Abstract
We recently showed that the γ-subunit of Kluyveromyces lactis killer toxin (γ-toxin) is a tRNA endonuclease that cleaves 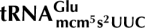 ,
, 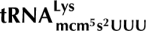 , and
, and 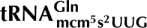 3′ of the wobble nucleoside 5-methoxycarbonylmethyl-2-thiouridine (mcm5s2U). The 5-methoxycarbonylmethyl (mcm5) side chain was important for efficient cleavage by γ-toxin, and defects in mcm5 side-chain synthesis correlated with resistance to γ-toxin. Based on this correlation, a genome-wide screen was performed to identify gene products involved in the formation of the mcm5 side chain. From a collection of 4826 homozygous diploid Saccharomyces cerevisiae strains, each with one nonessential gene deleted, 63 mutants resistant to Kluyveromyces lactis killer toxin were identified. Among these, eight were earlier identified to have a defect in formation of the mcm5 side chain. Analysis of the remaining mutants and other known γ-toxin resistant mutants revealed that sit4, kti14, and KTI5 mutants also have a defect in the formation of mcm5. A mutant lacking two of the Sit4-associated proteins, Sap185 and Sap190, displays the same modification defect as a sit4-null mutant. Interestingly, several mutants were found to be defective in the synthesis of the 2-thio (s2) group of the mcm5s2U nucleoside. In addition to earlier described mutants, formation of the s2 group was also abolished in urm1, uba4, and ncs2 mutants and decreased in the yor251c mutant. Like the absence of the mcm5 side chain, the lack of the s2 group renders
3′ of the wobble nucleoside 5-methoxycarbonylmethyl-2-thiouridine (mcm5s2U). The 5-methoxycarbonylmethyl (mcm5) side chain was important for efficient cleavage by γ-toxin, and defects in mcm5 side-chain synthesis correlated with resistance to γ-toxin. Based on this correlation, a genome-wide screen was performed to identify gene products involved in the formation of the mcm5 side chain. From a collection of 4826 homozygous diploid Saccharomyces cerevisiae strains, each with one nonessential gene deleted, 63 mutants resistant to Kluyveromyces lactis killer toxin were identified. Among these, eight were earlier identified to have a defect in formation of the mcm5 side chain. Analysis of the remaining mutants and other known γ-toxin resistant mutants revealed that sit4, kti14, and KTI5 mutants also have a defect in the formation of mcm5. A mutant lacking two of the Sit4-associated proteins, Sap185 and Sap190, displays the same modification defect as a sit4-null mutant. Interestingly, several mutants were found to be defective in the synthesis of the 2-thio (s2) group of the mcm5s2U nucleoside. In addition to earlier described mutants, formation of the s2 group was also abolished in urm1, uba4, and ncs2 mutants and decreased in the yor251c mutant. Like the absence of the mcm5 side chain, the lack of the s2 group renders 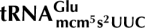 less sensitive to γ-toxin, reinforcing the importance of the wobble nucleoside mcm5s2U for tRNA cleavage by γ-toxin.
less sensitive to γ-toxin, reinforcing the importance of the wobble nucleoside mcm5s2U for tRNA cleavage by γ-toxin.
Keywords: K. lactisγ-toxin, 5-methoxycarbonylmethyl-2-thiouridine, SIT4, SAP185, SAP190, KTI14, KTI5, URM1, UBA4, NCS2, YOR251C
INTRODUCTION
Kluyveromyces lactis killer toxin (zymocin) is secreted by killer strains of K. lactis and inhibits growth of sensitive yeast strains, such as Saccharomyces cerevisiae (Stark et al. 1990; Schaffrath and Meinhardt 2005; Jablonowski and Schaffrath 2007). Zymocin consists of three subunits—α, β, and γ—that are encoded from a linear plasmid. Cytotoxicity of zymocin resides in the γ subunit, since intracellular expression of this subunit in S. cerevisiae mimics the action of zymocin (Butler et al. 1991a). The α and β subunits are required for the secretion of zymocin and docking of zymocin to other cells. In addition, the β subunit is possibly involved in the translocation of the γ subunit into the sensitive cells (Stark et al. 1990; Schaffrath and Meinhardt 2005).
Numerous S. cerevisiae mutants resistant to zymocin have been isolated (Kawamoto et al. 1993; Butler et al. 1994; Kishida et al. 1996; Frohloff et al. 2001). The zymocin-resistant mutants can be divided into two classes. Class I mutants are resistant to exterior zymocin but sensitive to intracellular expression of γ-toxin. Hence, they are defective in the docking/uptake of zymocin. Class II mutants are resistant both to exterior zymocin and endogenously expressed γ-toxin, and thus are proposed to be target mutants. So far, 14 class II mutants have been identified, namely, elp1, elp2, elp3 (Frohloff et al. 2001), elp4, elp5, elp6 (Jablonowski et al. 2001b), kti11, kti12, kti13 (Butler et al. 1994; Fichtner and Schaffrath 2002; Fichtner et al. 2002a), trm9 (Lu et al. 2005; Jablonowski et al. 2006), kti14 (Mehlgarten and Schaffrath 2003), sit4, a sap185 sap190 double mutant (Jablonowski et al. 2001a, 2004), and urm1 (Fichtner et al. 2003).
Ten of the class II zymocin-resistant mutants have been shown to be defective in the formation of modified uridine residues at the wobble position in tRNA. The Elp1p-Elp6p, Kti11p-Kti13p are all required for an early step in the formation of 5-methoxycarbonylmethyl (mcm5) and 5-carbamoylmethyl (ncm5) side chains on wobble uridines in 11 tRNA species (Huang et al. 2005; Lu et al. 2005; Johansson et al. 2008), whereas the Trm9p is required for the last step of mcm5 synthesis (Kalhor and Clarke 2003). Consistent with the correlation between class II zymocin resistance and tRNA wobble uridine modification deficiency (Huang et al. 2005), we showed that γ-toxin is an endonuclease that cleaves tRNAs containing the wobble nucleoside 5-methoxycarbonylmethyl-2-thiouridine (mcm5s2U) (Lu et al. 2005). In S. cerevisiae, the mcm5s2U nucleoside is present in 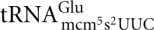 ,
, 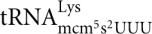 , and
, and 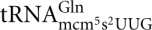 . The mcm5 side chain on the wobble uridine in tRNAs is required for efficient cleavage, explaining why the modification-deficient mutants elp1-elp6, kti11-kti13, and trm9 are resistant to γ-toxin. In addition to the strong stimulatory effect of the mcm5 side chain, we recently showed that the s2 group of mcm5s2U has a weak positive effect on in vitro cleavage of tRNA substrates by γ-toxin (Lu et al. 2008).
. The mcm5 side chain on the wobble uridine in tRNAs is required for efficient cleavage, explaining why the modification-deficient mutants elp1-elp6, kti11-kti13, and trm9 are resistant to γ-toxin. In addition to the strong stimulatory effect of the mcm5 side chain, we recently showed that the s2 group of mcm5s2U has a weak positive effect on in vitro cleavage of tRNA substrates by γ-toxin (Lu et al. 2008).
Since class II zymocin resistance correlates with mcm5 modification deficiency (Lu et al. 2005), it seemed possible that a screen for zymocin resistance would enable the isolation of mutants affecting the formation of the mcm5 side chain. Here we describe such a screen using a collection of S. cerevisiae homozygous diploid deletion strains, each of which has one nonessential gene deleted. This systematic screen identified not only proteins required for the formation of mcm5 side chain, but also proteins involved in the synthesis of the s2 group.
