109A invasion.
Fig. 4.
Effect of [6]-gingerol on the invasion of AH109A cells pre-cultured with HX–XO (a) or hydrogen peroxide (b). AH109A cells were cultured for 4 h in the absence or presence of 20 μM of [6]-gingerol and/or HX with XO or 25 μM of hydrogen peroxide (H2O2). After the treatment, AH109A cells were washed and applied onto the monolayer of M-cells in 10%CS/MEM. The invasive activity was determined as described in “Materials and methods” section. Data are the means ± SEM of 10 areas. Values not sharing a common letter are significantly different at p < 0.05 by Tukey–Kramer multiple comparisons test
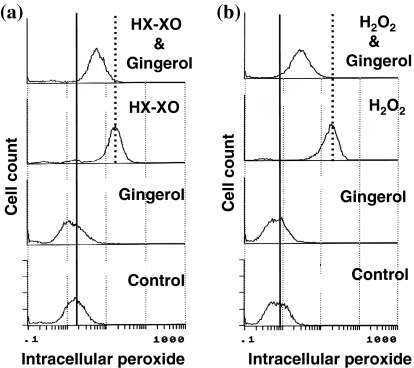
Intracellular peroxide levels of AH109A cells pre-treated with hypoxanthine and xanthine oxidase or hydrogen peroxide
As illustrated in Fig. 5, AH109A cells treated by HX–XO (Fig. 5a) or 50 μM hydrogen peroxide (Fig. 5b) for 4 h contained more intracellular peroxides than did control cells (control versus HX–XO, control versus H2O2) when analyzed with a flow cytometer using DCFH-DA as an indicator. [6]-Gingerol (20 μM) inhibited these rises in the intracellular peroxide levels of AH109A cells (HX–XO versus HX–XO & [6]-gingerol, H2O2 versus H2O2 & [6]-gingerol).
Fig. 5.
Flowcytometric analysis of intracellular peroxide in AH109A cells treated with exogenous reactive oxygen species (ROS) and [6]-gingerol. AH109A cells were treated with HX–XO (a) or 50 μM of hydrogen peroxide (H2O2) (b) as ROS resources. After ROS treatment for 4 h, DCFH-DA was added at a final concentration of 64 μM and the cells were incubated for 20 min. Basal and ROS activated intracellular peroxide levels were indicated as solid and dotted lines, respectively. Data are from a representative experiment repeated three times with similar results
Discussion
In this study, [6]-gingerol was demonstrated to inhibit both the proliferation and invasion of AH109A cells in vitro (Fig. 1). [6]-Gingerol concentrations that could significantly suppress the proliferation of AH109A cells (Fig. 1a, 12.50–200 μM) were lower than those which could suppress the invasion of AH109A cells (Fig. 1b, 50–200 μM). [6]-Gingerol commenced to significantly inhibit the hepatoma proliferation at a concentration of 12.5 μM and almost completely inhibited (6% of control) at 200 μM (Fig. 1a), whereas it significantly inhibited the invasion at 50 μM, which was four times as high as 12.5 μM, and the highest inhibition observed at 200 μM was 34% of control (Fig. 1b). These results suggest that the inhibition of proliferation by [6]-gingerol might not be a main cause of its inhibitory action against the invasion.
To elucidate the mechanisms of inhibition of hepatoma cell proliferation by [6]-gingerol, the effect of [6]-gingerol treatment on cell cycle and on the rate of apoptosis was examined by using flow cytometry. [6]-Gingerol was found to suppress proliferation from lower concentrations (25 μM∼) by increasing doubling time of AH109A cells through accumulation in the S phase (Fig. 2). Further, [6]-gingerol increased the rate of apoptosis at higher concentrations (100–200 μM) (Fig. 3). [6]-Gingerol has been reported to induce apoptosis in HL60 cells (Lee and Surh 1998). It is apparent that different cell lines have different sensitivities to [6]-gingerol but the primary target in cell cycle and apoptosis may be the same in these cell lines. Although the doses of [6]-gingerol which induced apoptosis were higher than those which induced cell cycle arrest in AH109A hepatoma cells, these results suggest that [6]-gingerol affects both cell proliferation and cell death which at least partly account for the inhibitory effect of [6]-gingerol on the proliferation of AH109A cells.
