Abstract
Background
Disorganization is a core dysfunction in schizophrenia. Coherent thought and behavior rely on the interactive neural responses to temporally discrete external events. Previous studies have demonstrated that a single visual stimulus (event) is abnormally affected by another (as in backward masking), but the integration (or ‘synthesis’) of temporally discrete events remains largely unexplored in schizophrenia. We examined the perceived interaction of two elementary visual events in schizophrenia patients, using a psychophysical approach.
Methods
Two brief, spatially-coincident foveal light pulses (5 msec) were presented with different inter-stimulus intervals (ISIs). At ISIs around 100 msec, a substantial proportion of the light pulse pairs was paradoxically perceived as three flashes, a known phenomenon in normal subjects. The subjects reported the number of flashes perceived (‘one’, ‘two’ or ‘three’).
Results
Schizophrenia patients (n=28) reported fewer ‘three flashes’ than normal controls (n=26) at the ISIs where ‘three flash’ reports were greatest in normal subjects (90 to 110 msec). On the other hand, at longer ISIs (130–310 msec) patients reported ‘three flashes’ in more trials than did normal subjects. The perception of three flashes in patients was correlated with certain aspects of clinical status, including the positive and general subscales of the PANSS.
Discussion
The alteration of the ‘three-flash’ illusion in schizophrenia suggests that the synthesis of discrete visual events is temporally ‘dilated’ or distorted, which might contribute to disorganized thought and behavior.
Introduction
Disorganized thought and behavior are prevalent in schizophrenia. For normal behavior, one must respond to a host of temporally discrete stimuli and integrate, or ‘synthesize’, the responses to these stimuli. Alteration of this synthesis might cause a coherent flow of events to become a barrage of more disjointed information. Studying the synthesis of temporally disparate events may thus shed light on the pathophysiology of schizophrenia.
Altered temporal processing of visual information in schizophrenia has been shown in studies on visual backward masking [1, 2] and temporal contrast detection [3–6]. These studies reveal two general effects in schizophrenia. First, there is a reduced sensitivity for contrast detection of temporally modulated stimuli. Second, there is temporal elongation in backward masking—one measure of how one visual event is affected by a later, masking event. The present study evaluated another type of temporal interaction in schizophrenia—the way in which two equivalent temporally discrete events interact to yield a ‘synthesized’ perceptual response.
We measured the synthesized perceptual response to two temporally discrete visual events (brief light pulses) in both patients and normal controls, and we compared this to the processing of single visual events (temporal contrast detection). The results were evaluated in terms of relevant clinical features.
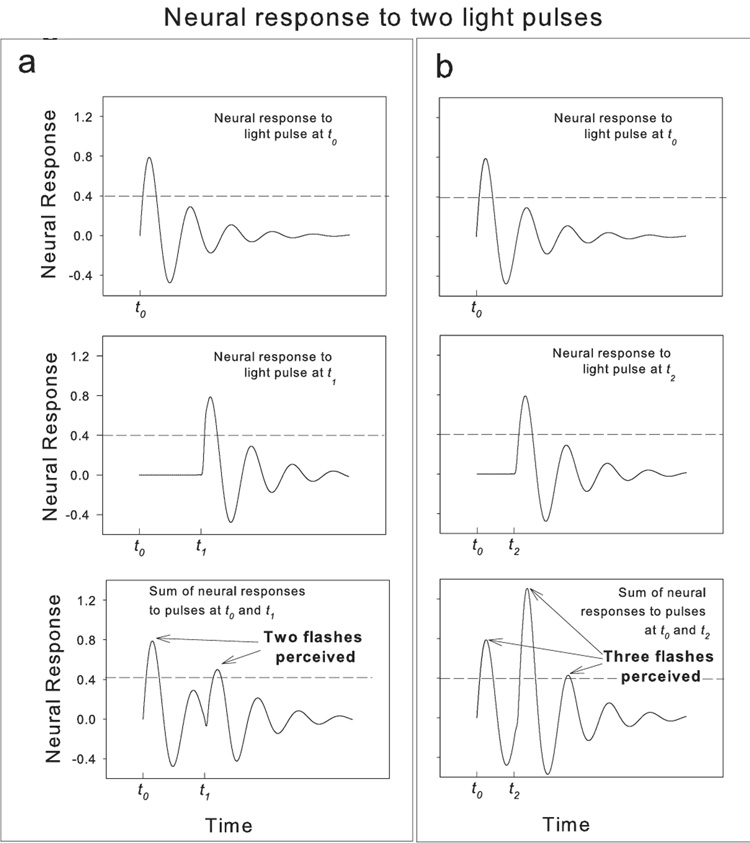
We adopted the paradigm of Bowen (1989). Two supra-threshold, spatially-coincident light pulses (5 msec) were presented to the fovea with varying inter-stimulus intervals (ISIs). For ISIs around 100 msec the two pulses are regularly mis-perceived as three flashes by normal subjects [7]. At this ISI, the ‘three-flash’ illusion occurs for 40–70% of the presentations. According to Bowen, the ‘three-flash’ illusion represents the interaction (the linear sum) of the multi-phase Impulse Response Functions (IRF) to each of the two flashes. The multi-phase IRFs have at least three alternating (or ‘oscillating’) lobes of excitation and inhibition that allow the perception of a third flash from only two pulse stimuli (see Figure 1 as well as Discussion for further explanation). These IRFs and their summation likely occur in early stages of the magnocellular pathway— a pathway largely responsible for temporal and motion detection [7–9].
