Abstract
Developmental endothelial locus-1 (Del-1) is a novel angiomatrix protein that has been shown to stimulate a potent angiogenic response and promote functional recovery in hind limb and cardiac ischemia in animal models; however, its impact on cerebral angiogenesis is unknown. In this study, we investigated whether Del-1 overexpression via gene transfer induces cerebral angiogenesis in a murine model, and examined Del-1 expression after ischemic stroke. Cerebral Del1-1 overexpression was achieved with AAV (adeno-associated virus) transduction system via stereotactic injection. Control mice were injected with AAV-lacZ. Del-1gene transduction led to a significant induction of cerebral angiogenesis compared to AAV-lacZ treatment at 4 weeks after gene transfer (Del-1: 97±7 vs lacZ: 64±28, vessels/field, p<0.05). Mice transduced with AAV-Del- 1 showed significantly elevated vascular densities for up to 6 weeks after gene delivery. In addition, double immunofluorescent staining showed co-localization of endothelial cell marker CD31 with BrdU (specific marker for proliferating cells), indicating that Del-1 promoted endogenous endothelial cell proliferation and angiogenesis. Our immunohistochemcial staining also showed that Del-1 expression was markedly upregulated in the peri-infarct area at 3 days after permanent focal cerebral ischemia compared to the sham-operated non-ischemic control. Our data suggest that Del-1 may participate in modulating cerebral angiogenesis, and that gene transfer of Del-1 may provide a novel and potent means for stimulating cerebral angiogenesis.
Keywords: Angiogenesis, Brain, Del-1, Gene transfer, Ischemia
1. Introduction
Angiogenesis, the sprouting of new capillaries from pre-existing vessels, is important in both health and disease (Carmeliet and Jain, 2000; Risau, 1997). Developmental endothelial locus-1 (Del-1) was a recently cloned and characterized unique matrix protein that is expressed by endothelial cells during embryological vascular development (Hidai et al., 1998). Del-1 is a 52-kD protein that contains 3 epidermal growth factor (EGF) repeats, and an arginine-glycine-aspartic acid (RGD) motif. The RGD motif of Del-1 binds αvβ5 integrin, which, in turn, leads to increased angiogenic transcription factor HoxD3 expression. HoxD3 activates αvβ3 and uPA, resulting in the transformation of resting endothelial cells to an angiogenic state (Penta et al., 1999; Rezaee et al., 2002). We have previously reported that HoxD3 overexpression can induce brain angiogenesis (Chen et al., 2004).
Del-1 becomes quiescent at the time of birth, and is no longer expressed in normal adult tissues. It has been found re-expressed in a number of human tumors as well as in ischemic muscles, which may play an important role in adult angiogenesis (Aoka et al., 2002; Ho et al., 2004). In addition to promoting adherence and migration of endothelial cells, Del-1 also acts as an endothelial cell survival agent through upregulating Bcl-2 expression (Pollman et al., 1999). Exogenous application of Del-1 has been demonstrated to augment angiogenesis and improve blood flow and tissue function in murine models of hind-limb ischemia (Ho et al., 2004; Zhong et al., 2003) and cardiac ischemia (Kown et al., 2003). The function of Del-1 in the normal and the ischemic brain has not been documented previously. In this study, we examined whether exogenous application of Del-1 can induce cerebral angiogenesis, and whether Del-1 is expressed after cerebral ischemia.
2. Results
2.1. Construction of AAV expression vectors
Gene transfer has the potential to maintain a sufficient concentration of transduced protein over a long period of time from a single administration (Burger et al., 2005; Monahan and Samulski, 2000). To investigate whether Del-1 might play a role in cerebral angiogenesis, we first developed adeno-associated viral (AAV) vectors with Del-1 or lacZ. AAV vector is a single-stranded DNA virus, and belongs to the nonpathogenic, helper-dependent member of the parvovirus family. It has several advantages over other viral vectors, including low immunogenicity, the ability to mediate long-term transgene, and infect both dividing and non-dividing cells (Zaiss et al., 2002). To construct AAV-Del-1, we inserted the human Del-1 cDNA between two ITRs of pAAV-MC plasmid to generate the pAAV-Del-1. CMV promoter was used to control gene expression in this vector. Fig. 1A shows the structure of the two AAV vectors with Del-1 or lacZ. Fig. 1B shows confirmation by gel electrophoresis of correct Del-1 cDNA inserted into pAAV-CMV after double-enzyme digest. After purification and concentration using CsCl gradient centrifuge, we obtained AAV-Del-1 as high as1013 gene copy determined by dot blot hybridization (Figs. 2A and Fig 2B). AAV-lacZ was generated as a control vector in the same fashion and at the same titer.
Fig. 1. Construction and production of AAV-Del-1.
