Abstract
The majority of modular polyketide synthase (PKS) systems which generate unsaturated products do so with trans double bonds. Phoslactomycin B (PLM B) presents a class of antitumor and antiviral natural polyketide products that have unique structural features, including a linear unsaturated backbone with one trans and three cis double bonds. There is substantial evidence that trans double bonds are established by ketoreductase-dehydratase (KR-DH) didomains within a PKS module. In cases where modules containing these didomains appear to generate product containing a cis double bond there is no experimental evidence to determine if they do so directly, or if they also form a trans double bond with a subsequent isomerization step. A critical step in addressing this issue is establishing the stereochemistry of the polyketide intermediate which passes to the subsequent module. Herein, we demonstrate through a series of experiments that an activated cis-3-cyclohexylpropenoic acid is the diketide intermediate which passes from module 1 to module 2 of the PLM PKS. The trans isomer of the diketide intermediate could not be processed directly into PLM B by module 2, but could be converted to PLM B by degradation to cyclohexanecarboxylic acid and elongation by the entire PLM PKS. These observations indicate not only that module 1 with a DH-KR didomain is responsible for establishing C14–C15 cis double bond of PLM B, but that the subsequent modules of the PKS clearly discriminate between the cis and trans-diketide intermediate and do not contain domains capable of catalyzing double bond isomerization.
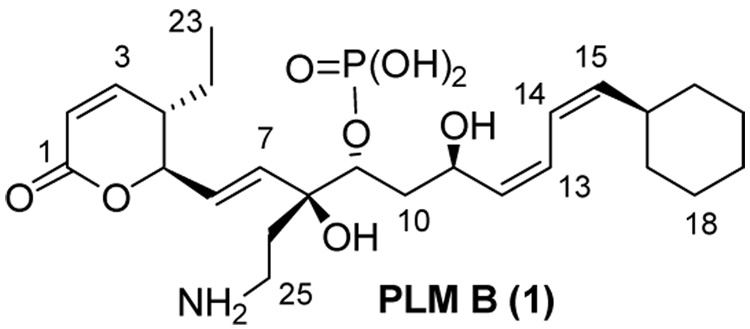
Phoslactomycins (PLMs), exemplified by PLM B (Figure 1), are a unique class of antitumor, antiviral, and antifungal polyketide natural products.1,2 The antitumor activity of PLMs is attributed to a potent and selective inhibition of protein Ser/Thr phosphatase 2A (PP2A).3 The PLM biosynthetic gene cluster from Streptomyces sp. HK803 has been cloned and sequenced.4 The PLM polyketide synthase (PKS) is a modular PKS comprised of a loading domain and seven extension modules which are responsible for the synthesis of a unique linear unsaturated polyketide structure containing three cis (Z) and one trans (E) double bonds.
Figure 1.
Phoslactomycin B (PLM B).
Modular PKSs which generate unsaturated products typically do so using trans double bonds.5 These double bonds are established by ketoreductase-dehydratase (KR-DH) domains which sequentially carry out ketoreduction and dehydration steps on the 3-ketoacyl-ACP products of the KS domains. The dehydration step makes the stereochemical course of the KR-catalyzed step cryptic. Recently in vitro work using a DH-inactivated module 2 of the pikromycin PKS, which establishes the single trans double bond of pikromycin and methymycin, have shown this KR generates the D-3-hydroxy product.6 A bioinformatic analysis of other cryptic KR-DH domains which generate trans-double bonds infers a D- hydroxyl configuration (this analysis is based on an established correlation of diagnostic residues in KR primary sequences and their known stereochemical products).5,7
Polyketide products containing cis double bonds are rare and appear to arise through a variety of mechanisms.8 In many cases such as modules 7 of PLM and module 4 of the epothilone PKS the required DH activity is absent from the module.4,9 Modules 1 and 2 of the PLM PKS are intriguing because they have combined KR-DH didomains which appear to establish two conjugated cis double bonds (C12–C13 and C14–C15 of PLM B, respectively).4 Bioinformatic analysis of the primary sequence of these KR domains does not clearly predict a D-hydroxyl configuration (which evidence indicates precedes trans double bond formation) or L-hydroxy configuration (which has been speculated might precede cis double bond formation).7 Thus in each case the combined activity of these KR-DH didomains might establish a trans double bond with a subsequent isomerization step to a cis double bond (epimerization domains, in both PKS10 and NRPS11 modules, as well as trans to cis double bond isomerization in retinoid cycle12 have been reported). Alternatively, these KR-DH domains might establish the cis double bond directly.