Figure 1.
Model of the neural response to single light flashes (top and middle panels) and their linear sum (bottom panels) —adopted from Bowen [7]. The horizontal dashed lines represent the hypothetical threshold that must be exceeded for perceiving a flash—as in Bowen. a. The top left panel shows the response to a single light pulse presented at time t0. The middle left panel shows the neural response to the second pulse, presented at time t1, (greater than ~100 msec after t0). The bottom left panel is the summed responses for the pair of pulses— at this ISI, two flashes are perceived. b. The right panels show the same schema, but the second flash occurs at t2, about 100 msec after t0, leading to a peak to peak summation of the two IRFs, — now three flashes are perceived.
Methods
Subjects
Subjects were 28 patients with schizophrenia or schizoaffective disorder and 26 non-psychiatric controls. Patients met criteria for schizophrenia or schizoaffective disorders based on standardized interviews by independent clinicians using the SCID-IV [10] and all available medical records. The schizophrenia group comprised inpatients from the Schizophrenia and Bipolar Disorder Program at McLean Hospital (n=6) and outpatients treated at McLean Hospital or local clinics (n=22). All were taking antipsychotic medication (mean chlorpromazine (CPZ) equivalent was 537.2 mg (SD=358.1 mg)) [11, 12]. Fifteen patients were taking antidepressant medication, 7 were taking anti-anxiety medications, and 17 were taking mood stabilizers. The patient group had mean PANSS [13] scores of 15.8 (SD=6.8), 13.6 (SD=5.9) and 29.4 (SD=10.6) for the positive, negative and general scales, respectively. Average duration of illness was 18.0 years (SD=10.4 years). Normal healthy controls were screened for axis-one disorders using an interview based on the SCID N/C [14]. Subjects from both groups were excluded if they had history of brain injury or neurological disorders, a verbal IQ [15] lower than 70, or any drug or alcohol abuse in the 6 months prior to the study. All subjects signed informed consent in accordance with McLean Hospital’s IRB guidelines. Clinical and demographic information are provided in Table 1. Patients and controls did not differ in age or sex, the standard deviation for age in the patient sample, however, was smaller than for controls. The two groups differed in education (t = 2.4, p = 0.02) and IQ (t = 2.3, p = 0.02).
Table 1.
Demographic Information of the Sample
| Demographic variable | Sex | Age | Verbal IQ* | Education* | Parental Education |
|---|---|---|---|---|---|
| Group | |||||
| Normal controls (NC) |
F = 16 M = 10 |
43.0 (13.8) |
108.0 (9.2) |
15.9 (2.3) |
13.5 (2.6) |
| Schizophrenia patients (SZ) |
F = 17 M = 11 |
40.3 (9.9) |
100.8 (12.3) |
14.1 (2.0) |
13.9 (2.1) |
Means are reported above standard deviations. Education and age are in years. Verbal IQ was measured using the WAIS – R [15].
Asterisk denotes significant group difference (p < 0.05).
Stimulus and Procedure
Perception of light pulse pairs
A diffuse red light emitting diode (LED) provided light pulses. Subjects viewed the LED target in a weakly-illuminated room—fixation was guided by the center of a small, circular neutral density filter placed over the LED. The target was a nearly uniform disc, subtending 17 min arc, with a peak luminance of 35 cd/m2. The LED has extremely high temporal bandwidth and provides precisely controlled flashes. Such flashes allow the visual events to be manipulated solely in the temporal domain. A Macintosh G3 computer controlled the presentation of the light pulses. For each trial, the light pulse was presented twice, each for 5 msec, as in Bowen’s paradigm. The inter-stimulus interval (ISI) of the two pulses ranged over 10 values (30, 70, 90, 110, 130, 150, 190, 230, 270 or 310 msec). Each ISI had 10 trials. A total of 100 trials were presented in a quasi-random order across ISIs.