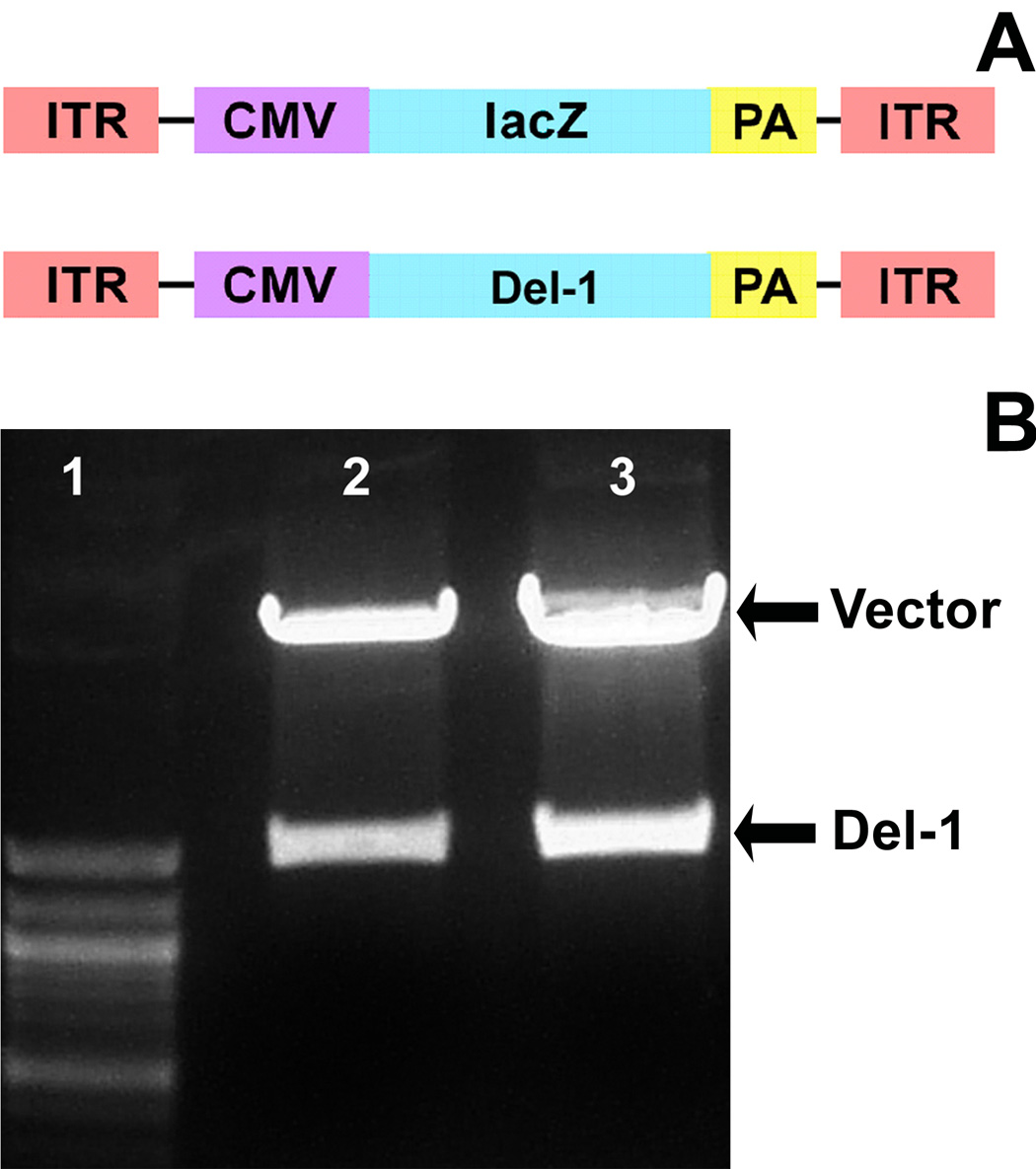
A. Structure of the 2 AAV vectors with Del-1 or lacZ (as a control). CMV promoter was used to control gene expression in this vector. B. The cDNA encoding human Del-1 was PCR-amplified by using the primers 5′ CG GAA TTC ATG AAG CGC TCG GTA GCC GT 3′ and 5′ CCC AAG CTT TC ATT CCT CCT CTG TGC AGC 3′. The fragment was cloned into the plasmid pAAV-MC with EcoR I/Hind III, and tested by double-enzyme cutting and sequencing. Gel image shows electrophoresis of recombinant pAAV-Del-1after EcoR I/Hind III digestion. Lane 1: molecular size marker. Lanes 2 and 3: Del-1 fragment at 1.2k base pairs.
Fig. 2. Dot blot analysis of AAV-Del-1 gene copies.
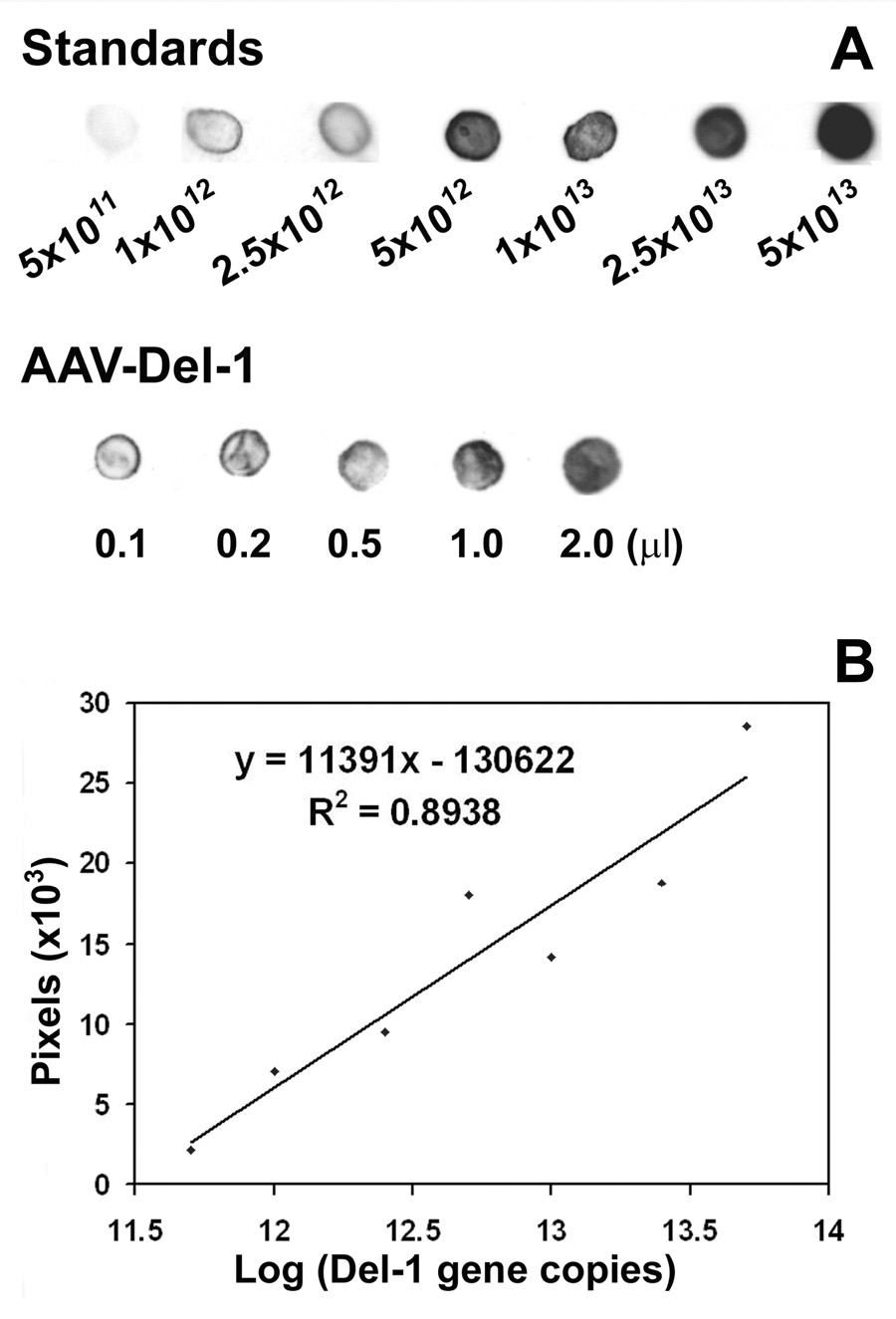
A. Photomicrograph shows AAV-Del-1 titers using dot blot hybridization. Del-1 gene fragment was amplified by PCR and used as standards. Upper panel in A shows the intensities of dot blot increases with the loading doses of the standards. Low panel in A is a representative blot image of AAV-Del-1 with different loading doses. After hybridization and exposure, the real pixels of dots were measured using software Image J. B. Standard curve, X-axis is log scale of gene copy, and Y-axis is linear scale of pixels. We obtained AAV-Del- 1 of 1.4×1013 /ml.
