Abstract
Background and Purpose
Insulin-like growth factor I (IGF-1) is a pleiotropic growth factor that has been demonstrated to protect against acute ischemic brain injury. Whether IGF-1 improves long-term functional outcome after ischemic stroke is not known. The aim of this study is to examine whether IGF-1 overexpression through adeno-associated virus (AAV) -mediated gene transfer enhances neurovascular remodeling and improves functional outcome in a mouse model of focal cerebral ischemia.
Methods
Long-term cerebral IGF-1 overexpression was achieved with the AAV transduction system through stereotaxic injection. Control mice were injected with AAV–green fluorescent protein or saline. Three weeks after gene transfer, the mice underwent permanent distal middle cerebral artery occlusion. Histological and behavioral analyses were performed at day 21 after middle cerebral artery occlusion.
Results
IGF-1 gene transfer compared with control treatment significantly improved motor performance assessed by sensorimotor tests. The functional recovery was accompanied by reduced volume of cerebral infarction. Immunohistochemical analysis with endothelial cell marker CD31 revealed that IGF-1 gene transfer potently increased neovessel formation in the periinfarct and injection needle tract area compared with AAV–green fluorescent protein transduction. Increased vascular density was associated with increased local vascular perfusion. Additionally, AAV-IGF-1 treatment enhanced neurogenesis in the subventricular zone compared with AAV–green fluorescent protein treatment.
Conclusions
These data demonstrate that IGF-1 overexpression promoted long-lasting functional recovery after cerebral infarction. The improved functional performance was paralleled by enhanced neovascularization and neurogenesis.
Keywords: angiogenesis, gene transfer, IGF-1, neurogenesis, stroke
Insulin growth factor-1 (IGF-1) plays an essential role in normal growth and brain development.1 Substantial data indicate that IGF-1 exerts potent neuroprotective effects against acute ischemic brain injury. Administration of IGF-1 protein protects the developing or adult brain from experimental ischemia2-5 and cultured neurons from diverse forms of injury.6,7 Low levels of IGF-1 are associated with poor outcome in elderly patients with stroke, suggesting that endogenous IGF-1 level impacts the evolution of cerebral infarction.8 In addition to its neuropotective activities, IGF-1 is also a potent angiogenic factor required for cerebral angiogenesis during development and in adulthood.9 IGF-1 has been reported to enhance endothelial function through antiinflammatory and antiapoptotic properties.10
Although IGF-1 has been established to protect neural cells against acute ischemic injury, a role for IGF-1 in long-term neurological recovery after ischemic stroke has not been demonstrated. In this study, we investigated whether IGF-1 overexpression enhances neurovascular remodeling and improves long-term functional outcome in a mouse model of permanent focal cerebral ischemia. Recombinant adeno-associated viral vector (AAV) was used to deliver IGF-1 to the brain and to achieve efficient IGF-1 production over long periods of time.
Materials and Methods
Adeno-Associated Viral Vector Delivery
This study was approved by the University of California, San Francisco Committee of Animal Research and conformed to the National Institutes of Health Guidelines for use of animals in research. Viral transduction was performed as described.11 Briefly, adult CD-1 mice weighing 30 to 35 g were placed in a stereotactic frame (Kopf, Tujunga, Calif) under anesthesia, and a burr hole was drilled 2.5 mm lateral to the sagittal suture and 1 mm posterior to the coronal suture. A 10-μL Hamilton syringe was slowly inserted into the left caudate nucleus (3.0 mm deep from the dura). Four microliters viral suspension (AAV-IGF-1 or AAV–green fluorescent protein [GFP]) containing 2×1010 particles was injected into the left hemisphere (injection sites are depicted in Figure 1A). Recombinant AAV serotype 2 (rAAV2) containing human IGF-1 or GFP was provided by Ceregene Inc (San Diego, Calif). AAV serotype 2 has been shown to only transfect postmitotic neurons.12 Systemic coadministration of mannitol has been demonstrated to profoundly amplify rAAV2 transduction efficiency.12 Thus, in our experiments, the mice were injected intraperitoneally with 3 mL of sterile 25% mannitol in 0.9% saline per 100 g body weight 15 minutes before intracerebral vector injection.
Figure 1.
