Abstract
Sequential carbonyl addition-conjugate addition of Grignard reagents to cyclic 5–7–membered oxoalkenenitriles efficiently generates cyclic magnesiated nitriles. Alkylations of these magnesiated nitriles exhibit diastereoselectivities that depend intimately on the size of the carbocyclic ring: 5-membered oxonitriles generate magnesiated nitriles whose alkylations are controlled by steric constraints whereas 6- and 7-membered oxonitriles generate internally coordinated, C-magnesiated nitriles whose alkylations are controlled by stereoelectronic effects. Reversing the alkylation selectivity of 6-membered C-magnesiated nitriles is achieved by conversion to an N-metalated nitrile in which steric, rather than electronic, effects direct the electrophile trajectory. Collectively, the conjugate addition-alkylation generates highly substituted, cyclic 5–7-membered nitriles containing three new stereocenters with selective access to diastereomers at the quaternary nitrile-bearing carbon.
Introduction
Conjugate additions to alkenenitriles are notoriously difficult.1 Highly nucleophilic organometallics are predisposed to add to the polarized nitrile group whereas more modest nucleophiles, such as cuprates, often fail to react.2 Three general strategies have emerged to address the difficulty of conjugate addition to alkenenitriles:1 activating the alkenenitrile by conjugation with an additional electron-withdrawing group,3 using radicaloid species for the conjugate addition,4 and employing highly nucleophilic organometallic reagents in intramolecular conjugate additions to E-alkenenitriles where addition to the nitrile group is geometrically prevented.5 The latter strategy is particularly effective with cyclic nitriles in which highly nucleophilic alkylmagnesium alkoxides are temporarily tethered to the proximal alkenenitrile, effectively using intramolecular delivery to achieve a formal intermolecular conjugate addition.6
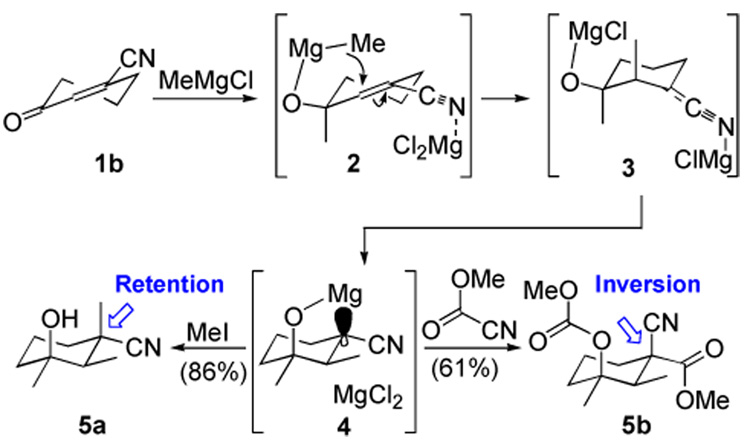
Cyclic γ-oxoalkenenitriles7 are excellent electrophiles for chelation-controlled conjugate addition reactions. Addition of excess MeMgCl to 3-oxo-1-cyclohexene-1-carbonitrile (1b)8 triggers a complex cascade (Scheme 1) in which carbonyl addition initially affords a halomagnesium alkoxide that suffers halogen-methyl exchange with a second equivalent of MeMgCl to generate the methylmagnesium alkoxide 2. Tethering the highly nucleophilic methylmagnesium alkoxide of 2 in close proximity to the alkenenitrile triggers a stereoelectronically controlled axial conjugate addition.6b Equilibration of the resulting N-magnesiated nitrile 3 to the more stable C-magnesiated nitrile 4 is favored by internal coordination and a conformation in which the two methyl groups adopt an equatorial orientation.
Scheme 1.
Electrophile-Dependent Alkylations of the C-Magnesiated Nitrile 4
Alkylations of the C-magnesiated nitrile 4 are highly unusual. Alkylating 4 with alkyl halides and sulfonates occurs with retention of the C-Mg configuration (4→5a), whereas aldehyde and acyl cyanide acylations proceed with inversion of stereochemistry (4→5b).9 The origin of this electrophile-dependent stereoselectivity stems from the size and accessibility of the electrophile's anti-bonding orbital. Highly reactive, planar electrophiles with large π* orbitals can achieve a favorable collinear overlap with the small orbital of the C-Mg bond in an invertive SE2 alkylation, whereas sterically demanding electrophiles with smaller anti-bonding orbitals can only access the C-Mg bond through a side-on overlap resulting in a retentive SE2 alkylation.10
The electrophile-dependent alkylations of the internally coordinated C-magnesiated nitrile 4 directly contrast with alkylations of comparable N-11 and C-metalated nitriles.12 In general, deprotonating cyclic nitriles with lithium amides generate planar N-lithiated nitriles (7, Scheme 2) whose alkylations are controlled by steric effects.13 For example, deprotonating the conformationally constrained nitrile 6 exposes two potentially nucleophilic faces with preferential interception of electrophiles occurring from the more sterically accessible equatorial direction (7→9). Alkylating the corresponding C-magnesiated nitrile 11, generated by bromine-magnesium exchange (10→11), generates the equatorially substituted nitrile 9 regardless of the nature of the electrophile.12
Scheme 2.
