Abstract
The relationship between pretreatment illicit benzodiazepine use (days of use in the last 30) assessed on the Addiction Severity Index (ASI) and treatment outcome was investigated by retrospective analysis of data from two controlled clinical trials in 361 methadone maintained cocaine/opiate users randomly assigned to 12-week voucher- or prize-based contingency management (CM) or control interventions. Based on screening ASI, participants were identified as nonusers (BZD-N; 0 days of use) or users (BZD-U; >0 days of use). Outcome measures were: urine drug screens (thrice weekly); quality of life and self-reported HIV-risk behaviors (every 2 weeks); and current DSM-IV diagnosis of cocaine and heroin dependence (study exit). In the CM group, BZD-U had significantly worse outcomes on in-treatment cocaine use, quality-of-life scores, needle-sharing behaviors, and current heroin dependence diagnoses at study exit compared to BZD-N. In the control group, BZD-U had significantly higher in-treatment cocaine use but did not differ from BZD-N on psychosocial measures. Thus, in a sample of non-dependent BZD users, self-reported illicit BZD use on the ASI, even at low levels, predicted worse outcome on cocaine use and blunted response to CM.
Keywords: contingency management, cocaine abuse, heroin abuse, opiate, benzodiazepine
1. Introduction
Opiate and cocaine dependence are highly responsive to contingency management (CM), a behavioral therapy in which abstinence (usually as measured by drug-negative biological specimens) is reinforced by the delivery of a monetary voucher, an opportunity to draw for a prize, or some other desired item or privilege (Higgins et al., 1991; Silverman et al., 1996; Bigelow et al., 1998; Silverman et al., 1998; Piotrowski et al., 1999; Downey et al., 2000; Higgins et al., 2000; Kellogg et al., 2005). CM has been particularly useful in decreasing the use of cocaine in patients in methadone maintenance (Piotrowski et al., 1999; Silverman et al., 1998; Silverman et al., 2004). Although methadone maintenance is a very effective treatment for opioid dependence, continued use of non-opioid drugs during treatment is a significant problem for many patients (Bleich et al., 2002; Drake et al., 1993; Stitzer et al., 1992). Co-ingestion of benzodiazepines (BZDs) and methadone augments the physiological and subjective opioid effects of methadone (Preston et al., 1984; Lintzeris et al., 2006), and methadone-maintenance patients have high rates of BZD abuse (Stitzer et al., 1981; Darke, 1994; Gossop et al., 2003). BZD use among injecting drug users has been associated with poorer psychosocial functioning, greater levels of polydrug use, and greater likelihood of HIV risk-taking behaviors (Darke et al., 1992; Drake et al., 1993; Darke, 1994; Chutuape et al., 1997; Bleich et al., 1999; Gelkopf et al., 1999; Bleich et al., 2002). However, it is not clear whether BZD use in methadone-maintenance patients affects their response to behavioral treatments designed to reduce use of other illicit drugs, such as CM for cocaine use. We have previously shown that treatment outcome in methadone-maintenance patients is not affected by cannabis use, as measured by the presence of cannabis-positive urine drug screens during treatment, in the absence of symptoms of cannabis dependence (Epstein and Preston, 2003).
In the present study, we evaluated whether treatment outcome was predicted by patients’ illicit benzodiazepine use in the thirty days prior to intake, as reported on the Addiction Severity Index (ASI) (McLellan et al., 1985), a standard assessment instrument widely used in both treatment and research. The following outcome measures were evaluated: (1) rates of cocaine or opiate use during treatment, (2) DSM-IV diagnoses of heroin or cocaine dependence at the end of treatment, (3) quality-of-life measures during treatment, and (4) HIV-risk behaviors during treatment. These outcome measures were assessed with validated instruments (Weissman et al., 1978; Weissman et al., 1981; Goldman et al., 1992). They have been shown to be important indicators of treatment success (Sorensen and Copeland, 2000; Teichner et al., 2001; Hudson et al., 2002; Kampman et al., 2004). To have adequate statistical power, we combined data from two of our clinical trials evaluating the efficacy of CM for heroin/cocaine abuse (Epstein et al., 2003; Ghitza et al., 2007, 2008). Each participant was enrolled in only one of the clinical trials. The first clinical trial used a CM procedure involving vouchers (N=252), and the second clinical trial used a CM procedure involving prize-draws (N=109). The BZD users in these studies were not individuals whose use rose to the level of physical dependence or who were seeking treatment for BZD abuse; such individuals would have been excluded during screening and referred for other treatment. Thus, the question of interest was whether treatment outcome was predicted by self-report of even relatively low levels of illicit BZD use.
2. Methods
2.1 Study Participants
The clinical trials in which we collected our data were approved by the local Institutional Review Board for human research and conducted at an outpatient inner-city treatment research clinic in Baltimore, MD between June, 1999 and August, 2005. Participants were recruited through advertisements in local newspapers and on television stations selected to ensure exposure to both sexes and all ethnicities in order to maximize generalizability (external validity). Participants gave informed written consent prior to participation. Participant screening included: medical, psychiatric, and drug-use histories; physical examination; standard laboratory screens; a battery of assessment instruments, including the ASI and the Diagnostic Interview Schedule (DIS-IV) (Robins, 1995). Eligibility criteria for enrollment in the study were: age 18–65, cocaine and opiate use (by self-report and urine screen), and physical dependence on opiates. Current DSM-IV diagnoses of heroin or cocaine dependence were not required. Exclusion criteria were: current psychotic, bipolar, or major depressive disorders; unstable serious medical illness; estimated IQ below 80 (Shipley Institute of Living Scale) (Zachary, 1986); urologic conditions that precluded urine collection; and current physical dependence on alcohol or sedatives as assessed by DIS-IV responses and clinical judgment (e.g. ability to provide a benzodiazepine-negative urine specimen without evincing signs of withdrawal). This exclusion criterion restricts the current sample to relatively nonproblematic users of benzodiazepines.
For analyses reported here, participants were classified as benzodiazepine users (BZD-U) if they reported at least 1 day of illicit or non-prescribed use in the last 30 days prior to treatment admission on the screening ASI. Participants who did not report BZD use over that time period were classified as benzodiazepine non-users (BZD-N). This nomenclature will be used throughout this paper; note that it refers only to pre-treatment self-reported use and not to in-treatment use of BZDs: not all BZD-U tested positive for BZDs during the study, and some BZD-N tested positive.