Our previous works have demonstrated that the invasion of AH109A cells is accelerated by ROS (Kozuki et al. 2000). In the present study, we therefore examined the effect of [6]-gingerol on the ROS-potentiated invasive activity using both HX–XO system and H2O2. [6]-Gingerol was found to inhibit the ROS-induced elevation of the AH109A invasion (Fig. 4). [6]-Gingerol was also found to scavenge intracellular peroxides (Fig. 5). We have found that ROS can induce gene expression of hepatocyte growth factor (HGF), which is known as a cell motility factor (Parr and Jiang 2001), in M-cells as well as AH109A cells (Miura Y et al. 2003b). Thus, HGF produced by both AH109A and M-cells may potentiate the motility of AH109A cells and also may induce the retraction of M-cells, leading to acceleration of the AH109A invasion. Provided that [6]-gingerol, like a polyphenol resveratrol (Miura D et al. 2004), suppresses the production of HGF through its anti-oxidative activity, [6]-gingerol may diminish the induction of the retraction of M-cells as well as the motility of AH109A cells, this leading to the effective reduction of the AH109A invasion by reducing the functions of both cells at the same time. Since prostaglandins are shown to enhance the invasion of hepatoma cells (Miura D et al. 2003), a possibility that [6]-gingerol, a cyclooxygenase inhibitor (Kim et al. 2004), interrupts prostaglandin synthesis and hence inhibits the invasion cannot be ruled out. Lee et al. have recently reported that [6]-gingerol inhibits cell adhesion, invasion, motility and activities of matrix metalloproteinase (MMP)-2 and 9 in MDA-MB-231 human breast cancer cells (Lee et al. 2007). AH109A cells are different from this breast cancer cells, for instance, in the productivity of MMPs; AH109A cells do not produce MMPs. Thus, different action sites as well as common action sites of [6]-gingerol may be existent between these two cancer cells. Further studies are required to clarify these aspects.
In summary, we clearly demonstrated that [6]-gingerol inhibited the proliferation and invasion of AH109A hepatoma cells in culture, the proliferation being more strongly suppressed than was the invasion. [6]-Gingerol induced cell cycle arrest at lower concentrations and apoptosis at higher concentrations in the hepatoma cells. It also suppressed the ROS-induced increases in invasive capacity and intracellular peroxide levels. These results suggest that [6]-gingerol affects both cell proliferation and cell death that account for, at least partly, the inhibitory effect of [6]-gingerol on the proliferation of AH109A cells. They also suggest that the anti-oxidative property of [6]-gingerol may be involved in its anti-invasive action. [6]-Gingerol may have promising beneficial effects in preventing tumor growth and metastasis and may be of significance from the aspect of nutritional control of cancers.
Acknowledgment
This work was supported by the Fund from the Yamazaki Spice Foundation, Tokyo, Japan.
Abbreviations
- CS
Calf serum
- HX
Hypoxanthine
- M-cells
Mesothelial cells
- MEM
Eagle’s minimum essential medium
- ROS
Reactive oxygen species
- XO
Xanthine oxidase
References
- Akedo H, Shinkai K, Mukai M, Mori Y, Tateishi R, Tanaka K, Yamamoto R, Morishita T (1986) Interaction of rat ascites hepatoma cells with cultured mesothelial cell layers: a model for tumor invasion. Cancer Res 46(5):2416–2422 [PubMed]
- Bass DA, Parce JW, Dechatelet LR, Szejda P, Seeds MC, Thomas M (1983) Flow cytometric studies of oxidative product formation by neutrophils: a guarded response to membrane stimulation. J Immunol 130(4):1910–1917 [PubMed]
- Hirakawa N, Miura Y, Yagasaki K (2005) Inhibitory effect of ascorbic acid on the proliferation and invasion of hepatoma cells in culture. Cytotechnology 47(1–3):133–138 [DOI] [PMC free article] [PubMed]
- Kim SO, Chun KS, Kundu JK, Surh YJ (2004) Inhibitory effects of [6]-gingerol on PMA-induced COX-2 expression and activation of NF-kappaB and p38 MAPK in mouse skin. Biofactors 21(1–4):27–31 [DOI] [PubMed]
- Kiyosawa K, Umemura T, Ichijo T, Matsumoto A, Yoshizawa K, Gad A, Tanaka E (2004) Hepatocellular carcinoma: recent trends in Japan. Gastroenterology 127(5 Suppl 1):S17-S26 [DOI] [PubMed]