On each trial the subject was informed that they would see one, two, or three flashes, and was asked to report the number of flashes they saw. The perceived number of flashes was the dependent variable. Education and IQ scores, the only two demographic variables that differed between groups, were used as covariates in analysis.
Contrast detection
Temporal contrast detection was also measured to provide an index for processing of a single visual stimulus. The target was a sinusoidal grating (0.5 cycles per degree) moving left or right at a temporal frequency of 0, 3, 6 or 12 Hz (or at a speed of 0, 6, 12 or 24 deg/sec). The stimulus field subtended 13 deg, at a mean luminance of 35 cd/m2. The threshold was measured separately at each temporal frequency, using a two alternative forced-choice staircase to determine the minimum contrast value for 79% accuracy. Each trial had two temporal intervals for the target and a blank respectively, ordered randomly, and the task was to judge which of the two intervals contained the target. The target and blank each lasted 300 msec, separated by a 500 msec gap. Contrast detection thresholds were converted to contrast sensitivity (1/threshold).
Results
Perception of the light pulse pair
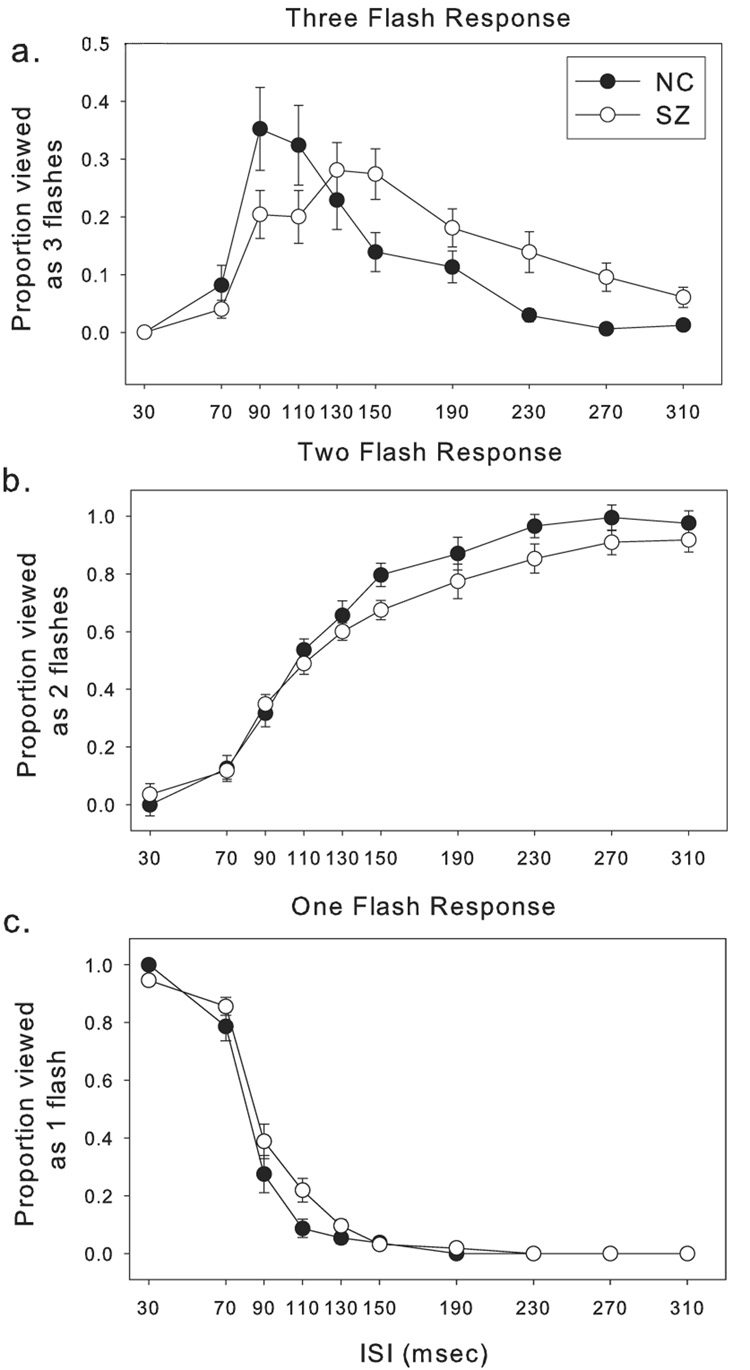
Perception of the ‘three-flash’ illusion occurred for schizophrenia patients (SZ) less frequently at short ISIs (90 to110 msec) and more frequently at intermediate and long ISIs (130 to 310 msec) (Figure 2). A two way ANOVA was performed on the proportion of ‘three-flash’ trials, using diagnosis and ISI as factors. The diagnosis X ISI interaction was significant (F = 2.94, p = 0.002), indicating that the ‘three-flash’ illusion for SZ and NC peaked at different ISIs (Figure 2a). There was a main effect for ISI (F = 13.97, p < 0.001), but not for diagnosis (F = 0.04, p = 0.85).