RESULTS
Identification of mutants resistant to zymocin and defective in wobble uridine modification
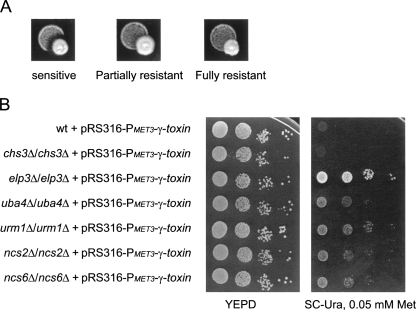
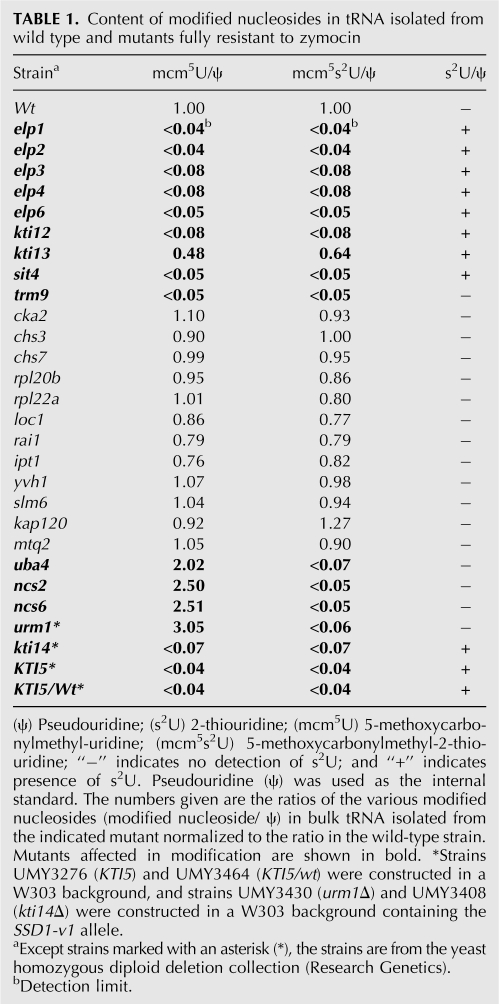
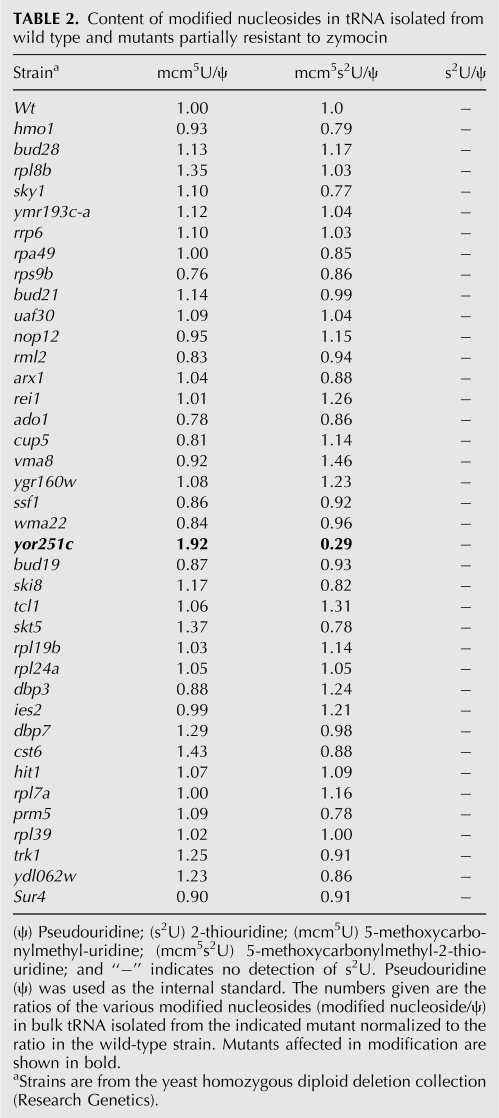
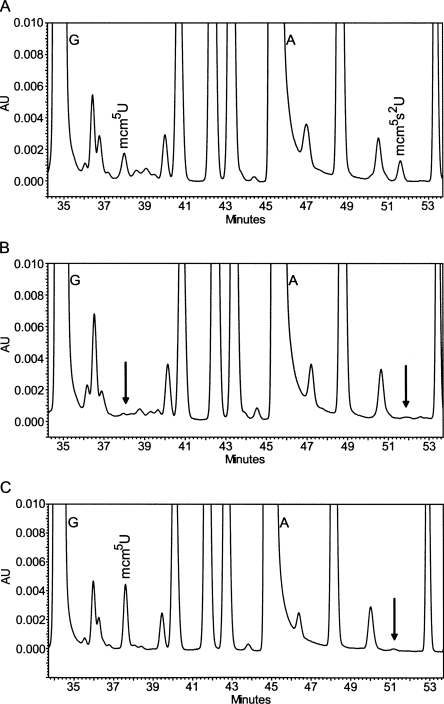
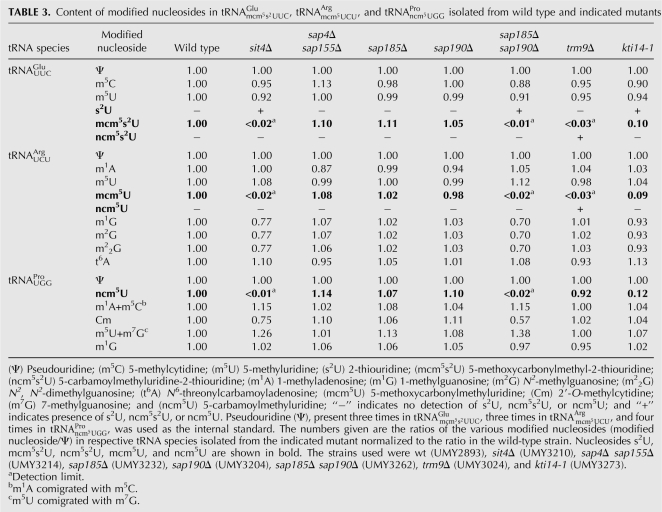
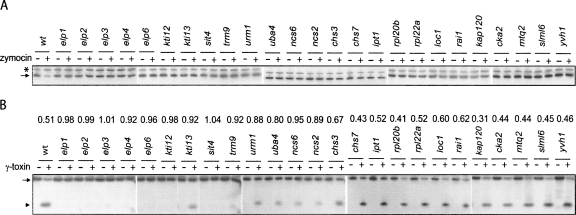
Zymocin resistance of 4826 homozygous diploid deletion strains of S. cerevisiae was examined by using the eclipse assay (Kishida et al. 1996). The S. cerevisiae deletion strains to be tested were spotted on rich plates, and the zymocin-producing K. lactis strain was inoculated on the edge of the spot. The size of the clearing zone caused by zymocin was compared between the mutants and the sensitive wild-type strain. Decreased size of clearing zone was scored for 63 mutants, showing that they are resistant to zymocin. According to the size of the clearing zone, the zymocin-resistant mutants were further divided into fully resistant and partially resistant groups (Fig. 1A; Tables 1, 2). As expected, the elp1-elp4, elp6, kti12, kti13 (Huang et al. 2005), and trm9 (Kalhor and Clarke 2003) mutants, which are all defective in the formation of the wobble mcm5 group in target tRNAs, were found among the fully resistant strains. To investigate if any of the other mutants had a defect in wobble uridine modifications, total tRNA prepared from the wild-type strain and zymocin-resistant mutants were analyzed by HPLC (Fig. 2; Tables 1, 2). In addition to those aforementioned mutants, a sit4 mutant was found to be deficient in the formation of mcm5 and ncm5 side chains (Tables 1, 3). Interestingly, in addition to the earlier described nfs1, cfd1, nbp35, cia1, an isu1 isu2 double mutant and the ncs6/tuc1 mutant (Nakai et al. 2004, 2007; Björk et al. 2007), we also found that urm1, uba4, ncs2, and yor251c mutants are affected in the formation of the s2 group in tRNA.
FIGURE 1.
Identification of mutants resistant to zymocin. (A) The S. cerevisiae deletion strains to be tested were spotted on YEPD plates, and the zymocin-producing K. lactis strain 2105-1D (Gunge and Sakaguchi 1981) was inoculated on the edge of the spot. After 24 h of incubation at 30°C, the size of the clearing zone caused by zymocin was compared between the mutants and the sensitive wild-type strain. (B) The indicated strains were transformed with a plasmid containing a PMET3-driven γ-toxin gene (pABY1728). Transformants were 10-fold serially diluted and grown on YEPD or synthetic complete plates lacking uracil and containing 0.05 mM methionine for 2 d at 30°C. All strains were from the S. cerevisiae deletion collection, except the urm1Δ/ urm1Δ strain (UMY3553).
TABLE 1.
Content of modified nucleosides in tRNA isolated from wild type and mutants fully resistant to zymocin
TABLE 2.
Content of modified nucleosides in tRNA isolated from wild type and mutants partially resistant to zymocin
FIGURE 2.
HPLC analysis of tRNA from zymocin-resistant strains. HPLC analysis of modified nucleosides of total tRNA from (A) wild type (BY4743) and its isogenic (B) elp1 and (C) ncs2 strain derivatives. (A,B) The 34.2–53.8-min and (C) the 33.6–53.8-min regions of the HPLC chromatograms monitored at 254 nm are shown. The arrows indicate expected retention times of mcm5U and mcm5s2U, respectively.
TABLE 3.
Content of modified nucleosides in  ,
,  , and
, and  isolated from wild type and indicated mutants
isolated from wild type and indicated mutants
A sit4-null mutant is deficient in the formation of mcm5 and ncm5 side chains in tRNA
The Sit4 protein is a protein phosphatase that plays a critical role in cell growth and proliferation (Sutton et al. 1991). Consistent with its class II zymocin resistance (Jablonowski et al. 2001a), a sit4-null mutant is deficient in the formation of mcm5 and ncm5 side chains. By analyzing total tRNA or purified tRNA species from the sit4-null mutant, we found that the mcm5 and mcm5s2U nucleosides were absent in total tRNA, and mcm5U, ncm5U, and mcm5s2U nucleosides were absent in purified 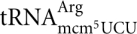 ,
, 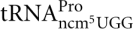 , and
, and 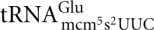 , respectively (Tables 1, 3). No intermediates of mcm5U and ncm5U were observed in tRNAArg and tRNAPro prepared from the sit4-null mutant, suggesting that the defect of the sit4 mutant is in an early step of mcm5U and ncm5U biosynthesis. In both total tRNA and purified
, respectively (Tables 1, 3). No intermediates of mcm5U and ncm5U were observed in tRNAArg and tRNAPro prepared from the sit4-null mutant, suggesting that the defect of the sit4 mutant is in an early step of mcm5U and ncm5U biosynthesis. In both total tRNA and purified 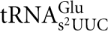 from a sit4-null mutant, 2-thiouridine (s2U) was observed, demonstrating that thiolation at position 2 is not affected (Tables 1, 3).
from a sit4-null mutant, 2-thiouridine (s2U) was observed, demonstrating that thiolation at position 2 is not affected (Tables 1, 3).