2.2. Del-1 gene transfer induced cerebral angiogenesis
To determine whether Del-1 increases vascular density in the brain, we performed intraparenchymal AAV injection. AAV-Del-1 or AAV-lacZ was stereotactically injected into the left caudate putamen of the mouse brain. The treated mice displayed normal behavior and showed no overt neurological deficits such as paresis or seizure activity for up to 6 weeks after injection of the viral vector. To determine whether intraparenchymal AAV injection leads to successful gene transduction and expression, we first examined the extent of lacZ expression after AAV-lacZ infection. As shown in Figure 3A, a robust lacZ signal was observed adjacent and distal to the injection tract 5 days post injection. LacZ positive staining was absent in the contralateral noninjected hemisphere. To determine whether Del-1 gene transduction induces brain angiogenesis, we assessed microvascular density by counting lectin-positive microvessels. Compared with AAV-lacZ-treated mice, mice transduced with Del-1gene showed increased vascular density at 4 weeks following the injection (Del-1: 97±7 vs lacZ: 64±28, vessels/field, p<0.05; Figure 3B and 3C). Del-1-treated mice showed elevated vascular densities for up to 6 weeks after Del-1 gene delivery (p<0.05). We have observed that the most dramatic increase in vascular density is at proximity of the needle track. The induced vascular density at distant sites from needle track is less pronounced, consistent with our previous reports (Fan et al., 2008; Shen et al., 2006a; Zhu et al., 2008). We have found that the transduced protein is expressed at 3 days, reaching maximal levels at 3 weeks after viral injection (Fan et al., 2008; Shen et al., 2006a; Zhu et al., 2008). AAV gene transfer did not cause neuronal death and inflammation at 6 days after transduction (Shen et al., 2006b, Shen, 2006 #19461). Despite the sustained increase in vascular density, we did not observe any evidence of abnormal vessel morphology for up to 6 weeks following administration of Del-1, using previously published criteria (Xu et al., 2004).
Fig. 3. Del-1 gene transfer enhances vascular density.
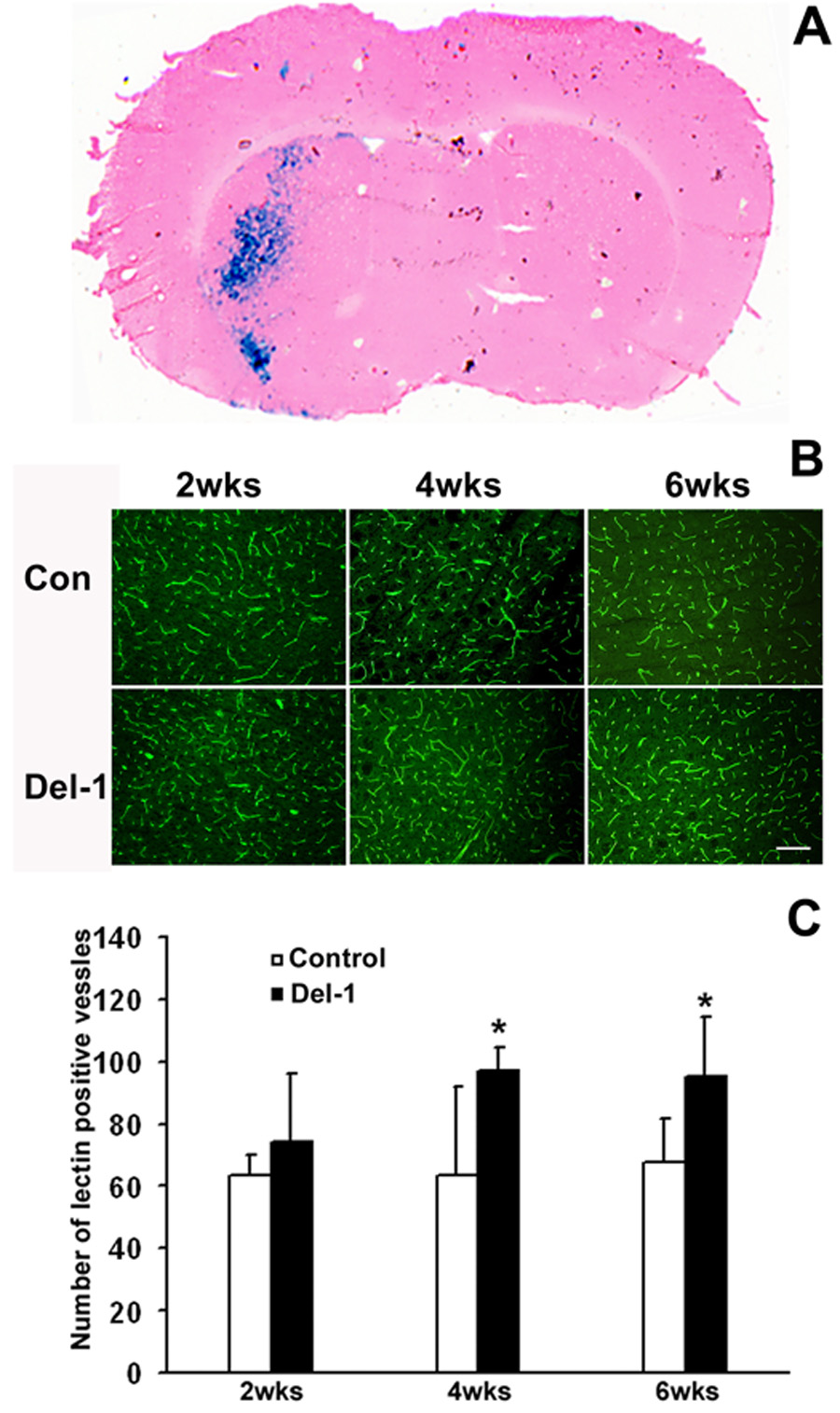
A. Distribution of X-gal positive staining (blue color) at 5 days following the injection of 5.6 × 1010 particles of AAV-lacZ. The brain section was counter-stained with H&E. B. Representative images of immunofluorescent staining of lectin positive blood vessels demonstrate that AAV-Del-1 transduction increased vascular density in the needle tract region compared to AAV-lacZ transduction. Size bar = 50µm. C. Quantification of microvessel numbers. *, p< 0.05 vs AAV-lacZ, n=8 for each group. The data are representative of 3 separate experiments.