Figure 2.
Perception of pulse pairs. a. The proportion of trials perceived as ‘three flashes’ at varying inter-stimulus intervals ISIs. b. The proportion of trials perceived as ‘two flashes’, and c. the proportion of trials perceived as ‘one flash’. In all panels, error bars represent ±1 standard error.
For the proportion of trials perceived as ‘one flash’, ANOVA with diagnosis and ISI as factors showed main effects for ISI (F = 277.44, p < 0.001) and diagnosis (F = 8.01, p = 0.005). There was also a trend towards a diagnosis X ISI interaction (F = 1.80, p = 0.07) (Figure 2b).
For the proportion of trials perceived as ‘two flashes’, there were significant effects for ISI (F = 122.3, p < 0.001) and group (F = 6.4, p = 0.012) (Figure 2c). The interaction was insignificant (F = 1.2, p = 0.29).
Contrast detection
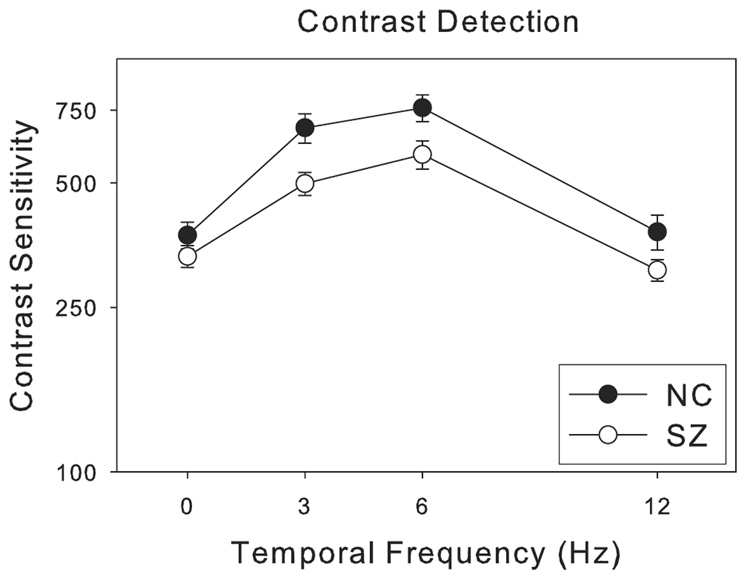
Contrast sensitivities for SZ were lower than for NC across all temporal frequencies (0, 3, 6 and 12 Hz, Figure 3) —the group difference reached a statistically significant level (F = 18.1, p < 0.001). There was also a main effect for temporal frequency (F = 36.2, p < 0.001), but the interaction between group and temporal frequency was not significant (F = 1.6, p = 0.18).
Figure 3.
Contrast sensitivity (inverse of contrast detection threshold) as a function of temporal frequency. The visual target was a sinusoidal grating (0.5 cycles per degree) moving at different velocities (0 to 24 degrees per second), or equivalently modulating at different temporal frequencies (0 to 12 Hz). The error bars represent ±1 standard error.
Relationship between ‘three-flash’ illusion and other variables
Visual variables
Table 2 shows correlation coefficients between the ‘three-flash’ illusion at peak ISIs (90 msec for NC, and 130 msec for SZ) and contrast sensitivity in patients and controls. Although none of the correlations were significant, normal controls approached significance for temporal frequencies of 6 (r = 0.36, p = 0.08) and 12 Hz (r = 0.38, p = 0.07). Patients showed such a pattern at 12 Hz (r = 0.30, p = 0.14).
Table 2.