2.2 Study Procedure
The study consisted of a 5-week baseline treatment period, a 12-week experimental (CM or control) intervention period, and an 8-week post-intervention period (i.e. a return to baseline conditions). Throughout the 25-week study, all participants received, without charge, daily methadone (70–100 mg/day) and weekly individual counseling and provided urine specimens under observation three times per week, usually Mondays, Wednesdays, and Fridays. Urine drug testing was conducted with an Enzyme Multiplied Immunoassay Technique (EMIT; Syva Corp., Palo Alto, California) system that provided qualitative results for cocaine (benzoylecgonine equivalents; BZE), opiates (morphine), marijuana, and benzodiazepines (oxazepam). Cutoffs were 300 ng/ml for cocaine, opiates, and benzodiazepines, and 50 ng/ml for marijuana. Breath alcohol was determined with an Alco-Sensor III (Intoximeters, Inc., St. Louis, MO).
At the end of a 5-week baseline period, participants whose urine specimens tested positive for heroin and cocaine (not necessarily on the same days) on at least four of 15 occasions were randomized to a contingency management (CM) or control intervention. Randomization was done by a technician who used a Microsoft Excel macro that stratified randomization by race, sex, employment status, probation status, and frequency of opiate- and cocaine-positive urine specimens during baseline. Group assignment in the clinical trials was unequal to maximize statistical power for pairwise comparisons of interest (Woods et al., 1998; Dumville et al., 2006). Because of the nature of the intervention, blinding of these conditions was not possible.
During the 12-week intervention, in addition to the EMIT testing, urine specimens from all participants were tested for the presence of cocaine metabolite (BE) and opiates (morphine) with an onsite dipstick-type drug screen (OnTrak TesTstik, Varian Products) that gave results in less than 15 minutes. All participants were told the results of these tests during the clinic visit. Participants in the CM condition earned either vouchers with monetary value or opportunities to draw for prizes for each negative cocaine screen (N=97). Some participants also received incentives for opioid abstinence (N=165). BZD use had no incentive or consequence contingencies attached to it within the research design or clinic program. Participants in the control condition (N=99) received vouchers or opportunities to draw for prizes independent of urine-test results, i.e., noncontingently, according to a schedule matched to earnings of participants in the CM groups. Prior studies have shown that delivery of noncontingent vouchers does not increase drug use (Schroeder et al., 2003). The voucher procedure was modeled after the method developed by Higgins and colleagues (Higgins et al., 1991; Silverman et al., 1996) Vouchers were given on the day they were earned (CM groups) or scheduled (noncontingent control groups); accrued vouchers were exchanged for goods and services that were consistent with a drug-free lifestyle and patients’ treatment goals, as described previously (Preston et al., 2002). The prize-based-reinforcement schedule was modeled after the method of Petry and Martin (Petry and Martin, 2002). Draws for prizes were made on the day they were earned (CM groups) or scheduled (noncontingent control groups); prizes were available on site for immediate dispensation.
Measures of psychosocial functioning were collected during and at the end of treatment. Quality of life was assessed with the Social Adjustment Scale – Self-Report (SAS-SR) (Weissman and Bothwell, 1976) at baseline and every two weeks throughout treatment. The SAS-SR is a widely used questionnaire with acceptable psychometric properties; it has good test-retest reliability, and its validity has been supported by robust intercorrelations among ratings by participants and interviewers in a wide variety of research and clinical contexts (Weissman et al., 1978; Weissman et al., 1981; Goldman et al., 1992). It measures adjustment and performance over the past 2 weeks in seven major areas of social functioning using seven individual subscales: work, social and leisure activities, relationship with extended family, parental role, marital role as a spouse, membership in the family unit, and financial status (Weissman and Bothwell, 1976). Each of the items on the SAS-SR is rated on a 5-point scale with 1 = no impairment in social functioning and 5 = greatest impairment in social functioning.
DSM-IV diagnoses of heroin or cocaine dependence at study exit were generated using the Substance Dependence Severity Scale (SDSS), a semistructured clinical interview consisting of items keyed to each criterion for DSM-IV dependence and abuse, covering the preceding 30 days (Miele et al., 2000).
Measures of HIV risk were collected every two weeks throughout the study using the HIV Risk-Taking Behaviour Scale (HRTBS) (Darke et al., 1991). Participants completed the HRTBS in written questionnaire form at 2-week intervals, from intake up until week 30. This instrument has been shown to have satisfactory psychometric properties for measuring HIV-risk behaviors in substance abusers (Petry, 2001).
2.3 Data Analysis
Demographic characteristics and intake measures were analyzed by analysis of variance (ANOVA) or Pearson χ2 to test comparability among groups. To address the issue of attrition, study retention as a function of drawing procedure and contingency was analyzed with a log-rank test (SAS LIFETEST procedure) of time until provision of the final urine sample.
Drug use, as measured by urine drug screens (EMIT), was analyzed by random-effects mixed-regression models (SAS GLIMMIX macro). Random-effects mixed-regression models have been widely accepted in the CM literature as appropriate analytical tools for longitudinal data since they were introduced in the late 1980s. They have been shown to compare favorably with traditional repeated-measures approaches (Nich and Carroll, 1997). These likelihood-based models use iterative methods that utilize all of the existing data, both on an individual and on a group level, to estimate treatment outcomes over time. They facilitate intent-to-treat analyses by interpolating missing values (with appropriate penalties reflected in larger standard errors) rather than deleting participants with missing values or coding all missing values identically. They also allow correlations between repeated measurements to be specified; in our case a first-order autoregressive covariance structure was used. This covariance structure allows the correlations of measurements taken further apart to be less than those taken closer to one another, a reasonable assumption for most clinical trials. The repeated outcome measures in our models were: (1) urines negative for opiates over time and (2) urines negative for cocaine over time. The independent variables were use of BZDs reported on screening ASI (yes, no), a covariate for baseline drug use (expressed as the percentage of urine specimens negative for the drug being analyzed, arcsine transformed to correct for heterogeneity of variance (Hogg and Craig, 1995)), treatment phase (Baseline, Intervention, Post-Intervention phases), and a covariate for dropout (number of last urine specimen collected during the study). Baseline was included as a covariate because, although baseline drug use was not significantly different across the groups, earlier work has shown that baseline drug use is a major predictor of treatment response (Preston et al., 1998). The inclusion of a term for dropout was based on the pattern-mixture approach to controlling for the nonrandom nature of missing data—i.e. for the possibility that dropouts differed in some systematic way from study completers (Hedeker and Gibbons, 1997).
Differences between BZD-U and BZD-N in SAS-SR quality-of-life data collected every 2 weeks throughout the study were analyzed in repeated-measures mixed-regression models (SAS Proc Mixed), controlling for each participant’s mean baseline cocaine use and dropout. A first-order autoregressive error structure was used.
Differences in DSM-IV diagnoses of current cocaine dependence or heroin dependence at study exit were tested with 2 multiple logistic regressions (SAS Proc Logistic), one for each DSM-IV diagnosis measure. Each analysis controlled for baseline drug use and dropout.