To verify that Del-1 treatment enhances endothelial cell proliferation, we performed dual-labeled immunohistochemcal staining using antibodies against the cell proliferation marker BrdU and endothelial cell marker CD31. As shown in Fig. 4A, BrdU positive cells co-localized with CD31 positive cells. Importantly, AAV-Del-1 gene transfer promoted endothelial cell proliferation (1.1 ± 0.3 vs 0.4 ± 0.1, p < 0.05, cells / field, Fig. 4B).
Fig. 4. Del-1 gene transfer enhances endothelial cell proliferation.
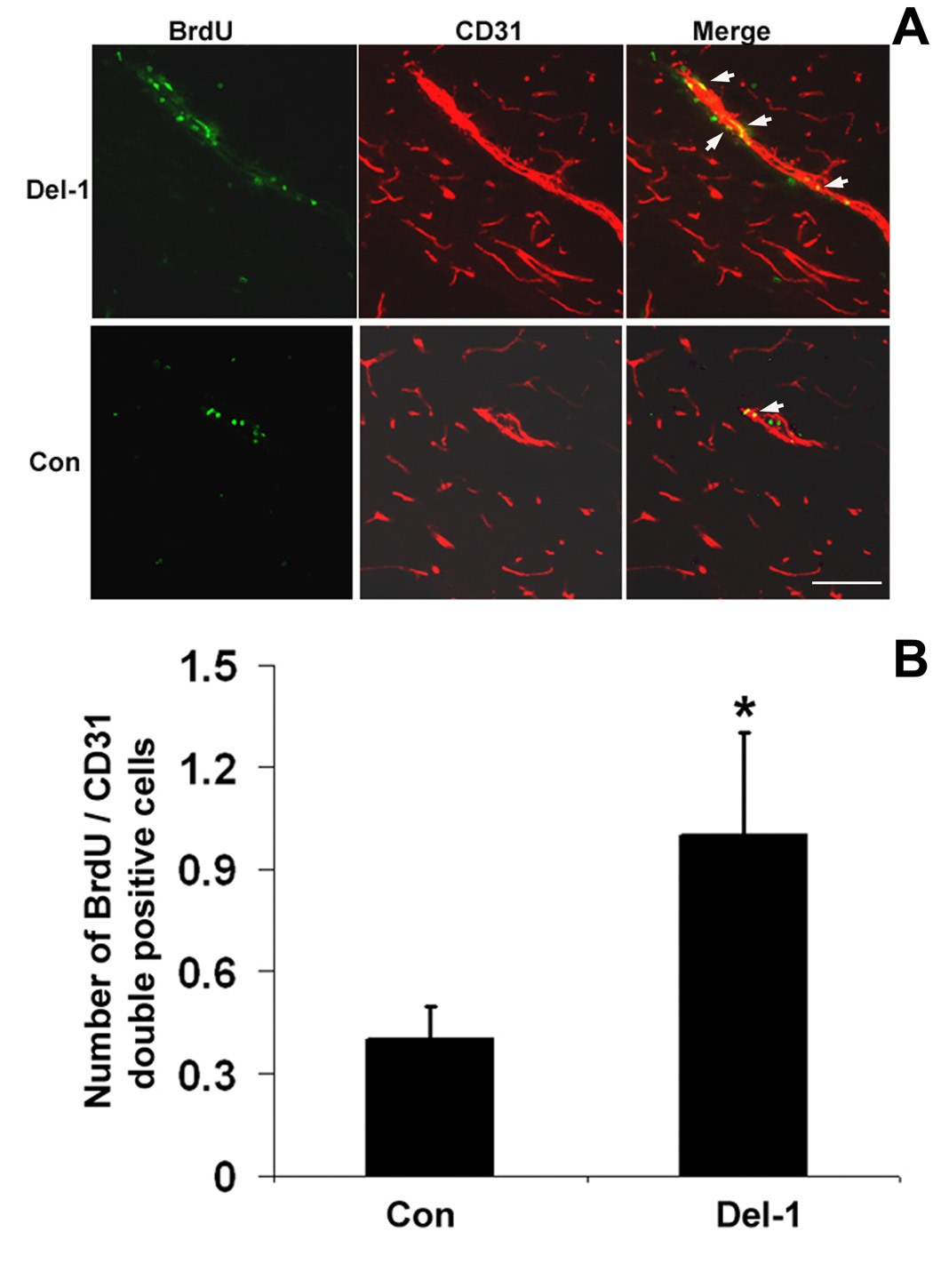
A. Representative images of co-localization of BrdU+ and CD31+ cells showing active endothelial cell proliferation induced by AAV-Del-1 transfection compared to AAV-lacZ treatment. The image is from a section around the needle track. B. Quantification of BrdU and CD31 co-labeled cells. *, p< 0.05 vs AAV-lacZ, n=8 for each group. Scale bar = 50 µm.
