Abstract
Clinical research suggests hormonal contraceptive use is associated with increased frequencies of herpes simplex virus (HSV) reactivation and shedding. We examined the effects of medroxyprogesterone acetate (MPA), the compound most commonly used for injectable hormonal contraception, on HSV-1 reactivation and CD8+ T cell function in murine trigeminal ganglia (TG). In ex vivo TG cultures, MPA dramatically inhibited canonical CD8+ T cell effector functions, including IFN-γ production and lytic granule release, and increased HSV-1 reactivation from latency. In vivo, MPA treatment of latently infected ovariectomized mice inhibited IFN-γ production and lytic granule release by TG resident CD8+ T cells stimulated directly ex vivo. RNA specific for the essential immediate early viral gene ICP4 as well as viral genome DNA copy number were increased in mice that received MPA during latency, suggesting that treatment increased in vivo reactivation. The increase in HSV-1 copy number appeared to be the result of a two-tine effect, as MPA induced higher reactivation frequencies from latently infected explanted TG neurons in the presence or absence of CD45+ cells. Our data suggest hormonal contraceptives that contain MPA may promote increased frequency of HSV reactivation from latency through the combinatory effects of inhibiting protective CD8+ T cell responses and by a leukocyte-independent effect on infected neurons.
Keywords: Rodent, T cells, Viral
Introduction
Herpes simplex virus (HSV) infection in humans are characterized by the establishment of viral latency (lifelong retention of functional viral genomes in the nuclei of infected neurons without production of infectious virions), as well as intermittent viral reactivation, anterograde axonal transport, and shedding at peripheral sites (1). HSV infections are highly prevalent worldwide (2, 3), and their intermittent reactivation from latency is associated with substantial morbidity. Clinical manifestations of disease include recurrent, painful epithelial and mucosal ulcerations, stromal keratitis, retinal necrosis, and encephalitis (4–8). Moreover, considerable evidence suggests HSV infections facilitate both sexual transmission and acquisition of HIV (9, 10). Intrapartum transmission of either HSV-1 or HSV-2 can cause severe, permanent neonatal neurological damage (11, 12). Because of the numerous adverse events associated with HSV infection, better understanding of the factors that promote viral reactivation and shedding is needed.
A key component for maintenance of HSV latency is host immunity. While the increased severity of disease reported among HSV infected immunocompromised patients suggests an integral role for cell-mediated immunity in the control of reactivation (13, 14), more recent data indicates that CD8+ T cells may be of particular importance in this process. In both experimental models and humans, CD8+ T cells retained in direct apposition to latently infected TG neurons remain persistently activated (15, 16). In murine models of infection, HSV-specific CD8+ T cells are essential for the prevention of reactivation from latency both in vivo and in ex vivo trigeminal ganglia (TG) cultures (15, 17–19). The HSV-specific CD8+ T cells retained in latently infected ganglia block reactivation through IFN-γ production (17, 20) and lytic granule release (our unpublished observation).
Many factors which induce HSV reactivation, such as ultraviolet radiation exposure and higher levels of physical or emotional stress, are also known to modulate the immune response (21–23). The female sex steroid progesterone modulates host immunity, inhibiting both T-cell activation (24, 25) and proliferation (26), but its effects on HSV reactivation are largely unknown. The use of depot medroxyprogesterone acetate (MPA), an injectable, progestin-only hormonal contraceptive, was recently shown to be a risk factor for increased detection of HSV-2 in the female genital tract (27), but the mechanism(s) responsible for this observation was not elucidated.
We utilized a murine model of ocular HSV-1, in which latency is established in TG neurons by 10 days post infection (dpi), and HSV-specific CD8+ T cells are retained in close apposition to neurons for the life of the animal (15), to investigate both the effects of MPA on CD8+ T cell function within the latently infected TG and on HSV-1 reactivation from latency. Our results demonstrate that MPA administration impairs viral specific CD8+ T cell effector function, and increases reactivation of HSV-1 from latency, at least in part, through a leukocyte-independent effect on infected neurons.
Materials and Methods
Mice and virus infection
All animal experiments were conducted according to the guidelines of the Institutional Animal Care and Use Committee of the University of Pittsburgh. Ovariectomized 5–6 week old female C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME), and provided a pellet diet devoid of estrogenic hormones (Verified Casein Diet 1 IF, LabDiet, St. Louis, MO). After a 1 week acclimation period, mice were anesthetized via intraperitoneal injection of 2.0 mg ketamine/0.04 mg xylazine (Phoenix Scientific; St. Joseph, MO). Corneas of the sedated mice were scarified bilaterally, and 3 μl of RPMI (BioWhittaker, Walkersville, MD) containing 1×105 PFU of wild-type HSV-1 RE was topically applied.
Reagents
TG culture medium consisted of DMEM (BioWhittaker) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA), 100 IU/ml penicillin G, 50 mg/ml streptomycin, and 10 U/ml recombinant IL-2 (R&D Systems, Minneapolis, MN). The antiviral drug acyclovir (American Pharmaceuticals Partners, Schaumburg, IL) was added, where indicated, to TG cultures at a concentration of 50 μg/ml. For ex vivo TG cultures, stock solutions of MPA (Spectrum, Gardena, CA) were solubilized in dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO) at a concentration of 10−2 M, and working solutions were diluted in TG culture medium (DMSO concentration in working solutions was < 0.001%). The final concentrations of MPA in the ex vivo TG cultures were within pharmacologic serum concentrations of the drug (28). For in vivo treatment, latently infected mice were implanted with 21 day sustained release pellets containing 10.0 or 50.0 mg MPA or matching placebo pellets (Innovative Research of America, Sarasota, FL). These mice were anesthetized by administration of ketamine/xylazine intraperitoneally, and pellets placed in subcutaneous tissue behind the neck via suprascapular incision. Surgical wounds were closed with 3–0 Vicryl (Ethicon, Somerville, NJ), and mice examined daily to confirm well-being and pellet retention. All antibodies were purchased from BD Pharmingen (San Diego, CA) unless otherwise indicated.