HIV risk-taking behaviors were assessed at 2-week increments throughout the study using the HRTBS and analyzed in models identical to those described above, using repeated-measures mixed regressions (SAS Proc Mixed). In order to rule out the possibility that decreases in drug-related risk behaviors among groups were solely due to decreases in injection drug use, analyses included only occasions when participants reported having injected in the last 2 weeks. Similarly, analyses of sex-related risk behaviors included only occasions when participants reported sexual activity in the last 2 weeks. Therefore, the question addressed in these analyses was the degree of risk associated with each potentially risky behavior when it occurred at all.
The alpha level for all data analyses was p ≤ 0.05. The analyses reported here were not specified in the original study protocols. However, the specific analytic strategies were chosen a priori to address conceptual questions we had formulated based on published studies of the adverse consequences of benzodiazepine use by patients in methadone maintenance and were not exploratory.
3. Results
3.1 Participant Characteristics and Retention
Among the 361 randomized participants, all were physically dependent on opiates, and 99% met DSM-IV criteria for current heroin dependence; 79% met criteria for current or remitted cocaine dependence, and another 6% met criteria for cocaine abuse. The percentage of participants that met criteria for both cocaine and heroin dependence was 70%. Sixteen percent (N=57) of participants reported using an illicit BZD on at least one day of the previous 30 on the screening ASI. Due to our exclusion criteria, which restricted our sample to BZD users with no signs of physical dependence on BZDs, only 3% of randomized participants met DSM-IV criteria for current or remitted BZD dependence, and another 1% met criteria for BZD abuse. Pearson χ2 and ANOVA analyses revealed that most demographic, ASI, and DIS-IV characteristics at intake did not differ significantly between BZD-U (N=57) and BZD-N (N=304) (Table 1). However, BZD-U were more likely to have used cannabis for a greater number of years than BZD-N and were more likely to be Caucasian. Mixed regression and logistic regression analyses investigating the relationship between cannabis use and the primary or secondary outcome measures revealed no significant relationships (data not shown). Similarly, regression analyses examining the relationship between race and the primary or secondary outcome measures revealed no significant relationships (data not shown). Study retention did not differ between BZD-U and BZD-N (log-rank χ2 =0.74, df=1, p>0.05). BZD-U were equally represented in the CM (15%; 40/262 participants) and the control (17%; 17/99) groups. Demographic characteristics for all 361 randomized participants are shown in Table 1.
Table 1.
Demographic characteristics and retention rates (mean and SD) of all participants and those who reported using benzodiazepines in the 30 days before treatment entry (BZD-U) and those who did not (BZD-N) on the ASI.
| Group of participants | ||||
|---|---|---|---|---|
| Variable | All Participants (N = 361) | BZD-U (N = 57) | BZD-N (N = 304) | p-value |
| Age (Years) | 38.3 (7.5) | 37.2 (8.0) | 38.5 (7.5) | n.s. |
| Sex (% male) | 50.8 | 43.4 | 52.0 | n.s. |
| Race % Caucasian | 43.5 | 61.4 | 39.5 | <0.05 |
| % Non-Caucasian | 56.5 | 38.6 | 60.5 | <0.05 |
| Heroin Use (Years) | 10.1 (7.4) | 8.7 (6.7) | 10.4 (7.5) | n.s. |
| Cocaine Use (Years) | 7.9 (6.5) | 9.6 (7.7) | 7.6 (6.3) | n.s. |
| BZD Use (Years) | 0.2 (0.4) | 1.3 (1.0) | 0.1 (0.4) | <0.05 |
| Cannabis Use (Years) | 3.5 (5.5) | 6.6 (7.0) | 3.0 (5.0) | <0.05 |
| Alcohol Use (Years) | 4.2 (7.3) | 3.9 (7.3) | 4.3 (7.3) | n.s. |
| Days Heroin Use* | 29.4 (3.3) | 29.7 (1.7) | 29.3 (3.6) | n.s. |
| Days Cocaine Use* | 17.6 (9.8) | 18.0 (9.3) | 17.5 (9.9) | n.s. |
| Days Cannabis Use* | 1.7 (4.5) | 4.1 (8.6) | 1.3 (3.1) | <0.05 |
| Days BZD Use* | 0.5 (2.0) | 2.9 (4.4) | 0 (0) | <0.05 |
| Days Alcohol Use* | 6.0 (8.6) | 7.3 (9.7) | 5.9 (8.4) | n.s. |
| Years of Education | 11.5 (1.7) | 11.4 (1.8) | 11.5 (1.7) | n.s. |
| Income Legal ($) | 452.5 (824.9) | 351.4 (651.5) | 471.2 (855.9) | n.s. |
| Income Illegal ($) | 1095.1 (1652.7) | 1143.7 (1686.3) | 1103.3 (1662.3) | n.s. |
| Retention (weeks) | 22.7 (5.7) | 23.0 (5.3) | 22.6 (5.2) | n.s. |
n.s. - not significant (p>0.05)
-number of days used in last 30 before admission
3.2 Drug Use During Treatment
Consistent with their self-reported use of benzodiazepines prior to entering treatment, BZD-U tested positive for benzodiazepines more frequently than BZD-N during treatment (Table 2). The overall percentage of BZD-positive urine specimens was 5% in BZD-N and 15% in BZD-U. GLIMMIX analysis of the CM and control groups combined showed that BZD use during treatment was significantly higher overall in the BZD-U than in the BZD-N (F(1,359) = 20.8, p<0.001). Analyses of the CM and control groups separately showed that BZD use was significantly higher overall in the BZD-U than in the BZD-N in the CM group (F(1,260) = 8.5, p<0.005) and in the control group (F(1,59) = 6.2, p<0.05).
Table 2.
Summary mean percentage of specimens positive for cocaine, opiates and benzodiazepines by participants who reported BZD use (BZD-U) or no BZD use (BZD-N) in the 30 days before treatment entry on the ASI in the CM (N=262) and Control (N=99) groups during the treatment phases.