2.3. Increased Del-1 expression after ischemic stroke
The expression of many angiogenic growth factors such as VEGF, angiopoietins, and integrins are up-regulated following ischemic stroke (del Zoppo and Mabuchi, 2003; Hayashi et al., 2003). We examined whether Del-1 expression was altered after ischemic stroke by immunohistochemical staining. Cerebral infarctions were confirmed by Nissl staining, and a representative brain coronal section at 3 days after MCAO is shown in Fig. 5. Normal brains stained blue with the Nissl staining method, but the cerebral infarcts in the brains of MCAO mice displayed decreased staining delineating the pale ischemic region. Del-1 expression was strongly increased in the peri-infarct region of the ischemic cortex at 3 days after ischemic stroke (Figs. 5A and 5B). Del-1 signal was weak in the sham-operated non-ischemic hemisphere (Fig. 5C and 5D).
Fig. 5. Increased Del-1 signal after ischemic stroke.
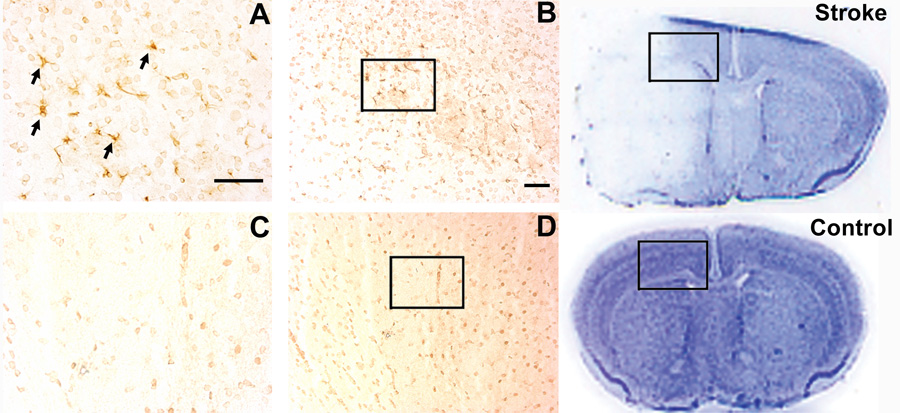
Upper right panel is a representative Nissl-stained brain coronal section showing ischemic infarct at 3 days after MCAO. Lower right panel shows a Nissl-stained normal brain coronal section. Images A and B show representative Del-1 immunostaining at 3 days after ischemic stroke. Arrows indicate positive Del-1 signal. Boxed area indicates location of Del-1 immunostaining in the peri-infarct cortex. Del-1 ποσιτιω̄ε σιγναλσ ωερε νοτ οβσερω̄εδ ιν τηε νον–ισχηεμιχ σηαμ–οπερατεδ μιχε βραινσ (Χ, Δ). Scale bar = 50 µm.
3. Discussion
In the present study, we demonstrated that gene transfer of Del-1 using AAV vector significantly promoted cerebral angiogenesis, and that ischemic stroke dramatically induced Del-1 expression.
Angiogenesis is essential for development, normal growth and repair. It is complete around postnatal day 20 and is suppressed in adult tissues. However, angiogenesis is triggered in many pathological processes, including tumor progression and ischemic diseases (Bikfalvi and Bicknell, 2002; Hayashi et al., 2003; Zhang and Chopp, 2002). The role of angiogenesis in pathophysiology is different in each disorder, and manipulation of new vessel growth can be effective treatments for some diseases. Whereas suppression of angiogenesis can block tumor growth (Bikfalvi and Bicknell, 2002; Folkman, 1995), potentiation of new vessel formation may represent effective means to reduce ischemic injury, as ischemic tissue is dependent upon restoration of microvascular circulation. Brain ischemia induces angiogenesis (Carmeliet, 2003; Hayashi et al., 2003), and the number of new vessels produced in the ischemic penumbra is correlated to decreased morbidity and longer survival in stroke patients (Cramer et al., 1997; Krupinski et al., 1994; Weiller et al., 1993), suggesting that restoration of cerebral microvascular circulation is important for functional recovery following ischemic attack.
The formation of a functioning vasculature requires the orchestrated interaction of endothelial cells, extracellular matrix and surrounding cells, which are coordinately regulated by multiple angiogenic and angiostatic factors. Regulation of these factors in both temporal and spatial manners is fundamental for efficient angiogenesis, and different biological activities are required in the different phases of neovascularization from initiation to maturation (Epstein et al., 2001; Ferrara and Alitalo, 1999). Following ischemic stroke, the expression of many angiogenic growth factors such as VEGF, angiopoietins and integrins are up-regulated, possibly playing a role in ischemic-induced angiogenesis (del Zoppo and Mabuchi, 2003; Hayashi et al., 2003). Our data show that Del-1 expression is low in normal brain tissue and is enhanced after cerebral ischemia, suggesting that Del-1 may participate in ischemia-induced angiogenesis. The positive cells are of glia morphology. We speculate that glia cells participate in the angiogenic process after ischemic stroke by secreting angiogenic growth factors such as VEGF and integrin. In line with our findings, Hanayama et al have reported that Del-1 expression is induced in macrophages during apoptosis (Hanayama et al., 2004). Endogenous angiogenesis may not be sufficient to compensate for the hypoperfusion state. Therefore, potentiation of the functional vascularization is necessary to enhance long-term survival of the penumbra. Experimental and clinical studies have provided evidence that therapeutically-induced angiogenesis can reduce ischemic injury in endangered territories of the peripheral and cardiovascular system (Arras et al., 1998; Cao et al., 2003; Chen et al., 2003; Simons and Ware, 2003).