Stereoselectivity Differences between N- and C-Metalated Nitriles
The disparate alkylation stereoselectivities of typical N- and C-metalated nitriles such as 7 and 11, when compared with the internally coordinated C-magnesiated nitrile 4, stimulated a series of alkylations with analogous 5- and 7-membered magnesiated nitriles. Surprisingly, sequential Grignard additions to 5-membered oxonitriles generate magnesiated nitriles whose alkylations are controlled by steric constraints whereas 6- and 7-membered oxonitriles generate internally coordinated C-magnesiated nitriles whose alkylations are controlled by stereoelectronic effects. Surveying an array of addition-alkylations with cyclic oxonitriles of varying ring size generates highly substituted 5–7–membered nitriles, establishes fundamental stereoselectivity preferences for alkylations of the intermediate magnesiated nitriles, and provides a strategy for changing the stereochemistry at the quaternary nitrile-bearing carbon.
Results and Discussion
Grignard addition-alkylations to oxonitrile 1b rapidly assembles highly substituted nitriles (Scheme 1). The unusual alkylation stereoselectivity of the intermediate magnesiated nitrile was investigated by an extensive series of alkylations with the magnesiated nitrile 4 derived by sequential carbonyl and conjugate additions with MeMgCl.9 Probing the alkylation stereoselectivity for the analogous 5-membered nitrile (Scheme 3) therefore employed MeMgCl for the carbonyl and conjugate additions to oxonitrile 1a14 to provide a direct point of comparison. Adding MeMgCl to the 5-membered oxonitrile 1a causes rapid carbonyl addition, with the subsequent conjugate addition via the methylmagnesium alkoxide 12 being significantly more challenging than for the 6-membered oxonitrile 1b.15 The difficulty arises, in part, because the rehybridization required during the intramolecular conjugate addition requires a pyramidalization of the β-carbon that is less readily accommodated in a 5-membered ring than a 6-membered ring.6b The reactivity difference is apparent in the efficiency differences for the addition-alkylations of the 5- and 6-membered oxonitriles 1a and 1b (49% and 86% yield, respectively: Table 1, entries 1 and 7).
Scheme 3.
Grignard Addition-Alkylation with the 5-Membered Oxonitrile 1a.
Table 1.
Grignard Addition-Alkylations to Cyclic Oxonitriles
 | ||||
|---|---|---|---|---|
| entry | oxonitrile | electrophile | cyclic nitrile | yielda (ratio)b |
| 1 |  |
Mel |  |
49%a (3.4:1) |
| 2 |  |
 |
 |
55%a |
| 3 |  |
 |
 |
51%a |
| 4 |  |
 |
 |
47%a |
| 5 |  |
 |
 |
49%c |
| 6 |  |
 |
 |
61%d (1.8:1) |
| 7 |  |
Mel |  |
86%e |
| 8 |  |
 |
 |
61%e |
| 9 |  |
 |
 |
71%e (1.7:1) |
| 10 |  |
Mel |  |
50%f |
| 11 |  |
 |
 |
24% |
| 12 |  |
 |
 |
28% |
Stereochemistry assigned by x-ray crystallography.19
Diastereomeric ratio at the nitrile-bearing carbon with the major isomer shown.
The stereochemical assignment is made by analogy to the acylation with t- BuCOCl, entry 4.
The stereochemistry of the major isomer is unknown and is made by analogy.
Included for comparison from reference 9.