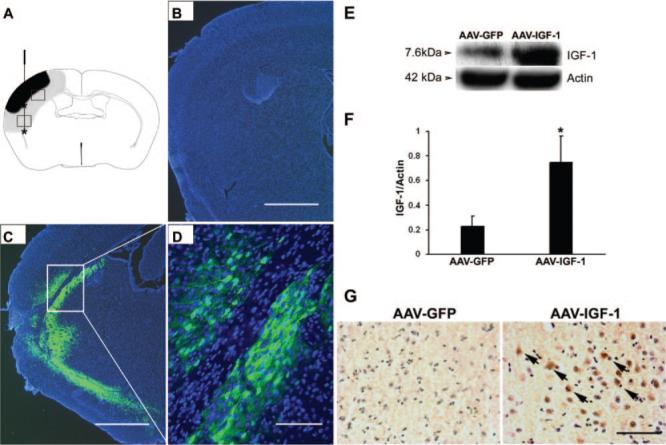
A, Schematic diagram of a mouse brain coronal section. AAV vector was delivered at 2 different sites for each injection. Asterisk designates sites of viral injection. The purpose of injecting the cortex is to protect and rescue the ischemic brain with IGF-1 overexpression. The purpose of injecting the caudate nucleus is to maximize the transduced brain area and IGF-1 expression. Ischemic core is shown as black area, and periinfarct region is shown as gray-shaded area. Two white squares, upper and lower, denote area of interest for immunohistochemical analysis and angiogenesis assessment. B–D, Distribution of AAV vector mediated gene transduction. Intraparenchymal injection of AAV-GFP resulted in an intense GFP signal at day 21 poststroke (C–D). D, Higher magnification of inset in C. GFP signal was absent in the noninjected, contralateral hemisphere (B). Blue color shows the nuclei stained with DAPI. E–F, Western blot analysis. The upper gel shows a representative IGF-1 blot, and the lower gel shows a β-actin blot for control of protein load. Histogram indicates quantification of IGF-1 level. *P<0.05, versus AAV-GFP, n=8 animals per group. G, Immunohistochemical staining of IGF-1. Strong IGF-1 immunoreactivity was observed in AAV-IGF-1-treated mice (brown color indicated with arrows), but not in AAV-GFP-treated mice. Sections were counterstained with hematoxylin to show nuclei. Scale bars: B and C, 1 mm; D, 50 μmol/L; G, 50 μmol/L.
Animal Stroke Model
Three weeks after gene transfer, the mice were subjected to permanent focal ischemia by distal middle cerebral artery occlusion (MCAO).13 The left middle cerebral artery was occluded by electric coagulation just proximal to the pyriform branch. Body temperature was maintained at 37±0.5°C by using a thermal blanket throughout the surgical procedure. Surface cerebral blood flow was monitored during MCAO using a laser Doppler flowmeter (Vasamedics Inc). Mice with surface cerebral blood flow that was more than 15% of the baseline were excluded from the experiment.
5-bromo-2-deoxyuridine-5-monophosphate–Labeling
5-bromo-2-deoxyuridine-5-monophosphate (BrdU), a thymidine analog incorporated into the DNA of dividing cells, was used to track proliferating cells. Before euthanization, mice were intraperitoneally injected twice daily with BrdU (50 mg/kg; Sigma) for 7 consecutive days.
Western Blotting
The protein concentration was determined using the BCA protein assay kits (Pierce, Ill). Equal amounts of proteins were loaded on 10% acrylamide gel for electrophoresis and were electroblotted onto a polyvinyl difluoride membrane. The membranes were then probed with mouse anti-IGF-1 antibody, 1:500 (Upstate, the antibody reacts with IGF-1 from human and mouse origin) followed by incubation with horseradish–peroxidase-conjugated sheep antimouse IgG (Bio-Rad Laboratories). Protein expression was detected with an enhanced chemiluminescence detection system (Amersham Pharmacia Biotech Inc). Beta-actin was used as a loading control.
Immunohistochemistry
Immunohistochemical staining was performed as described.11 Briefly, sections were incubated with primary antibodies at the following concentrations: mouse anti-IGF-1, 1:100 (Upstate), rat anti-CD31, 1:200 (BD Bioscience), mouse anti-BrdU, 1:1000 (Sigma), and rabbit antidoublecortin (DCX), 1:200 (Cell Signaling). After incubating at 4°C overnight and washing, the sections were incubated with biotinylated secondary antibody (Vector Laboratories) at 1:5000 dilution. The sections were treated with the ABC streptavidin detection system. For dual fluorescent staining, after incubating with primary antibodies, sections were incubated with Alexa Fluor 594-conjugated or Alexa Fluor 488-conjugated IgG (Molecular Probes) at 1:500 dilution. Negative controls were performed by omitting the primary antibodies during the immunostaining.
Behavioral Tests
A set of behavioral tests was performed 1 day before MCAO (as baseline values) and 21 days after MCAO by an investigator masked to the experimental groups.
Corner test was used to detect sensorimotor and postural asymmetries.14 Mice were placed between the 2 angled boards facing the corner. When entering deep into the corner, the vibrissae on both sides were stimulated simultaneously. The mouse would then rear forward and upward, and then turn back to face the open end. The nonischemic animals would turn back from either left or right randomly. The ischemic animals would preferentially turn toward the ipsilateral side. The number of turns taken to each side was recorded from 10 trials for each test. Turning movements that were not part of a rearing movement were not scored. Data are presented as normalized turn ratio out of 10 trials.