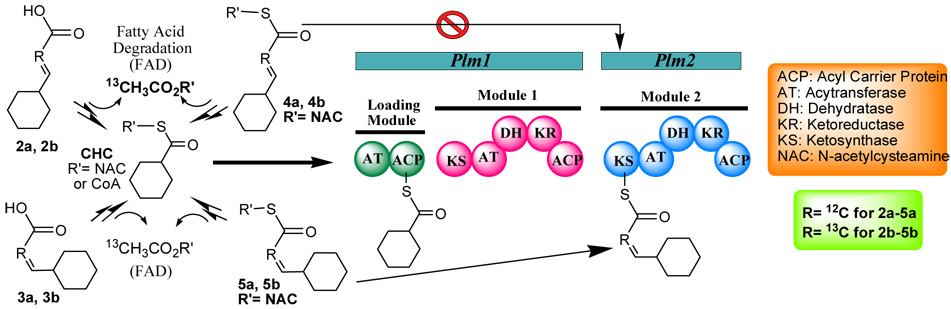
In this work, we have distinguished between these two possibilities by determining the stereochemistry of the polyketide intermediate which is transferred from module 1 to module 2. PLM1 contains a loading domain and the first extension module of the PKS and is predicted to generate either cis or trans 3-cyclohexylpropenoic acid (Figure 2) from an activated cyclohexanecarboxylic acid (CHC) starter unit. We generated a ΔchcA mutant (NP3), blocked in biosynthesis of the starter unit, and demonstrated that it only produces PLM B when grown in the presence of CHC (Table 1). The trans and cis diketide products of PLM1 were synthesized in both the acid (2a and 3a, Figure 2) and N-acetylcysteamine (SNAC) thioester (4a and 5a, Figure 2) forms and added to separate fermentations of this ΔchcA mutant. Surprisingly, compounds 2a–5a all restored PLM B production. PLM B production levels were the highest for the trans-products (2a and 4a) and were 40% higher than that observed with either CHC supplementation or the cis-SNAC (5a) (Table 1). The lowest level of PLM B production was observed with the cis-acid (3a). Interestingly, the PLM B isolated from feeding trans-acid 2a had the C14–C15 double bond in the cis configuration, as confirmed by 1H NMR and NOESY experiments. This initial result suggested that the trans-diketide intermediate might be the preferred substrate for PLM2, with a subsequent trans to cis isomerization step.
Figure 2.
Incorporation of CHC, compounds 2a–5a, and 2b–5b into Plm1 and Plm2 of PLM B PKS.
Table 1.
Relative % of PLM B production by feeding CHC and compounds 2a–5a to ΔchcA and Δplm1 mutants.
| Substrate | ΔchcA mutant | Δplm1 mutant |
|---|---|---|
| control | 0 | 0 |
| CHC | 68 ± 3.9 | 0 |
| 2a | 100 ± 7 | 0 |
| 3a | 50 ± 3 | 0 |
| 4a | 98 ± 6 | ~ 0.5 a |
| 5a | 72 ± 7 | 100 |
LC-MS analysis demonstrated 4a contained trace levels of 5a (<1%).
Alternatively, the trans-compounds might be converted efficiently to the activated CHC starter unit by fatty acid degradation and subsequently elongated by the entire PLM PKS (in this way the trans double bond would be lost through degradation and reintroduced as a cis double bond by PLM1) (Figure 2). To distinguish between these two hypotheses we synthesized and fed the [2-13C] labeled analogs 2b–5b (Figure 2) to the ΔchcA mutant. Mass spectroscopy revealed that isotopic enrichment over natural abundance for the PLM B product was only observed with the cis-SNAC 5b (20% isotope enrichment, Table 2). These data showed that both cis- and trans-compounds undergo degradation to form the activated CHC starter unit, and that this is the primary route for PLM B production in these experiments. Furthermore, the experiments established that only cis SNAC (5a,5b) could prime PLM2 directly. The cis-acid (3a,3b) which gives the lowest levels of PLM B restoration levels can be transported into the mutant and degraded to the activated CHC (at about 50% the efficiency of the corresponding trans-diketides) but cannot be activated intact such that it can prime PLM2.
Table 2.
% of 13C isotope enrichment in produced PLM B generated by feeding CHC and compounds 2b–5b to ΔchcA and Δplm1 mutants.
| Substrate | ΔchcA mutant | Δplm1 mutant |
|---|---|---|
| control | 0 | ND |
| CHC | 0 | ND |
| 2b | 0 | ND |
| 3b | 0 | ND |
| 4b | 0 | 99% a |
| 5b | ~ 20% | 99% |
LC-MS analysis demonstrated 4b contained trace levels of 5b (<1%).
