Abstract
Propionibacterium acnes is a gram-positive, non-spore-forming, rod-shaped bacterium that is often detected in normal human skin flora. P. acnes has been associated with many diseases. In this study, we attempted to generate anti-P. acnes human monoclonal antibodies. A phage antibody library was first generated from human peripheral blood mononuclear cells immunized in vitro with P. acnes using the phage display method, and P. acnes-specific phage antibodies were obtained using solid phase panning. Antigen-specific variable region genes were then amplified and recombined into vectors expressing human IgG antibodies. The results indicated that the recombinant human IgG antibodies exhibited P. acnes-specific binding. This study demonstrates that the combined use of an in vitro immunization protocol and the phage display method enables the generation of human monoclonal antibodies against pathogenic bacteria and toxic antigens.
Keywords: Human monoclonal antibody, In vitro immunization, Phage display, Propionibacterium acnes
Introduction
Phage display is a powerful method for screening peptide sequences bound to a specific protein by in vitro selection and obtaining its respective DNA (Smith 1985). Thus, the phage display method is considered to be useful for obtaining antigen-specific antibody genes. Using the phage display method, heavy chain and light chain immunoglobulin variable region (VH and VL, respectively) genes were amplified and assembled to form a single-chain Fv (scFv) via two-step polymerase chain reaction (overlap extension PCR) (Lerner et al. 1992). After the ligation of this scFv fragment into a phagemid vector, scFv was expressed as a phage antibody by infecting the harboring vector with the helper phage M13KO7 (Hellmuth et al. 1994). Antigen-specific phage antibodies can be obtained after several rounds of panning using specific antigens. Thus far, antigen-specific phage antibodies and their respective variable region genes have been cloned using the phage display method.
The phage display method is useful for obtaining antigen-specific antibody genes. However problems are still encountered when the generated antibodies are used in the treatment of human diseases (Bruggemann 2005; Kellermann and Green 2002). Mouse-derived antibodies are immunogenic to humans and often cause anaphylactic reactions. Therefore, we attempted to obtain Propionibacterium acnes-specific human monoclonal antibody genes by employing in vitro immunization to expand B cells producing P. acnes-specific antibodies (Ichikawa et al. 1999) and by using the phage display method to select variable region (V region) genes of P.acnes-specific antibodies.
Materials and methods
Preparation of P. acnes
P. acnes was purchased from the American Type Culture Collection (ATCC; bacterial strain, #11827) and cultured in GAM broth (Nissui, Tokyo, Japan) at 37 °C under anaerobic conditions for 3 weeks.
In vitro immunization of human peripheral blood mononuclear cells with heat-killed P. acnes
Human peripheral blood mononuclear cells (PBMCs) were obtained from a healthy donor and were separated by density-gradient centrifugation using a lymphocyte separation medium (LSM; Organon Teknika, Durham, NC, USA), as described previously (Xu et al. 2004; Yamashita et al. 2002). Experiments throughout this study were carried out in accordance with the principles of the Declaration of Helsinki and the regulations of the ethics committee of Kyushu University’s Faculty of Agriculture. Isolated PBMCs were first treated with 0.25 mM l-leucyl-l-leucine methyl ester (LLME; Bachem, Torrance, CA, USA) for 20 min at room temperature. After washing with an eRDF medium (Invitrogen, Carlsbad, CA, USA), the cells were sensitized with killed P. acnes (10 μg mL−1) in the presence of interleukin-2 (IL-2) (1 unit·mL−1), IL-4 (10 ng mL−1), and D-type CpG ODN (1 μM; 5′-ggTGCATCGATGCAGGGGggG-3′, uppercase and lowercase letters indicate bases with phosphodiester- and phosphorothioate-modified backbones, respectively). The cells were then cultured in an eRDF medium supplemented with 2-mercaptoethanol (50 μM) and 10% heat-inactivated fetal bovine serum (FBS). After 3 days of culture, K-type CpG ODN (1 μM; 5′-tcgagcgttctcC-3′) was added, and the cells were cultured for an additional 3 days.