Mutants defective in the formation of 2-thio group of mcm5s2U are resistant to zymocin
In addition to the mcm group at position 5 of wobble uridines, γ-toxin substrate tRNAs, i.e., 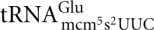 ,
, 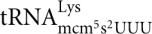 , and
, and 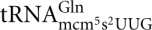 contain an s2 group on the wobble uridine. Interestingly, besides mutants deficient in the mcm5 formation, the present screen also identified five mutants affecting the s2 modification, including the already defined ncs6/tuc1 mutant (Björk et al. 2007). As shown in Table 1 and Figure 2, the nucleoside mcm5s2U was absent, while the levels of mcm5U increased in total tRNA from urm1, uba4, ncs2, and ncs6/tuc1 mutants. In addition, a reduced level of mcm5s2U and an increased level of mcm5U were seen in the yor251c mutant (Table 2).
contain an s2 group on the wobble uridine. Interestingly, besides mutants deficient in the mcm5 formation, the present screen also identified five mutants affecting the s2 modification, including the already defined ncs6/tuc1 mutant (Björk et al. 2007). As shown in Table 1 and Figure 2, the nucleoside mcm5s2U was absent, while the levels of mcm5U increased in total tRNA from urm1, uba4, ncs2, and ncs6/tuc1 mutants. In addition, a reduced level of mcm5s2U and an increased level of mcm5U were seen in the yor251c mutant (Table 2).
It has been shown that an urm1-null strain is resistant to intracellular expression of γ-toxin (Fichtner et al. 2003). To test if all mutants lacking the s2 group (urm1, uba4, ncs2, and ncs6/ tuc1) are resistant to γ-toxin, these mutants were transformed with a PGAL1-driven γ-toxin plasmid (pABY1472), and growth on galactose plates was examined. In contrast to the previous study, the urm1 mutant, as well as other mutants lacking the s2 group, were sensitive to γ-toxin expressed from the PGAL1 promoter (data not shown). This discrepancy is likely caused by the different expression levels of γ-toxin, since this study used a different γ-toxin expression construct from that of the previous report (Fichtner et al. 2003). In accordance with this assumption, all mutants lacking the s2 group (urm1, uba4, ncs2, and ncs6/ tuc1) did show resistance to low intracellular γ-toxin expression when transformed with a PMET3-driven γ-toxin plasmid (pABY1728) and grown on plates with 0.05 mM methionine (Fig. 1B). The MET3 promoter, a weaker promoter compared to the GAL1 promoter, is partially turned off in the presence of 0.05 mM methionine (Mao et al. 2002). Notably, mutants lacking the s2 group were less resistant than the elp3 mutant (Fig. 1B), which lacks the mcm5 side chain (Huang et al. 2005).
Lack of the mcm5 group renders tRNA less sensitive to γ-toxin cleavage, explaining the γ-toxin resistance of mutants deficient in the formation of the mcm5 group (Lu et al. 2005). To test if this explanation applies to the mutants identified in the present screen, we analyzed the effect of in vivo zymocin treatment and in vitro γ-toxin treatment on one of the substrate tRNAs (tRNAGlu UUC). The level of tRNAGlu UUC was assayed by Northern blot after adding crude zymocin to log-phase cultures of strains to be tested. As shown in Figure 3A, the level of tRNAGlu UUC decreased in the wild-type cells after zymocin treatment, but remained unchanged in all mutants fully resistant to zymocin. The in vitro tRNAGlu UUC cleavage was examined by treating total RNA isolated from wild-type and zymocin-resistant strains with purified γ-toxin-GST and assaying the level of tRNAGlu UUC using Northern blot. According to the level of tRNAGlu UUC after in vitro γ-toxin treatment, zymocin-resistant mutants can be divided into two groups (Fig. 3B). The first group consists of mutants defective in the formation of the mcm5 or s2 group, that is, elp1-elp4, elp6, kit12, kti13, sit4, trm9, urm1, uba4, ncs2, and ncs6/tuc1. The tRNAGlu UUC isolated from these mutants showed robust or partial resistance to γ-toxin cleavage. The second group contains all the remaining mutants including several known barrier mutants (chs3, chs7, and ipt1) that showed a similar extent of in vitro tRNA cleavage to the wild-type strain. Presumably, similar to the known barrier mutants, the remaining mutants in the second group could be defective in either the uptake of γ-toxin or other events prior to γ-toxin cleavage of substrate tRNAs.
FIGURE 3.
Reactivity of tRNAGlu UUC from different strains to zymocin (in vivo) and γ-toxin (in vitro). (A) Crude zymocin was added to log-phase cultures of indicated strains. Total RNA was isolated and the level of tRNAGlu UUC was analyzed by Northern blot using oligonucleotides specific for (*) tRNASer CGA and (arrow) tRNAGlu UUC. (B) Total RNA from indicated strains was mixed with purified γ-toxin-GST at a concentration of 5 nM and incubated for 15 min at 30°C. The level of tRNAGlu UUCwas analyzed by Northern blot using oligonucleotides specific for tRNAGlu UUC. (Arrowhead) The 3′ cleavage product of tRNAGlu UUC. (Arrow) The full-length tRNAGlu UUC signals were quantified, and the ratio between γ-toxin treated and untreated samples was calculated and shown in the graph. All strains were from the S. cerevisiae deletion collection.
Other mutants with defects in mcm5 and ncm5 group formation
The systematic screen using the yeast deletion collection reinforced the correlation between class II zymocin resistance and the defects in wobble uridine modification. We extended our investigation to other previously identified class II zymocin-resistant mutants to investigate if they also had a defect in formation of mcm5s2U.
The function of the Sit4 protein is regulated by various sit4-associated proteins (SAPs): Sap185, Sap190, Sap4, and Sap155 (Luke et al. 1996). While deletion of SAP185 or SAP190 individually does not affect zymocin resistance, a strain lacking both Sap190 and Sap185 proteins displays class II zymocin resistance (Jablonowski et al. 2001a, 2004). We investigated if a sap185Δ mutant, a sap190Δ mutant, or a sap185Δ sap190Δ double mutant is impaired in the formation of mcm5s2U. No defect was observed in the single sap185Δ or sap190Δ mutants, but a sap185Δ sap190Δ double mutant lacked mcm5U, ncm5U, and mcm5s2U in purified 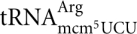 ,
, 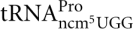 , and
, and 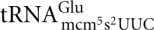 , respectively (Table 3). On the contrary, a strain deleted for genes encoding the other two Sit4-associated proteins (sap4Δ sap155Δ), which is not resistant to zymocin (Jablonowski et al. 2001a), did not show any defect in wobble uridine modification (Table 3). The formation of s2 group is not affected in the sap185Δ sap190Δ double mutant, since s2U was detected in purified
, respectively (Table 3). On the contrary, a strain deleted for genes encoding the other two Sit4-associated proteins (sap4Δ sap155Δ), which is not resistant to zymocin (Jablonowski et al. 2001a), did not show any defect in wobble uridine modification (Table 3). The formation of s2 group is not affected in the sap185Δ sap190Δ double mutant, since s2U was detected in purified 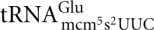 (Table 3).
(Table 3).
A mutation in the KTI14 gene causes class II zymocin resistance (Butler et al. 1994). The KTI14 gene was found to be allelic to HRR25 (Mehlgarten and Schaffrath 2003), which encodes a casein kinase homolog involved in diverse cellular processes, for example, DNA repair, vesicular trafficking, calcineurin signaling, ribosome maturation, and chromosome segregation (Hoekstra et al. 1991; DeMaggio et al. 1992; Ho et al. 1997; Murakami et al. 1999; Kafadar et al. 2003; Petronczki et al. 2006; Schafer et al. 2006). Monitoring the wobble uridine modifications in tRNA isolated from the kti14-1 mutant revealed a decreased level of mcm5s2U, mcm5U, and ncm5U nucleosides (∼10% of wild type) in 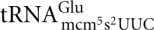 ,
, 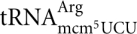 , and
, and 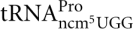 , respectively (Table 3). A deletion of the KTI14 gene abolished the formation of mcm5U and mcm5s2U in total tRNA (Table 1). Thiolation at position 2 is not affected in the kti14-null and the kti14-1 mutants, since s2U was detected in both total tRNA and purified
, respectively (Table 3). A deletion of the KTI14 gene abolished the formation of mcm5U and mcm5s2U in total tRNA (Table 1). Thiolation at position 2 is not affected in the kti14-null and the kti14-1 mutants, since s2U was detected in both total tRNA and purified 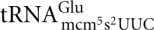 (Tables 1, 3).
(Tables 1, 3).