Correlations between perception of ‘three flashes’ and contrast sensitivity
| Contrast detection temporal frequency | 0 Hz | 3 Hz | 6 Hz | 12 Hz |
|---|---|---|---|---|
| Group (ISI) | ||||
| SZ (90ms) | −0.15 | −0.22 | 0.13 | 0.30 |
| SZ (130ms) | −0.15 | −0.02 | 0.01 | 0.05 |
| NC (90ms) | 0.03 | −0.08 | 0.36 | 0.38 |
| NC (130ms) | −0.11 | −0.08 | 0.34 | 0.37 |
Demographic variables
Table 3 shows correlation coefficients between the ‘three-flash’ illusion and several demographic variables. In normal controls, the prevalence of the ‘three-flash’ illusion was not significantly correlated with IQ, education, parental education or age. In patients, the ‘three-flash’ illusion was correlated with age (r = −0.51, p < 0.01 at 90 msec, r = −0.41, p < 0.05 at 130 msec), but not with IQ, education or parental education.
Table 3.
Correlations between demographic variables and the perception of the ‘three flashes’
| Demographic variable | Verbal IQ | Education | Parental Education | Age |
|---|---|---|---|---|
| Group (ISI) | ||||
| SZ (90ms) | −0.14 | 0.05 | 0.13 | −0.51* |
| SZ (130ms) | −0.29 | −0.23 | 0.05 | −0.41* |
| NC (90ms) | −0.03 | −0.30 | 0.14 | −0.22 |
| NC (130ms) | 0.13 | −0.16 | 0.27 | −0.30 |
Asterisk denotes p < 0.05.
Clinical variables
The prevalence of the ‘three-flash’ illusion in patients at 130 msec was correlated with positive (r = 0.65, p < 0.001) and general (r = 0.64, p < 0.001) subscales of the PANSS. To a lesser extent, the negative subscale of the PANSS was also correlated with the ‘three-flash’ illusion measure at 130 msec (r = 0.46, p < 0.05). To evaluate the relationship between the ‘three-flash’ illusion and clinical disorganization, we used a cluster score composed of three PANSS items (Conceptual Disorganization, Difficulty in Abstract Thinking and Disorientation [16]). The cluster score was correlated with the perceptual measure at the 130 msec ISI (r = 0.58, p < 0.01) but not at the 90msec one (r = 0.25, p > 0.10).
Neither CPZ equivalents (r = 0.17) nor illness duration (r = −0.18) were correlated with the ‘three-flash’ illusion at 130 msec. We also compared the three flash illusion in the presence versus the absence of other drugs (antidepressant, anti-anxiety and mood stabilizing medications) across ISIs; each drug by a separate ANOVA. No medication group effects or interactions were significant (p > 0.05).
Discussion
We observed that the perceptual response to two simple visual events (light pulses) was altered in schizophrenia. The perception of the ‘three-flash’ illusion, typically occurring for ISI’s near 100 msec in healthy subjects, shifted to longer intervals in patients. At longer intervals (beyond 110 msec), patients perceived three flashes more frequently, and two flashes less frequently. Also, patients perceived one flash more frequently than controls at intervals from 90 to 130 msec. This pattern of results suggests that the underlying temporal impulse response function is prolonged (or ‘dilated’) in schizophrenia.
Temporal interactions
The temporal interactions that give rise to the ‘three-flash’ illusion likely begin at the magnocellular ganglion cells of the retina and lateral geniculate nucleus of the thalamus (LGN) [8, 9]. The magnocellular pathway largely mediates temporal and motion processing, and this pathway is affected by contrast gain control [8, 9]. Contrast gain control is manifested as a speeding-up of the IRF (and temporal processing) as stimulus contrast is raised in the magnocellular system [reviewed in 8].
The perception of the ‘three-flash’ illusion (with the double-pulse stimulus) requires that the IRF has at least three phases of alternating excitation and inhibition—modeled by a dampened sine function [7] (Figure 1). The perceived number of flashes is predicted by the sum of a tri-phasic [7] IRF to each of the two light pulses. This sum depends on the magnitude of each IRF (controlled by flash intensity) and the delay between the two IRFs (controlled by ISI). The perception of the third flash arises (at the appropriate ISI) by a linear summation between the third positive, excitatory lobe of the first IRF (from the first pulse) and the second excitatory lobe of the second IRF (from the second pulse). It is the ‘ringing’ of the extra lobes that results in the perception of the extra flash.