Single cell TG suspensions
TG of mice latently infected with HSV-1 were excised and dissociated with 400 U/ml collagenase type I (Sigma-Aldrich) into single-cell suspensions as previously described (29). Where indicated, TG cell suspensions were depleted of CD45+ cells by sequential treatment with monoclonal antibodies specific for CD45 (30-F11) and rabbit complement (Cedarlane Laboratories, Hornby, Ontario, Canada) (30, 31). Depletion of CD45+ cells was confirmed by flow cytometric analysis.
Analysis of the TG inflammatory cell infiltrates
At >35 dpi, TG were excised, dispersed into single cell suspensions, and stained with PerCP-conjugated anti-CD45 (30-F11), Pacific Blue conjugated anti-CD8α (53-6.7, eBioscience, San Diego, CA), and PE-conjugated H-2Kb dimers (BD Pharmingen) or tetramers (NIAID Tetramer Facility) containing the immunodominant gB498–505 (SSIEFARL) peptide (Invitrogen) (32).
Cytokine/Chemokine detection in TG culture supernatant
At 35–40 dpi, TG cultures (1.0 TG equivalent per well of a 24-well culture plate) were incubated in TG culture medium containing acyclovir for 5 days at 37°C/5% CO2. Cytokine arrays were performed on collected supernatants using Beadlyte® Mouse 21-Plex Cytokine Detection (Upstate Biotechnology, Lake Placid, NY) and Luminex® 100™ xMAP® (Luminex Corporation, Austin, TX) systems according to manufacturers’ instructions. Quantification of cytokine/chemokine concentrations was performed by regression analyses from standard curves generated from standards supplied by the manufacturer.
Ex vivo CD8+ T cell responses
At >35 dpi, single cell TG suspensions were stimulated for 6 h with 5×105 HSV-1 infected targets (B6WT3 fibroblast cell line infected at a multiplicity of infection = 5) at 37°C/5% CO2 in the presence of FITC-conjugated anti-CD107a (1D4B) and Golgi-Plug (BD Pharmingen). Following stimulation, cells were stained with PerCP-conjugated anti-CD45 (30-F11) and Pacific Blue-conjugated anti-CD8α (53-6.7, eBioscience). Following treatment with Cytofix/Cytoperm (BD Pharmingen), intracellular cytokine staining with APC-conjugated anti-IFN-γ (XMG1.2) and PE-conjugated anti-TNF (MP6-XT22) was performed. Appropriate isotype control antibodies were included.
CD8+ T cell response to latently infected neurons
At 35–40 dpi, pooled TG cells were suspended in TG medium supplemented with acyclovir, or vehicle only, and incubated at 37°C/5% CO2 (1.0 TG equivalent per well of a 24-well plate). FITC-conjugated anti-CD107a (1D4B) and Golgi-Plug (BD Pharmingen) were added to the cultures during the last 6 h of a 96-h incubation, and staining was performed as described above.
Quantification of TG ICP4 HSV-1 transcripts
At 4 dpi (acute infection) or 32 dpi (latent infection), TG were excised and disassociated into single cell suspensions. Total RNA was extracted from the cells using QIAshredder and RNeasy kits (QIAGEN, Valencia, CA) according to manufacturer’s instructions. cDNAs were then generated from the extracted RNA using a high-capacity cDNA archive kit (Applied Biosystems Inc.[ABI], Foster City, CA), also according to manufacturer’s instructions. Quantitative real-time PCR (QRT-PCR) analysis for message transcripts used ABI primer-probe kits for the mouse housekeeping gene encoding pyruvate carboxylase (PC: catalog # Mm0050092_m1) and for the HSV-1 immediate early gene ICP4. The custom-synthesized ICP4 sequences were: forward primer (5′-GCAGCAGTACGCCCTGA-3′), reverse primer (5′-CGGCGCCTCTGCGT-3′), and probe [5′-(FAM) CACGCGGCTGCTGTACA (NFQ)-3′]. QRT-PCR assays were performed as previously described (29), and ICP4 viral transcript levels were determined by the mean difference between levels measured from samples in which reverse transcriptase (RT) was included (positive RT) and samples in which RT was excluded (no RT controls).
Quantification of TG HSV-1 genome copy number
HSV-1 genome copy number per TG was determined by real-time PCR quantification of the HSV-1 glycoprotein H (gH) gene (18). Total DNA per TG was isolated using DNeasy columns (Qiagen), and quantified spectrophotometrically. Using real-time PCR, 25 ng of DNA was amplified using TaqMan Universal PCR Master Mix (Roche) and a gH-specific primer-probe set (Applied Biosystems’ Assays-by-Design Service). Samples were assayed in 96-well plates using an Applied Biosystems Prism 7700 sequence detector. The number of viral gH copies per sample was determined from standard curves generated from known standards, and the number of copies of viral genome per TG was calculated based on the total DNA extracted from each TG. The gH sequences were: forward primer (5′-CGACCACCAGAAAACCCTCTTT-3′), reverse primer (5′-ACGCTCTCGTCTAGATCAAAGC-3′), and probe (5′-(FAM) TCCGGACCATTTTC (NFQ)-3′.
HSV-1 reactivation kinetics
TG cell suspensions were incubated in 96-well plates (0.1 TG equivalents per well) at 37°C (5% CO2). Culture supernatants were sampled after 4 and 8 days of incubation, and assayed for infectious HSV-1 using a standard viral plaque assay on a monolayer of Vero cells as previously described (29).
Statistical Analysis
Statistical analysis of the effects of MPA on cytokine detection in TG culture supernatants was performed using a two-tailed unpaired t test with 95% confidence intervals. Differences in CD8 T cell number and function and viral genome copy number were determined by one-way ANOVA and subsequent Tukey’s multiple comparisons. For quantification of ICP4 viral transcripts, results were analyzed using Student’s Newman-Keuls test (t test among multiple sample groups).
Results
In vivo MPA does not affect the number of CD8+ T cell maintained in the latently infected TG
After the establishment of HSV-1 latency (35 dpi), ovariectomized C57BL/6 mice were treated with sustained release MPA (10.0 or 50.0 mg) or matching placebo pellets. Fourteen days after pellet placement, TG from individual mice were excised and dispersed with collagenase. The entire cell suspension was then processed and analyzed for total number of CD8+ T cells as well as the percentage of CD8+ T cells specific for HSV-1 (H2-Kb/gB498–505 dimer positive). Neither MPA treatment concentration significantly affected the total number of TG CD8+ T cells or the percentage of CD8+ T cells that were HSV-specific (Fig. 1).