The KTI5 mutant displays resistance to both exogenous zymocin and intracellular γ-toxin expression. Interestingly, its resistance to exogenous zymocin is a dominant phenotype (Butler et al. 1991a). In the effort to identify the mutation in this strain, a recessive temperature-sensitive (ts) phenotype was found to be tightly linked to zymocin resistance. By complementing this ts phenotype, we found that KTI5 is allelic to the KTI11 gene (data not shown). When the KTI5 allele was sequenced, a G-to-C mutation was observed at position 80 in the KTI11 gene, which results in cysteine-to-serine substitution at residue 27 (data not shown). Cys27 is one of the four cysteines in the Kti11 protein that form a zinc-binding domain (Sun et al. 2005). Similar to a kti11 mutant, a KTI5 mutant lacked mcm5s2U and mcm5U in total tRNA (Table 1). When the KTI5 mutant was crossed with a wild-type strain, the resulting diploid strain displayed the identical modification defect as a haploid KTI5 mutant strain (Table 1), demonstrating that the modification defect in KTI5 is also dominant. In addition to the wobble uridine modification in tRNA, the Kti11 protein is required for the synthesis of diphthamide, a post-translationally modified histidine residue present in the elongation factor 2 (eEF2) in eukaryotes (Liu and Leppla 2003). Diphthamide is the target of diphtheria toxin and Pseudomonas exotoxin A (Collier 2001). Similar to a kti11 mutant, a KTI5 mutant was resistant to diphtheria toxin (data not shown), suggesting that the zinc-binding motif in the Kti11 protein is important for both tRNA modification and diphthamide biosynthesis.
DISCUSSION
The formation of mcm5 and ncm5 groups at wobble uridines requires many gene products (Huang et al. 2005). In this study, we report the screening of a yeast homozygous diploid deletion collection for mutants defective in mcm5s2U formation. Because lack of mcm5 modification in substrate tRNAs causes zymocin resistance (Lu et al. 2005), which is easy to score, the initial screen was based on this phenotype. In the collection of 4826 deletion mutant strains, 63 mutants were found to be resistant to zymocin. Transfer RNA from these zymocin-resistant mutants was further analyzed by HPLC to identify mutants impaired in the formation of mcm5s2U. The validity of the screen was confirmed by the identification of deletion strains known to be affected in the synthesis of mcm5, that is, elp1-elp4, elp6, kti12, kti13, and trm9 (null strains of elp5 and kti11 are not present in the deletion collection). Interestingly, we also identified additional mutants that are affected in the formation of the mcm5 (sit4) or s2 group (urm1, uba4, ncs2, and yor251c). Analysis of other previously known class II zymocin-resistant mutants showed that the KTI5 is a dominant allele of KTI11 and that KTI5, kti14 mutants and a sap185Δ sap190Δ double mutant are defective in the formation of mcm5U.
Involvement of a protein phosphatase and a kinase in the formation of mcm5 and ncm5 groups at wobble uridines
The Sit4 protein is a type 2A-related serine-threonine phosphatase involved in the regulation of cell growth and proliferation (for review, see Jiang 2006). Sit4 is required for proper G1 to S-phase transition, plays an important role in nutrient response mediated by TOR pathway, and modulates functions mediated by protein kinase C (Pkc1p) including cell wall and actin cytoskeleton organization (Sutton et al. 1991; Di Como and Arndt 1996; Angeles de la Torre-Ruiz et al. 2002). The function of the Sit4 protein is regulated, at least partly, by various Sit4-associated proteins (Sap185, Sap190, Sap155, and Sap4) (Luke et al. 1996). Similar to a sit4 mutant, cells lacking Sap185p and Sap190p display class II zymocin resistance (Jablonowski et al. 2001a, 2004). Consistently, a sap185, sap190 double mutant displayed the same defect in the synthesis of the mcm5 and ncm5 groups as a sit4 mutant (Table 3). On the contrary, a strain with deletions of the genes encoding the other two Sit4-associated proteins Sap4p and Sap 155p, which do not cause zymocin resistance (Jablonowski et al. 2001a), had no effect on mcm5 and ncm5 side-chain formation. This suggests that the role of Sit4p in tRNA modification is modulated by or acting through the association with Sap185p and Sap190p.
In addition to the Sit4 phosphatase, we also identified the kinase Kti14p in the present study. Consistent with its class II zymocin resistance (Butler et al. 1994), the kti14-1 mutant was found to be defective in the formation of mcm5 and ncm5 side chains (Table 3). Kti14p/ Hrr25p, a homolog of mammalian casein kinase 1δ, is involved in diverse cellular events including DNA repair (Hoekstra et al. 1991; DeMaggio et al. 1992; Murakami et al. 1999; Ho et al. 2002; Kafadar et al. 2003; Petronczki et al. 2006; Schafer et al. 2006). Interestingly, a kti14-2 mutant, which is zymocin resistant, is not hypersensitive to the DNA damage reagent drug methylmethane sulfonate (MMS) like other kti14 alleles, suggesting that the function of the Kti14 protein in DNA repair is uncoupled from its function in zymocin action (or rather tRNA modification) (Mehlgarten and Schaffrath 2003). So how does defective Kti14p lead to impaired tRNA modification? Given that the Kti14 protein physically interacts with proteins in the core Elongator complex (Elp2 and Elp3 proteins) and with the Sit4p-Sap185p-Sap190p complex (Ho et al. 2002; Schafer et al. 2003; Gavin et al. 2006), it is tempting to propose that Sit4 and Kti14 proteins may cooperate to regulate the phosphorylation status of proteins required for the formation of mcm5 and ncm5 side chains. One such candidate substrate that links tRNA modification to the Sit4p/Kti14p regulation is the Elp1 protein. It has been shown that the phosphorylation status of Elp1 protein is affected by Sit4p, Sap185p, Sap190p, and Kti12p (Jablonowski et al. 2004). The dephosphorylation of Elp1p depends on the Sit4 phosphatase and two of its associated proteins, Sap185p and Sap190p, while Kti12p can antagonize Sit4-dependent dephosphorylation of Elp1p (Jablonowski et al. 2004). Kti12p is associated with the Elongator complex (Frohloff et al. 2001, 2003; Fichtner et al. 2002b, 2003; Petrakis et al. 2005; Krogan et al. 2006). Lack of Kti12p causes class II zymocin resistance (Frohloff et al. 2001) and abolishes the formation of mcm5 and ncm5 side chains (Huang et al. 2005). Presumably, the modification defect in the sit4 mutant is elicited through the deregulation of Elp1p phosphorylation, as in the case of the kti12 mutant (Jablonowski et al. 2004). However, it remains to be seen if the phosphorylation of Elp1 involves Kti14p and Kti12p and whether dephosphorylation of Elp1p is a direct effect of Sit4p.
In accordance with the role of Trm9 in methylation (Kalhor and Clarke 2003), modified nucleosides mcm5s2U and mcm5U were absent in 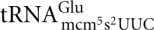 and
and 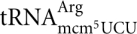 , respectively, in a trm9-null mutant (Table 3). Interestingly, in the trm9 mutant, we noticed the occurrence of ncm5s2U in tRNAGlu
UUC and ncm5U in tRNAArg
UCU, instead of the expected cm5s2U and cm5U nucleosides (Kalhor and Clarke 2003). This surprising finding presently is being investigated in our laboratory.
, respectively, in a trm9-null mutant (Table 3). Interestingly, in the trm9 mutant, we noticed the occurrence of ncm5s2U in tRNAGlu
UUC and ncm5U in tRNAArg
UCU, instead of the expected cm5s2U and cm5U nucleosides (Kalhor and Clarke 2003). This surprising finding presently is being investigated in our laboratory.
Proteins involved in the formation of the 2-thio group on wobble uridines
All three γ-toxin target tRNAs, 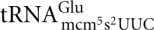 ,
, 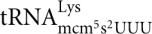 , and
, and 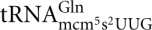 , contain a 2-thio modification at the wobble uridine. Earlier, seven proteins were known to be involved in the thiolation of cytosolic tRNAs in the yeast S. cerevisiae (Nakai et al. 2004, 2007; Björk et al. 2007). The NFS1 gene encodes a cysteine desulfurase involved in Fe-S cluster biogenesis. An nfs1 mutant has a defect in 2-thio modification of both mitochondrial and cytoplasmic tRNAs (Nakai et al. 2004). Most likely, Nfs1 serves as sulfur donor for tRNA thiolation, a function shared with its bacterial homolog IscS (Zheng et al. 1998). Thiolation of cytosolic tRNAs is iron-sulfur (Fe/S) protein dependent and requires the mitochondrial and cytosolic Fe/S cluster assembly machineries termed ISC and CIA. Three components of CIA (Cfd1, Nbp35, and Cia1) and two mitochondria scaffold proteins (Isu1 and Isu2) are required for the 2-thio modification of the cytosolic tRNAs but are dispensable for thiolation of the mitochondrial tRNAs (Nakai et al. 2007). Recently, the Ncs6/Tuc1 protein was shown to be required for 2-thio modification of cytosolic tRNAs, and based on its homology with the bacterial TtcA protein, which is required for 2-thiocytidine synthesis, Ncs6/Tuc1 was proposed to be responsible for making 2-thiouridine in yeast (Björk et al. 2007).
, contain a 2-thio modification at the wobble uridine. Earlier, seven proteins were known to be involved in the thiolation of cytosolic tRNAs in the yeast S. cerevisiae (Nakai et al. 2004, 2007; Björk et al. 2007). The NFS1 gene encodes a cysteine desulfurase involved in Fe-S cluster biogenesis. An nfs1 mutant has a defect in 2-thio modification of both mitochondrial and cytoplasmic tRNAs (Nakai et al. 2004). Most likely, Nfs1 serves as sulfur donor for tRNA thiolation, a function shared with its bacterial homolog IscS (Zheng et al. 1998). Thiolation of cytosolic tRNAs is iron-sulfur (Fe/S) protein dependent and requires the mitochondrial and cytosolic Fe/S cluster assembly machineries termed ISC and CIA. Three components of CIA (Cfd1, Nbp35, and Cia1) and two mitochondria scaffold proteins (Isu1 and Isu2) are required for the 2-thio modification of the cytosolic tRNAs but are dispensable for thiolation of the mitochondrial tRNAs (Nakai et al. 2007). Recently, the Ncs6/Tuc1 protein was shown to be required for 2-thio modification of cytosolic tRNAs, and based on its homology with the bacterial TtcA protein, which is required for 2-thiocytidine synthesis, Ncs6/Tuc1 was proposed to be responsible for making 2-thiouridine in yeast (Björk et al. 2007).