Stereochemical assignment is based on the characteristic 13C shift of the nitrile carbon.20
The chelation-controlled conjugate addition to oxonitrile 1a affords the 5-membered magnesiated nitrile 13 whose alkylations reveal an immediate point of departure with comparable alkylations of the 6-membered nitrile 4. The methylation of 4 occurs from the axial direction and is completely selective (Scheme 1) whereas intercepting 13 with MeI is only modestly selective (Scheme 3), installing the new methyl group predominantly from the pseudo-equatorial orientation (Table 1, compare the configuration of the nitrile-bearing carbon in entries 1 and 7). The stereoselectivity differences suggest that the initially formed bis-magnesiated nitrile 13a does not equilibrate to an internally coordinated nitrile analogous to 4, but exists as a diastereomeric mixture of C-magnesiated nitriles 13b and 13c (Scheme 3).16 Presumably internal coordination between the proximal oxygen and magnesium atoms is prevented in 13b because of strain within the ensuing bicyclic scaffold. The C-magnesiated nitrile 13c is likely to be the more favored diastereomer because the small nitrile17 is positioned in a sterically compressed environment while the large solvated magnesium occupies the less demanding pseudo equatorial orientation. Retentive alkylation from 13c leads to the major diastereomer 14a.
Alkylations of 13 with electrophiles larger than MeI are completely selective (Table 1, entries 2–5). Intercepting 13 with cyclohexanone is an exception that likely stems from a facile retro-aldol-like fragmentation18 causing rapid equilibration to a mixture of diastereomers (Table 1, entry 6). The otherwise consistent preference of the 5-membered nitrile 13 for alkylation opposite to the hydroxyl group, contrasts with alkylations of the 6-membered, C-magnesiated nitrile 4 where alkylation with MeI proceeds with stereochemical retention (Table 1, entry 7), inversion for acylation with MeOCOCN (Table 1, entry 8), and essentially no stereocontrol with CH2=CHCH2Br (Table 1, entry 9).9 Collectively, the stereoselectivity differences underscore the structural differences between the magnesiated 5- and 6-membered nitriles 4 and 13.
Grignard addition-alkylations with the 7-membered oxonitrile 1c14 bear a striking similarity to those of the 6-membered oxonitrile 1b (Table 1, entries 10–12). Addition of MeMgCl to oxonitrile 1c and alkylating the magnesiated nitrile intermediate with MeI installs a methyl substituent in an axial orientation, although the efficiency of the carbonyl- and conjugate additions are diminished relative to the 6-membered oxonitrile (Table 1, compare entries 7 and 10).21 Intercepting the magnesiated nitrile derived from 1c with methyl cyanoformate causes acylation from the equatorial direction, consistent with stereochemical inversion akin to that of the 6-membered oxonitrile (Table 1, compare entries 8 and 11). Collectively these alkylation preferences suggest formation of the internally coordinated, C-magnesiated nitrile 16 which intercepts methyl cyanoformate by an initial invertive acylation leading to 17 (Scheme 4). Subsequent internal attack of the magnesium alkoxide on the proximal nitrile is facilitated in the flexible 7-membered ring22 with the resulting imino lactone reacting with excess methyl cyanoformate to afford 15b. The overall conversion installs 5 new bonds in a remarkable cascade.
Scheme 4.
Grignard Addition-Acylations with the 7-Membered Oxonitrile 1c.
Intercepting the C-magnesiated nitrile 16 with pivaloyl chloride and MeMgCl results in the formation of the enamide 15c (Table 1, entry 12). The unusual conversion to an enamide upon alkylation with pivaloyl chloride is a signature of internally coordinated, C-magnesiated nitriles.23 Mechanistically, N-acylation of the magnesiated nitrile 16 (Scheme 4) is favored with the large, highly polarized electrophile pivaloyl chloride which affords the reactive acyl ketenimine 18. Rapid addition to the acyl ketenimine 18 is facilitated by performing the acylation in the presence of the Grignard reagent which attacks from the more sterically accessable face opposite the neighboring secondary methyl group. Protonation of the resulting bis-metalated enamide intermediate ultimately affords the enamide 15c.
Comparative alkylations and acylations with 6- and 7-membered magnesiated nitriles are consistent with forming internally coordinated C-magnesiated nitriles. Analogous alkylations with the corresponding 5-membered magnesiated nitrile 13 implicate a structurally different metalated nitrile. The reactivity difference is evident on intercepting 13 with pivaloyl chloride which affords only the pseudo-equatorially oriented ketone 14d (Table 1, entry 4) as does acylation with the sterically more demanding triethylacetyl chloride24 (Table 1, entry 5). The complete absence of enamides in the acylations of the 5-membered magnesiated nitrile 1325 is consistent with the intermediacy of a C-magnesiated nitrile devoid of internal coordination.