| BZD User status |
|||||
|---|---|---|---|---|---|
| BZD-U (N=57) | BZD-N (N=304) | ||||
| Variable | Mean | SD | Mean | SD | p-value |
| Contingency Management group: | |||||
| N | 40 | 222 | |||
| Percentage of urine specimens positive for: | |||||
| Cocaine - | |||||
| Study Phase | |||||
| Baseline* | 86.2 | 21.4 | 84.9 | 21.5 | n.s. |
| Intervention* | 73.2 | 34.9 | 62.2 | 41.0 | p<0.05 |
| Post-intervention* | 68.5 | 36.7 | 63.9 | 39.9 | n.s. |
| Opiates - | |||||
| Study Phase | |||||
| Baseline | 82.4 | 23.5 | 80.7 | 23.8 | n.s. |
| Intervention | 59.0 | 36.3 | 54.1 | 39.2 | n.s. |
| Post-intervention | 52.9 | 37.0 | 47.9 | 39.0 | n.s. |
| Benzodiazepines - | |||||
| Study Phase | |||||
| Baseline | 11.2 | 22.6 | 3.9 | 11.1 | p<0.05 |
| Intervention | 14.2 | 24.0 | 5.8 | 16.4 | p<0.05 |
| Post-intervention | 10.5 | 17.1 | 6.6 | 19.4 | n.s. |
| Control group: | |||||
| N | 17 | 82 | |||
| Percentage of urine specimens positive for: | |||||
| Cocaine - | |||||
| Study Phase | |||||
| Baseline | 91.0 | 14.1 | 83.5 | 24.0 | n.s. |
| Intervention | 87.7 | 23.0 | 76.8 | 31.2 | p<0.05 |
| Post-intervention | 85.8 | 27.8 | 74.7 | 36.3 | p<0.05 |
| Opiates - | |||||
| Study Phase | |||||
| Baseline | 83.9 | 21.4 | 78.1 | 25.6 | n.s. |
| Intervention | 61.0 | 30.4 | 57.2 | 35.2 | n.s. |
| Post-intervention | 50.1 | 34.9 | 46.2 | 37.8 | n.s. |
| Benzodiazepines - | |||||
| Study Phase | |||||
| Baseline | 14.5 | 29.6 | 3.6 | 9.4 | p<0.05 |
| Intervention | 21.8 | 32.7 | 3.6 | 7.5 | p<0.05 |
| Post-intervention | 18.4 | 29.8 | 7.0 | 17.3 | p<0.05 |
n.s. - not significant (p>0.05)
Baseline - first 5 weeks of treatment; Intervention - 12 weeks following baseline in which the Abstinence-Reinforcement or control intervention was in place; Post-intervention- 12 weeks following Intervention; daily methadone and weekly counseling were given throughout the entire study.
A GLIMMIX analysis assessing cocaine abstinence revealed a significant interaction between treatment group and BZD group (F(1,314) = 5.19, p<0.05). Results for each group are therefore presented separately. Percentages of cocaine positive urine specimens by group and treatment phase are shown in Table 2.
In the CM group, a GLIMMIX analysis assessing cocaine abstinence showed a significant interaction between BZD group and treatment phase (F(2,330)=3.53, p<0.05). There were no differences in cocaine use during either the Baseline (t=0.39, p>0.05) or Post-Intervention phases (t=−0.73, p>0.05). During the Intervention phase, cocaine use was lower in BZD-N than in BZD-U (t=−2.10, p<0.05), with adjusted percentages and 95% confidence intervals of 79.3% (61.4–90.2%) cocaine-positive urines for BZD-U and 61.7% (54.3–68.7%) for BZD-N.1 (Adjusted percentages from GLIMMIX analyses are not readily interpretable in absolute terms, but are useful for comparing groups while controlling for all covariates in the model.)
In the control group, a GLIMMIX analysis showed significant main effect of BZD use (F(1,57)=5.24, p<0.05) and a non-significant trend for an interaction between BZD group and treatment phase (F(2,102)=2.80, p=0.07). There was no significant difference in cocaine use during baseline (t=−0.70, p>0.05). Cocaine use was lower in BZD-N than in BZD-U during the Intervention (t=−2.28, p<0.05) and Post-Intervention phases (t=−2.78, p<0.01). During Intervention, the adjusted percentages and 95% confidence intervals were 96.5% (89.6–98.9%) cocaine-positive urines for BZD-U and 86.1% (79.1–91.0%) for BZD-N. During Post-Intervention, the adjusted percentages and 95% confidence intervals were 97.7% (90.8–99.4%) for BZD-U and 82.3% (73.6–88.6%) for BZD-N.
BZD-U and BZD-N did not differ in terms of opiate use in either the CM or control groups (Table 2).
3.3 DSM-IV diagnoses of current cocaine or heroin dependence at the end of treatment
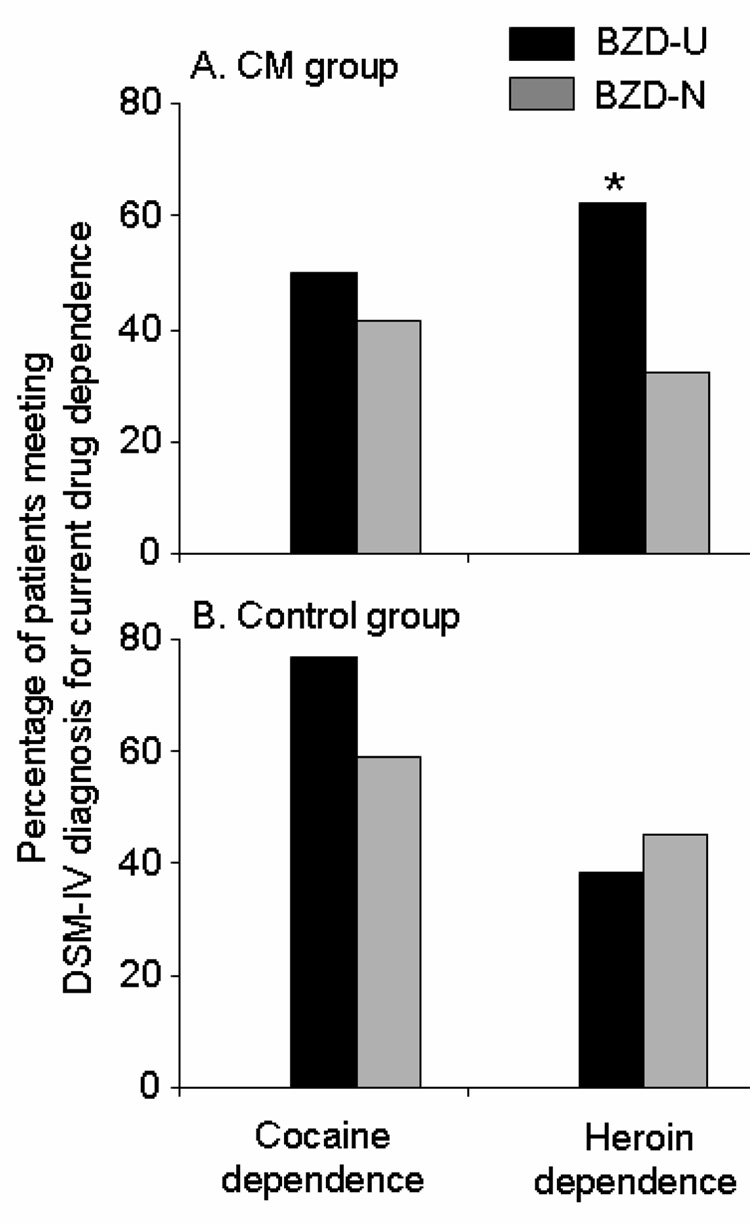
In the CM group at the end of treatment (N=262), BZD-N were significantly more likely than BZD-U to be in remission of DSM-IV diagnoses of heroin dependence (Wald χ2=6.00, p<0.05; adjusted odds ratio=4.01; 95% confidence intervals: 1.32–12.21) (Figure 1). However, there was no relationship between pretreatment BZD use and DSM-IV diagnoses of current cocaine dependence at the end of treatment (Wald χ2=0.86, p>0.05; adjusted odds ratio=1.65; 95% confidence intervals: 0.57–4.79) (Figure 1).