Therapeutic angiogenesis represents a novel approach to rescue ischemic injured brain tissue. Among the many known classes of angiogenic growth factors, VEGF is a prototypical angiogenic growth factor, and is unregulated in the ischemic brain after stroke (Beck et al., 2000; Hayashi et al., 1997). VEGF delivery has been reported to enhance vascular density and improve cerebral blood flow in ischemic brain regions (Jin et al., 2000; Marti, 2002; Sun et al., 2003). The potential therapeutic value of VEGF, however, is compromised by associated detrimental effects such as vascular leakage and edema (Thurston et al., 1999; Zhang et al., 2000). We demonstrate in this study that Del-1 overexpression, via AAV-mediated gene delivery, potently enhances cerebral angiogenesis. Del-1 is a unique angiogenic factor that possesses a distinct mechanism of action from other known angiogenic factors such as VEGF and FGF. Del-1 stimulates a potent angiogenic response through activation of integrin signaling cascade (Boudreau et al., 1997; Gorski and Walsh, 2000). Del-1 binds to integrin αvβ5 on resting endothelial cells, leading to the activation of transcription factor HoxD3. HoxD3 then induces the expression of proangiogenic factors such as integrin αvβ3, and proteinase uPA/uPAR, thereby resulting in angiogenesis. αVβ3 is a heterodimeric receptor consisting of αV and β3 subunits that bind to a variety of extracellular matrix proteins, including vitronectin, tenascin and fibrinogen. This integrin promotes angiogenesis by regulating cell adhesion and migration (Carmeliet, 2002; Travis et al., 2003). Although αVβ3 expression is low in resting vessels, its expression is rapidly induced following exposure to angiogenic cytokines that include Del-1 (Brooks et al., 1994; Penta et al., 1999; Sepp et al., 1994).
Our study provides the first evidence that Del-1 overexpression via gene transfer may yield a means to induce vascular density in the adult brain. In this study, we assessed vascular density by counting the number of lectin-positive vessels. It would be ideal to employ detailed methods to characterize the neovessel formations, such as the number of vascular branch, the diameter and length of capillary, or the capillary area measure (Chen et al., 2003). Also, the number of vessels in 2-D plane varies according to the direction of the section plane. Nevertheless, the counting error caused by this phenomenon should be distributed equally among different groups. We believe it would not significantly impact our vascular measurement and conclusion. Future study is needed to examine whether Del-1 gene transduction-mediated neovascularization promotes long-term neurological functional recovery after ischemic stroke.
4. Experimental Procedure
4.1. AAV vector construction and production
AAV vectors were generated according to a previous method with minor modifications (Su et al., 2000). We first inserted the human Del-1 cDNA (Origene, Rockville, MD) between two ITRs of pAAV-MC plasmid (Strategene, La Jolla, CA) to generate the pAAV-Del-1. CMV promoter was used to control gene expression in this vector (Fig. 1A). The recombinant plasmid pAAV-Del-1 was co-transfected with 2 helper plasmids into HEK293 cells by the calcium phosphate precipitation method. One helper plasmid, pHelper, contains adenoviral VA, E2A, and E4 regions, which mediate AAV vector replication. The other, pAAV-RC, has rep gene and cap gene of AAV serotype 1. Cell lysates were produced using 3 freeze-thaw cycles 3 days after gene transfection. AAV vectors were purified by CsCl density gradient centrifugation. Viral titers were determined by dot blot analysis of the DNA content. AAV-lacZ was prepared as a control.
4.2. Adeno-associated viral vector delivery
The study was approved by the University of California, San Francisco Committee of Animal Research, and conformed to the NIH Guidelines for use of animals in research. Viral transduction was performed as previously described (Zhu et al., 2008). Adult CD-1 mice weighing 30–35 g were placed in a stereotactic frame (Kopf, Tujunga, CA) under anesthesia, and a burr hole was drilled 2.5 mm lateral to the sagittal suture and 1 mm posterior to the coronal suture. A 10µl Hamilton syringe was slowly inserted into the left caudate nucleus (3.0 mm deep from the dura). Four µl viral suspension (AAV-Del-1 or AAV-lacZ) containing 5.6 × 1010 particles were injected into the left hemisphere.
4.3. Immunohistochemistry
Immunohistochemical staining was performed as previously described. (Chen et al., 2006) Sections were incubated with primary antibodies at the following concentrations: mouse anti-Del-1, 1:200 (a gift from Valentis Inc., Burlingame CA); rat anti-CD31, 1:200 (BD bioscience); mouse anti-BrdU, 1:1000 (Sigma). After incubating at 4°C overnight and washing, the sections were incubated with biotinylated secondary antibody (Vector Laboratories) at 1:5000 dilution. The sections were treated with ABC streptavidin detection system. For dual fluorescent staining, after incubating with primary antibodies, sections were incubated with Alexa Fluor 594-conjugated or Alexa Fluor 488-conjugated IgG (Molecular probes) at 1:500 dilution. Negative controls were performed by omitting the primary antibodies during the immunostaining.
4.4. Vascular density assessment
Microvessel counting was performed as previously described (Shen et al., 2006a). Brain sections were stained with fluorescein conjugated lectin (Vector laboratories, Burlingame, CA). Lectin is a commonly used marker to label blood vessels. Six brain coronal sections from the lectin-stained brain sections, 1, 1.5, 2 mm anterior and 1, 1.5, 2 mm posterior from the needle track, were chosen. Three areas of microvessels, immediately to the left, right, and bottom of the needle track, were photographed using a 10x objective. Microvessel counting was performed on these photographs. Vessels with a diameter between 3 and 8 µM were counted. The number of microvessels was calculated as the mean of the vascular counts obtained from 3 pictures, and the counting was conducted in a blinded fashion.
4.5. 5-bromo-2-deoxyuridine-5-monophosphate (BrdU) labeling
BrdU, a thymidine analogue incorporated into the DNA of dividing cells, was used to label proliferating cells. Before sacrifice, mice were i.p. injected twice daily with BrdU (50mg/kg, Sigma) for 7 consecutive days.
4.6. Animal stroke model
The study was approved by the University of California, San Francisco Committee of Animal Research and conformed to NIH Guidelines for use of animals in research. Adult male CD-1 mice (30–35g) were subjected to permanent focal ischemia by intraluminal middle cerebral artery blockade (Zhu et al., 2008). Surface cerebral blood flow (sCBF) was monitored during MCAO using laser Doppler flowmetry (Vasamedics). Mice were excluded from the experiment if sCBF in the ischemic core region was more than 15% of the baseline. Stroke mice and sham-operated control mice were sacrificed at 3 days after MCAO, and serial coronal sections (200 µm apart) were stained with cresyl violet for assessing brain infarction (Shen et al., 2006a).