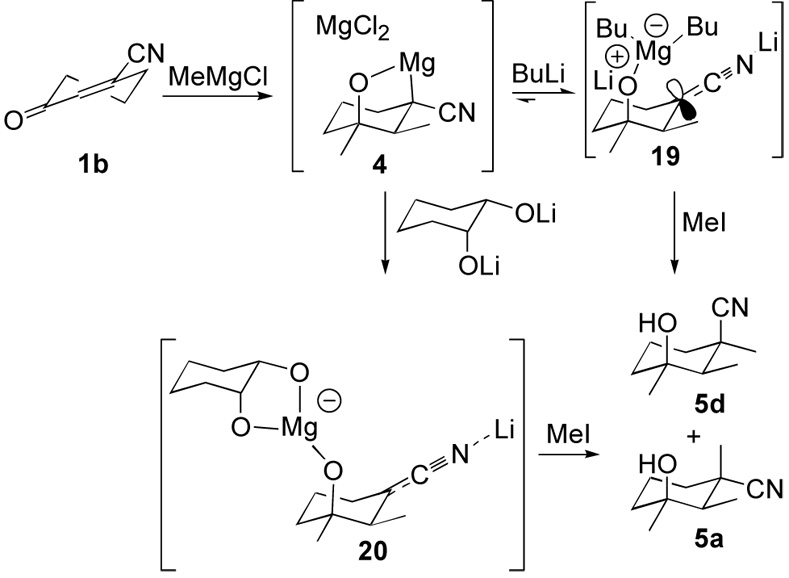
Divergent stereoselectivities in multi-component addition-alkylations of 5–7–membered oxonitriles reveals the key role of internal chelation in alkylations of C-magnesiated nitriles. The stereoselectivity differences suggested an intriguing strategy for reversing the alkylation stereoselectivity of the C-magnesiated nitrile 4 by conversion to the corresponding N-metalated nitrile 19 (Scheme 5). Brief optimization identified a procedure in which the sequential addition of 6 equivalents of BuLi and MeI, preferentially affords the equatorially methylated nitrile 5d and diastereomer 5a in a 12:1 ratio (Scheme 5).26 The stereoselectivity is consistent with an initial conversion of 4 to the magnesiate 19,27 followed by alkylation from the more accessible equatorial face. Extending the protocol to alkylations with allyl bromide and propyl iodide similarly favored equatorial alkylation, although to a more modest extent (Table 2, compare entry 1 with entries 2 and 3).
Scheme 5.
Stereodivergent Alkylations of N-Metalated Nitriles
Table 2.
Stereodivergent Oxonitrile Addition-Alkylations
 | ||||
|---|---|---|---|---|
| entry | R1M | electrophile | cyclic nitrilea | yield (ratio)b |
| 1 | BuLi | Mel |  |
65% (12:1) |
| 2 | BuLi |  |
 |
60% (3:1) |
| 3 | BuLi |  |
 |
69% (1.4:1) |
| 4 | LiF |  |
 |
61% (0:1) |
| 5 | BuOLi |  |
 |
52% (1:1) |
| 6 |  |
 |
50% (1.6:1) |
|
| 7 |  |
 |
 |
73% (6:1) |
| 8 |  |
 |
 |
79% (7.9:1) |
| 9 |  |
Mel |  |
72% (3.3:1) |
| 10 |  |
 |
69% (5.2:1) |
|
| 11 |  |
Mel |  |
62%c (4:1) |
| 12 |  |
 |
 |
58% (5i:5g, 1.8:1)d |
Stereochemical assignments are based on x-ray crystallography.
Diastereomeric ratio at the nitrile-bearing carbon with the major isomer shown.
MeMgCl was employed for the carbonyl addition (1.1 equiv) and CH2CHMgBr for the conjugate addition.
The major diastereomer 5i predominates by 4.9:1 for the configuration shown and 5g predominates over the diastereomer by 2.6:1).