Enzyme-linked immunosorbent assay
The frequency of B cell producing P. acnes-specific antibody was determined using an enzyme-linked immunosorbent (ELISPOT) assay. Multiscreen HA filtration plates (Millipore, Bedford, MA, USA) were coated with 10 μg of killed P. acnes per well and incubated for 2 h at 37 °C; thereafter, they were blocked with 2% fish gelatin (FG) in phosphate-buffered saline (PBS) at 4 °C overnight. After washing the plates with PBS, in vitro-immunized PBMCs in an eRDF medium supplemented with 10% FBS were added to the plates in triplicate at a density of 1 × 105 cells·well−1 and cultured for 18 h in a humidified atmosphere at 37 °C and 5% CO2. After culturing, the plates were washed with PBS containing 0.05% Tween 20 (PBST) and incubated with diluted goat anti-human antibody conjugated with horseradish peroxidase (IgM-HRP; Biosource, Camarillo, CA, USA) for 2 h at 37 °C. After washing the plates with PBST, TrueBlue substrate solution (KPL, Gaithersburg, MD, USA) was added and the plates were incubated at 37 °C for 10 min.
Generation of antigen-specific phage antibody by phage display
Total RNA was prepared from the in vitro-immunized PBMCs by using a Total RNA Extraction Kit (Sigma, St. Louis, MO, USA). Total RNA (1 μg) was used as a template for the cDNA synthesis reaction using M-MLV reverse transcriptase (Promega, Madison, WI, USA). The VH and VL genes were amplified using KOD-plus-DNA polymerase (Toyobo, Osaka, Japan) and appropriate family-specific primers (Marks et al. 1991; Wang and Stollar 2000). Amplification involved 25 to 35 PCR cycles (94 °C for 15 s, 55 °C for 30 s, and 68 °C for 1 min). The amplified VH and VL genes were connected using a DNA linker coding a (Gly4Ser)3 peptide linker sequence in the direction of 5′-VH-linker-VL-3′, and the recombinant scFv fragment was then inserted into the phagemid vector pCANTAB5E using a recombinant phage antibody system kit (GE Healthcare UK Ltd., Amersham Place, UK). Phage antibodies were produced according to the method described in the manufacturer’s protocol.
P. acnes-specific phage antibodies were then selected via solid phase panning using P. acnes fixed onto a T-flask. After the phage library was incubated in the T-flask fixed with P. acnes and was thoroughly washed, the captured phage was amplified in E. coli TG1. This selection and amplification cycle was repeated 2 times after which the panning efficiency was evaluated. The colony-forming unit (cfu) of the acquired phage antibodies before and after each round of panning was measured by transforming log phase E.coli TG1 cells with phage.
Evaluation antigen-specificity of phage antibody by ELISA
ELISA was performed using the culture supernatants containing phage antibodies in order to examine their binding affinity to P. acnes. Microtiter plates (Nunc, Naperville, IL, USA) were coated with 10 μL well−1 of killed P. acnes in a carbonate buffer overnight at 4 °C and were then blocked with 1% FG in PBS for 2 h at 37 °C. After washing the plates, 100 μL of culture supernatants was added to the wells and incubated for 2 h at 37 °C. After washing the plates with PBST, diluted horseradish peroxidase-conjugated mouse anti-M13 mAb (GE Healthcare) was added, and the plates were incubated for 2 h at 37 °C. The plates were once again washed with PBST, and a substrate solution containing 0.3 mg mL−1 ABTS (2, 2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid)) (Sigma) was added. Absorbance at 405 nm was measured using a microtiter plate reader (Wako, Osaka, Japan).