Body Asymmetry Test
To measure motor asymmetry, mice were examined by elevated body swing test as described.15 Mice were examined for head swings while being suspended by their tails. The direction of the swing, either right or left, was recorded when the mouse turned its head sideways by approximately at a 10° angle to the body's midline. After each swing, the mouse was allowed to move freely in a Plexiglas box for at least 30 seconds before taking the next test; the trials were repeated 20 times for each animal. The frequency of head swings toward the contralateral side was counted and normalized as follows:
Beam Test
Beam walking across a bridge was used to assess motor coordination and balance after stroke injury.16 Mice were trained for 5 days before MCAO to traverse a narrow round beam (5 mm diameter and 900 mm in length) to reach an enclosed escape platform. They were placed on one end of the beam and the latency to traverse the central 80% of the beam toward the enclosed escape platform at the other end was recorded. Data are expressed as mean latency to cross the beam of 3 trials.
Evaluation of Cerebral Infarction
Twenty-one days after MCAO, mice were euthanized so their brains could be examined histologically for infarct volume and secondary end points (see subsequently). Serial coronal sections (200 μm apart) were stained with cresyl violet for assessing infarct volume. Sections were digitized, and the area of infarct and noninfarct tissues was outlined using National Institutes of Health Image J. Infarct volume was calculated by multiplying the appropriate area by the section interval thickness. Infarct volume was indirectly determined by subtracting the volume of intact tissue in the ipsilateral hemisphere from that in the contralateral hemisphere.17
Vascular Density Assessment
Microvessel counting was performed as described.11 Briefly, 2 brain coronal sections from the CD31-stained brain sections, 1 mm anterior and 1 mm posterior from the needle track, were chosen. Three areas of microvessels, immediate to the left, right, and bottom of the needle track, were photographed using a 10× objective. Three random areas in the perifocal region were also photographed. Microvessel counting and cell counting (BrdU and CD31 dual-labeled cells) were performed on these photographs. Vessels with a diameter between 3 and 8 μmol/L were counted. The number of microvessels was calculated as the mean of the vascular counts obtained from 3 pictures; the number of cells was calculated in the same manner. The counting was conducted in a blinded fashion.
Cerebral Blood Flow Measurement
For surface cerebral blood flow measurement, a laser Doppler flowmetry monitor equipped with a small-caliber probe 0.7 mm in diameter was used. The laser Doppler probe was in contact with the surface of the mice's skull bone during measurement. Blood flow was recorded in the ischemic penumbra area, which is 1.0 mm lateral to the sagittal suture and 1 mm posterior to the coronal suture. Baseline blood flow was recorded 5 minutes before MCAO. Changes in cerebral blood flow were measured immediately, 1 week, and 3 weeks after MCAO. Blood flow values are calculated and expressed as percentages of baseline values.11
Cell Counting
Quantification of DCX+ and BrdU+ cells was performed as described.18 DCX+ and BrdU+ cells in the ipsilateral subventricular zone (the area that lines the lateral walls of the lateral ventricle and is a predominant source of neuronal progenitors) were digitized under a 20× objective with a spot camera (Leica) equipped with National Institutes of Health Image J software. To clearly differentiate positively stained cells from the background, the digitalized images were contrast-enhanced and a threshold parameter was established to assess the proportion of immunoreactive region within a fixed field of view. The thresholds were selected with a “set color threshold” feature in the Image J software, which permits the user to select pixel regions that were considered positive. After establishment, the same parameter was applied to all images acquired under the same magnification and light intensities on slides that were processed identically. The signals were counted using 4 sections from each mouse and the numbers were averaged. The data are presented as numbers of positive cells per microscopic field.
Statistical Analysis
Data are presented as mean±SD. Parametric data from the AAV-IGF-1, AAV-GFP, and saline-treated groups were compared using a one-way analysis of variance followed by Fisher protected least significant difference test, as appropriate. A probability value <0.05 is considered statistically significant.
Results
Adeno-Associated Viral Vector-Mediated Gene Transduction and Expression
To determine whether intraparenchymal AAV injection leads to successful gene transduction and expression, we first examined the extent of GFP expression after AAV-GFP infection. As shown in Figures 1C and 1D, a robust GFP signal was observed adjacent and distal to the injection tract as well as in the periinfarct region 3 weeks after MCAO (6 weeks postinjection). Previous experiments showed that GFP signal could be detected as early as 2 weeks after AAV transduction (data not shown). In contrast, GFP signal was absent in the contralateral noninjected hemisphere (Figure 1B). AAV-IGF-1 administration resulted in increased IGF-1 protein levels compared with AAV-GFP-administered mice as assessed by Western blot analysis (Figure 1E–F, P<0.01) 3 weeks after MCAO. IGF-1 expression was also present in GFP-injected mice, which might be reflective of endogenous IGF-1 protein expressed after the ischemia. Furthermore, immunohistochemical staining revealed intense IGF-1 immunoreactivity in IGF-1-transduced mice, but not in GFP-injected mice (Figure 1G). These data indicate that AAV efficiently delivered IGF-1 to a large area of the ischemic penumbra, not limited to the injection sites, with a prolonged expression period.