Figure 1.
Percentage of participants meeting DSM-IV diagnosis for heroin dependence or cocaine dependence at study exit by benzodiazepine user and contingency management group; * = p<0.05. BZD-U are participants who reported BZD use in the 30 days prior to treatment on the screening ASI; BZD-N are participants who reported no BZD use in the 30 days prior to treatment on the screening ASI.
In the control group at the end of treatment (N=99), there was no significant relationship between pretreatment BZD use and DSM-IV diagnoses for heroin dependence (Wald χ2=0.23, p>0.05; adjusted odds ratio=0.73; 95% confidence intervals: 0.21–2.60), or cocaine dependence (Wald χ2=0.77, p>0.05; adjusted odds ratio=1.91; 95% confidence intervals: 0.45–8.05) (Figure 1).
3.4 SAS-SR quality-of-life
In the CM group, BZD-N had significantly lower scores than BZD-U on the SAS-SR during treatment (indicating higher quality of life). This was true for scores on the Marital (F(1,182)=4.78, p<0.05), Extended Family (F(1,182)=7.93, p<0.01), and Family Unit (F(1,194)=6.41, p<0.05) subscales as well as on the Overall Adjustment scale (F(1,225)=7.28, p<0.01) of the SAS-SR (Table 3). In contrast, SAS-SR scores during treatment did not differ between BZD-N and BZD-U in the control group (Table 3).
Table 3.
Summary Mean SAS-SR quality-of-life scores during study (scale 1 to 5; 1 = no impairment in social functioning, 5 = greatest impairment in social functioning) by BZD user and contingency management (CM) group
| BZD User status |
|||||
|---|---|---|---|---|---|
| BZD-U (N=57) | BZD-N (N=304) | ||||
| Variable: SAS-SR Scale | Mean | SD | Mean | SD | p-value |
| Contingency Management group: | |||||
| N | 40 | 222 | |||
| Work | 2.23 | 1.03 | 2.13 | 1.08 | n.s. |
| Social and Leisure | 2.61 | 0.64 | 2.42 | 0.71 | n.s. |
| Parental | 1.39 | 0.49 | 1.42 | 0.61 | n.s. |
| Marital | 2.16 | 0.81 | 1.89 | 0.67 | p<0.05 |
| Extended Family | 2.00 | 0.60 | 1.68 | 0.57 | p<0.05 |
| Family Unit | 2.46 | 1.21 | 1.94 | 0.95 | p<0.05 |
| Financial | 2.82 | 1.55 | 2.92 | 1.49 | n.s. |
| Overall Adjustment | 2.25 | 0.49 | 2.02 | 0.52 | p<0.05 |
| Control group: | |||||
| N | 17 | 82 | |||
| Work | 2.42 | 1.17 | 2.27 | 0.12 | n.s. |
| Social and Leisure | 2.40 | 0.56 | 2.41 | 0.08 | n.s. |
| Parental | 1.52 | 0.80 | 1.45 | 0.08 | n.s. |
| Marital | 2.12 | 0.85 | 1.99 | 0.09 | n.s. |
| Extended Family | 1.99 | 0.63 | 1.78 | 0.07 | n.s. |
| Family Unit | 2.34 | 1.19 | 2.35 | 0. | n.s. |
| Financial | 3.33 | 1.30 | 3.26 | 1.37 | n.s. |
| Overall Adjustment | 2.17 | 0.50 | 2.11 | 0.52 | n.s. |
n.s. - not significant (p>0.05)
BZD-U - participants who reported BZD use in the 30 days prior to treatment on the screening ASI
BZD-N - participants who reported no BZD use in the 30 days prior to treatment on the screening ASI
For significant SAS-SR differences between BZD-U and BZD-N in the CM group, we list below the adjusted means and their corresponding 95% confidence intervals from the mixed-regression model, taking into account baseline drug use and dropout. For the Marital Subscale, adjusted means and 95% confidence intervals were 2.22 (1.94–2.49) for BZD-U and 1.88 (1.77–2.00) for BZD-N. For the Extended Family Subscale, adjusted means and 95% confidence intervals were 2.01 (1.80–2.23) for BZD-U and 1.68 (1.59–1.77). For the Family Unit Subscale, adjusted means and 95% confidence intervals were 2.45 (2.09–2.81) for BZD-U and 1.95 (1.81–2.10) for BZD-N. For the Overall Adjustment scale, adjusted means and 95% confidence intervals were 2.28 (2.11–2.46) for BZD-U and 2.02 (1.95–2.09) for BZD-N.
3.5 HIV-risk behaviors
In the CM group, BZD-U were more likely than BZD-N to report the following in the last 2 weeks: 1) using a needle after someone else had already used it, 2) a greater number of people using a needle before they used it, and 3) using a needle and subsequently letting someone else use it (combined score on these three items: F(1,159)=7.29, p<0.01). These items are needle-sharing HIV risk-taking behaviors; the rationale for combining them was that prior studies had shown a greater likelihood of needle sharing among BZD users than among non-users (Bleich et al., 1999; Darke et al., 1993; Darke, 1994). The adjusted means and 95% confidence intervals from the mixed-regression model, after taking into account differences in baseline drug use, in injection drug use (i.e., whether participants were injection drug users) and in dropout, were 3.12 (2.49–3.76) for BZD-U and 2.17 (1.89–2.45) for BZD-N, with higher values indicating more HIV-risk behaviors. (The inclusion of a control term for dropout enabled us to show that BZD use had predictive value beyond that associated with differences in treatment retention.) Statistical analyses of these three individual items showed that BZD-U were more likely than BZD-N to report using a needle after someone else had already used it (F(1,158)=7.51, p<0.01), a greater number of people using a needle before they used it (F(1,155)=4.39, p<0.05), and using a needle and subsequently letting someone else use it (F(1,155)=10.46, p<0.01). BZD-U and BZD-N did not differ on items assessing needle cleaning (combined score on needle-cleaning items: F(1,155)=0.04, p>0.05), nor on items assessing sexual risk behaviors (combined score on sexual-risk items: F(1,225)=0.22, p>0.05).