Figure 1.
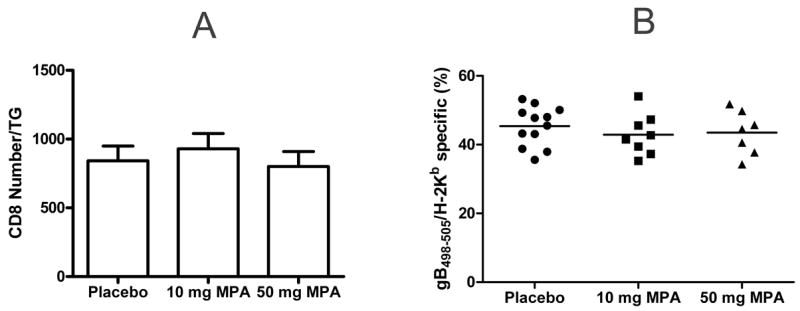
In vivo MPA treatment does not affect the number of CD8+ T cells detected directly ex vivo from latently infected TG. Latently infected ovariectomized mice (35 dpi) were treated with 21 day sustained release pellets containing 10.0 or 50.0 mg MPA or matching placebo pellets. After 14 days, TG were excised, dispersed into single-cell suspensions, and simultaneously stained with anti-CD8α mAb and dimer or tetramer containing the immunodominant HSV-1 gB498–505 epitope (gB498–505 H2-Kb). A, The mean absolute number of CD8+ T cells per TG (±SEM) in MPA and placebo treated mice. B, Percentage of CD8+ T cells that recognize the gB498–505 epitope in MPA and placebo treated mice. No group differences were statistically significant as evaluated by ANOVA and Tukey’s multiple comparison tests.
MPA inhibits cytokine/chemokine production in ex vivo cultures of latently infected TG
TG from mice latently infected with HSV-1 (>35 dpi) were excised, dispersed into single cell suspensions and incubated in TG medium containing 10−10 M MPA or vehicle alone. After 5 d incubation, individual culture supernatants were removed and assayed for cytokines/chemokines using multiplex detection system technology. Compared to controls, supernatants from MPA treated cultures contained significantly lower levels of IFN-γ and TNF (Figure 2). HSV-specific CD8+ T cells are considered necessary for the maintenance of HSV-1 latency (17), and these two cytokines are important mediators of T cell effector function.
Figure 2.
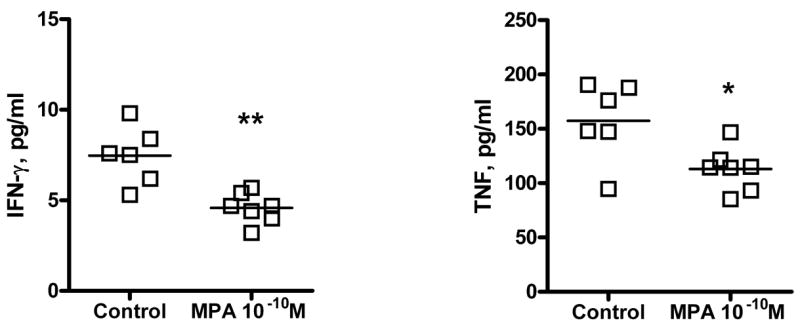
MPA decreases supernatant concentrations of IFN-γ and TNF in ex vivo cultures from latently infected TG. TG from mice latently infected with HSV-1 (>35 dpi) were excised, dispersed into single cell suspensions and incubated in TG medium containing acyclovir and 10−10 M MPA or vehicle alone. After 5 day incubation, the supernatants from ex vivo cultures of latently infected TG were collected for the measurement of a variety of cytokines and chemokines using multiplex detection system technology. The supernatants from cultures treated with 10−10 M MPA concentrations (n = 7) contained significantly lower levels of the IFN-γ and TNF compared to the levels detected in vehicle-treated cultures (n = 6). (**, p = 0.0015, *, p = 0.0168, respectively).
In vivo MPA impairs the function of TG-resident CD8+ T cells
Since CD8+ T cells produce the majority of IFN-γ detected in latently infected TG cultures (20), we next sought to determine the effect of MPA treatment on the function of TG resident HSV-specific CD8+ T cells. Ovariectomized mice harboring latent HSV-1 (35 dpi) were treated for 14 d with sustained release pellets containing MPA (10.0 or 50.0 mg) or matching placebo pellets. TG were then excised, dispersed into single cell suspensions, and stimulated directly ex vivo with HSV-1 infected targets. CD8+ T cells were analyzed for IFN-γ and TNF production and lytic granule release (CD107a surface expression) (33). As shown in Figure 3, both in vivo doses of MPA significantly reduced IFN-γ and TNF production and lytic granule release by TG resident HSV-1 specific CD8+ T cells stimulated directly ex vivo.
Figure 3.
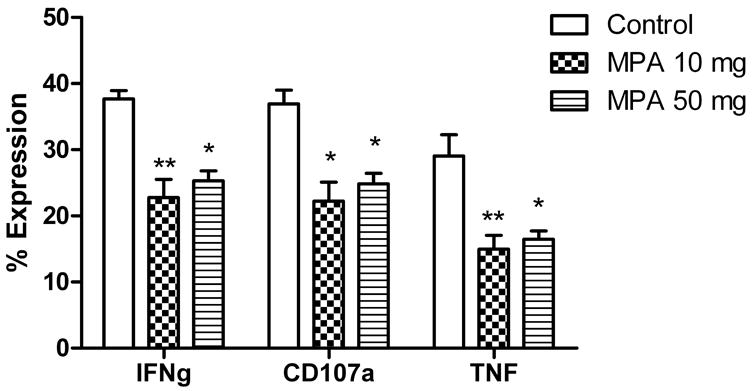
In vivo MPA treatment decreases CD8+ T cell function in response to infected targets. Latently infected ovariectomized mice (35 dpi) were treated with MPA or matching placebo pellets as described in Fig. 1. 14 d after pellet implantation, single-cell suspensions of TG from individual mice were stimulated directly ex vivo for 6 hr with histocompatible fibroblasts infected with HSV-1, and CD8+ T cells were analyzed for lytic granule release (CD107a) and INF-γ and TNF production. Data are presented as the mean (±SEM) percentage of IFN-γ, CD107a, or TNF positive CD8+ T cells in each treatment group. *, p < 0.05, **, p <0.01, as evaluated by ANOVA and Tukey’s multiple comparison tests.