Insulin Growth Factor-1 Gene Transduction Confers Improved Neurological Behavior and Reduced Hemispheric Atrophy After Cerebral Ischemia
After demonstrating efficient gene transfer, we investigated whether IGF-1 overexpression would improve neurological functions after ischemic stroke. Three weeks after AAV-mediated gene transfer, mice were subjected to ischemic injury by permanent MCAO. Compared with the stroke mice with AAV-GFP or saline treatment, those with AAV-IGF-1 transduction showed dramatically improved motor performance as assessed by corner test, body asymmetry trials, and beam test (Figure 2, P<0.05). Furthermore, AAV-IGF-1 treatment significantly reduced the infarct volume compared with AAV-GFP or saline treatment (Figure 3; AAV-IGF-1 versus AAV-GFP versus saline: 16.6±5.3, 29.2±7.8, 30.5±5.2; mm3; P<0.05).
Figure 2.
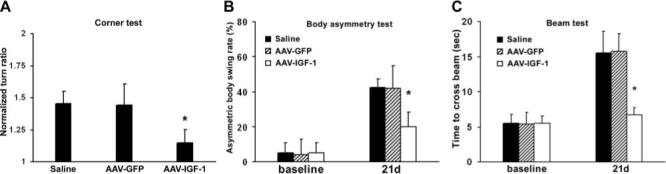
IGF-1 overexpression improves sensorimotor functions after stroke. A, In the corner test, ischemia increased the turn frequency toward the ipsilateral side, which was significantly attenuated by the AAV-IGF-1 treatment. B, Assessed by the body asymmetry test, mice displayed more frequent turn toward contralateral side after MCAO. Stroke mice with AAV-IGF-1 transduction showed significantly decreased biased swing activity compared with stroke mice with AAV-GFP or saline injection. C, In the beam test, ischemic injury caused mice to use much longer time to transverse the beam. Compared with treatment with saline or AAV-GFP, AAV-IGF-1 administration dramatically improved the crossing speed. *P<0.05 versus AAV-GFP or saline, n=8 animals per group.
Figure 3.
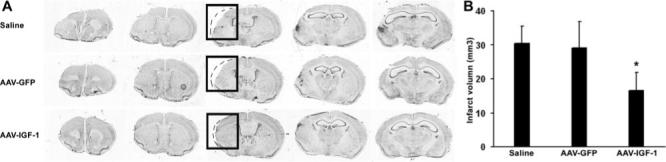
IGF-1 overexpression attenuates cerebral lesion volumes after stroke. A, Representative Nissl staining of coronal brain sections sliced rostral to caudal shows AAV-IGF-1 treatment decreased infarct volume determined 3 weeks after MCAO as compared with AAV-GFP or saline treatment. B, Quantification of the volume of infarction. *P<0.05 versus AAV-GFP or saline, n=8 animals per group.
Insulin Growth Factor-1 Overexpression Increases Vascular Density and Cerebral Blood Flow
Immunohistochemical analysis using an antibody against endothelial cell marker CD31 showed increased vascular density in the penumbra of AAV-IGF-1-treated hemispheres (112±12, number of microvessels/field) compared with AAV-GFP (54±6) or saline-injected hemispheres (51±5; Figure 4A–B, P<0.05). IGF-1 gene transduction also increased the number of microvessels in the needle tract region (91±22, P<0.05) compared with GFP gene transduction (44±12) or saline treatment (45±10). Dual-labeled immunofluorescence showed that BrdU+ cells colocalized with CD31 with higher numbers of BrdU and CD31 dual-labeled cells in IGF-1-transduced groups compared with AAV-GFP administration (Figure 4C–D, 1.8±1.3 versus 0.6±0.3, P<0.05, cells/field). The data indicate that IGF-1 promoted endogenous endothelial cell proliferation and ongoing angio-genesis. Importantly, IGF-1 overexpression significantly increased vascular perfusion detected at 3 weeks after MCAO (21±7) compared with AAV-GFP (7±1) or saline (7±2) treatment (Table; percentages of baseline values, P<0.05). There was no difference in blood flow among the 3 groups assessed immediately or 1 week after MCAO.
Figure 4.