In schizophrenia the ‘three-flash’ illusion (Figure 2a) was reduced at briefer ISIs and increased at longer ISIs (compared to normal subjects). This shift of the illusion to longer intervals could be explained by a temporal dilation of the IRF in schizophrenia. Such dilation would cause the summation of the second and high-order phases of the responses to be delayed accordingly, causing the ‘three-flash’ illusion to shift to longer ISIs.
The prolongation of the IRF in schizophrenia is consistent with earlier results on backward masking [17, 18], for the patients there also required a longer temporal separation between mask and target to elicit optimal backward masking. The main difference between the two types of studies is that the backward masking studies examined the effect of a large mask on a subsequent different stimulus whereas the present study examined simple interactions of two virtually identical stimuli (equal pulses). The pulse interaction, occurring at the exactly same spatial location and with no cognitive demands (such as object recognition), may be much simpler to interpret, as shown by the simple linear summation model in Figure 1.
Temporal interaction and processing of individual stimuli
In the present study, temporal contrast sensitivity was measured as an index of visual processing of individual stimuli. Temporal contrast sensitivity was reduced in schizophrenia patients (Figure 3). The reduced contrast sensitivity might indicate that contrast gain control is reduced in the magnocellular pathway for these patients, and this might lead to dilated IRFs (since contrast gain acts to speed-up the IRF [9]). Reduced contrast sensitivity at low temporal frequency could be consistent with the dysfunction in the parvocellular pathway. Yet neither group showed any correlation between the ‘three-flash’ illusion and contrast sensitivity for the static gratings (0 Hz) (Table 2). On the other hand, the three flash illusion was modestly correlated contrast sensitivity at intermediate and high temporal frequencies in controls, and to a lesser degree in patients. This may hint at an association of the altered temporal interaction with the functioning of the magnocellular pathway, but less so with the parvocellular pathway [19, 20]. However, the relatively small magnitudes of the correlations between the ‘three-flash’ illusion and contrast sensitivity suggest that the altered ‘three-flash’ illusion cannot be solely attributed to the processing of individual stimuli within the magnocellular pathway.
The role of the magnocellular pathway in schizophrenia is complex [21–23]. Adding to this complexity are the inherent attentional demands involved in behavioral assessment of visual functioning [24]. The impaired ability to attend to behavioral tasks in schizophrenia patients [25, 26] could be a factor for the altered temporal interaction studied here. However, the ‘three-flash’ illusion in patients is not universally dampened or amplified across conditions, rather it is shifted to specific temporal intervals. The temporal-interval-specific result cannot be easily reconciled with the attention interpretation, which would likely alter the ‘three-flash’ illusion across temporal intervals.
Temporal interaction and clinical manifestations
Altered temporal interactions of simple visual responses may give rise to more complex disorganized behavior. In the present study, the ‘three-flash’ illusion was correlated with the positive and general subscales of the PANSS (Table 3) —but was less correlated with the negative subscale. Also, the cluster score used to measure clinical disorganization was significantly and positively correlated with the ‘three-flash’ illusion at 130 msec, but not at 90 msec. This pattern of correlations suggests that those patients whose ‘three-flash’ illusion had migrated to longer stimulus intervals exhibit more positive symptoms such as clinical disorganization. No causal link can be drawn from the correlations between altered temporal interactions and clinical measures of disorganization. Yet, since the temporal processing in the ‘three-flash’ illusion occurs early on in the visual stream, it is reasonable to speculate that altered temporal interaction at the sensory stage contributes to higher order cognitive disorganization such as Difficulty in Abstract Thinking and Conceptual Disorganization. It is worth noting that in previous studies, backward masking performance was not correlated with clinical symptoms in patients [27, 28]. Clearly, more studies are needed to better determine what visual measures are related to disorganized symptomatology.
Further studies
One area for future studies should be the characterization of physiological correlates of temporal response functions and the interaction between them. While some evidence suggests neuronal number and volume are not altered in early visual areas such as LGN [29], electrophysiological studies would provide complementary information about neural organization of temporally discrete events in schizophrenia.
Another unanswered question concerns how the altered temporal processing in schizophrenia is related to other visual and cognitive processes requiring temporal organization [30–36]. Many cognitive activities such as reading [37] and language processing [38] depend on organization of events that occur at different points of time. It is conceivable that the sensory temporal interaction studied here may contribute to poor performance in such common activities.
Summary
Our study demonstrated that the synthesis of temporal interaction is lengthened in schizophrenia, as measured by the ‘three-flash illusion’. This perceptual abnormality may be partially linked to the patients’ decreased contrast sensitivity for temporal modulation. The altered ‘three-flash illusion’ represents a genuine abnormality of temporal organization at the basic sensory level. Future studies in schizophrenia may reveal whether these temporal alterations affect other cognitive activities requiring temporal processing.