In the present screen of the yeast gene deletion collection, we identified four mutants, urm1, uba4, ncs2, and ncs6/ tuc1, which lacked the 2-thio modification on wobble uridines. Another mutant, yor251c, shows a reduced level of s2-containing modified uridines. Strains with deletions of the NFS1, CFD1, NBP35, or CIA1 genes were not found as they are nonviable, and the ISU1 and ISU2 are functionally redundant (Nakai et al. 2001, 2007; Gerber et al. 2004). Urm1 and Uba4 proteins function in the urmylation pathway, one of the ubiquitin-like protein conjugation systems in S. cerevisiae (Furukawa et al. 2000). Ubiquitin and ubiquitin-like modifier proteins are attached covalently to target proteins and alter their function and localization (Hochstrasser 2000; Schnell and Hicke 2003). Although the urmylation pathway has been suggested to be involved in multiple processes, for example, invasive growth, budding, and antioxidant stress response, only one target protein (Ahp1p) has been identified so far (Goehring et al. 2003). It is possible that any of the Ncs2, Ncs6/Tuc1 proteins or components of the Fe/S cluster assembly machineries ISC and CIA may function only after being urmylated.
Lack of the mcm5 side chain in 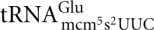 ,
, 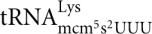 , and
, and 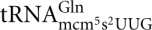 causes inefficient cleavage by γ-toxin and renders cells resistant to zymocin (Lu et al. 2005). Mutants lacking the s2 group displayed a weak γ-toxin resistance, consistent with the observation that 2-thio modification on tRNAs is also required for efficient γ-toxin cleavage (Lu et al. 2008).
causes inefficient cleavage by γ-toxin and renders cells resistant to zymocin (Lu et al. 2005). Mutants lacking the s2 group displayed a weak γ-toxin resistance, consistent with the observation that 2-thio modification on tRNAs is also required for efficient γ-toxin cleavage (Lu et al. 2008).
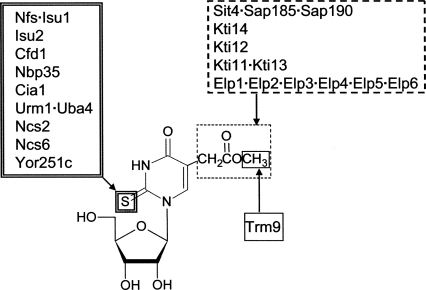
In conclusion, by screening 4826 yeast strains with deletions in nonessential genes, we have identified gene products involved in the formation of the mcm5s2U nucleoside at wobble positions in tRNA. It is still possible that additional genes representing essential genes in yeast are required for the formation of the mcm5s2U nucleoside. The number of proteins so far identified to be required for the formation of the mcm5 side chain and the s2 group is surprisingly large (Fig. 4). Knowing that a kinase, a phosphatase, and a ubiquitin-like protein-modifying system is required for formation of mcm5s2U suggests that biosynthesis of mcm5s2U is a complex process likely to involve regulation by signal transduction.
FIGURE 4.
Proteins involved in the formation of wobble nucleoside mcm5s2U. So far 14 and 11 gene products have been found to be involved in the formation of the mcm5and s2 group of mcm5s2U, respectively. Note that the former group of gene products is also involved in the formation of ncm5U. Trm9p is required for the last step, formation of the esterified methyl constituent in mcm5U and mcm5s2U. Physical interaction between proteins is indicated by a dot (Luke et al. 1996; Otero et al. 1999; Wittschieben et al. 1999; Furukawa et al. 2000; Krogan and Greenblatt 2001; Winkler et al. 2001; Ho et al. 2002; Gavin et al. 2006; Krogan et al. 2006). Other interactions not shown in the figure are Kti14p, Kti12p, and Kti11p, which all interact with subunits of Elongator subcomplex (Elp1p, Elp2p, Elp3p) (Fichtner et al. 2002a, 2003; Frohloff et al. 2003; Schafer et al. 2003; Petrakis et al. 2005; Gavin et al. 2006; Krogan et al. 2006). There is also interaction between the Sit4 complex and Kti14p (Ho et al. 2002). Note that Nfs1p, Isu1p, and Isu2p are located in the mitochondria. Figure modified from Lu (2007).
MATERIALS AND METHODS
Strains, medium, and genetic procedure
Yeast media, transformation, and genetic procedures have been described (Burke et al. 2000). The yeast cells were grown in YEPD medium at 30°C, unless otherwise stated. All the yeast strains used in this study, except those from the yeast diploid homozygous deletion collection (Research Genetics), are listed in Table 4.
TABLE 4.
Yeast strains used in this study
A deletion of the SIT4 gene in S. cerevisiae is lethal in strains with a W303 background but not in strains with an S288C background, which is attributed to a polymorphic gene, SSD1. Strains with a W303 background carry the ssd1-d allele, while strains with an S288C background carry the SSD1-v1 allele (Sutton et al. 1991). To make a deletion of the SIT4 gene in a W303 background, the SSD1-v1 allele was introduced into UMY2893 (W303-1B SUP4) (Huang et al. 2005) by transforming a linearized pRS306–SSD1-v1 plasmid, generating UMY3292 (W303-1B SUP4 ssd1-d∷URA3∷SSD1-v1). The strain UMY3292 was crossed with CY3938 (W303-1A sit4∷HIS3 SSD1-v1) (Luke et al. 1996), and the resulting diploid was sporulated to generate UMY3210 (W303-1B sit4∷HIS3 SSD1-v1 SUP4). UMY3204 (W303-1B sap190∷TRP1 SSD1-v1 SUP4) and UMY3214 (W303-1B sap4∷LEU2 sap155∷HIS3 SSD1-v1 SUP4) were constructed in a similar way from the crosses between UMY3292 and CY4380 (W303-1A sap190∷TRP1 SSD1-v1) (Luke et al. 1996) or CY5220 (W303-1A sap4∷LEU2 sap155∷HIS3 SSD1-v1) (Luke et al. 1996), respectively. The strain UMY3292 was crossed with CY4029 (W303-1A SSD1-v1), and the resulting diploid was sporulated to generate UMY3200 (W303-1A SSD1-v1 SUP4) and UMY3201 (W303-1B SSD1-v1 SUP4). To delete the SAP185 gene, chromosomal DNA from the corresponding null mutant in the yeast deletion collection (Research Genetics) was used as template to amplify DNA fragments containing the KanMX4 cassette and 300–400 nucleotides (nt) of flanking sequences. The PCR product was transformed into strains UMY3200 and UMY3201. Transformants were selected on YEPD plates containing 200 μg/mL G418, and the deletion of the SAP185 gene was confirmed by PCR. The strains generated were UMY3231 (W303-1A SSD1-v1 SUP4 sap185∷KanMX4) and UMY3232 (W303-1B SSD1-v1 SUP4 sap185∷KanMX4). The strain UMY3231 was crossed with UMY3204, and the resulting diploid was sporulated to generate UMY3262 (W303-1B SSD1-v1 SUP4 sap185∷KanMX4 sap190∷TRP1).
The homozygous diploid strains deleted for each nonessential gene were from Research Genetics. The control strain used in the zymocin resistance assay, with an isogenic genetic background, was BY4743 (MATa/MATα his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 met15Δ0/MET15 ura3Δ0/ura3Δ0 LYS2/lys2Δ0) (Research Genetics). The mutations in sit4, trm9, uba4, ncs2, ncs6/ tuc1, and yor251c in the yeast deletion collection were confirmed by PCR. The urm1Δ strain from the deletion collection was found to contain wild-type alleles of URM1. To delete the URM1 gene, oligonucleotides containing 50-nt homology with the flanking sequences of URM1 gene were used to amplify the KanMX6 cassette (Longtine et al. 1998). The PCR product was transformed into strain UMY3385 (W303-1A SSD1-v1) or UMY3386 (W303-1B SSD1-v1), and transformants were selected on YEPD plates containing 200 μg/mL G418. UMY3385 and UMY3386 were segregants in a cross between CY4029 and CY4102 (W303-1B cln3∷URA3 SSD1-v1). Deletion of the URM1 gene was confirmed by PCR, and the strains generated were UMY3429 (W303-1A urm1∷KanMX6 SSD1-v1) and UMY3430 (W303-1B urm1∷KanMX6 SSD1-v1). A diploid strain, UMY3546, was obtained by crossing UMY3429 with UMY3430. To delete the URM1 gene in the BY4743 background, the chromosomal DNA of UMY3430 was used as template to amplify DNA fragments containing the KanMX6 cassette and 200 nt of flanking sequences of the URM1 gene. The PCR product was transformed into BY4743, and transformants were selected on YEPD plates containing 200 μg/mL G418. The deletion of the URM1 gene was confirmed by PCR, and the heterozygous URM1/urm1 deletion strain (UMY3548) was sporulated to obtain UMY3549 (MATα his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 urm1∷KanMX6) and UMY3552 (MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 urm1∷KanMX6). UMY3549 was crossed with UMY3552 to generate the homozygous urm1Δ/urm1Δ diploid UMY3553.