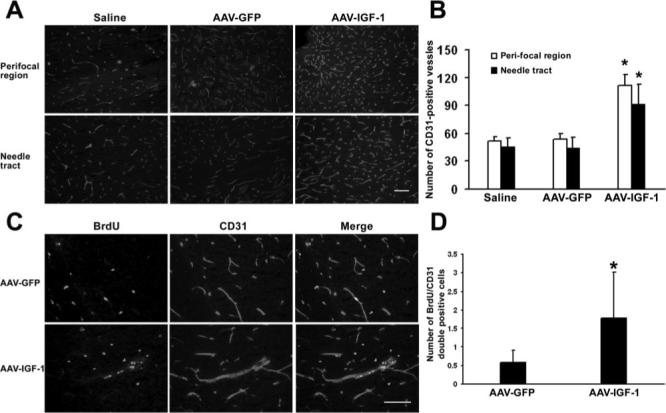
IGF-1 gene transfer enhances neovascularization. A, Representative images of immunofluorescent staining of endothelial cell marker CD31 demonstrate AAV-IGF-1 transduction increased vascular density in the ischemic penumbra and needle tract region compared with AAV-GFP transduction or saline injection. B, Quantification of microvessel numbers. *P<0.05 versus AAV-GFP or saline, n=8 for each group. C, Representative images of colocalization of BrdU+ and CD31+ cells showing active endothelial cell proliferation induced by AAV-IGF-1 transfection. D, Quantification of BrdU and CD31 colabeled cells. *P<0.05 versus AAV-GFP, n=8 for each group. Scale bar=50 μmol/L.
Table.
Local Blood Flow in the Ischemic Cortex
| Time After MCAO |
||||
|---|---|---|---|---|
| Group | Baseline | 0 Week | 1 Week | 3 Weeks |
| Saline | 97 ± 8 | 4 ± 1 | 6 ± 2 | 7 ± 2 |
| AAV-GFP | 100 ± 9 | 4 ± 1 | 6 ± 2 | 7 ± 1 |
| AAV-IGF-1 | 96 ± 13 | 5 ± 1 | 6 ± 3 | 21 ± 7* |
Baseline indicates local blood flow before MCAO; local blood flow values are expressed as percentages of baseline values; data are mean±SD.
P<0.05 versus saline or AAV-GFP, n=8 for each group.
Transfer of Insulin Growth Factor-1 Gene Enhances Neurogenesis
To examine whether IGF-1 overexpression enhances neuro-genesis, we performed immunohistochemcal analysis using antibodies against the cell proliferation marker BrdU and DCX, a specific marker for immature neurons. As shown in Figures 5A and 5B, IGF-1 gene transfer resulted in pronounced elevation of BrdU+ cells in the subventricular zone (SVZ) with AAV-GFP treatment (for SVZ: 100±21 versus 28±16, P<0.05, cells/field). In SVZ, there was a significant increase in DCX+ cells in AAV-IGF-1-treated mice compared with those treated with AAV-GFP (Figure 5C–5D, 52±31 versus 11±8, P<0.05, cells/field). Both BrdU+ and DCX+ cells were present in the AAV-GFP-transduced mice, indicating endogenous neurogenesis induced by ischemic injury. Furthermore, double-fluorescent staining showed that cells positive for both BrdU and DCX were significantly enhanced in AAV-IGF-1-transduced mice compared with AAV-GFP-transduced mice in the SVZ (Figure 5E–5F, 25±4 versus 6±4, P<0.05, cells/field). These data demonstrate that IGF-1 administration accelerated endogenous neurogenesis.
Figure 5.
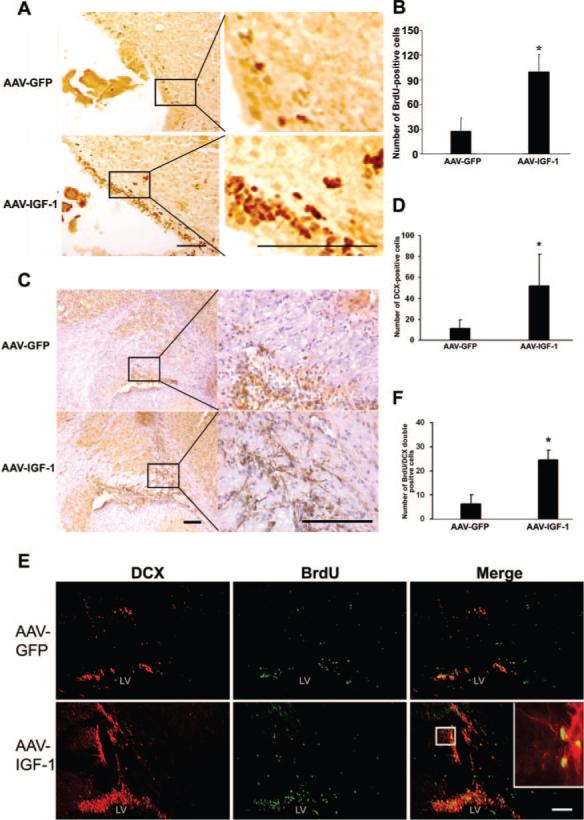
IGF-1 gene transfer promotes neurogenesis. A, Representative images of BrdU staining show AAV-IGF-1 administration increased cell proliferation in the SVZ area compared with AAV-GFP injection. Higher magnification image of BrdU+ cells is shown in the inset. B, Quantitation of BrdU-labeled cells. C, Representative images of DCX staining show AAV-IGF-1 administration increased the number of newborn neurons in SVZ compared with AAV-GFP injection. Right panel images are higher magnifications of the inset depicted in the left panel images. Brown color indicates DCX-positive signal, and blue color is hematoxylin counter staining of nuclei. D, Quantification of DCX+ cells. E, Double immunofluorescent staining shows colocalization of DCX+ and BrdU+ cells, and IGF-1 overexpression increased the number of DCX and BrdU dual-labeled cells. Image of DCX and BrdU-colabeled cells at higher magnification is shown in the inset. F, Quantification of DCX and BrdU-colabeled cells. *P<0.05 versus AAV-GFP, n=8 for each group. Scale bar=100 μmol/L.