4.7. Statistical analysis
All data are presented as mean ± SD. Parametric data among different groups were analyzed using a one-way ANOVA followed by Fisher’s PLSD Test. A probability value of less than 5% was considered to be statistically significant.
Acknowledgments
This work was supported by NIH grants R21 NS053943 (YC), R01 NS27713 (WLY) and P01 NS44145 (WLY, GYY). The authors thank Dr. Xiaozhu Huang for her advice regarding the study, Voltaire Gungab for editorial assistance, and the staff of the Center for Cerebrovascular Research (http://avm.ucsf.edu/) for their collaborative support.
Abbreviations
- AAV
adeno-associated virus
- AAV-Del-1
adeno-associated viral Del-1 vector
- Del-1
developmental endothelial locus-1
- MCAO
middle cerebral artery occlusion
- sCBF
surface cerebral blood flow
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoka Y, Johnson FL, Penta K, Hirata Ki K, Hidai C, Schatzman R, Varner JA, Quertermous T. The embryonic angiogenic factor Del1 accelerates tumor growth by enhancing vascular formation. Microvasc Res. 2002;64:148–161. doi: 10.1006/mvre.2002.2414. [DOI] [PubMed] [Google Scholar]
- Arras M, Mollnau H, Strasser R, Wenz R, Ito WD, Schaper J, Schaper W. The delivery of angiogenic factors to the heart by microsphere therapy. Nat Biotechnol. 1998;16:159–162. doi: 10.1038/nbt0298-159. [DOI] [PubMed] [Google Scholar]
- Beck H, Acker T, Wiessner C, Allegrini PR, Plate KH. Expression of angiopoietin-1, angiopoietin-2, and tie receptors after middle cerebral artery occlusion in the Rat. Am J Pathol. 2000;157:1473–1483. doi: 10.1016/S0002-9440(10)64786-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikfalvi A, Bicknell R. Recent advances in angiogenesis, anti-angiogenesis and vascular targeting. Trends Pharmacol Sci. 2002;23:576–582. doi: 10.1016/s0165-6147(02)02109-0. [DOI] [PubMed] [Google Scholar]
- Boudreau NJ, Andrews C, Srebrow A, Ravanpay A, Cheresh DA. Induction of the angiogenic phenotype by Hox D3. J Cell Biol. 1997;139:257–264. doi: 10.1083/jcb.139.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Burger C, Nash K, Mandel RJ. Recombinant adeno-associated viral vectors in the nervous system. Hum Gene Ther. 2005;16:781–791. doi: 10.1089/hum.2005.16.781. [DOI] [PubMed] [Google Scholar]
- Cao R, Brakenhielm E, Pawliuk R, Wariaro D, Post MJ, Wahlberg E, Leboulch P, Cao Y. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med. 2003;9:604–613. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Integrin indecision. Nat Med. 2002;8:14–16. doi: 10.1038/nm0102-14. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, Lu M, Zhu Z, Chopp M. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003;92:692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- Chen Y, Xu B, Arderiu G, Hashimoto T, Young WL, Boudreau NJ, Yang GY. Retroviral delivery of homeobox d3 gene induces cerebral angiogenesis in mice. J Cereb Blood Flow Metab. 2004;24:1280–1287. doi: 10.1097/01.WCB.0000141770.09022.AB. [DOI] [PubMed] [Google Scholar]
- Chen Y, Pawlikowska L, Yao JS, Shen F, Zhai W, Achrol AS, Lawton MT, Kwok PY, Yang GY, Young WL. Interleukin-6 involvement in brain arteriovenous malformations. Ann Neurol. 2006;59:72–80. doi: 10.1002/ana.20697. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Nelles G, Benson RR, Kaplan JD, Parker RA, Kwong KK, Kennedy DN, Finklestein SP, Rosen BR. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke. 1997;28:2518–2527. doi: 10.1161/01.str.28.12.2518. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003;23:879–894. doi: 10.1097/01.WCB.0000078322.96027.78. [DOI] [PubMed] [Google Scholar]
- Epstein SE, Kornowski R, Fuchs S, Dvorak HF. Angiogenesis therapy: amidst the hype, the neglected potential for serious side effects. Circulation. 2001;104:115–119. doi: 10.1161/01.cir.104.1.115. [DOI] [PubMed] [Google Scholar]
- Fan Y, Shen F, Chen Y, Liu W, Su H, Young WL, Yang GY. Overexpression of Netrin-1 induces neovascularization in the adult mouse brain. J Cereb Blood Flow Metab. 2008 doi: 10.1038/jcbfm.2008.39. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat Med. 1999;5:1359–1364. doi: 10.1038/70928. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- Gorski DH, Walsh K. The role of homeobox genes in vascular remodeling and angiogenesis. Circ Res. 2000;87:865–872. doi: 10.1161/01.res.87.10.865. [DOI] [PubMed] [Google Scholar]
- Hanayama R, Tanaka M, Miwa K, Nagata S. Expression of developmental endothelial locus-1 in a subset of macrophages for engulfment of apoptotic cells. J Immunol. 2004;172:3876–3882. doi: 10.4049/jimmunol.172.6.3876. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Abe K, Suzuki H, Itoyama Y. Rapid induction of vascular endothelial growth factor gene expression after transient middle cerebral artery occlusion in rats. Stroke. 1997;28:2039–2044. doi: 10.1161/01.str.28.10.2039. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Noshita N, Sugawara T, Chan PH. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab. 2003;23:166–180. doi: 10.1097/01.WCB.0000041283.53351.CB. [DOI] [PubMed] [Google Scholar]
- Hidai C, Zupancic T, Penta K, Mikhail A, Kawana M, Quertermous EE, Aoka Y, Fukagawa M, Matsui Y, Platika D, Auerbach R, Hogan BL, Snodgrass R, Quertermous T. Cloning and characterization of developmental endothelial locus-1: an embryonic endothelial cell protein that binds the alphavbeta3 integrin receptor. Genes Dev. 1998;12:21–33. doi: 10.1101/gad.12.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho HK, Jang JJ, Kaji S, Spektor G, Fong A, Yang P, Hu BS, Schatzman R, Quertermous T, Cooke JP. Developmental endothelial locus-1 (Del-1), a novel angiogenic protein: its role in ischemia. Circulation. 2004;109:1314–1319. doi: 10.1161/01.CIR.0000118465.36018.2D. [DOI] [PubMed] [Google Scholar]