Production of anti-P.acnes recombinant human monoclonal IgG in Chinese hamster ovary cells
VH and VL genes were amplified using vectors expressing scFv specific for P. acnes as templates and family-specific primers described elsewhere (paper in preparation) (Fig. 2). VH and VL genes were cloned into pSecTag2A/hIgH bearing the heavy chain constant region gene and pSecTag2A/hIgL bearing the light chain constant region gene, respectively. CHO cells were transfected with these expression vectors using Lipofectamine (Invitrogen), as described in the manufacturer’s instructions. Culture supernatants were collected and used for further analysis.
Fig. 2.
Schematic representation of scFv library generation. The variable region (V region) genes of the antibodies produced by in vitro-immunized peripheral blood mononuclear cells (PBMCs) were amplified by reverse transcription-polymerase chain reaction (RT-PCR) using a V region family-specific primer. Next, a single chain Fv (scFv) library was assembled by combining the heavy chain variable region (VH), linker, and the light chain variable region (VL) genes using overlap extension PCR and cloning this construct into the pCANTAB5E vector
Evaluation of P. acnes-specific binding of recombinant human IgG by ELISA
Microtiter plates (Nunc) were coated with 10 μg of antigens (P. acnes, Lactobacillus acidophilus, Listeria monocytogenes, FG, skim milk, or β-LG), incubated at 4 °C overnight, and then blocked with 1% ovalbumin, skim milk, or FG in PBS for 2 h at 37 °C. After washing the plate, recombinant human IgG was added to the plates that were then incubated for 2 h at 37 °C. After washing the plates 3 times with PBST, diluted goat anti-human IgG-HRP (Biosource) was added and the plates were incubated at 37 °C for 2 h. After washing the plates once again with PBST, a substrate solution containing ABTS was added. The absorbance at 405 nm was measured using a microtiter plate reader.
Results
The number of B cells producing anti-P. acnes antibodies increased using in vitro immunization
LLME-treated PBMCs were immunized in vitro with killed P. acnes in the presence of IL-2, IL-4, and D- and K-type CpG ODN. After culture for 6 days, an ELISPOT assay was performed using the cultured cells to count the number of B cells producing anti-P. acnes antibodies and to evaluate the efficiency of in vitro immunization as a method of inducing B cell responses specific to killed bacteria. The results indicated that the number of B cells producing P. acnes-specific antibodies increased when using in vitro immunization (Fig. 1).
Fig. 1.
In vitro immunization augmented the number of B cells secreting P. acnes-specific antibodies. Peripheral blood mononuclear cells (PBMCs) treated with l-leucyl-l-leucine methyl ester (LLME) were sensitized with killed P. acnes and were cultured in eRDF supplemented with 10% fetal bovine serum (FBS), interleukin-2 (IL-2), IL-4, and CpG ODN, as described in the Materials and methods. After 6 days of culture, the PBMCs were seeded into Multiscreen-HA plates coated with P. acnes or fish gelatin (FG). B cells secreting antigen-specific antibodies were detected as blue spots using TrueBlue staining. The number of spots was denoted at the lower right of each photograph
Construction of scFv phage library and selection of P.acnes-specific phage antibodies by panning
The V region genes of antibodies produced from the in vitro immunized PBMCs were amplified by reverse transcription (RT)-PCR. Next, we constructed an scFv phage library by combining the VH, linker, and VL genes using PCR, cloning this construct into the pCANTAB5E vector (Fig. 2), and infecting the vector with the M13KO7 helper phage, as described elsewhere (paper in preparation). We then attempted to select P. acnes-specific phage antibodies via solid phase panning using P. acnes fixed onto a T-flask, as described in Materials and methods. After the second round of panning, the antigen specificity of the phage antibodies was tested using solid-phase ELISA. Among the 60 selected clones, several phage antibodies demonstrated antigen-specific binding (Fig. 3).
Fig. 3.