Discussion
The present study is the first to demonstrate that IGF-1 gene transfer effectively improved the functional outcome, which was coupled with enhanced angiogenesis and neurogenesis, assessed in the chronic stage of cerebral infarction.
Endogenous IGF-1 level is upregulated after cerebral ischemia, suggesting that IGF-1 may mediate endogenous protection or repair in the ischemic lesion.2,19 Endogenously elevated IGF-1 may not be sufficient to counteract the injury, and therapeutic administration is needed to augment the trophic support to sustain long-term stroke protection. Several studies have reported that exogenous IGF-1 protein delivery effectively reduces the extent of neuronal damage and neurological deficits in the acute phase of ischemic injury assessed from 24 hours to 7 days.20,21 However, the effect of IGF-1 treatment on the long-term neurological functions after ischemic stroke has not been reported. In this study, we used AAV to achieve sustained IGF-1 overexpression in the ischemic hemisphere. The advantage of gene transfer is that a single injection of vector can lead to efficient production of proteins over a widely distributed target area for an extended period of time. Thus, gene transfer offers significant advantages over approaches that rely on administration of protein from a single point source (whether through multiple injections over time or chronic infusions involving indwelling hardware); these advantages include more widespread coverage and simplicity of long-term protein exposures. In addition, compared with other viral vectors such as adenoviral vector, AAV vector has several advantages such as the ability to mediate long-term transgene expression and low immunogenicity.22 IGF-1 overexpression was sustained for at least 6 weeks after AAV transfer as shown by Western blot and immunohistochemical analysis. After synthesis and secretion, IGF-1 could diffuse readily through tissues, a property that permits it to signal over long distances.23 We demonstrated here that IGF-1 overexpression effectively enhanced motor function, assessed at 3 weeks after MCAO, compared with GFP or saline treatment. The improved behavior performance correlated with a reduction in stroke-induced brain atrophy.
Although IGF-1 is an angiogenic factor and reported to mediate vascular growth in developing and adult brain,9 its role in vascular remolding after cerebral ischemia has not been studied. Our data show that IGF-1 treatment produced increased microvessels in the periinfarct region along with increased local blood flow. Improved microcirculation is likely to enhance neural cell survival, support neurogenesis, and help the removal of necrotic brain tissue to facilitate the repair process ultimately leading to long-term functional recovery. Consistent with this notion, several studies have shown that restoration of perfusion by collateral growth or new capillaries facilitates functional improvement after stroke.24-26
Neurogenesis persists in the adult brain and could be enhanced by ischemic injury and various growth factors.27 Inhibiting endogenous IGF-1 activity prevents focal ischemia-induced neurogenesis.19 We demonstrated here that IGF-1 gene transfer increased the number of newly formed neurons in the SVZ area of infarcted hemispheres compared with ischemic injured hemispheres with GFP gene transfer. Our finding is consistent with the report that IGF-1 protein infusion through osmotic minipump increases stroke-induced progenitor cell proliferation in hypertensive rats.28 Together with these studies, our data indicate that neurogenesis can be amplified by administration of exogenous growth factors such as IGF-1 to promote the healing of the ischemic brain. Angiogenesis and neurogenesis are linked in a temporal and spatial manner.29,30 Their interdependence emerges in development with concurrent growth and remodeling. Vascular cells secrete trophic factors such as SDF-1 or Ang1 to promote neural progenitor cells to proliferate and migrate to regions of damage.30 Taken together, enhanced neovascularization and neurogenesis could be the essential mechanisms by which IGF-1 enhances functional recovery after MCAO.