MPA suppresses CD8+ T cell function in ex vivo TG cultures
To determine if MPA inhibits CD8+ T cell effector function in response to latently infected neurons, single cell TG suspensions from mice latently infected with HSV-1 were incubated for 96 h in TG medium supplemented with 10−8 or 10−10 M MPA. In these cultures, HSV-1 typically does not reactivate from latency in neurons until 4 d after culture initiation, and in the absence of reactivation, neurons are the only cells in which viral gene expression is detectable (29). To further insure that latency was maintained, and that neurons were the only source of HSV-1 proteins, half of the cultures were additionally supplemented with the antiviral drug acyclovir. During the last 6 h of incubation, CD8+ T cell lytic granule exocytosis and IFN-γ and TNF production were assayed. MPA treatment significantly reduced production of IFN-γ and TNF and lytic granule exocytosis by CD8+ T cells at both concentrations tested (Fig. 4). CD8+ T cell viability, however, was unaffected by MPA treatment (data not shown). Nearly identical results were obtained in parallel cultures that did not receive acyclovir (data not shown).
Figure 4.
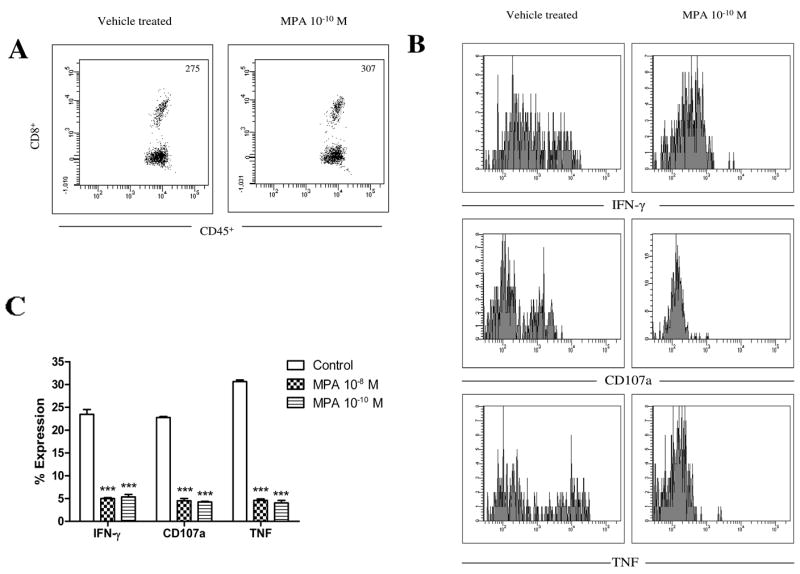
MPA impairs effector function of CD8+ T cells interacting with HSV-1 latently infected neurons in ex vivo TG cultures. Single-cell suspensions of pooled TG (1.0 TG equivalents) from mice latently infected with HSV-1 (35–40 dpi) were incubated for 96 h in TG medium supplemented with acyclovir and 10−8 or 10−10 M MPA or vehicle alone. Endogenous CD8+ T cells were assayed for lytic granule release (CD107a) and IFN-γ and TNF production. A, Representative dot plots demonstrate similar numbers of CD8+ T cells from TG cultures treated with vehicle or MPA. B, Representative histograms of CD8+ T cell effector function are demonstrated for the response to latently infected neurons seen in TG cultures treated with vehicle or 10−10 MPA. C, Bar graphs representing three experiments are shown illustrating the percentage of CD107a expression and IFN-γ and TNF production by CD8+ T cells for each of the culture conditions tested. Error bars denote the standard error of the mean. ***, p < 0.001, as evaluated by ANOVA and Tukey’s multiple comparison tests.
In vivo MPA treatment increases HSV-1 genome copy number and induces lytic gene expression in TG
To determine if the inhibition of CD8+ T cell effector function produced by MPA treatment was associated with higher HSV-1 viral genome copies in the latently infected TG, ovariectomized mice harboring latent HSV-1 (≥ 32 dpi) were treated for 14 d with sustained release MPA or matching placebo pellets. At sacrifice, pellet retention was confirmed. TG were excised, dispersed into single cell suspensions, and DNA was analyzed for HSV-1 genome copy number by real time PCR. Placebo treated mice established HSV-1 latency with TG viral burdens quite similar to those observed in wild type B6 mice (19). However, MPA treatment caused a dramatic, dose-dependent increase in HSV-1 genome copy number (Fig. 5A).
Figure 5.