Assuming that an equilibrium exists between the C- and N-magnesiated nitriles 4 and 19, several additives were screened with the aim of generating a more stable magnesiate analogous to 19. Although LiF was ineffective (Table 1, entry 4), lithium butoxide led to equal amounts of the two diastereomers (Table 1, entry 5). Formation of a strong Mg-O bond28 and the entropic advantage of forming an ate complex with a bifunctional ligand, provided the impetus to use lithium alkoxides derived from 1,2-diols. Adding the lithium alkoxide obtained by treating ethylene glycol with BuLi improved the preference for equatorial alkylation although the reagent derived from 1,2-cyclohexanediol is significantly more effective (Table 2, entries 6 and 7, respectively). The latter protocol provides an effective method for equatorial alkylations. Addition of the alkoxide and alkylation with allyl bromide favors equatorial alkylation by 7.9:1 whereas alkylating 4 with allyl bromide is essentially non-selective (compare Table 1, entry 9 with Table 2, entries 2 and 8). The stereodivergent cyclohexanediol alkylation strategy is also effective for the sequential addition of two different Grignard reagents followed by alkylation (Table 2, entry 11).
Employing the lithium alkoxide derived from cyclohexanediol and alkylating with cyclopropylmethyl iodide affords the direct alkylation product 5i and the ring-opened nitrile 5g in a 1.8:1 ratio (Table 2, entry 12). This deployment of cyclopropylmethyl iodide as a single electron transfer probe29 suggests that SN2 and single electron transfer mechanisms are of roughly equal efficiency.9 In both cases the preferred diastereomer results from an equatorial alkylation, consistent with the steric influence of a bulky axial magnesiate. Effectively, the conversion of the C-magnesiated nitrile 4 to the N-metalated nitrile 20 (Scheme 5) extends the carbonyl addition-conjugate addition-alkylation strategy by providing selective access to diastereomers at the quaternary, nitrile-bearing stereocenter.
The Grignard addition-alkylation of 5–7 membered oxonitriles efficiently installs contiguous quaternary-tertiary-quaternary stereocenters. Unfortunately, introduction of an additional quaternary center through conjugate addition to a tetra-substituted alkenenitrile appears not to be possible. The strategy was pursued with the bromooxonitrile 21, prepared by bromination of 1b (Scheme 6). Addition of excess MeMgCl to 21 triggers sequential carbonyl- and conjugate additions to afford a metalated nitrile postulated to be 22,30 which ejects bromide to ultimately generate 24. Despite the use of excess MeMgCl, to promote a chelation-controlled conjugate addition from the magnesiate 23,31 no conjugate addition is observed. The inability to coax the conjugate addition is consistent with the deactivating effect of β-alkyl substituents in alkenenitriles.1
Scheme 6.
1,2-1,4-Grignard Addition to Bromooxonitrile 21
Conclusion
Grignard addition-alkylations to cyclic 5–7–membered oxonitriles efficiently generate highly substituted cyclic nitriles. The strategy installs contiguous quaternary-tertiary-quaternary centers imbedded within diverse cyclic nitriles. Stereoselective alkylations of the intermediate magnesiated nitriles depend intimately on the size of the carbocyclic ring: 5-membered oxonitriles generate magnesiated nitriles whose alkylations are controlled by steric constraints whereas 6- and 7-membered oxonitriles generate internally coordinated, C-magnesiated nitriles whose alkylations are controlled by stereoelectronic effects. Conversion of 6-membered C-magnesiated nitriles to the corresponding N-metalated nitrile is readily achieved by adding lithium alkoxides which allows steric, rather than electronic, effects to control the alkylation stereoselectivity. Synthetically, this interconversion between C-magnesiated and N-lithiated nitriles allows stereodivergent alkylations at the quaternary nitrile-bearing carbon. Collectively, the conjugate addition-alkylation installs three new stereocenters in cyclic 5–7-membered nitriles, establishes fundamental stereoselectivity preferences for alkylations of the intermediate magnesiated nitriles, and provides a strategy for changing the stereochemistry at the quaternary nitrile-bearing carbon.
Experimental
General Procedure for Oxonitrile Synthesis
Two individual watchglasses having a thin (< 3mm) layer of powdered chromium trioxide (12 equiv) or 2,3-dimethylpyrazole (12 equiv) were dried overnight (> 16 h) in a vacuum desiccator (< 5mm/Hg) containing P2O5. Dry 2,3-dimethylpyrazole was added to a magnetically stirred, −20 °C, CH2Cl2 solution (1.2 M) of dry CrO3. After stirring at −20 °C for 15 min, neat nitrile (1 equiv) was added. The mixture was stirred between −10 °C to −12 °C for 3 h and then the temperature raised to 0 °C. Aqueous NaOH (5M) was then added and stirring continued at 0 °C for 1 h. The crude reaction mixture was then refrigerated (−10 °C) overnight causing the aqueous phase to freeze and the organic phase to be removed by decanting. The aqueous phase was further extracted twice with CH2Cl2, the organic extracts were then combined, and the resulting organic material was then washed sequentially with HCl, H2O and brine. The organic extract was dried (Na2SO4) and then concentrated to give a dark yellow oil that was purified by radial chromatography to afford pure oxonitrile.