In this study, AAV-IGF-1 was injected before MCAO to allow maximal expression of IGF-1. This experimental paradigm minimizes the usefulness of clinical applications. However, pretreatment is also of interest primarily as proof of concept that IGF-1 transduction could promote long-lasting functional recovery after cerebral infarction. In addition to enhanced angiogenesis and neurogenesis, the improved neurological function and reduced brain atrophy could also be contributed by the neuroprotective effects of IGF-1 in the acute phase of stroke. Because the acute neuroprotective action of IGF-1 has been well demonstrated, in the present study, we focused our effort on studying the neural regenerative effect of IGF-1. Targeting the neurovascular unit with neurotrophic and angiogenic IGF-1 may represent a novel regenerative paradigm for patients with stroke. Future experiments are needed to assess the restorative effects of IGF-1 by postischemic gene transfer.
Acknowledgments
We thank Jialing Liu for advice on animal stroke model and behavioral tests, Voltaire Gungab for editorial assistance, and the staff of the Center for Cerebrovascular Research (http://avm.ucsf. edu/) for their collaborative support.
Sources of Funding This work was supported by National Institutes of Health grants R21 NS053943 (Y.C.), P01 NS44145 (W.L.Y., G.Y.Y.), and P39-05-A50-05 of the Else-Kroener-Fresenius-Stiftung, Germany (T.F.). AAV-IGF-1 and AAV-GFP were provided by Ceregene Inc.
Footnotes
Disclosures None.
References
- 1.Leinninger GM, Feldman EL. Insulin-like growth factors in the treatment of neurological disease. Endocr Dev. 2005;9:135–159. doi: 10.1159/000085763. [DOI] [PubMed] [Google Scholar]
- 2.Loddick SA, Liu XJ, Lu ZX, Liu C, Behan DP, Chalmers DC, Foster AC, Vale WW, Ling N, De Souza EB. Displacement of insulin-like growth factors from their binding proteins as a potential treatment for stroke. Proc Natl Acad Sci U S A. 1998;95:1894–1898. doi: 10.1073/pnas.95.4.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schabitz WR, Hoffmann TT, Heiland S, Kollmar R, Bardutzky J, Sommer C, Schwab S. Delayed neuroprotective effect of insulin-like growth factor-I after experimental transient focal cerebral ischemia monitored with MRI. Stroke. 2001;32:1226–1233. doi: 10.1161/01.str.32.5.1226. [DOI] [PubMed] [Google Scholar]
- 4.Liu XF, Fawcett JR, Thorne RG, DeFor TA, Frey WH., II Intranasal administration of insulin-like growth factor-I bypasses the blood–brain barrier and protects against focal cerebral ischemic damage. J Neurol Sci. 2001;187:91–97. doi: 10.1016/s0022-510x(01)00532-9. [DOI] [PubMed] [Google Scholar]
- 5.Guan J, Bennet L, George S, Wu D, Waldvogel HJ, Gluckman PD, Faull RL, Crosier PS, Gunn AJ. Insulin-like growth factor-1 reduces postischemic white matter injury in fetal sheep. J Cereb Blood Flow Metab. 2001;21:493–502. doi: 10.1097/00004647-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Dore S, Kar S, Quirion R. Insulin-like growth factor I protects and rescues hippocampal neurons against beta-amyloid- and human amylin-induced toxicity. Proc Natl Acad Sci U S A. 1997;94:4772–4777. doi: 10.1073/pnas.94.9.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng B, Mattson MP. IGF-I and IGF-II protect cultured hippocampal and septal neurons against calcium-mediated hypoglycemic damage. J Neurosci. 1992;12:1558–1566. doi: 10.1523/JNEUROSCI.12-04-01558.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denti L, Annoni V, Cattadori E, Salvagnini MA, Visioli S, Merli MF, Corradi F, Ceresini G, Valenti G, Hoffman AR, Ceda GP. Insulin-like growth factor 1 as a predictor of ischemic stroke outcome in the elderly. Am J Med. 2004;117:312–317. doi: 10.1016/j.amjmed.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Lopez C, LeRoith D, Torres-Aleman I. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc Natl Acad Sci U S A. 2004;101:9833–9838. doi: 10.1073/pnas.0400337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conti E, Carrozza C, Capoluongo E, Volpe M, Crea F, Zuppi C, Andreotti F. Insulin-like growth factor-1 as a vascular protective factor. Circulation. 2004;110:2260–2265. doi: 10.1161/01.CIR.0000144309.87183.FB. [DOI] [PubMed] [Google Scholar]