The strain ARB97 (MATα leu2-3,112 his3-11,15 kti14-1) (Butler et al. 1994) was backcrossed with the strain W303-1A three times, generating UMY3273 (W303-1A kti14-1). Similar to the sit4 mutant, the phenotypes of the kti14 mutation were more severe in strains carrying the ssd1-d allele (data not shown). The SSD1-v1 allele was introduced into UMY3273 by transforming a linearized pRS306–SSD1-v1 plasmid, generating UMY3323 (W303-1A kti14-1 ssd1-d∷URA3∷SSD1-v1). The strain UMY3323 was crossed with UMY3386, and the resulting diploid was sporulated to generate UMY3326 (W303-1B kti14-1 SSD1-v1). To delete the KTI14 gene, oligonucleotides containing 50-nt homology with the flanking sequences of KTI14 gene were used to amplify the KanMX6 cassette (Longtine et al. 1998). The PCR product was transformed into strain UMY3387 (a diploid from the cross between UMY3385 and UMY3386), and transformants were selected on YEPD plates containing 200 μg/mL G418. Deletion of the KTI14 gene was confirmed by PCR, and the strain was sporulated to obtain UMY3408 (W303-1B kti14∷KanMX6 SSD1-v1).
Identification of the mutation in the dominant KTI5 mutant
The KTI5 allele was moved into W303-1A by backcrossing the KTI5 mutant (ARB15) (Butler et al. 1991a) with W303-1A three times, generating UMY3275 (W303-1A, KTI5) and UMY3276 (W303-1B, KTI5). During the cross, a recessive temperature-sensitive (ts) phenotype cosegregated with the zymocin-resistant phenotype of KTI5 mutation. A genomic library was introduced into strain UMY3275, and plasmids complementing the ts phenotype were isolated. The restriction pattern and sequence analysis showed that all these complementing plasmids contained the KTI11 gene. The KTI5 allele in UMY3275 was PCR-amplified and sequenced. The heterozygous KTI5/wt diploid UMY3464 was generated by crossing UMY3275 with W303-1B.
Zymocin resistance assay and intracellular induction of γ-toxin
The resistance to zymocin was analyzed using the killer eclipse assay (Kishida et al. 1996). The S. cerevisiae strains of interest were resuspended in autoclaved sterile water, yielding 0.15–0.2 OD600 units. Five microliters of the cells was spotted on the YEPD plate. After the spots were dried, the K. lactis killer strain (2105-1D) (Gunge and Sakaguchi 1981) was inoculated on the edge of the spots. Clearing zones formed by the secreted zymocin were observed after incubation for 24 h at 30°C. For some slow-growing mutants, the incubation time was extended.
To test resistance to intracellular expression of γ-toxin, strains to be tested were transformed with the plasmid pRS316-PGAL1-γ-toxin (pABY1472) (Lu et al. 2005) or pRS316-PMET3-γ-toxin (pABY1728). Transformants were grown overnight in SC-Ura medium and resuspended in sterile water to a concentration of 3 × 106 cells/mL. For strains carrying the plasmid pRS316-PGAL1-γ-toxin, 10-fold serial dilutions of cells were spotted onto synthetic complete plates lacking uracil and containing either 2% galactose or 2% glucose, respectively. For strains carrying the plasmid pRS316-PMET3-γ-toxin, 10-fold serial dilutions of cells were spotted onto YEPD plates or synthetic complete plates lacking uracil and containing 0.05 mM methionine, respectively.
Plasmid constructions
DNA manipulations, plasmid preparations, and bacterial transformations were performed according to standard protocols. To construct the plasmid pRS306–SSD1-v1 (pABY1617), a DNA fragment containing the SSD1-v1 allele from plasmid YCp50-SSD1-v1 (Luke et al. 1996) was released by PvuII digestion and moved into the corresponding site in plasmid pRS306. The DNA fragment containing the MET3 promoter was PCR-amplified from chromosomal DNA using oligonucleotides 5′-ACGTGAATTCCGTTTAATTTAGTACTAACAGAG-3′ (EcoRI site underlined) and 5′-ACGTGGATCCGTTAATTATACTTTATTCTTGTTATT-3′ (BamHI site underlined). The PCR product was cut with EcoRI/BamHI and used to replaced the PGAL1 in the plasmid pRS316-PGAL1-γ-toxin (pABY1472) (Lu et al. 2005), generating pRS316-PMET3-γ-toxin (pABY1728).
HPLC analysis of tRNA
Total tRNA and single tRNA species prepared as previously described (Björk et al. 2001) were digested to nucleosides and analyzed by HPLC. The levels of modified nucleosides mcm5U/mcm5s2U and ncm5U were examined using the Develosil C30 and C18 reverse-phase columns, respectively (Gehrke et al. 1982; Gehrke and Kuo 1990). Identifications of modified nucleosides mcm5s2U, mcm5U, ncm5U, and s2U have previously been described (Huang et al. 2005).
In vivo and in vitro cleavage of tRNA by γ-toxin
Overnight cultures of strains to be tested were diluted in pre-warmed YEPD, grown for three generations to OD600 ∼0.1, and split into two. To one-half, crude zymocin (Butler et al. 1991b) was added to a concentration of 7% (vol/vol); the other untreated half served as a control. The cells were harvested 3 h after treatment, and total RNA was prepared using hot phenol (Ausubel et al. 2001). For the in vitro cleavage assay, total tRNA was isolated from exponentially growing cultures and treated with purified γ-toxin-GST or GST at a concentration of 5 nM as described earlier (Lu et al. 2005).
Samples of ∼5 μg of total RNA were separated on 8% polyacrylamide, 8 M urea gels and transferred to Zeta-Probe membranes (Bio-Rad). The oligonucleotides used to detect tRNAs were 5′-GCCCAAGAGATTTCGAGTCTCT-3′ (tRNASer
CGA) and 5′-CTCCGCTACGGGGAGTCGAAC-3′ (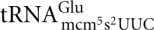 , 3′ probe). Oligonucleotides were labeled using adenosine [γ-32P]triphosphate (5000 Ci/mmol; Amersham Biosciences) and polynucleotide kinase (Roche Applied Science). Northern blots were visualized and quantified by PhosphorImager analysis.
, 3′ probe). Oligonucleotides were labeled using adenosine [γ-32P]triphosphate (5000 Ci/mmol; Amersham Biosciences) and polynucleotide kinase (Roche Applied Science). Northern blots were visualized and quantified by PhosphorImager analysis.
ACKNOWLEDGMENTS
We thank Drs. K.T. Arndt, M.J. Stark, S.Y. Roth, and N. Gunge for strains and plasmids. We acknowledge Drs. M.J.O. Johansson, G.R. Björk, and T.G. Hagervall for critical reading of the manuscript. This work was financially supported by grants from the Swedish Cancer Foundation (07 0637), Swedish Research Council (621-2006-4269), and Bernhard and Signe Bäckström Foundation (223-438-07).
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.1184108.