In the control group, there was no significant difference between BZD-U and BZD-N with respect to the three injection-related risk behaviors that had differed in the CM group (score on these items: F(1,35)=0.13, p>0.05), needle-cleaning items (score on these items: F(1,33)=0.54, p>0.05), or sexual risk behaviors (score on these items: F(1,88)=0.02, p>0.05).
4. Discussion
The present study showed that methadone-maintained outpatients reporting illicit BZD use on the ASI in the 30 days prior to treatment had a greater proportion of BZD-positive urine specimens and a greater proportion of cocaine-positive urine specimens compared to those who reported no illicit BZD use. In addition, among those randomized to a cocaine abstinence-reinforcement contingency, those reporting pre-treatment BZD use were more likely at the end of treatment to meet DSM-IV diagnoses for current heroin dependence and to have worse quality-of-life scores and higher likelihood of engaging in needle sharing during treatment, compared to those who did not report pretreatment BZD use. In contrast, in the control group, BZD non-users performed as poorly as BZD users with respect to psychosocial outcome measures (Figure 1 and Table 3) including HIV-risk taking behaviors. These results pertaining to psychosocial functioning suggest that the benefits of CM are blunted in patients who self-report BZD use during screening.
This is the first study, to our knowledge, showing that reporting use of illicit BZDs on the ASI prior to treatment predicts greater cocaine use and a blunted response to CM, including increased severity of psychosocial problems and more HIV risk behaviors in methadone-maintained outpatients relative to individuals who report no BZD use. This effect was seen in spite of our having enrolled only individuals with relatively modest BZD use and without physical dependence, and it contrasts with our prior finding that cannabis use appeared to have no adverse effect on treatment outcome in general or on response to CM specifically (Epstein and Preston, 2003). We speculate that pretreatment BZD use is related to a blunting of CM, in part, due to BZD users’ increased likelihood of heroin dependence. Dependence severity has been associated with worse treatment outcome (Kampman et al., 2004). Greater baseline drug use has also been shown to be a robust predictor of treatment response (Preston et al., 1998; Stitzer et al., 2007). These results extend previous findings that BZD use among injecting drug users is associated with poorer psychosocial functioning, greater levels of polydrug use, and greater likelihood of HIV risk-taking behaviors (Darke et al., 1992; Drake et al., 1993; Darke, 1994; Chutuape et al., 1997; Bleich et al., 1999; Gelkopf et al., 1999; Bleich et al., 2002). These findings also complement previous work showing that co-use of BZDs and methadone in methadone-maintained outpatients is associated with performance deficits on a variety of measures, relative to methadone alone (Preston et al., 1984; Lintzeris et al., 2006). Not surprisingly, those who reported BZD use on the screening ASI also used more BZDs during treatment. It is not clear whether the poorer treatment response seen in the present study is related to actual BZD use during treatment (there was too little use for statistical power to analyze).
The following are potential limitations of this study. First, there is a possibility of method variance: classification of BZD users and assessment of many of our outcome variables was based on self-report, and some patients may simply have been more prone to disclose information about which they were asked, in this case BZD use and adverse psychosocial outcomes. One mitigating factor is that our findings were not uniform across treatment groups and time, suggesting a limited role of method variance. Another mitigating factor is that self-reported BZD use at intake predicted objective measures of drug use during treatment. Second, participants were not representative of all cocaine- or opiate-dependent individuals because we excluded those with current dependence on other illicit substances (except tobacco dependence). This was done to minimize possible effects of other substance-use disorders on the primary treatment-outcome measures assessed in the two clinical trials. However, there is no reason to believe that the underlying relationships observed in our sample would not also hold for participants with current dependence on substances other than cocaine or opiates. Third, the relationships between illicit BZD use and treatment outcome are correlative, and they do not imply a causal effect of use on outcome. Further work is needed to understand the nature of self-reported BZD use as a marker and to understand the relationship between actual BZD use and treatment outcome.
The clinical significance of the outcome differences in drug use, DSM-IV diagnoses of current heroin dependence, quality of life measures, and HIV-risk taking behaviors warrants comment. The diagnostic criteria for cocaine or heroin dependence in the DSM-IV focus on the adverse consequences of cocaine or heroin use, e.g., disruption of psychosocial functioning and lack of ability to control use in spite of problems (American Psychiatric Association, 1994). Moreover, severity of drug dependence, quality of life, and HIV risk taking behaviors have been shown to have an important impact on the success of treatment interventions for substance abuse patients and on the social adjustment of the patients and their family members (Sorensen and Copeland, 2000; Teichner et al., 2001; Hudson et al., 2002; Kampman et al., 2004). For researchers, our findings suggest that BZD use prior to admission should be considered as a covariate in analyses of treatment outcome in clinical trials. Moreover, our findings may justify the use of BZD use prior to treatment admission as a stratification variable in randomized clinical trials. For clinicians, our findings suggest that BZD users, even those with relatively modest BZD use, represent high-need, treatment-resistant patients. BZD users in methadone maintenance may benefit from more intensive psychosocial interventions as alternatives, supplements, or prerequisites to CM targeted at other drug use. Such interventions could include individualized case management (McLellan et al., 1998; McLellan et al., 1999) and need-service matching (Friedmann et al., 2004; McLellan et al., 1997). The utility of these findings is increased by the fact that they were based on self-reported BZD use collected using the ASI, an assessment tool widely used in both community and research treatment programs. Due to our exclusion criteria, which restricted our sample to BZD users with no signs of physical dependence on BZDs, it is likely that our findings are an underestimate of the problems associated with BZD abuse and dependence in methadone-maintained outpatients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The overlap in confidence intervals is not inconsistent with the significant difference (Cumming and Finch, 2005; Wolfe and Hanley, 2002).