General Grignard Addition-Alkylation Procedure with Oxonitrile 1a
A THF solution of MeMgCl (2.5 equiv) was added to a −78 °C THF solution (0.1M) of oxonitrile 1a, and the resulting solution was stirred at −78 °C for 1 h and then warmed to room temperature. After 0.5 h, the solution was cooled to 0 °C and the electrophile (2–10 equiv) was then added neat. After 1 h, saturated aqueous NH4Cl was added and the resulting mixture was extracted with EtOAc. The extracts were combined, washed with brine and dried (NaSO4). Concentration and purification of the crude product by radial chromatography afforded the pure nitrile.
General Grignard Addition-Alkylation Procedure with Oxonitrile 1c
A THF solution of MeMgCl (2.5–6 equiv) was added to a −78 °C THF solution (0.1 M) of oxonitrile 1c, and the resulting solution was stirred at −78 °C for 1 h and then warmed to room temperature. After 1.5 h, the electrophile (2.5–6equiv) was added neat either at room temperature or with prior cooling to −78 °C. Subsequent addition of saturated NH4Cl and extraction with EtOAc afforded a crude product that was washed with brine and dried (MgSO4), concentrated, and purified by radial chromatography to afford the pure product.
General Procedure for the Addition-Alkylation with Stereochemical Inversion
A THF solution of MeMgCl (1.05–1.1 equiv) was added to a −15 °C THF solution (0.1 M) of the oxonitrile 1b. After 2 h, a second Grignard reagent (1.2–1.5 equiv), if different from MeMgCl, was added and the solution was then allowed to warm to room temperature. After 2h, the solution was transferred by syringe to a 0 °C THF solution (0.3 M) of cis-cyclohexane-1,2-diol (3 equiv) to which BuLi (3.3 equiv) had been added. The resulting solution was cooled to −78 °C and after 0.5 h, the electrophile (3.0–10 equiv) was added neat. After 1 h, the solution was allowed to warm to room temperature, and after 16 h, saturated aqueous NH4Cl was added, and the resulting mixture was extracted with EtOAc. The extracts were combined, washed with brine and dried (NaSO4). Concentration and purification of the crude product by radial chromatography afforded the pure nitrile.
2-Bromo-3-oxocyclohex-1-ene-1-carbonitrile (21)
A 1 mL CH2Cl2 solution of the bromine (240 mg, 1.5 mmol) was added to a 0 °C, 4 mL CH2Cl2 solution of 1b (181 mg, 1.5 mmol). After 1.5 h at 0 °C, Et3N (0.32 mL, 2.25 mmol) was added dropwise. The solution was stirred at room temperature for 1.5 h and was washed with 3% HCl twice and saturated brine once and dried (MgSO4). Concentration and radial chromatography (stepped gradient 1:9, 3:17, 1:4, 1:3 EtOAc/Hexanes) to afford 21 (210 mg, 70%) as a light yellow oil. IR (film) 2221, 1706, 1692 cm−1; 1H NMR (300 MHz, CDCl3): δ 2.10–2.18 (m, 2H), 2.62–2.70 (m, 4H); 13C NMR (300 MHz, CDCl3): δ 21.9, 30.6, 37.4, 116.2, 131.1, 134.6, 188.7; HRMS (EI) calcd for (M+), C7H6BrNO+ 198.9627, found 198.9633.
3-Hydroxy-2, 3-dimethylcyclohex-1-ene-1-carbonitrile (24)
A THF solution of MeMgCl (3 M, 3 mmol) was added to a −78 °C THF solution of 21 (0.1 M). After 1 h at −78 °C and 2.5 h at room temperature, MeI was added neat and the resulting solution was stirred overnight. Saturated aqueous NH4Cl was added and the resultant mixture was extracted with EtOAc. The extracts were combined, washed with brine, and dried (NaSO4). Concentration and purification of the crude product by radial chromatography (stepped gradient 1:4, 3:7 EtOAc/hexanes) furnished 46 mg (51%) of 24 as a waxy solid. IR (film) 3438, 2213, 1646 cm−1; 1H NMR (300 MHz, CDCl3): δ 1.31 (s, 3H), 1.60–1.75 (m, 4H), 2.06 (s, 3H), 2.20–2.22 (m, 2H); 13C NMR (300 MHz, CDCl3): δ 16.7, 19.0, 26.9, 27.8, 38.2, 70.0, 108.7, 118.7, 156.0.