REFERENCES
- Angeles de la Torre-Ruiz, M., Torres, J., Arino, J., Herrero, E. Sit4 is required for proper modulation of the biological functions mediated by Pkc1 and the cell integrity pathway in Saccharomyces cerevisiae . J. Biol. Chem. 2002;277:33468–33476. doi: 10.1074/jbc.M203515200. [DOI] [PubMed] [Google Scholar]
- Ausubel, F.M., Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.A., Struhl, K. Current protocols in molecular biology. John Wiley and Sons; New York: 2001. [Google Scholar]
- Björk, G.R., Jacobsson, K., Nilsson, K., Johansson, M.J., Byström, A.S., Persson, O.P. A primordial tRNA modification required for the evolution of life? EMBO J. 2001;20:231–239. doi: 10.1093/emboj/20.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk, G.R., Huang, B., Persson, O.P., Byström, A.S. A conserved modified wobble nucleoside (mcm5s2U) in lysyl-tRNA is required for viability in yeast. RNA. 2007;13:1245–1255. doi: 10.1261/rna.558707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke, D., Dawson, D., Stearns, T. Methods in yeast genetics. Cold Spring Harbor Laboratory Press; New York: 2000. [Google Scholar]
- Butler, A.R., Porter, M., Stark, M.J. Intracellular expression of Kluyveromyces lactis toxin γ subunit mimics treatment with exogenous toxin and distinguishes two classes of toxin-resistant mutant. Yeast. 1991a;7:617–625. doi: 10.1002/yea.320070610. [DOI] [PubMed] [Google Scholar]
- Butler, A.R., White, J.H., Stark, M.J. Analysis of the response of Saccharomyces cerevisiae cells to Kluyveromyces lactis toxin. J. Gen. Microbiol. 1991b;137:1749–1757. doi: 10.1099/00221287-137-7-1749. [DOI] [PubMed] [Google Scholar]
- Butler, A.R., White, J.H., Folawiyo, Y., Edlin, A., Gardiner, D., Stark, M.J. Two Saccharomyces cerevisiae genes which control sensitivity to G1 arrest induced by Kluyveromyces lactis toxin. Mol. Cell. Biol. 1994;14:6306–6316. doi: 10.1128/mcb.14.9.6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier, R.J. Understanding the mode of action of diphtheria toxin: A perspective on progress during the 20th century. Toxicon. 2001;39:1793–1803. doi: 10.1016/s0041-0101(01)00165-9. [DOI] [PubMed] [Google Scholar]
- DeMaggio, A.J., Lindberg, R.A., Hunter, T., Hoekstra, M.F. The budding yeast HRR25 gene product is a casein kinase I isoform. Proc. Natl. Acad. Sci. 1992;89:7008–7012. doi: 10.1073/pnas.89.15.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Como, C.J., Arndt, K.T. Nutrients, via the Tor proteins, stimulate the association of Tap42 with type 2A phosphatases. Genes & Dev. 1996;10:1904–1916. doi: 10.1101/gad.10.15.1904. [DOI] [PubMed] [Google Scholar]
- Fichtner, L., Schaffrath, R. KTI11 and KTI13, Saccharomyces cerevisiae genes controlling sensitivity to G1 arrest induced by Kluyveromyces lactis zymocin. Mol. Microbiol. 2002;44:865–875. doi: 10.1046/j.1365-2958.2002.02928.x. [DOI] [PubMed] [Google Scholar]
- Fichtner, L., Frohloff, F., Burkner, K., Larsen, M., Breunig, K.D., Schaffrath, R. Molecular analysis of KTI12/TOT4, a Saccharomyces cerevisiae gene required for Kluyveromyces lactis zymocin action. Mol. Microbiol. 2002a;43:783–791. doi: 10.1046/j.1365-2958.2002.02794.x. [DOI] [PubMed] [Google Scholar]
- Fichtner, L., Frohloff, F., Jablonowski, D., Stark, M.J., Schaffrath, R. Protein interactions within Saccharomyces cerevisiae Elongator, a complex essential for Kluyveromyces lactis zymocicity. Mol. Microbiol. 2002b;45:817–826. doi: 10.1046/j.1365-2958.2002.03055.x. [DOI] [PubMed] [Google Scholar]
- Fichtner, L., Jablonowski, D., Schierhorn, A., Kitamoto, H.K., Stark, M.J., Schaffrath, R. Elongator's toxin-target (TOT) function is nuclear localization sequence dependent and suppressed by post-translational modification. Mol. Microbiol. 2003;49:1297–1307. doi: 10.1046/j.1365-2958.2003.03632.x. [DOI] [PubMed] [Google Scholar]
- Fiorentini, P., Huang, K.N., Tishkoff, D.X., Kolodner, R.D., Symington, L.S. Exonuclease I of Saccharomyces cerevisiae functions in mitotic recombination in vivo and in vitro. Mol. Cell. Biol. 1997;17:2764–2773. doi: 10.1128/mcb.17.5.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohloff, F., Fichtner, L., Jablonowski, D., Breunig, K.D., Schaffrath, R. Saccharomyces cerevisiae Elongator mutations confer resistance to the Kluyveromyces lactis zymocin. EMBO J. 2001;20:1993–2003. doi: 10.1093/emboj/20.8.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohloff, F., Jablonowski, D., Fichtner, L., Schaffrath, R. Subunit communications crucial for the functional integrity of the yeast RNA polymerase II elongator [γ-toxin target (TOT)] complex. J. Biol. Chem. 2003;278:956–961. doi: 10.1074/jbc.M210060200. [DOI] [PubMed] [Google Scholar]
- Furukawa, K., Mizushima, N., Noda, T., Ohsumi, Y. A protein conjugation system in yeast with homology to biosynthetic enzyme reaction of prokaryotes. J. Biol. Chem. 2000;275:7462–7465. doi: 10.1074/jbc.275.11.7462. [DOI] [PubMed] [Google Scholar]
- Gavin, A.C., Aloy, P., Grandi, P., Krause, R., Boesche, M., Marzioch, M., Rau, C., Jensen, L.J., Bastuck, S., Dumpelfeld, B., et al. Proteome survey reveals modularity of the yeast cell machinery. Nature. 2006;440:631–636. doi: 10.1038/nature04532. [DOI] [PubMed] [Google Scholar]
- Gehrke, C.W., Kuo, K.C.T. Chromatography and modification of nucleosides. Elsevier; Amsterdam, New York: 1990. [Google Scholar]
- Gehrke, C.W., Kuo, K.C., McCune, R.A., Gerhardt, K.O., Agris, P.F. Quantitative enzymatic hydrolysis of tRNAs: Reversed-phase high-performance liquid chromatography of tRNA nucleosides. J. Chromatogr. 1982;230:297–308. [PubMed] [Google Scholar]
- Gerber, J., Neumann, K., Prohl, C., Muhlenhoff, U., Lill, R. The yeast scaffold proteins Isu1p and Isu2p are required inside mitochondria for maturation of cytosolic Fe/S proteins. Mol. Cell. Biol. 2004;24:4848–4857. doi: 10.1128/MCB.24.11.4848-4857.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehring, A.S., Rivers, D.M., Sprague G.F., Jr Attachment of the ubiquitin-related protein Urm1p to the antioxidant protein Ahp1p. Eukaryot. Cell. 2003;2:930–936. doi: 10.1128/EC.2.5.930-936.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunge, N., Sakaguchi, K. Intergeneric transfer of deoxyribonucleic acid killer plasmids, pGKl1 and pGKl2, from Kluyveromyces lactis into Saccharomyces cerevisiae by cell fusion. J. Bacteriol. 1981;147:155–160. doi: 10.1128/jb.147.1.155-160.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, Y., Mason, S., Kobayashi, R., Hoekstra, M., Andrews, B. Role of the casein kinase I isoform, Hrr25, and the cell cycle-regulatory transcription factor, SBF, in the transcriptional response to DNA damage in Saccharomyces cerevisiae . Proc. Natl. Acad. Sci. 1997;94:581–586. doi: 10.1073/pnas.94.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, Y., Gruhler, A., Heilbut, A., Bader, G.D., Moore, L., Adams, S.L., Millar, A., Taylor, P., Bennett, K., Boutilier, K., et al. Systematic identification of protein complexes in Saccharomyces cerevisiae by mass spectrometry. Nature. 2002;415:180–183. doi: 10.1038/415180a. [DOI] [PubMed] [Google Scholar]
- Hochstrasser, M. Evolution and function of ubiquitin-like protein-conjugation systems. Nat. Cell Biol. 2000;2:E153–E157. doi: 10.1038/35019643. [DOI] [PubMed] [Google Scholar]
- Hoekstra, M.F., Liskay, R.M., Ou, A.C., DeMaggio, A.J., Burbee, D.G., Heffron, F. HRR25, a putative protein kinase from budding yeast: association with repair of damaged DNA. Science. 1991;253:1031–1034. doi: 10.1126/science.1887218. [DOI] [PubMed] [Google Scholar]
- Huang, B., Johansson, M.J., Byström, A.S. An early step in wobble uridine tRNA modification requires the Elongator complex. RNA. 2005;11:424–436. doi: 10.1261/rna.7247705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonowski, D., Schaffrath, R. Zymocin, a composite chitinase and tRNase killer toxin from yeast. Biochem. Soc. Trans. 2007;35:1533–1537. doi: 10.1042/BST0351533. [DOI] [PubMed] [Google Scholar]
- Jablonowski, D., Butler, A.R., Fichtner, L., Gardiner, D., Schaffrath, R., Stark, M.J. Sit4p protein phosphatase is required for sensitivity of Saccharomyces cerevisiae to Kluyveromyces lactis zymocin. Genetics. 2001a;159:1479–1489. doi: 10.1093/genetics/159.4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonowski, D., Frohloff, F., Fichtner, L., Stark, M.J., Schaffrath, R. Kluyveromyces lactis zymocin mode of action is linked to RNA polymerase II function via Elongator. Mol. Microbiol. 2001b;42:1095–1105. doi: 10.1046/j.1365-2958.2001.02705.x. [DOI] [PubMed] [Google Scholar]
- Jablonowski, D., Fichtner, L., Stark, M.J., Schaffrath, R. The yeast Elongator histone acetylase requires Sit4-dependent dephosphorylation for toxin-target capacity. Mol. Biol. Cell. 2004;15:1459–1469. doi: 10.1091/mbc.E03-10-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonowski, D., Zink, S., Mehlgarten, C., Daum, G., Schaffrath, R. tRNA wobble uridine methylation by Trm9 identifies Elongator's key role for zymocin-induced cell death in yeast. Mol. Microbiol. 2006;59:677–688. doi: 10.1111/j.1365-2958.2005.04972.x. [DOI] [PubMed] [Google Scholar]