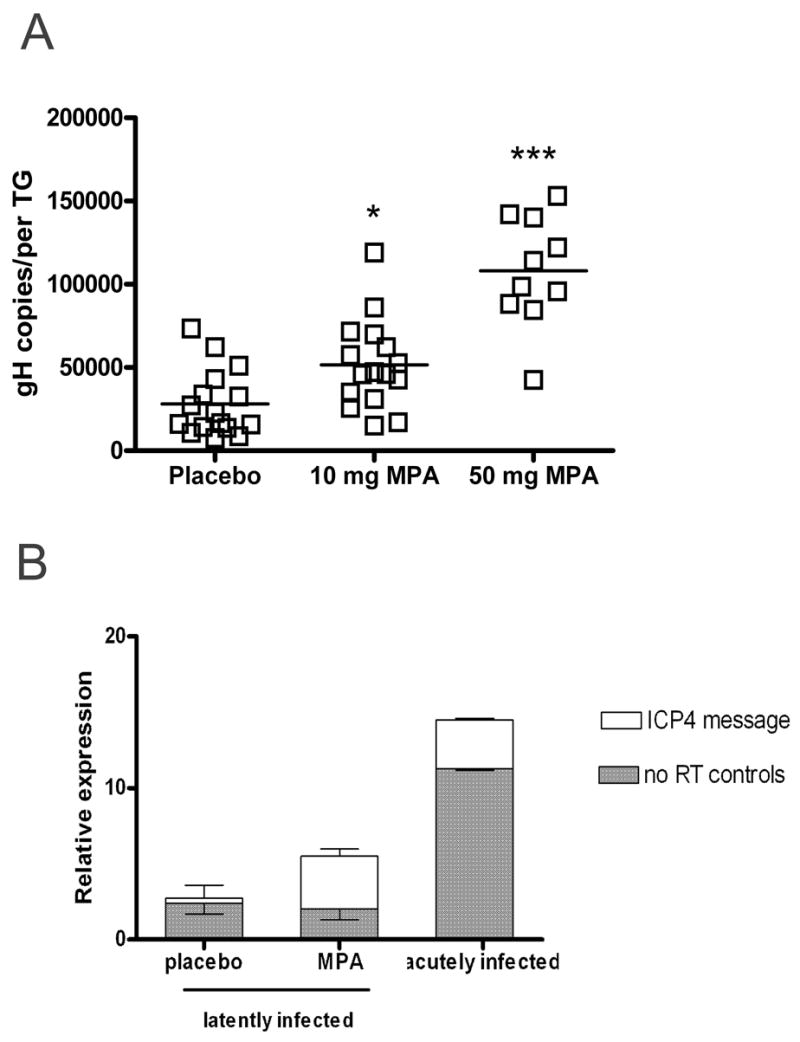
MPA treatment of latently infected mice increases HSV-1 genome copy number and induces lytic gene expression in the TG. A, Ovariectomized mice latently infected with HSV-1 (≥ 32 dpi) were treated with 21 d sustained release pellets containing 10.0 or 50.0 mg MPA or matching placebo pellets for 14 d prior to TG excision. HSV-1 genome copy number per TG was determined by real-time PCR. *, p < 0.05, ***, p < 0.001, as evaluated by ANOVA and Tukey’s multiple comparison tests. B, Untreated acutely infected (4 dpi) and latently infected (≥ 32 dpi) ovariectomized mice treated with 21 d sustained release pellets containing 50.0 mg MPA or matching placebo pellets for 5 d prior to TG excision were used for real-time PCR analysis of ICP4 expression. Samples with a CT value of 40 were considered to lack ICP4 transcripts, and the relative amounts of transcript per group are displayed as 40 − CT. Positive RT samples from MPA-treated, latently infected mice had a mean CT lower than their no RT equivalents (p < 0.01) and lower than samples from mice that received placebo pellets (p < 0.05), as evaluated by the Student Newman-Keuls test (t test among multiple sample groups) (n = 13).
Ovariectomized latently infected with HSV-1 (≥ 32 dpi) were treated for 5 d with sustained release MPA or matching placebo pellets to measure expression of the HSV-1 lytic gene ICP4 during the course of MPA exposure. Additional ovariectomized mice were sacrificed 4 dpi to determine the level of expression in the acutely infected TG. Transcripts for ICP4 were detected by QRT-PCR in TG of latently infected mice treated with MPA, but not in the TG of latently infected mice that received matching placebo pellets (Fig 5B). Although the control (no RT) data suggests that viral DNA content during acute infection is as much as 1000-fold higher than the amount present in mice latently infected with HSV-1, we found that the latently infected MPA treated mice had an ICP4 transcript level comparable to that found in the acutely infected TG (Figure 5B). The increased viral genome copy number and ICP4 expression detected during MPA exposure suggests that treatment during latency induced in vivo HSV-1 reactivation, at least to the point of DNA replication.
MPA increases HSV-1 reactivation in ex vivo TG cultures
Following establishment of HSV-1 latency (35 dpi) TG were excised, dispersed with collagenase, and aliquots of TG cells were either depleted of CD45+ (bone marrow-derived) cells by treatment with antibody and complement or mock depleted. The TG cells were then suspended in TG medium supplemented with 10−10 M MPA or vehicle only (0.1 TG equivalent per well of a 96-well tissue culture plate). Supernatant fluids were serially sampled at 4 d and 8 d of culture. Among mock depleted TG cultures treated with vehicle alone, we observed a reactivation frequency of approximately 10%. This frequency of reactivation in cultures that contained 0.1 TG cell equivalents was compatible with the 100% reactivation frequency we previously saw in cultures that contained 1.0 TG cell equivalents (20). MPA treatment significantly increased the reactivation frequency in mock depleted TG cultures (Fig 6). CD45-depletion had a similar effect on HSV-1 reactivation frequency, consistent with the well-established capacity of CD8+ T cells to block HSV-1 reactivation from latency in ex vivo TG cultures, and the dramatic impairment of CD8+ T cell function in MPA treated cultures (Fig. 4). However, the reactivation frequency of CD45-depleted cultures was further increased by MPA treatment, demonstrating an additional leukocyte-independent effect of MPA on HSV-1 reactivation in ex vivo TG cultures.
Figure 6.
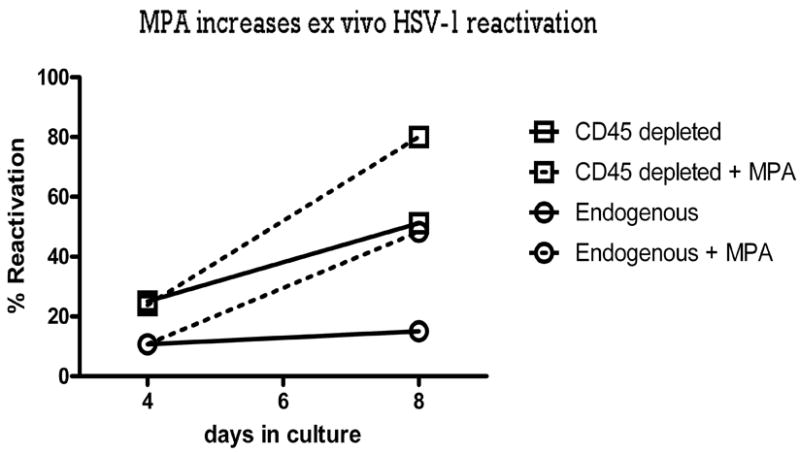
MPA increases ex vivo HSV-1 reactivation. Latently infected ganglia from ovariectomized mice latently infected with HSV-1 (35 dpi) were excised, dispersed into single-cell suspensions, and either depleted of CD45+ (bone marrow-derived) cells by treatment with antibody and complement or mock depleted. TG cells (0.1 TG equivalents) were then suspended in TG medium supplemented with 10−10 M MPA or vehicle. Culture supernatants were tested for live virus by plaque assay on d4 and d8 of the incubation. Pooled data from two assays (n = 81) are presented as the percentage of cultures in which HSV-1 reactivated.