Supplementary Material
Experimental procedures, 1H NMR, and 13C NMR spectra for all new compounds and ORTEPs for all of the crystal structures. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgment
Financial support of this research from the National Institutes of Health (2R15AI051352), and in part, from the National Science Foundation (CHE 0515715, CRIF 024872 for x-ray facilities, and CHE 0421252 for HRMS instrumentation) are gratefully acknowledged as is assistance from Dr. Derek Beauchamp with the crystallographic analyses.
References
- 1.Fleming FF, Wang Q. Chem. Rev. 2003;103:2035. doi: 10.1021/cr020045d. [DOI] [PubMed] [Google Scholar]
- 2.(a) Totleben MJ, Curran DP, Wipf P. J. Org. Chem. 1992;57:1740. [Google Scholar]; (b) Yoneda R, Harusawa S, Kurihara T. J. Chem. Soc., Perkin Trans. 1. 1988:3163. [Google Scholar]; (c) House HO. Acc. Chem. Res. 1976;9:59. [Google Scholar]; (d) House HO, Umen MJ. J. Org. Chem. 1973;38:3893. [Google Scholar]
- 3.(a) Gololobov YG, Gruber W. Russ. Chem. Rev. 1997;66:953. [Google Scholar]; (b) Fleming FF, Pu Y, Tercek F. J. Org. Chem. 1997;62:4883. [Google Scholar]; (c) Kung L-R, Tu C-H, Shia K-S, Liu H-J. Chem. Commun. 2003:2490. doi: 10.1039/b307114f. [DOI] [PubMed] [Google Scholar]; (d) Pan L-R, Tokoroyama T. Chem. Lett. 1990;1999 [Google Scholar]; (e) Wallenfels K, Friedrich K, Reiser J, Ertel W, Thieme HK. Angew. Chem. Int. Ed. 1976;15:261. [Google Scholar]
- 4.(a) Fleming FF, Gudipati S. Org. Lett. 2006;8:1557. doi: 10.1021/ol060010q. [DOI] [PubMed] [Google Scholar]; (b) Blanchard P, Da Silva AD, El Kortbi MS, Fourrey J-L, Machado AS, Robert-Gero M. J. Org. Chem. 1993;58:6517. [Google Scholar]; (c) Blanchard P, Da Silva AD, Fourrey J-L, Machado AS, Robert-Gero M. Tetrahedron Lett. 1992;33:8069. [Google Scholar]; (d) Blanchard P, El kortbi MS, Fourrey J-L, Robert-Gero M. Tetrahedron Lett. 1992;33:3319. [Google Scholar]; (e) Sarandeses LA, Mourino A, Luche J-L. J. Chem. Soc. Chem. Commun. 1992:798. [Google Scholar]; (f) Dupuy C, Petrier C, Sarandeses LA, Luche JL. Synth. Commun. 1991;21:643. [Google Scholar]; (g) Shono T, Nishiguchi I, Sasaki M. J. Am. Chem. Soc. 1978;100:4314. [Google Scholar]
- 5.(a) Jamison TF, Shambayati S, Crowe WE, Schreiber SL. J. Am. Chem. Soc. 1997;119:4353. [Google Scholar]; (b) Fleming FF, Hussain Z, Weaver D, Norman RE. J. Org. Chem. 1997;62:1305. [Google Scholar]; (c) Brattesani DN, Heathcock CH. J. Org. Chem. 1975;40:2165. [Google Scholar]
- 6.(a) Fleming FF, Zhang Z, Wang Q, Steward OW. Angew. Chem. Int. Ed. 2004;43:1126. doi: 10.1002/anie.200352920. [DOI] [PubMed] [Google Scholar]; (b) Fleming FF, Zhang Z, Wang Q, Steward OW. J. Org. Chem. 2003;68:7646. doi: 10.1021/jo0345291. [DOI] [PubMed] [Google Scholar]; (c) Fleming FF, Gudipati V, Steward OW. Tetrahedron. 2003;59:5585. [Google Scholar]
- 7.Fleming FF, Iyer PS. Synthesis. 2006:893. [Google Scholar]
- 8.Fleming FF, Zhang Z, Wei G. Synthesis. 2005:3179. [Google Scholar]
- 9.Fleming FF, Zhang Z, Wei G, Steward OW. J. Org. Chem. 2006;71:1430. doi: 10.1021/jo052102j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.For an excellent overview of terms, steric constraints, and orbital overlap see:Gawley RE. Tetrahedron Lett. 1999;40:4297.