- Jin KL, Mao XO, Greenberg DA. Vascular endothelial growth factor: direct neuroprotective effect in in vitro ischemia. Proc Natl Acad Sci U S A. 2000;97:10242–10247. doi: 10.1073/pnas.97.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kown MH, Suzuki T, Koransky ML, Penta K, Sakamoto G, Jahncke CL, Carter AJ, Quertermous T, Robbins RC. Comparison of developmental endothelial locus-1 angiogenic factor with vascular endothelial growth factor in a porcine model of cardiac ischemia. Ann Thorac Surg. 2003;76:1246–1251. doi: 10.1016/s0003-4975(03)00721-5. [DOI] [PubMed] [Google Scholar]
- Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794–1798. doi: 10.1161/01.str.25.9.1794. [DOI] [PubMed] [Google Scholar]
- Marti HH. Vascular endothelial growth factor. Adv Exp Med Biol. 2002;513:375–394. doi: 10.1007/978-1-4615-0123-7_14. [DOI] [PubMed] [Google Scholar]
- Monahan PE, Samulski RJ. Adeno-associated virus vectors for gene therapy: more pros than cons? Mol Med Today. 2000;6:433–440. doi: 10.1016/s1357-4310(00)01810-4. [DOI] [PubMed] [Google Scholar]
- Penta K, Varner JA, Liaw L, Hidai C, Schatzman R, Quertermous T. Del1 induces integrin signaling and angiogenesis by ligation of alphaVbeta3. J Biol Chem. 1999;274:11101–11109. doi: 10.1074/jbc.274.16.11101. [DOI] [PubMed] [Google Scholar]
- Pollman MJ, Naumovski L, Gibbons GH. Endothelial cell apoptosis in capillary network remodeling. J Cell Physiol. 1999;178:359–370. doi: 10.1002/(SICI)1097-4652(199903)178:3<359::AID-JCP10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Rezaee M, Penta K, Quertermous T. Del1 mediates VSMC adhesion, migration, and proliferation through interaction with integrin alpha(v)beta(3) Am J Physiol Heart Circ Physiol. 2002;282:H1924–H1932. doi: 10.1152/ajpheart.00921.2001. [DOI] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Sepp NT, Li LJ, Lee KH, Brown EJ, Caughman SW, Lawley TJ, Swerlick RA. Basic fibroblast growth factor increases expression of the alpha v beta 3 integrin complex on human microvascular endothelial cells. J Invest Dermatol. 1994;103:295–299. doi: 10.1111/1523-1747.ep12394617. [DOI] [PubMed] [Google Scholar]
- Shen F, Su H, Fan Y, Chen Y, Zhu Y, Liu W, Young WL, Yang GY. Adeno-associated viral vector-mediated hypoxia-inducible vascular endothelial growth factor gene expression attenuates ischemic brain injury after focal cerebral ischemia in mice. Stroke. 2006a;37:2601–2606. doi: 10.1161/01.STR.0000240407.14765.e8. [DOI] [PubMed] [Google Scholar]
- Shen F, Su H, Liu W, Kan YW, Young WL, Yang GY. Recombinant adeno-associated viral vector encoding human VEGF165 induces neomicrovessel formation in the adult mouse brain. Front Biosci. 2006b;11:3190–3198. doi: 10.2741/2042. [DOI] [PubMed] [Google Scholar]
- Simons M, Ware JA. Therapeutic angiogenesis in cardiovascular disease. Nat Rev Drug Discov. 2003;2:863–871. doi: 10.1038/nrd1226. [DOI] [PubMed] [Google Scholar]
- Su H, Lu R, Kan YW. Adeno-associated viral vector-mediated vascular endothelial growth factor gene transfer induces neovascular formation in ischemic heart. Proc Natl Acad Sci U S A. 2000;97:13801–13806. doi: 10.1073/pnas.250488097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- Travis MA, Humphries JD, Humphries MJ. An unraveling tale of how integrins are activated from within. Trends Pharmacol Sci. 2003;24:192–197. doi: 10.1016/S0165-6147(03)00069-5. [DOI] [PubMed] [Google Scholar]
- Weiller C, Ramsay SC, Wise RJ, Friston KJ, Frackowiak RS. Individual patterns of functional reorganization in the human cerebral cortex after capsular infarction. Ann Neurol. 1993;33:181–189. doi: 10.1002/ana.410330208. [DOI] [PubMed] [Google Scholar]
- Xu B, Wu YQ, Huey M, Arthur HM, Marchuk DA, Hashimoto T, Young WL, Yang GY. Vascular endothelial growth factor induces abnormal microvasculature in the endoglin heterozygous mouse brain. J Cereb Blood Flow Metab. 2004;24:237–244. doi: 10.1097/01.WCB.0000107730.66603.51. [DOI] [PubMed] [Google Scholar]
- Zaiss AK, Liu Q, Bowen GP, Wong NC, Bartlett JS, Muruve DA. Differential activation of innate immune responses by adenovirus and adeno-associated virus vectors. J Virol. 2002;76:4580–4590. doi: 10.1128/JVI.76.9.4580-4590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Chopp M. Vascular endothelial growth factor and angiopoietins in focal cerebral ischemia. Trends Cardiovasc Med. 2002;12:62–66. doi: 10.1016/s1050-1738(01)00149-9. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen N, Chopp M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Eliceiri B, Stupack D, Penta K, Sakamoto G, Quertermous T, Coleman M, Boudreau NJ, Varner JA. Neovascularization of ischemic tissues by gene delivery of the extracellular matrix protein Del-1. J Clin Invest. 2003;112:30–41. doi: 10.1172/JCI17034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Fan Y, Frenzel T, Gasmi M, Bartus RT, Young WL, Yang GY, Chen Y. Insulin growth factor-1 gene transfer enhances neurovascular remodeling and improves long-term stroke outcome in mice. Stroke. 2008;39:1254–1261. doi: 10.1161/STROKEAHA.107.500801. [DOI] [PMC free article] [PubMed] [Google Scholar]