Discussion
Ganglionic sensory neurons infected with HSV are the primary reservoir for latent virus, and intermittent reactivation from latency can cause recurrent oral and genital epithelial ulcerations, herpetic stromal keratitis, encephalitis, and a number of other manifestations of disease. HSV reactivation from latency may also be fueling the HIV pandemic (34). Extrication of viral genome from latently infected neurons can not be performed, and is unlikely to be available as a treatment option for HSV-infected individuals any time soon. Accordingly, a priority of current HSV research is a more complete elucidation of the mechanisms regulating latency, as this may permit the development of approaches that inhibit reactivation.
Previous research has shown that viral, hormonal, and immunological factors influence the likelihood of HSV reactivation. For example, the capacity of resident HSV-specific CD8+ T cells to inhibit HSV-1 reactivation from latency in TG neurons has been clearly established (17, 35). Increased stress and stress-related hormone levels are also associated with inhibition of CD8+ T cell function and increased HSV reactivation. These observations were linked in a recent study in which exposure to restraint stress reduced the number and inhibited the function of murine HSV-specific CD8+ T cells within latently infected TG, and was associated with increased viral reactivation from latency (19).
The current study similarly investigated the effects of MPA on number and function of CD8+ T cells in latently infected TG as well as viral reactivation from latency. We found that MPA treatment of ex vivo TG cultures dramatically reduced the capacity of the endogenous CD8+ T cells to produce IFN-γ and to release lytic granules. The reduced CD8+ T cell function did not appear to be related to toxic effects of treatment as the number of viable CD8+ T cells in the TG cultures were not altered by MPA. CD8+ T cell function was measured in these cultures in the presence or absence of acyclovir, a drug that inhibits viral DNA synthesis and reactivation. Prior to reactivation latent virus is harbored only in TG neurons (29), so latently infected neurons were the most probable source of viral antigens in these acyclovir-treated TG cultures. The observation that the level of IFN-γ production and lytic granule release by CD8+ T cells was nearly identical in acyclovir treated and non-treated ex vivo TG cultures, suggests that the T cell response to antigen was produced prior to viral DNA synthesis. Our finding is consistent with the observation that most HSV-specific CD8+ T cells in latently infected TG are specific for the gB498–505 epitope that is derived from the HSV-1 γ1 protein glycoprotein B (36). Moreover, γ1 proteins are produced at low levels prior to viral DNA synthesis, and HSV-1 infected targets gain the capacity to stimulate gB498–505-specific CD8+ T cells within 2 h of infection (37). The ability of CD8+ T cells to respond to latently infected neurons prior to full reactivation and virion formation is essential to their role in regulating HSV-1 latency.
Depletion of CD8+ T cells or CD45+ cells increases to a similar extent the reactivation frequency in cultures of latently infected TG, suggesting that CD8+ T cells are the leukocyte population primarily responsible for inhibiting reactivation (20). In the current study, we observed a 10% reactivation frequency from cultures containing 0.1 TG cell equivalents, signifying the presence of at least one reactivating neuron per TG. Both MPA treatment and CD45+ cell depletion of ex vivo TG increased HSV-1 reactivation from latency nearly five-fold. These results are consistent with a view that within ex vivo TG cultures, MPA may induce reactivation solely via inhibition of CD8+ T cell function. However, our observation that MPA further increased the reactivation frequency in TG cultures depleted of CD45+ cells demonstrated that MPA may also induce HSV-1 reactivation by a leukocyte-independent mechanism. In our hands, depletion of CD8+ or CD45+ cells from ex vivo TG cultures results in reactivation frequencies ranging from 30–60%. The much higher reactivation frequency (~ 80%) in MPA treated TG cultures depleted of CD45+ cells suggests that a subset of neurons that do not normally require immune system protection from reactivation are induced to reactivate by MPA. Overall, our findings are consistent with the concept that MPA induces HSV-1 reactivation from latency both by inhibiting protective CD8+ T cell responses and by directly or indirectly inducing HSV-1 reactivation in neurons by a leukocyte-independent mechanism.
Since direct exposure to MPA in ex vivo TG cultures dramatically inhibited HSV-specific CD8+ T cell function and enhanced HSV-1 reactivation, it was of interest to determine if a similar effect would be observed in vivo. Corneas of ovariectomized mice were infected with HSV-1, and viral latency was established before treatment with sustained release MPA pellets. We found that mice treated with matching placebo pellets established HSV-1 latency with TG viral burdens similar to those observed in wild type mice (19). We also found that in vivo MPA treatment significantly inhibited IFN-γ and TNF production and lytic granule release by resident TG CD8+ T cells when stimulated directly ex vivo with HSV-infected targets, and that these CD8+ T cell functions were similarly affected by either concentration of MPA tested. In addition, treatment did not influence the total number of CD8+ T cells in latently infected TG or the percentage of CD8+ T cells that were HSV-specific. These results are consistent with our findings from ex vivo TG cultures where treatment inhibited CD8+ T cell function but produced no observable toxicity. Although both concentrations of MPA pellets tested had a similar effect on CD8+ T cell function, we did observe a dose-dependent increase in viral genome copy number in treated mice. Based on our in vitro studies, the dose-dependent effect on viral genome copy number could reflect both inhibition of the protective CD8+ T cell response and a leukocyte-independent induction of reactivation in neurons that do not normally require maintenance of viral latency. The possibility that inhibition of CD8+ T cell function is exerted at a lower dose of MPA than is required for the leukocyte independent induction of HSV-1 reactivation is currently under investigation.