- 11.Bare TH, Hershey ND, House HO, Swain CG. J. Org. Chem. 1972;37:997. [Google Scholar]
- 12.Fleming FF, Gudipati S, Zhang Z, Liu W, Steward OW. J. Org. Chem. 2005;70:3845. doi: 10.1021/jo0501184. [DOI] [PubMed] [Google Scholar]
- 13.Fleming FF, Zhang Z. Tetrahedron. 2005;61:747. [Google Scholar]
- 14.Prepared by oxidation of the corresponding cycloalkenecarbonitrile with CrO3-3,5-dimethylpyrazole.8
- 15.Repetitive alkylations consistently proceed with the same yield, accompanied by the hydroxyalkenenitrile 12 (OMgMe = OH) resulting from carbonyl addition.
- 16.Comparable alkylations of 11 imply that magnesiated nitriles prefer coordination on carbon rather than on nitrogen (Scheme 2).12
- 17.Nitriles have an extremely small A-value of only 0.2 kcal mol−1:Eliel Ernest L, Wilen Samuel H, Mander Lewis N. Stereochemistry of Organic Compounds. Wiley, NY: 1994. pp. 696–697.
- 18.The fragmentation of β-alkoxynitriles is often facile and in this instance the formation of a dioxide likely facilitates the fragmentation to an even greater extent:Carlier PR, Lo CW-S, Lo MM-C, Wan NC, Williams ID. Org. Lett. 2000;2:2443. doi: 10.1021/ol006099w.Carlier PR, Lam WW-F, Wan NC, Williams ID. Angew. Chem., Int. Ed. 1998;37:2252. doi: 10.1002/(SICI)1521-3773(19980904)37:16<2252::AID-ANIE2252>3.0.CO;2-Z.
- 19.X-ray crystallography of 14b, and derivatives of 14a, 14c, 14d, confirmed the stereochemical assignments. The authors have deposited the crystallographic data with the Cambridge Crystallographic Data Center. The data can be obtained, on request, from the Director, Cambridge Crystallographic Data Center, 12 Union Road, Cambridge, CB2 1EZ, UK.
- 20.Bailey WF, Cioffi A. Magn. Reson. Chem. 1987;25:181. [Google Scholar]
-
21.Considerable amounts of starting oxonitrile 1c, the hydroxyalkenenitrile i, and the hydroxyalkanenitrile ii are generated, which result from incomplete conjugate addition and alkylation, respectively.

- 22.Intercepting the magnesiated nitrile 4 with cyclopropanecarboxaldehyde triggers an analogous cyclization when the reaction is allowed to warm to room temperature.9
- 23.Fleming FF, Wei G, Zhang Z, Steward OW. Org. Lett. 2006;8:4903. doi: 10.1021/ol0619765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beak P, Zajdel WJ. J. Am. Chem. Soc. 1984;106:1010. [Google Scholar]
- 25.1H NMR analysis of the crude reaction mixture failed to identify any enamide.
- 26.For a preliminary account see:Fleming FF, Zhang Z, Wei G, Steward OW. Org. Lett. 2005;7:447. doi: 10.1021/ol047598q.
- 27.Inoue A, Kitagawa K, Shinkubo H, Oshima K. J. Org. Chem. 2001;66:4333. doi: 10.1021/jo015597v. [DOI] [PubMed] [Google Scholar]
- 28.Simanek E, Huang NL. Phys. Rev. Lett. 1966;17:699. [Google Scholar]
- 29.Gawley RE, Low E, Zhang Q, Harris R. J. Am. Chem. Soc. 2000;122:3344.Butenyl alkylation is conceptually possible by prior, catalytic radical atom transfer rearrangement to 3-butenyl iodide or through SN2' alkylation:Alnajjar MS, Smith GF, Kuivila HG. J. Org. Chem. 1984;49:1271.
- 30.Presumably the ejection of bromide is faster than reorganization to a C-magnesiated nitrile followed by loss of bromide.
- 31.Fleming FF, Wang Q, Zhang Z, Steward OW. J. Org. Chem. 2002;67:5953. doi: 10.1021/jo0258150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures, 1H NMR, and 13C NMR spectra for all new compounds and ORTEPs for all of the crystal structures. This material is available free of charge via the Internet at http://pubs.acs.org.