- 11.Shen F, Su H, Fan Y, Chen Y, Zhu Y, Liu W, Young WL, Yang G-Y. Adeno-associated viral vector-mediated hypoxia-inducible vascular endothelial growth factor gene expression attenuates ischemic brain injury after focal cerebral ischemia in mice. Stroke. 2006;37:2601–2606. doi: 10.1161/01.STR.0000240407.14765.e8. [DOI] [PubMed] [Google Scholar]
- 12.Burger C, Nguyen FN, Deng J, Mandel RJ. Systemic mannitol-induced hyperosmolality amplifies RAAV2-mediated striatal transduction to a greater extent than local co-infusion. Mol Ther. 2005;11:327–331. doi: 10.1016/j.ymthe.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Potrovita I, Tarabin V, Herrmann O, Beer V, Weih F, Schneider A, Schwaninger M. Neuronal activation of NF-kappaB contributes to cell death in cerebral ischemia. J Cereb Blood Flow Metab. 2005;25:30–40. doi: 10.1038/sj.jcbfm.9600004. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Schallert T, Zhang ZG, Jiang Q, Arniego P, Li Q, Lu M, Chopp M. A test for detecting long-term sensorimotor dysfunction in the mouse after focal cerebral ischemia. J Neurosci Methods. 2002;117:207–214. doi: 10.1016/s0165-0270(02)00114-0. [DOI] [PubMed] [Google Scholar]
- 15.Borlongan CV, Tajima Y, Trojanowski JQ, Lee VM, Sanberg PR. Transplantation of cryopreserved human embryonal carcinoma-derived neurons (NT2N cells) promotes functional recovery in ischemic rats. Exp Neurol. 1998;149:310–321. doi: 10.1006/exnr.1997.6730. [DOI] [PubMed] [Google Scholar]
- 16.Feeney DM, Gonzalez A, Law WA. Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science. 1982;217:855–857. doi: 10.1126/science.7100929. [DOI] [PubMed] [Google Scholar]
- 17.Swanson RA, Morton MT, Tsao WG, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, Katakowski M, Lu M, Chopp M. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan YP, Sailor KA, Vemuganti R, Dempsey RJ. Insulin-like growth factor-1 is an endogenous mediator of focal ischemia-induced neural progenitor proliferation. Eur J Neurosci. 2006;24:45–54. doi: 10.1111/j.1460-9568.2006.04872.x. [DOI] [PubMed] [Google Scholar]
- 20.Mackay KB, Loddick SA, Naeve GS, Vana AM, Verge GM, Foster AC. Neuroprotective effects of insulin-like growth factor-binding protein ligand inhibitors in vitro and in vivo. J Cereb Blood Flow Metab. 2003;23:1160–1167. doi: 10.1097/01.WCB.0000087091.01171.AE. [DOI] [PubMed] [Google Scholar]
- 21.Dluzniewska J, Sarnowska A, Beresewicz M, Johnson I, Srai SK, Ramesh B, Goldspink G, Gorecki DC, Zablocka B. A strong neuroprotective effect of the autonomous C-terminal peptide of IGF-1 EC (MGF) in brain ischemia. Faseb J. 2005;19:1896–1898. doi: 10.1096/fj.05-3786fje. [DOI] [PubMed] [Google Scholar]
- 22.Burger C, Nash K, Mandel RJ. Recombinant adeno-associated viral vectors in the nervous system. Hum Gene Ther. 2005;16:781–791. doi: 10.1089/hum.2005.16.781. [DOI] [PubMed] [Google Scholar]
- 23.Fraidenraich D, Stillwell E, Romero E, Wilkes D, Manova K, Basson CT, Benezra R. Rescue of cardiac defects in id knockout embryos by injection of embryonic stem cells. Science. 2004;306:247–252. doi: 10.1126/science.1102612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei L, Boyle MP, Yu SP. Roles of VEGF in angiogenesis and functional recovery after a focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2001;21:S315. [Google Scholar]
- 25.Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gertz K, Priller J, Kronenberg G, Fink KB, Winter B, Schrock H, Ji S, Milosevic M, Harms C, Bohm M, Dirnagl U, Laufs U, Endres M. Physical activity improves long-term stroke outcome via endothelial nitric oxide synthase-dependent augmentation of neovascularization and cerebral blood flow. Circ Res. 2006;99:1132–1140. doi: 10.1161/01.RES.0000250175.14861.77. [DOI] [PubMed] [Google Scholar]
- 27.Gross CG. Neurogenesis in the adult brain: death of a dogma. Nat Rev Neurosci. 2000;1:67–73. doi: 10.1038/35036235. [DOI] [PubMed] [Google Scholar]
- 28.Dempsey RJ, Sailor KA, Bowen KK, Tureyen K, Vemuganti R. Stroke-induced progenitor cell proliferation in adult spontaneously hypertensive rat brain: effect of exogenous IGF-1 and GDNF. J Neurochem. 2003;87:586–597. doi: 10.1046/j.1471-4159.2003.02022.x. [DOI] [PubMed] [Google Scholar]
- 29.del Zoppo GJ. Stroke and neurovascular protection. N Engl J Med. 2006;354:553–555. doi: 10.1056/NEJMp058312. [DOI] [PubMed] [Google Scholar]
- 30.Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