Acknowledgment
We thank Ken Nakayama for help in the initial phase of the study. We also thank Ryan McBain for comments on the early version of the paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Green MF, Nuechterlein KH, Mintz J. Backward masking in schizophrenia and mania. II. Specifying the visual channels. Archives of General Psychiatry. 1994;51:945–951. doi: 10.1001/archpsyc.1994.03950120017004. [DOI] [PubMed] [Google Scholar]
- 2.Butler PD, et al. Backward masking in schizophrenia: relationship to medication status, neuropsychological functioning, and dopamine metabolism. Biol Psychiatry. 1996;40(4):295–298. doi: 10.1016/0006-3223(96)00007-8. [DOI] [PubMed] [Google Scholar]
- 3.Butler PD, et al. Dysfunction of early-stage visual processing in schizophrenia. Am J Psychiatry. 2001;158(7):1126–1133. doi: 10.1176/appi.ajp.158.7.1126. [DOI] [PubMed] [Google Scholar]
- 4.Slaghuis WL. Contrast sensitivity for stationary and drifting spatial frequency gratings in positive- and negative-symptom schizophrenia. J Abnorm Psychol. 1998;107:49–62. doi: 10.1037//0021-843x.107.1.49. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, et al. Contrast detection in schizophrenia. Archives of General Psychiatry. 2000;57(10):995. [PubMed] [Google Scholar]
- 6.Keri S, et al. Contrast detection in schizophrenia. Arch Gen Psychiatry. 2000;57(10):995–996. doi: 10.1001/archpsyc.57.10.995. [DOI] [PubMed] [Google Scholar]
- 7.Bowen RW. Two pulses seen as three flashes: a superposition analysis. Vision Research. 1989;29(4):409–417. doi: 10.1016/0042-6989(89)90005-9. [DOI] [PubMed] [Google Scholar]
- 8.Stromeyer CF, Martini P. Human temporal impulse response speeds up with increased stimulus contrast. Vision Research. 2003;43(2003):285–298. doi: 10.1016/s0042-6989(02)00412-1. [DOI] [PubMed] [Google Scholar]
- 9.Stromeyer CF, 3rd, et al. Colour adaptation modifies the long-wave versus middle-wave cone weights and temporal phases in human luminance (but not red-green) mechanism. J Physiol. 1997;499(Pt 1):227–254. doi: 10.1113/jphysiol.1997.sp021923. (Pt 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.First MB, et al. Structured clinical interview for DSM-IV-TR Axis I Disorders -Patient Edition (SCID - I/P, 11/2002 revision) New York, NY: Biometrics Research Department; 2002. [Google Scholar]
- 11.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. Journal of Clinical Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 12.Davis JM. Dose equivalence of the antipsychotic drugs. Journal of Psychiatric Research. 1974;11:65–69. doi: 10.1016/0022-3956(74)90071-5. [DOI] [PubMed] [Google Scholar]
- 13.Kay S, Fiszbein A, Opler L. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophria Bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 14.First MB, S R, Gibbon M, William JBW. Structure Clinical Interview for DSM -IV-TR Axis I Disorders - Non-patient Edition (SCID-I/NP, 11/2002 revision) New York, NY: Biometric Research Department, New York State Psychiatric Institute; [Google Scholar]
- 15.Wechsler D. Manual for the Adult Intelligence Scale-Revised. New York: Psychological Corporation; 1981. [Google Scholar]
- 16.Lepine JP, Piron JJ, Chapotot E. Factor analysis of the PANSS in schizophrenic patients, in Psychiatry Today: Accomplishments and Promises. In: Stefanis CN, Soldatos CR, Rabavilas AD, editors. Proceedings of the 8th international Congress of Psychiatry. Amsterdam: Athens; 1989. p. Abstract 3232. [Google Scholar]
- 17.Green MF, Nuechterlein KH, Mintz J. Backward masking in schizophrenia and mania. I. Specifying a mechanism. Archives of General Psychiatry. 1994;51(12):939–944. doi: 10.1001/archpsyc.1994.03950120011003. [DOI] [PubMed] [Google Scholar]
- 18.Butler PD, Schechter I, Zemon V, Schwartz SG, Greenstein VC, Gordon J, Schroeder CE, Javitt DC. Dysfunction of early-stage visual processing in schizophrenia. American Journal of Psychiatry. 2001;158(7):1126–1133. doi: 10.1176/appi.ajp.158.7.1126. [DOI] [PubMed] [Google Scholar]