Antigen-specificity of phage antibodies. Sixty randomly selected phage antibodies were tested for antigen specificity (black bar, P. acnes; white bar, fish geratin (FG)) using solid-phase enzyme-linked immunosorbent assay (ELISA)
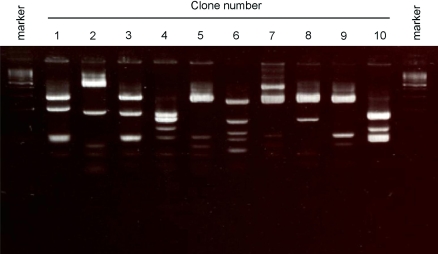
Clone diversity analysis
The digestion pattern of the V region gene with BstNI is reported to be inherent in respective clones (Marks et al. 1991). To investigate the diversity of the 10 selected clones, scFv DNA was amplified and digested with BstNI (Fig. 4). The results showed diverse digestion patterns, demonstrating that in vitro immunization expanded a diverse repertoire of antigen-specific B cells, suggesting that antigen-specific B cells with various antigen specificity can be obtained from this in vitro-immunized PBMC pool.
Fig. 4.
BstNI fingerprint analysis. Single chain Fv (ScFv) DNA was amplified using a plasmid isolated from P. acnes-specific clones as a template and was digested using BstNI. The digestion pattern was analyzed by 4% agarose gel electrophoresis
Production of anti-P. acnes human IgG antibodies in CHO cells
Three clones—D9, F6, and F10—were selected for production in CHO cells. The VH and VL genes of these scFv antibodies were PCR-amplified and cloned into the expression vectors pSecTag2A/hIgH and pSecTag2A/hIgL, respectively. CHO cells were transfected with these vectors and then cultured for several weeks. Supernatants containing recombinant human IgG were collected and subjected to ELISA in order to investigate the antigen-specificity of these antibodies. The results showed that the human IgG antibody derived from the D9 scFv clone demonstrated P. acnes-specific binding (Fig. 5), while the F6 and F10 antibodies antibodies demonstrated weak antigen specificity.
Fig. 5.
Antigen-specific binding of recombinant human IgG. Antigen-specific binding of human IgG monoclonal antibodies was evaluated by direct enzyme-linked immunosorbent assay (ELISA). The heavy chain and light chain variable region (VH and VL, respectively) genes of the phage antibodies (a, D9; b, F6; and c, F10) were amplified and inserted into their respective human IgG expression vectors. Chinese hamster ovary cells (CHO) cells were transfected with these expression vectors and cultured for several weeks. ELISA was used to evaluate the antigen specificity of these antibodies in the culture supernatants. Propionibacterium acnes, Lactobacillus acidophilus, Listeria monocytogenes, fish gelatin, skim milk, and β-lactoglobulin were used as antigens. Fish gelatin, skim milk, and ovalbumin were used as blocking reagents
Discussion
The phage display method offers many advantages for preparing antigen-specific mouse and human-derived scFv antibodies; however, it also presents many difficulties (Watanabe et al. 2002). In order to obtain antigen-specific antibody fragments by using the phage display method, a large library containing more than 109 clones is required. However, constructing a large phage display library from human PBMCs is very difficult (Perelson and Oster 1979). Thus, we attempted to generate a phage antibody library by using PBMCs immunized in vitro with specific antigens (Ichikawa et al. 1999; Matsumoto et al. 2006). We have reported that the in vitro immunization of PBMCs enables the induction of antigen-specific B cells and the expansion of a diverse repertoire of B cells, and that only a small number of PBMCs are required to obtain antigen-specific B cells. The present study showed that by using PBMCs immunized in vitro with killed P. acnes as a template for the phage display method, P. acnes-specific antibody fragments could be efficiently obtained from a relatively small phage library. Moreover, these results suggest that our strategy based on the use of in vitro immunization and the phage display method is useful for generating antibodies specific for pathogenic bacteria and toxic antigens.