- Kozuki Y, Miura Y, Yagasaki K (2000) Inhibitory effects of carotenoids on the invasion of rat ascites hepatoma cells in culture. Cancer Lett 151(1):111–115 [DOI] [PubMed]
- Kozuki Y, Miura Y, Yagasaki K (2001) Resveratrol suppresses hepatoma cell invasion independently of its anti-proliferative activity. Cancer Lett 167(2):151–156 [DOI] [PubMed]
- Lee E, Surh YJ (1998) Induction of apoptosis in HL-60 cells by pungent vanilloids, [6]-gingerol and [6]-paradol. Cancer Lett 134(2):163–168 [DOI] [PubMed]
- Lee HS, Seo EY, Kang NE, Kim WK (2007) [6]-Gingerol inhibits metastasis of MDA-MB-231 human breast cancer cells. J Nutr Biochem. doi:10.1016/j.jnutbio.2007.05.008 [DOI] [PubMed]
- Liotta LA, Wewer U, Rao NC, Schiffmann E, Stracke M, Guirguis R, Thorgeirsson U, Muschel R, Sobel M (1988) Biochemical mechanisms of tumor invasion and metastases. Adv Exp Med Biol 233:161–169 [DOI] [PubMed]
- Miura D, Miura Y, Yagasaki K (2003) Restoration by prostaglandins E2 and F2α of resveratrol-induced suppression of hepatoma cell invasion in culture. Cytotechnology 43(1–3):155–159 [DOI] [PMC free article] [PubMed]
- Miura D, Miura Y, Yagasaki K (2004) Resveratrol inhibits hepatoma cell invasion by suppressing gene expression of hepatocyte growth factor via its reactive oxygen species-scavenging property. Clin Exp Metastasis 21(5):445–451 [DOI] [PubMed]
- Miura D, Saarinen N, Miura Y, Santti R, Yagasaki K (2007) Hydroxymatairesinol and its mammalian metabolite enterolactone reduce the growth and metastasis of subcutaneous AH109A hepatomas in rats. Nutr Cancer 58(1):49–59 [DOI] [PubMed]
- Miura Y, Tsukamoto S, Yagasaki K (2003a) Blockade of endogenous reactive oxygen species by N-acetyl-l-cysteine suppresses the invasive activity of rat hepatoma cells by modulating the expression of hepatocyte growth factor gene. Cytotechnology 43(1–3):121–126 [DOI] [PMC free article] [PubMed]
- Miura Y, Kozuki Y, Yagasaki K (2003b) Potentiation of invasive activity of hepatoma cells by reactive oxygen species is mediated by autocrine/paracrine loop of hepatocyte growth factor. Biochem Biophys Res Commun 305(1):160–165 [DOI] [PubMed]
- Miura Y, Ono K, Okauchi R, Yagasaki K (2004) Inhibitory effect of coffee on hepatoma proliferation and invasion in culture and on tumor growth, metastasis and abnormal lipoprotein profiles in hepatoma-bearing rats. J Nutr Sci Vitaminol 50(1):38–44 [DOI] [PubMed]
- Parr C, Jiang WG (2001) Expression of hepatocyte growth factor/scatter factor, its activator, inhibitors and the c-Met receptor in human cancer cells. Int J Oncol 19(4):857–863 [PubMed]
- Shinkai K, Mukai M, Akedo H (1986) Superoxide radical potentiates invasive capacity of rat ascites hepatoma cells in vitro. Cancer Lett 32(1):7–13 [DOI] [PubMed]
- Surh YJ (2002) Anti-tumor promoting potential of selected spice ingredients with antioxidative and anti-inflammatory activities: a short review. Food Chem Toxicol 40(8):1091–1097 [DOI] [PubMed]
- Suzuki F, Kobayashi M, Komatsu Y, Kato A, Pollard RB (1997) Keishi-ka-kei-to, a traditional Chinese herbal medicine, inhibits pulmonary metastasis of B16 melanoma. Anticancer Res 17(2A):873–878 [PubMed]
- Tanaka M, Kogawa K, Nishihori Y, Kuribayashi K, Nakamura K, Muramatsu H, Koike K, Sakamaki S, Niitsu Y (1997) Suppression of intracellular Cu–Zn SOD results in enhanced motility and metastasis of Meth A sarcoma cells. Int J Cancer 73(2):187–192 [DOI] [PubMed]
- Wang CC, Chen LG, Lee LT, Yang LL (2003) Effects of 6-gingerol, an antioxidant from ginger, on inducing apoptosis in human leukemic HL-60 cells. In Vivo 17(6):641–645 [PubMed]
- Yagasaki K, Tanabe T, Ishihara K, Funabiki R (1992) Modulation of the proliferation of cultured hepatoma cells by urea cycle-related amino acids. In: Murakami H, Shirahata S, Tachibana H (eds) Animal Cell Technology: Basic and Applied Aspects, vol. 4. Kluwer Academic Publishers, Dordrecht , pp 257–263
- Zhang G, Miura Y, Yagasaki K (2000) Induction of apoptosis and cell cycle arrest in hepatoma cells by in vivo metabolites of teas. Nutr Cancer 38(2):265–273 [DOI] [PubMed]