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Bigelow GE, Brooner RK, Silverman K. Competing motivations: drug reinforcement vs non-drug reinforcement. J. Psychopharmacol. 1998;12:8–14. doi: 10.1177/026988119801200102. [DOI] [PubMed] [Google Scholar]
- Bleich A, Gelkopf M, Schmidt V, Hayward R, Bodner G, Adelson M. Correlates of benzodiazepine abuse in methadone maintenance treatment. A 1 year prospective study in an Israeli clinic. Addiction. 1999;94:1533–1540. doi: 10.1046/j.1360-0443.1999.941015339.x. [DOI] [PubMed] [Google Scholar]
- Bleich A, Gelkopf M, Weizman T, Adelson M. Benzodiazepine abuse in a methadone maintenance treatment clinic in Israel: characteristics and a pharmacotherapeutic approach. Isr. J. Psychiatry Relat. Sci. 2002;39:104–112. [PubMed] [Google Scholar]
- Chutuape MA, Brooner RK, Stitzer M. Sedative use disorders in opiate-dependent patients: association with psychiatric and other substance use disorders. J. Nerv. Ment. Dis. 1997;185:289–297. doi: 10.1097/00005053-199705000-00002. [DOI] [PubMed] [Google Scholar]
- Cumming G, Finch S. Inference by eye: Confidence intervals, and how to read pictures of data. Am. Psychol. 2005;60:170–180. doi: 10.1037/0003-066X.60.2.170. [DOI] [PubMed] [Google Scholar]
- Darke S, Hall W, Heather N, Ward J, Wodak A. The reliability and validity of a scale to measure HIV risk-taking behaviour among intravenous drug users. Aids. 1991;5:181–185. doi: 10.1097/00002030-199102000-00008. [DOI] [PubMed] [Google Scholar]
- Darke S, Hall W, Ross M, Wodak A. Benzodiazepine use and HIV risk-taking behaviour among injecting drug users. Drug Alcohol Depend. 1992;31:31–36. doi: 10.1016/0376-8716(92)90005-w. [DOI] [PubMed] [Google Scholar]
- Darke S. Benzodiazepine use among injecting drug users: problems and implications. Addiction. 1994;89:379–382. doi: 10.1111/j.1360-0443.1994.tb00909.x. [DOI] [PubMed] [Google Scholar]
- Downey KK, Helmus TC, Schuster CR. Treatment of heroin-dependent polydrug abusers with contingency management and buprenorphine maintenance. Exp. Clin. Psychopharmacol. 2000;8:176–184. doi: 10.1037//1064-1297.8.2.176. [DOI] [PubMed] [Google Scholar]
- Drake S, Swift W, Hall W, Ross M. Drug use, HIV risk-taking and psychosocial correlates of benzodiazepine use among methadone maintenance clients. Drug Alcohol Depend. 1993;34:67–70. doi: 10.1016/0376-8716(93)90047-t. [DOI] [PubMed] [Google Scholar]
- Dumville JC, Hahn S, Miles JN, Torgerson DJ. The use of unequal randomisation ratios in clinical trials: a review. Contemp. Clin. Trials. 2006;27:1–12. doi: 10.1016/j.cct.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL. Does cannabis use predict poor outcome for heroin-dependent patients on maintenance treatment? Past findings and more evidence against. Addiction. 2003;98:269–279. doi: 10.1046/j.1360-0443.2003.00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann PD, Hendrickson JC, Gerstein DR, Zhang Z. The effect of matching comprehensive services to patients' needs on drug use improvement in addiction treatment. Addiction. 2004;99:962–972. doi: 10.1111/j.1360-0443.2004.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelkopf M, Bleich A, Hayward R, Bodner G, Adelson M. Characteristics of benzodiazepine abuse in methadone maintenance treatment patients: a 1 year prospective study in an Israeli clinic. Drug Alcohol Depend. 1999;55:63–68. doi: 10.1016/s0376-8716(98)00175-6. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Epstein DH, Schmittner J, Vahabzadeh M, Lin JL, Preston KL. Randomized trial of prize-based reinforcement density for simultaneous abstinence from cocaine and heroin. J. Consult. Clin. Psychol. 2007;75:765–774. doi: 10.1037/0022-006X.75.5.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Epstein DH, Preston KL. Contingency management reduces injection-related HIV risk behaviors in heroin and cocaine using outpatients. Addict. Behav. 2008;33:593–604. doi: 10.1016/j.addbeh.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman HH, Skodol AE, Lave TR. Revising axis V for DSM-IV: a review of measures of social functioning. Am. J. Psychiatry. 1992;149:1148–1156. doi: 10.1176/ajp.149.9.1148. [DOI] [PubMed] [Google Scholar]
- Gossop M, Marsden J, Stewart D, Kidd T. The National Treatment Outcome Research Study (NTORS): 4–5 year follow-up results. Addiction. 2003;98:291–303. doi: 10.1046/j.1360-0443.2003.00296.x. [DOI] [PubMed] [Google Scholar]
- Hedeker D, Gibbons RD. Application of random-effects pattern-mixture models for missing data in longitudinal studies. Psychol. Methods. 1997;2:64–78. [Google Scholar]
- Higgins ST, Delaney DD, Budney AJ, Bickel WK, Hughes JR, Foerg F, Fenwick JW. A behavioral approach to achieving initial cocaine abstinence. Am. J. Psychiatry. 1991;148:1218–1224. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Badger GJ, Budney AJ. Initial abstinence and success in achieving longer term cocaine abstinence. Exp. Clin. Psychopharmacol. 2000;8:377–386. doi: 10.1037//1064-1297.8.3.377. [DOI] [PubMed] [Google Scholar]
- Hogg RV, Craig AT. Introduction to Mathematical Statistics. New York: Macmillan; 1995. [Google Scholar]
- Hudson CR, Kirby KC, Firely ML, Festinger DS, Marlowe DB. Social adjustment of family members and significant others (FSOs) of drug users. J. Subst. Abuse Treat. 2002;23:171–181. doi: 10.1016/s0740-5472(02)00245-3. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Pettinati HM, Volpicelli JR, Oslin DM, Lipkin C, Sparkman T, O'Brien CP. Cocaine dependence severity predicts outcome in outpatient detoxification from cocaine and alcohol. Am. J. Addict. 2004;13:74–82. doi: 10.1080/10550490490265389. [DOI] [PubMed] [Google Scholar]