Further, we succeeded in producing human IgG antibodies that exhibited P. acnes-specific binding in CHO cells. These antibodies, including the phage antibody scFv and the human IgG antibody specific for P. acnes, have the potential to be used as an in vitro reagents in the preparation of cosmetic and pharmaceutical products used for the diagnosis or treatment of skin problems caused by P. acnes. We predict that the industrial applications of these antibodies will broaden when their target proteins are identified.
Abbreviations
- IVI
In vitro immunization
- LLME
l-leucyl-l-leucine methyl ester
- PBMCs
Peripheral blood mononuclear cells
- P. acnes
Propionibacterum acnes
- VH
Heavy chain variable region
- VL
Light chain variable region
- ScFv
Single chain Fv.
References
- Bruggemann H (2005) Insights in the pathogenic potential of Propionibacterium acnes from its complete genome. Semin Cutan Med Surg 24:67–72 [DOI] [PubMed]
- Hellmuth K, Korz DJ, Sanders EA, Deckwer WD (1994) Effect of growth rate on stability and gene expression of recombinant plasmids during continuous and high cell density cultivation of Escherichia coli TG1. J Biotechnol 32:289–298 [DOI] [PubMed]
- Ichikawa A, Katakura Y, Teruya K, Hashizume S, Shirahata S (1999) In vitro immunization of human peripheral blood lymphocytes: establishment of B cell lines secreting IgM specific for cholera toxin B subunit from lymphocytes stimulated with IL-2 and IL-4. Cytotechnology 31:131–139 [DOI] [PMC free article] [PubMed]
- Kellermann SA, Green LL (2002) Antibody discovery: the use of transgenic mice to generate human monoclonal antibodies for therapeutics. Curr Opin Biotechnol 13:593–597 [DOI] [PubMed]
- Lerner RA, Kang AS, Bain JD, Burton DR, Barbas CF 3rd (1992) Antibodies without immunization. Science 258:1313–1314 [DOI] [PubMed]
- Marks JD, Hoogenboom HR, Bonnert TP, McCafferty J, Griffiths AD, Winter G (1991) By-passing immunization. Human antibodies from V-gene libraries displayed on phage. J Mol Biol 222:581–597 [DOI] [PubMed]
- Matsumoto SE, Yamashita M, Katakura Y, Noguchi E, Aiba Y, Ichikawa A, Teruya K, Shirahata S (2006) In vitro immunization can elicit the expansion of diverse repertoire of B cells from peripheral blood mononuclear cells. Cytotechnology 52:227–233 [DOI] [PMC free article] [PubMed]
- Perelson AS, Oster GF (1979) Theoretical studies of clonal selection: minimal antibody repertoire size and reliability of self-non-self discrimination. J Theor Biol 81:645–670 [DOI] [PubMed]
- Smith GP (1985) Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science 228:1315–1317 [DOI] [PubMed]
- Wang X, Stollar BD (2000) Human immunoglobulin variable region gene analysis by single cell RT-PCR. J Immunol Methods 244:217–225 [DOI] [PubMed]
- Watanabe H, Tsumoto K, Asano R, Nishimiya Y, Kumagai I (2002) Selection of human antibody fragments on the basis of stabilization of the variable domain in the presence of target antigens. Biochem Biophys Res Commun 295:31–36 [DOI] [PubMed]
- Xu Q, Katakura Y, Yamashita M, Fang S, Tamura T, Matsumoto SE, Aiba Y, Teruya K, Osada K, Nishikawa R, Shirahata S (2004) IL-10 augments antibody production in in vitro immunized lymphocytes by inducing a Th2-type response and B cell maturation. Biosci Biotechnol Biochem 68:2279–2284 [DOI] [PubMed]
- Yamashita M, Katakura Y, Shim S-Y, Matsumoto SE, Tamura T, Morihara K, Aiba Y, Teruya K, Tsuchiya T, Shirahata S (2002) Differential individual immune responses elicited by in vitro immunization. Cytotechnology 40:161–165 [DOI] [PMC free article] [PubMed]