- 19.Watson AB, et al. Patterns of temporal interaction in the detection of gratings. Vision Res. 1977;17(8):893–902. doi: 10.1016/0042-6989(77)90063-3. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Bedell HE, Frishman LJ. Temporal-contrast discrimination and its neural correlates. Perception. 1996;25(5):505–522. doi: 10.1068/p250505. [DOI] [PubMed] [Google Scholar]
- 21.Skottun BC, Skoyles JR. Contrast sensitivity and magnocellular functioning in schizophrenia. Vision Res. 2007;47(23):2923–2933. doi: 10.1016/j.visres.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Delord S, et al. Psychophysical assessment of magno- and parvocellular function in schizophrenia. Vis Neurosci. 2006;23(3–4):645–650. doi: 10.1017/S0952523806233017. [DOI] [PubMed] [Google Scholar]
- 23.Butler PD, Javitt DC. Early-stage visual processing deficits in schizophrenia. Curr Opin Psychiatry. 2005;18(2):151–157. doi: 10.1097/00001504-200503000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joseph JS, Chun MM, Nakayama K. Attentional requirements in a 'preattentive' feature search task. Nature. 1997;387(6635):805–807. doi: 10.1038/42940. [DOI] [PubMed] [Google Scholar]
- 25.Nestor PG, et al. Attentional networks and cingulum bundle in chronic schizophrenia. Schizophr Res. 2007;90(1–3):308–315. doi: 10.1016/j.schres.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stip E, et al. Atypical neuroleptics and selective attention. Encephale. 1999;25(3):260–264. [PubMed] [Google Scholar]
- 27.Green M, Walker E. Symptom correlates of vulernability to backward masking in schizophrenia. American Journal of Psychiatry. 1986;143(2):181–186. doi: 10.1176/ajp.143.2.181. [DOI] [PubMed] [Google Scholar]
- 28.Butler PDD, L.A.Maddox J, Herkavy-Friedman JM, Amador XF, Raymond RG, Javitt DC, Gorman JM. Visual-backward masking deficits in schizophrenia: relationship to visual pathway function and symptomatology. Schizophrenia Research. 2003;59:199–209. doi: 10.1016/s0920-9964(01)00341-3. [DOI] [PubMed] [Google Scholar]
- 29.Selemon LD, Begovic A. Stereologic analysis of the lateral geniculate nucleus of the thalamus in normal and schizophrenic subjects. Psychiatry Res. 2007;151(1–2):1–10. doi: 10.1016/j.psychres.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Donnell BF, et al. Selective deficits in visual perception and recognition in schizophrenia. Am J Psychiatry. 1996;153(5):687–692. doi: 10.1176/ajp.153.5.687. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, et al. Processing of global, but not local, motion direction is deficient in schizophrenia. Schizophrenia Research. 2003;61(2–3):215–227. doi: 10.1016/s0920-9964(02)00222-0. [DOI] [PubMed] [Google Scholar]
- 32.Kim D, et al. Magnocellular contributions to impaired motion processing in schizophrenia. Schizophrenia Research. 2006;82(1):1–8. doi: 10.1016/j.schres.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keri S, et al. Spatiotemporal visual processing in schizophrenia. Journal of Neuropsychiatry Clinical Neuroscience. 2002;14(2):190–196. doi: 10.1176/jnp.14.2.190. [DOI] [PubMed] [Google Scholar]
- 34.Amado I, Olie JP. Effects of disorganization on choice reaction time and visual orientation in untreated schizophrenics. Dialogues Clin Neurosci. 2006;8(1):53–58. doi: 10.31887/DCNS.2006.8.1/iamado. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bedwell JS, Brown JM, Miller LS. The magnocellular visual system and schizophrenia: what can the color red tell us? Schizophr Res. 2003;63(3):273–284. doi: 10.1016/s0920-9964(02)00356-0. [DOI] [PubMed] [Google Scholar]
- 36.Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Arch Gen Psychiatry. 1992;49(12):975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- 37.Revheim N, et al. Reading impairment and visual processing deficits in schizophrenia. Schizophr Res. 2006;87(1–3):238–245. doi: 10.1016/j.schres.2006.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adams J, et al. ERP abnormalities during semantic processing in schizophrenia. Schizophr Res. 1993;10(3):247–257. doi: 10.1016/0920-9964(93)90059-r. [DOI] [PMC free article] [PubMed] [Google Scholar]