- Kellogg SH, Burns M, Coleman P, Stitzer M, Wale JB, Kreek MJ. Something of value: the introduction of contingency management interventions into the New York City Health and Hospital Addiction Treatment Service. J. Subst. Abuse Treat. 2005;28:57–65. doi: 10.1016/j.jsat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Lintzeris N, Mitchell TB, Bond A, Nestor L, Strang J. Interactions on mixing diazepam with methadone or buprenorphine in maintenance patients. J. Clin. Psychopharmacol. 2006;26:274–283. doi: 10.1097/01.jcp.0000219050.33008.61. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, O'Brien CP. New data from the Addiction Severity Index. Reliability and validity in three centers. J. Nerv. Ment. Dis. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Grissom GR, Zanis D, Randall M, Brill P, O'Brien CP. Problem-service 'matching' in addiction treatment: A prospective study in 4 programs. Arch. Gen. Psychiatry. 1997;54:730–735. doi: 10.1001/archpsyc.1997.01830200062008. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Hagan TA, Levine M, Gould F, Meyers K, Bencivengo M, Durell J. Supplemental social services improve outcome in public addiction treatment. Addiction. 1998;93:1489–1499. doi: 10.1046/j.1360-0443.1998.931014895.x. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Hagan TA, Levine M, Meyers K, Gould F, Bencivengo M, Durell J, Jaffe J. Does clinical case management improve outpatient addiction treatment. Drug Alcohol Depend. 1999;55:91–103. doi: 10.1016/s0376-8716(98)00183-5. [DOI] [PubMed] [Google Scholar]
- Miele GM, Carpenter KM, Smith Cockerham M, Trautman KD, Blaine J, Hasin DS. Substance Dependence Severity Scale (SDSS): reliability and validity of a clinician-administered interview for DSM-IV substance use disorders. Drug Alcohol Depend. 2000;59:63–75. doi: 10.1016/s0376-8716(99)00111-8. [DOI] [PubMed] [Google Scholar]
- Nich C, Carroll K. Now you see it, now you don't: a comparison of traditional versus random-effects regression models in the analysis of longitudinal follow-up data from a clinical trial. J. Consult. Clin. Psychol. 1997;65:252–261. doi: 10.1037//0022-006x.65.2.252. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B. Low-cost contingency management for treating cocaine- and opioid-abusing methadone patients. J. Consult. Clin. Psychol. 2002;70:398–405. doi: 10.1037//0022-006x.70.2.398. [DOI] [PubMed] [Google Scholar]
- Piotrowski NA, Tusel DJ, Sees KL, Reilly PM, Banys P, Meek P, Hall SM. Contingency contracting with monetary reinforcers for abstinence from multiple drugs in a methadone program. Exp. Clin. Psychopharmacol. 1999;7:399–411. doi: 10.1037//1064-1297.7.4.399. [DOI] [PubMed] [Google Scholar]
- Preston KL, Griffiths RR, Stitzer ML, Bigelow GE, Liebson IA. Diazepam and methadone interactions in methadone maintenance. Clin. Pharmacol. Ther. 1984;36:534–541. doi: 10.1038/clpt.1984.215. [DOI] [PubMed] [Google Scholar]
- Preston KL, Silverman K, Higgins ST, Brooner RK, Montoya I, Schuster CR, Cone EJ. Cocaine use early in treatment predicts outcome in a behavioral treatment program. J. Consult. Clin. Psychol. 1998;66:691–696. doi: 10.1037//0022-006x.66.4.691. [DOI] [PubMed] [Google Scholar]
- Preston KL, Umbricht A, Epstein DH. Abstinence reinforcement maintenance contingency and one-year follow-up. Drug Alcohol Depend. 2002;67:125–137. doi: 10.1016/s0376-8716(02)00023-6. [DOI] [PubMed] [Google Scholar]
- Robins LN, Cottler LB, Bucholz KK, Compton WM., III . The Diagnostic Interview Schedule, version IV. St. Louis, MO: Washington University; 1995. [Google Scholar]
- Schroeder JR, Gupman AE, Epstein DH, Umbricht A, Preston KL. Do noncontingent vouchers increase drug use? Exp. Clin. Psychopharmacol. 2003;11:195–201. doi: 10.1037/1064-1297.11.3.195. [DOI] [PubMed] [Google Scholar]
- Silverman K, Wong CJ, Higgins ST, Brooner RK, Montoya ID, Contoreggi C, Umbricht-Schneiter A, Schuster CR, Preston KL. Increasing opiate abstinence through voucher-based reinforcement therapy. Drug Alcohol Depend. 1996;41:157–165. doi: 10.1016/0376-8716(96)01246-x. [DOI] [PubMed] [Google Scholar]
- Silverman K, Wong CJ, Umbricht-Schneiter A, Montoya ID, Schuster CR, Preston KL. Broad beneficial effects of cocaine abstinence reinforcement among methadone patients. J. Consult. Clin. Psychol. 1998;66:811–824. doi: 10.1037//0022-006x.66.5.811. [DOI] [PubMed] [Google Scholar]
- Silverman K, Robles E, Mudric T, Bigelow GE, Stitzer ML. A randomized trial of long-term reinforcement of cocaine abstinence in methadone-maintained patients who inject drugs. J. Consult. Clin. Psychol. 2004;72:839–854. doi: 10.1037/0022-006X.72.5.839. [DOI] [PubMed] [Google Scholar]
- Sorensen JL, Copeland AL. Drug abuse treatment as an HIV prevention strategy: a review. Drug Alcohol Depend. 2000;59:17–31. doi: 10.1016/s0376-8716(99)00104-0. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Griffiths RR, McLellan AT, Grabowski J, Hawthorne JW. Diazepam use among methadone maintenance patients: patterns and dosages. Drug Alcohol Depend. 1981;8:189–199. doi: 10.1016/0376-8716(81)90061-2. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Iguchi MY, Felch LJ. Contingent take-home incentive: effect on drug use of methadone-maintenance patients. J. Consult. Clin. Psychol. 1992;60:927–934. doi: 10.1037//0022-006x.60.6.927. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Petry N, Peirce J, Kirby K, Killeen T, Roll J, Hamilton J, Stabile PQ, Sterling R, Brown C, Kolodner K, Li R. Effectiveness of abstinence-based incentives: interaction with intake stimulant test results. J. Consult. Clin. Psychol. 2007;75:805–811. doi: 10.1037/0022-006X.75.5.805. [DOI] [PubMed] [Google Scholar]
- Teichner G, Horner MD, Harvey RT. Neuropsychological predictors of the attainment of treatment objectives in substance abuse patients. Int. J. Neurosci. 2001;106:253–263. doi: 10.3109/00207450109149753. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Bothwell S. Assessment of social adjustment by patient self-report. Arch. Gen. Psychiatry. 1976;33:1111–1115. doi: 10.1001/archpsyc.1976.01770090101010. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Prusoff BA, Thompson WD, Harding PS, Myers JK. Social adjustment by self-report in a community sample and in psychiatric outpatients. J. Nerv. Ment. Dis. 1978;166:317–326. doi: 10.1097/00005053-197805000-00002. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Sholomskas D, John K. The assessment of social adjustment. An update. Arch. Gen. Psychiatry. 1981;38:1250–1258. doi: 10.1001/archpsyc.1981.01780360066006. [DOI] [PubMed] [Google Scholar]
- Wolfe R, Hanley J. If we're so different, why do we keep overlapping? When 1 plus 1 doesn't make 2. CMAJ. 2002;166:65–66. [PMC free article] [PubMed] [Google Scholar]
- Woods SW, Sholomskas DE, Shear MK, Gorman JM, Barlow DH, Goddard AW, Cohen J. Efficient allocation of patients to treatment cells in clinical trials with more than two treatment conditions. Am. J. Psychiatry. 1998;155:1446–1448. doi: 10.1176/ajp.155.10.1446. [DOI] [PubMed] [Google Scholar]
- Zachary R. Shipley Institute of Living Scale. Revised Manual. Los Angeles: Western Psychological Services; 1986. [Google Scholar]