- Jiang, Y. Regulation of the cell cycle by protein phosphatase 2A in Saccharomyces cerevisiae . Microbiol. Mol. Biol. Rev. 2006;70:440–449. doi: 10.1128/MMBR.00049-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson, M.J., Esberg, A., Huang, B., Björk, G.R., Byström, A.S. Eukaryotic wobble uridine modifications promote a functionally redundant decoding system. Mol. Cell. Biol. 2008;28:3301–3312. doi: 10.1128/MCB.01542-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafadar, K.A., Zhu, H., Snyder, M., Cyert, M.S. Negative regulation of calcineurin signaling by Hrr25p, a yeast homolog of casein kinase I. Genes & Dev. 2003;17:2698–2708. doi: 10.1101/gad.1140603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalhor, H.R., Clarke, S. Novel methyltransferase for modified uridine residues at the wobble position of tRNA. Mol. Cell. Biol. 2003;23:9283–9292. doi: 10.1128/MCB.23.24.9283-9292.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto, S., Sasaki, T., Itahashi, S., Hatsuyama, Y., Ohno, T. A mutant allele skt5 affecting protoplast regeneration and killer toxin resistance has double mutations in its wild-type structural gene in Saccharomyces cerevisiae . Biosci. Biotechnol. Biochem. 1993;57:1391–1393. doi: 10.1271/bbb.57.1391. [DOI] [PubMed] [Google Scholar]
- Kishida, M., Tokunaga, M., Katayose, Y., Yajima, H., Kawamura-Watabe, A., Hishinuma, F. Isolation and genetic characterization of pGKL killer-insensitive mutants (iki) from Saccharomyces cerevisiae . Biosci. Biotechnol. Biochem. 1996;60:798–801. doi: 10.1271/bbb.60.798. [DOI] [PubMed] [Google Scholar]
- Krogan, N.J., Greenblatt, J.F. Characterization of a six-subunit holo-elongator complex required for the regulated expression of a group of genes in Saccharomyces cerevisiae . Mol. Cell. Biol. 2001;21:8203–8212. doi: 10.1128/MCB.21.23.8203-8212.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogan, N.J., Cagney, G., Yu, H., Zhong, G., Guo, X., Ignatchenko, A., Li, J., Pu, S., Datta, N., Tikuisis, A.P., et al. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae . Nature. 2006;440:637–643. doi: 10.1038/nature04670. [DOI] [PubMed] [Google Scholar]
- Liu, S., Leppla, S.H. Retroviral insertional mutagenesis identifies a small protein required for synthesis of diphthamide, the target of bacterial ADP-ribosylating toxins. Mol. Cell. 2003;12:603–613. doi: 10.1016/j.molcel.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Longtine, M.S., McKenzie A., III, Demarini, D.J., Shah, N.G., Wach, A., Brachat, A., Philippsen, P., Pringle, J.R. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae . Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Lu, J. Umeå University; Umeå, Sweden: 2007. The Kluyveromyces lactis killer toxin is a transfer RNA endonuclease. Ph.D. thesis, [Google Scholar]
- Lu, J., Huang, B., Esberg, A., Johansson, M.J., Byström, A.S. The Kluyveromyces lactis γ-toxin targets tRNA anticodons. RNA. 2005;11:1648–1654. doi: 10.1261/rna.2172105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, J., Esberg, A., Huang, B., Byström, A.S. Kluyveromyces lactis γ-toxin, a ribonuclease that recognizes the anticodon stem loop of tRNA. Nucleic Acids Res. 2008;36:1072–1080. doi: 10.1093/nar/gkm1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke, M.M., Della Seta, F., Di Como, C.J., Sugimoto, H., Kobayashi, R., Arndt, K.T. The SAP, a new family of proteins, associate and function positively with the SIT4 phosphatase. Mol. Cell. Biol. 1996;16:2744–2755. doi: 10.1128/mcb.16.6.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, X., Hu, Y., Liang, C., Lu, C. MET3 promoter: A tightly regulated promoter and its application in construction of conditional lethal strain. Curr. Microbiol. 2002;45:37–40. doi: 10.1007/s00284-001-0046-0. [DOI] [PubMed] [Google Scholar]
- Mehlgarten, C., Schaffrath, R. Mutant casein kinase I (Hrr25p/Kti14p) abrogates the G1 cell cycle arrest induced by Kluyveromyces lactis zymocin in budding yeast. Mol. Genet. Genomics. 2003;269:188–196. doi: 10.1007/s00438-003-0807-5. [DOI] [PubMed] [Google Scholar]
- Murakami, A., Kimura, K., Nakano, A. The inactive form of a yeast casein kinase I suppresses the secretory defect of the sec12 mutant. Implication of negative regulation by the Hrr25 kinase in the vesicle budding from the endoplasmic reticulum. J. Biol. Chem. 1999;274:3804–3810. doi: 10.1074/jbc.274.6.3804. [DOI] [PubMed] [Google Scholar]
- Nakai, Y., Nakai, M., Hayashi, H., Kagamiyama, H. Nuclear localization of yeast Nfs1p is required for cell survival. J. Biol. Chem. 2001;276:8314–8320. doi: 10.1074/jbc.M007878200. [DOI] [PubMed] [Google Scholar]
- Nakai, Y., Umeda, N., Suzuki, T., Nakai, M., Hayashi, H., Watanabe, K., Kagamiyama, H. Yeast Nfs1p is involved in thio-modification of both mitochondrial and cytoplasmic tRNAs. J. Biol. Chem. 2004;279:12363–12368. doi: 10.1074/jbc.M312448200. [DOI] [PubMed] [Google Scholar]
- Nakai, Y., Nakai, M., Lill, R., Suzuki, T., Hayashi, H. Thio modification of yeast cytosolic tRNA is an iron-sulfur protein-dependent pathway. Mol. Cell. Biol. 2007;27:2841–2847. doi: 10.1128/MCB.01321-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero, G., Fellows, J., Li, Y., de Bizemont, T., Dirac, A.M., Gustafsson, C.M., Erdjument-Bromage, H., Tempst, P., Svejstrup, J.Q. Elongator, a multisubunit component of a novel RNA polymerase II holoenzyme for transcriptional elongation. Mol. Cell. 1999;3:109–118. doi: 10.1016/s1097-2765(00)80179-3. [DOI] [PubMed] [Google Scholar]
- Petrakis, T.G., Sogaard, T.M., Erdjument-Bromage, H., Tempst, P., Svejstrup, J.Q. Physical and functional interaction between Elongator and the chromatin-associated Kti12 protein. J. Biol. Chem. 2005;280:19454–19460. doi: 10.1074/jbc.M413373200. [DOI] [PubMed] [Google Scholar]
- Petronczki, M., Matos, J., Mori, S., Gregan, J., Bogdanova, A., Schwickart, M., Mechtler, K., Shirahige, K., Zachariae, W., Nasmyth, K. Monopolar attachment of sister kinetochores at meiosis I requires casein kinase 1. Cell. 2006;126:1049–1064. doi: 10.1016/j.cell.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Schafer, T., Strauss, D., Petfalski, E., Tollervey, D., Hurt, E. The path from nucleolar 90S to cytoplasmic 40S pre-ribosomes. EMBO J. 2003;22:1370–1380. doi: 10.1093/emboj/cdg121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer, T., Maco, B., Petfalski, E., Tollervey, D., Bottcher, B., Aebi, U., Hurt, E. Hrr25-dependent phosphorylation state regulates organization of the pre-40S subunit. Nature. 2006;441:651–655. doi: 10.1038/nature04840. [DOI] [PubMed] [Google Scholar]
- Schaffrath, R., Meinhardt, F. Kluyveromyces lactis zymocin and other plasmid-encoded yeast killer toxins. In: Schmitt M., editor. Microbial protein toxins. Topics in current genetics 11. Springer; New York: 2005. pp. 133–155. [Google Scholar]
- Schnell, J.D., Hicke, L. Nontraditional functions of ubiquitin and ubiquitin-binding proteins. J. Biol. Chem. 2003;278:35857–35860. doi: 10.1074/jbc.R300018200. [DOI] [PubMed] [Google Scholar]
- Stark, M.J., Boyd, A., Mileham, A.J., Romanos, M.A. The plasmid-encoded killer system of Kluyveromyces lactis: A review. Yeast. 1990;6:1–29. doi: 10.1002/yea.320060102. [DOI] [PubMed] [Google Scholar]
- Sun, J., Zhang, J., Wu, F., Xu, C., Li, S., Zhao, W., Wu, Z., Wu, J., Zhou, C.Z., Shi, Y. Solution structure of Kti11p from Saccharomyces cerevisiae reveals a novel zinc-binding module. Biochemistry. 2005;44:8801–8809. doi: 10.1021/bi0504714. [DOI] [PubMed] [Google Scholar]
- Sutton, A., Immanuel, D., Arndt, K.T. The SIT4 protein phosphatase functions in late G1 for progression into S phase. Mol. Cell. Biol. 1991;11:2133–2148. doi: 10.1128/mcb.11.4.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler, G.S., Petrakis, T.G., Ethelberg, S., Tokunaga, M., Erdjument-Bromage, H., Tempst, P., Svejstrup, J.Q. RNA polymerase II elongator holoenzyme is composed of two discrete subcomplexes. J. Biol. Chem. 2001;276:32743–32749. doi: 10.1074/jbc.M105303200. [DOI] [PubMed] [Google Scholar]
- Wittschieben, B.O., Otero, G., de Bizemont, T., Fellows, J., Erdjument-Bromage, H., Ohba, R., Li, Y., Allis, C.D., Tempst, P., Svejstrup, J.Q. A novel histone acetyltransferase is an integral subunit of elongating RNA polymerase II holoenzyme. Mol. Cell. 1999;4:123–128. doi: 10.1016/s1097-2765(00)80194-x. [DOI] [PubMed] [Google Scholar]
- Zheng, L., Cash, V.L., Flint, D.H., Dean, D.R. Assembly of iron-sulfur clusters. Identification of an iscSUA-hscBA-fdx gene cluster from Azotobacter vinelandii . J. Biol. Chem. 1998;273:13264–13272. doi: 10.1074/jbc.273.21.13264. [DOI] [PubMed] [Google Scholar]