In large groups of latently infected female mice, we have previously observed a relatively narrow range of CD8+ T cell number, CD8+ T cell function, and viral genome copy number. Thus, changes in progesterone concentrations during the murine estrous cycle do not appear to produce the inhibition of CD8+ T cell function and increase in viral genome copy number observed with MPA treatment. These results are consistent with clinical investigations that demonstrated that while the frequency of HSV shedding does not vary by stage of menstrual cycle (38), it was increased among women who used injectable contraceptives containing MPA (27). Further work is needed to determine if there are differences in the chemical makeup of MPA versus progesterone or if there are differential effects on host or viral gene expression that may account for the increased numbers of HSV-1 copies observed in latently infected mice treated with MPA. For example, prior investigation has demonstrated that the in vitro immunosuppressive effects produced by MPA are, in fact, more pronounced than those associated with progesterone (39). Determining these differences may be important for current use and future design of hormonal contraceptives that do not increase the likelihood of HSV shedding.
Acknowledgments
This work was supported by National Institutes of Health Grants K23 AI064396 (T.L.C), T32 AI 060525 (B.S.S), R01 EY05945 and P30EY08098 (R.L.H), an unrestricted grant from Research to Prevent Blindness (New York, NY), and the Eye and Ear Foundation of Pittsburgh
The authors would like to thank Dawn Maker and Nancy Zurowski for excellent technical assistance and the National Institute of Allergy and Infectious Diseases Tetramer Core Facility (Emory Vaccine Center, Atlanta, GA) for tetramers.
Footnotes
Disclosures
The authors have no financial conflicts of interest to report.
References
- 1.Roizman B, Knipe DM. Herpes simplex viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. 4. Lippincott Williams & Wilkins; Philadelphia, PA: 2001. pp. 2399–2459. [Google Scholar]
- 2.Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, Berman SM, Markowitz LE. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296:964–973. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- 3.Malkin JE, Morand P, Malvy D, Ly TD, Chanzy B, de Labareyre C, El Hasnaoui A, Hercberg S. Seroprevalence of HSV-1 and HSV-2 infection in the general French population. Sex Transm Infect. 2002;78:201–203. doi: 10.1136/sti.78.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaye S, Choudhary A. Herpes simplex keratitis. Prog Retin Eye Res. 2006;25:355–380. doi: 10.1016/j.preteyeres.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Curtis TH, Mandava N. Acute retinal necrosis as a late sequelae of herpes simplex type 1 encephalitis in a child. J AAPOS. 2007;11:509–510. doi: 10.1016/j.jaapos.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Itoh N, Matsumura N, Ogi A, Nishide T, Imai Y, Kanai H, Ohno S. High prevalence of herpes simplex virus type 2 in acute retinal necrosis syndrome associated with herpes simplex virus in Japan. Am J Ophthalmol. 2000;129:404–405. doi: 10.1016/s0002-9394(99)00391-8. [DOI] [PubMed] [Google Scholar]
- 7.Klein A, Lefebvre P. Three consecutive episodes of acute retinal necrosis due to herpes simplex-1 over twelve years following herpes encephalitis. Ocul Immunol Inflamm. 2007;15:411–413. doi: 10.1080/09273940701662510. [DOI] [PubMed] [Google Scholar]
- 8.Tyler KL. Update on herpes simplex encephalitis. Rev Neurol Dis. 2004;1:169–178. [PubMed] [Google Scholar]
- 9.Ramaswamy M, Geretti AM. Interactions and management issues in HSV and HIV coinfection. Expert Rev Anti Infect Ther. 2007;5:231–243. doi: 10.1586/14787210.5.2.231. [DOI] [PubMed] [Google Scholar]
- 10.Corey L. Synergistic copathogens – HIV-1 and HSV-2. N Engl J Med. 2007;356:854–856. doi: 10.1056/NEJMe068302. [DOI] [PubMed] [Google Scholar]
- 11.Brown ZA, Wald A, Morrow RA, Selke S, Zeh J, Corey L. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA. 2003;289:203–209. doi: 10.1001/jama.289.2.203. [DOI] [PubMed] [Google Scholar]
- 12.Knezevic A, Martic J, Stanojevic M, Jankovic S, Nedeljkovic J, Nikolic L, Pasic S, Jankovic B, Jovanovic T. Disseminated neonatal herpes caused by herpes simplex virus types 1 and 2. Emerg Infect Dis. 2007;13:302–304. doi: 10.3201/eid1302.060907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanberry LR, Floyd-Reising SA, Connelly BL, Alter SJ, Gilchrist MJ, Rubio C, Myers MG. Herpes simplex viremia: report of eight pediatric cases and review of the literature. Clin Infect Dis. 1994;18:401–7. doi: 10.1093/clinids/18.3.401. [DOI] [PubMed] [Google Scholar]
- 14.Stewart JA, Reef SE, Pellett PE, Corey L, Whitley RJ. Herpesvirus infections in persons infected with human immunodeficiency virus. Clin Infect Dis. 1995;21:S114–120. doi: 10.1093/clinids/21.supplement_1.s114. [DOI] [PubMed] [Google Scholar]