ND: No PLM B production was detected
A consistent and predictable set of results was obtained by generation and analysis of a plm1 deletion mutant [NP9, see supplementation data] (Figure 2). PLM B production was abrogated in this mutant and was only significantly restored by growth in the presence of the cis-SNAC compounds 5a and its 13C-labeled counterpart 5b (Table 1). In the case of 5b the PLM B now contained the same level of isotopic enrichment (>99%) as the diketide substrate (Table 2). No restoration of PLM B was seen with cis or trans acids (2a,2b,3a,3b) and low levels of PLM B were observed with the trans SNAC diketides (4a,4b) and correlated with LC-MS detection of trace levels of the corresponding cis-SNAC diketides (5a,5b) in these samples (Table 1 and 2).
These observations unequivocally demonstrate that only the SNAC derivative of the cis-diketide can prime PLM2 directly and that all other diketides give rise to PLM B production only through degradation to an activated CHC and elongation using PLM1. The product of PLM1 must therefore be the cis-3-cyclohexylpropenoic acid. These experiments also demonstrate that the PLM biosynthetic process cannot process the trans-diketide intermediate either into PLM B (ruling out an isomerization domain in the subsequent PKS modules) or a PLM analog with trans C14–C15 double bond. This last observation indicates significant challenges to successful alteration of the stereochemistry of unsaturated polyketide products through either directed biosynthesis or KR-DH didomain switches.
Supplementary Material
Experimental procedures, spectroscopic data. This material is available free of charge via theInternet at http://pubs.acs.org
Acknowledgment
Funding for this research was generously provided by the National Institutes of Health (AI51629).
References
- 1.Fushimi S, Nishikawa S, Shimazu A, Seto H. J. Antibiot. 1989;42:1019–1025. doi: 10.7164/antibiotics.42.1019. [DOI] [PubMed] [Google Scholar]
- 2.Fushimi S, Furihata K, Seto H. J. Antibiot. 1989;42:1026–1036. doi: 10.7164/antibiotics.42.1026. [DOI] [PubMed] [Google Scholar]
- 3.Usui T, Marriott G, Inagaki M, Swarup G, Osada H. J. Biochem. 1999;125:960–965. doi: 10.1093/oxfordjournals.jbchem.a022375. [DOI] [PubMed] [Google Scholar]
- 4.Palaniappan N, Kim BS, Sekiyama Y, Osada H, Reynolds KA. J. Biol. Chem. 2003;278:35552–35557. doi: 10.1074/jbc.M305082200. [DOI] [PubMed] [Google Scholar]
- 5.Reid R, Piagentini M, Rodriguez E, Ashley G, Viswanathan N, Carney J, Santi DV, Hutchinson CR, McDaniel R. Biochemistry. 2003;42:72–79. doi: 10.1021/bi0268706. [DOI] [PubMed] [Google Scholar]
- 6.Wu J, Zaleski TJ, Valenzano C, Khosla C, Cane DE. J. Am. Chem. Soc. 2005;127:17393–17404. doi: 10.1021/ja055672+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caffrey P. Chembiochem. 2003;4:654–657. doi: 10.1002/cbic.200300581. [DOI] [PubMed] [Google Scholar]
- 8.August PR, Lang T, Yoon YJ, Ning S, Muller R, Yu TW, Taylor M, Hoffman D, Kim CG, Zhang X, Hutchinson CR, Floss HG. Chem. Biol. 1998;5:69–79. doi: 10.1016/s1074-5521(98)90141-7. [DOI] [PubMed] [Google Scholar]
- 9.Tang L, Shah S, Chung L, Carney J, Katz L, Khosla C, Julien B. Science. 2000;287:640–642. doi: 10.1126/science.287.5453.640. [DOI] [PubMed] [Google Scholar]
- 10.Holzbaur IE, Ranganathan A, Thomas IP, Kearney DJ, Reather JA, Rudd BA, Staunton J, Leadlay PF. Chem. Biol. 2001;8:329–340. doi: 10.1016/s1074-5521(01)00014-x. [DOI] [PubMed] [Google Scholar]
- 11.Patel HM, Tao J, Walsh CT. Biochemistry. 2003;42:10514–10527. doi: 10.1021/bi034840c. [DOI] [PubMed] [Google Scholar]
- 12.Golczak M, Kuksa V, Maeda T, Moise AR, Palczewski K. Proc. Natl. Acad. Sci. U S A. 2005;102:8162–8167. doi: 10.1073/pnas.0503318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures, spectroscopic data. This material is available free of charge via theInternet at http://pubs.acs.org