- 15.Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity. 2003;18:593–603. doi: 10.1016/s1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Theil D, Derfuss T, Paripovic I, Herberger S, Meinl E, Schueler O, Strupp M, Arbusow V, Brandt T. Latent herpesvirus infection in human trigeminal ganglia causes chronic immune response. Am J Path. 2003;163:2179–84. doi: 10.1016/S0002-9440(10)63575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lui TK, Khanna KM, Chen DJ, Fink DJ, Hendricks RL. CD8+ T cells can block herpes simplex virus (HSV-1) reactivation from latency in sensory neurons. J Ex Med. 2000;191:1459–1466. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoshino Y, Pesnicak L, Cohen JI, Straus SE. Rates of reactivation of latent herpes simplex virus from mouse trigeminal ganglia ex vivo correlate directly with the viral load and inversely with the number of infiltrating CD8+ T cells. J Virol. 2007;81:8157–8164. doi: 10.1128/JVI.00474-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freeman ML, Sheridan BS, Bonneau RH, Hendricks RL. Psychological stress compromises CD8+ T cell control of latent herpes simplex virus type 1 infections. J Immunol. 2007;179:322–328. doi: 10.4049/jimmunol.179.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lui T, Khanna KM, Carriere BN, Hendricks RL. Gamma interferon can prevent herpes simplex virus type 1 reactivation from latency in sensory neurons. J Virol. 2001;75:11178–11184. doi: 10.1128/JVI.75.22.11178-11184.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goade DE, Nofchissey RA, Kusewitt DF, Hjelle B, Kreisel J, Moore J, Lyons CR. Ultraviolet light induces reactivation in a murine model of cutaneous herpes simplex virus-1 infeciton. Photochem Photobiol. 2001;74:108–114. doi: 10.1562/0031-8655(2001)074<0108:uliria>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 22.Perna JJ, Mannix ML, Rooney JF, Notkins AL, Straus SE. Reactivation of latent herpes simplex virus infection by ultraviolet light: a human model. J Am Acad Dermatol. 1987;17:473–478. doi: 10.1016/s0190-9622(87)70232-1. [DOI] [PubMed] [Google Scholar]
- 23.Padgett DA, Sheridan JF, Dorne J, Berntson GG, Candelora J, Glaser R. Social stress and the reactivation of latent herpes simplex virus type 1. Proc Natl Acad Sci USA. 1998;95:7231–7235. doi: 10.1073/pnas.95.12.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mori T, Kobayashi H, Nishimura T, Mori TS, Fujii G, Inou T. Inhibitory effect of progesterone on the phytohaemagglutinin-induced transformation of human peripheral lymphocytes. Immunol Commun. 1975;4:519–527. doi: 10.3109/08820137509055790. [DOI] [PubMed] [Google Scholar]
- 25.Clemens LE, Sitteri PK, Stites DP. Mechanism of immunosuppression of progesterone on maternal lymphocyte activation during pregnancy. J Immunol. 1979;122:1978–1985. [PubMed] [Google Scholar]
- 26.Van Voorhis BJ, Anderson DJ, Hill JA. The effects of RU 486 on immune function and steroid-induced immunosuppression in vitro. J Clin Endocrinol Metab. 1989;69:1195–1199. doi: 10.1210/jcem-69-6-1195. [DOI] [PubMed] [Google Scholar]
- 27.Cherpes TL, Melan MA, Kant JA, Cosentino LA, Meyn LA, Hillier S. Genital tract shedding of herpes simplex virus type 2 in women: effects of hormonal contraception, bacterial vaginosis, and vaginal Group B Streptococcus colonization. Clin Infect Dis. 2005;40:1422–1428. doi: 10.1086/429622. [DOI] [PubMed] [Google Scholar]
- 28.Mishell DR., Jr Pharmacokinetics of depot medroxyprogesterone acetate contraception. J Reprod Med. 1996;41:381–390. [PubMed] [Google Scholar]
- 29.Decman V, Kinchington PR, Harvey SAK, Hendricks RL. Gamma interferon can block herpes simplex virus type reactivation from latency, even in the presence of late gene expression. J Virol. 2005;79:10339–10347. doi: 10.1128/JVI.79.16.10339-10347.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coulombe M, Gill RG. T lymphocyte indifference to extrathymic islet allografts. J Immunol. 1996;156:1998–2003. [PubMed] [Google Scholar]
- 31.Nakamoto Y, Suda T, Momoi T, Kaneko S. Different procarcinogenic potentials of lymphocyte subsets in a transgenic mouse model of chronic hepatitis B. Cancer Res. 2004;64:3326–3233. doi: 10.1158/0008-5472.can-03-3817. [DOI] [PubMed] [Google Scholar]
- 32.Sheridan BS, Khanna KM, Frank GM, Hendricks RL. Latent virus influences the generation and maintenance of CD8+ T cell memory. J Immunol. 2006;177:8356–8364. doi: 10.4049/jimmunol.177.12.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Betts MR, Koup RA. Detection of T-cell degranulation: CD107a and b. Methods Cell Biol. 2004;75:497–512. doi: 10.1016/s0091-679x(04)75020-7. [DOI] [PubMed] [Google Scholar]
- 34.Corey L, Wald A, Celum CL, Quinn TC. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J Acquir Immune Defic Syndr. 2004;35:435–445. doi: 10.1097/00126334-200404150-00001. [DOI] [PubMed] [Google Scholar]
- 35.Hoshino Y, Pesnicak L, Cohen JI, Straus SE. Rates of reactivation of latent herpes simplex virus from mouse trigeminal ganglia ex vivo correlate directly with viral load and inversely with number of infiltrating CD8+ T cells. J Virol. 2007;81:8157–8164. doi: 10.1128/JVI.00474-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallace ME, Keating R, Heath WR, Carbone FR. The cytotoxic T-cell response to herpes simplex virus type 1 infection of C57BL/6 mice is almost entirely directed against a single immunodominant determinant. J Virol. 1999;73:7619–7626. doi: 10.1128/jvi.73.9.7619-7626.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mueller SN, Jones CM, Chen W, Kawaoka Y, Castrucci MR, Heath WR, Carbone FR. The early expression of glycoprotein B from herpes simplex virus can be detected by antigen-specific CD8+ T cells. J Virol. 2003;77:2445–2451. doi: 10.1128/JVI.77.4.2445-2451.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mostad SB, Kreiss JK, Ryncarz A, Chohan B, Mandaliya K, Ndinya-Achola J, Bwayo JJ, Corey L. Cervical shedding of herpes simplex virus and cytomegalovirus throughout the menstrual cycle in women infected with human immunodeficiency virus type 1. Am J Obstet Gynecol. 2000;183:948–955. doi: 10.1067/mob.2000.106589. [DOI] [PubMed] [Google Scholar]
- 39.Elovitz M, Wang Z. Medroxyprogesterone acetate, but not progesterone, protects against inflammation-induced parturition and intrauterine fetal demise. Am J Obstet Gynecol. 2004;190:693–701. doi: 10.1016/j.ajog.2003.10.693. [DOI] [PubMed] [Google Scholar]


