Abstract
Vaccinia virus has recently been used as an expression vector for gene delivery and an oncolytic agent for cancer therapy. Although it has been established that interferon-induced double-stranded RNA (dsRNA)-activated protein kinase (PKR) and RNase L interfere with viral replication, little else is known about the other host factors that might affect viral replication and virus-mediated host cell killing. In this study, we evaluated the roles of c-Jun NH2-terminal kinase (JNK) in oncolytic vaccinia virus replication and vaccinia virus-mediated host cell killing. We found that JNK knockout mouse embryonic fibroblasts (MEFs) were more susceptible to oncolytic vaccinia virus infection than wild-type MEFs. Moreover, viral replication and the production of infectious viral progeny were up to 100 -fold greater in JNK-deficient MEFs than in wild-type MEFs. A similar result was observed for wild-type vaccinia virus. The increased killing of infected cells and the production of viral progeny was also observed in wild-type MEFs that had been treated with JNK inhibitors and in human colon cancer cells that had been transfected with dominant-negative JNK constructs. Moreover, testing on several human lung cancer cell lines and HeLa cells showed an inverse correlation between levels of JNK expression and susceptibility to oncolytic vaccinia virus. Our study also revealed that oncolytic virus infection-mediated PKR activation was blocked or diminished in JNK-deficient MEFs. The adenovirus-mediated ectopic expression of human PKR in JNK-deficient MEFs reduced vaccinia virus replication to the levels observed in wild-type MEFs, indicating that JNK is required for vaccinia virus to efficiently activate PKR. Our results demonstrated that the cellular status of JNK function can dramatically affect oncolytic vaccinia virus replication and vaccinia virus-mediated host cell killing. This finding may enable further improvements in oncolytic virotherapy using vaccinia virus.
Introduction
Unlike other DNA viruses, which replicate in the nuclei of infected cells, Orthopoxvirus vaccinia virus, which has been used as a smallpox vaccine for more than 150 years, replicates in the cytoplasm. This characteristic is important, because it will never integrate into host genome. For this reason, it has been used as an expression vector in the development of vaccines against various cancers and infectious diseases 1-3 , for gene delivery and therapy 2,4 , and for oncolytic virotherapy in cancer 5,6. The advantages of using vaccinia virus in particular as an expression vector or as an oncolytic virotherapy agent include its short life cycle, rapid cell-to-cell spread, strong lytic activity, large capacity to accommodate foreign DNA fragments, and well-defined molecular biology 6. In addition, vaccinia virus can replicate effectively in host cells derived from a wide range of species, including both mice and humans, whereas human adenoviruses can replicate only in cells from humans and a few other species.
Evidence has established that vaccinia virus requires the participation of host cell transcriptional apparatus to replicate 7 and that interferons can suppress replication by activating downstream enzymes, such as double-stranded RNA-activated protein kinase (PKR) 8, nitric oxide synthase 9,10, and RNase L 11. However, the roles of other signaling pathways in vaccinia virus replication and other host factors that may determine susceptibility to the virus have not been well-characterized.
One potentially important focus of attention in vaccinia virus replication is the c-Jun NH2-terminal kinase (JNK) signaling cascade, which can be activated by viral infection and has been implicated in the replication of herpesviruses 12 and rotaviruses 13. JNK activation has been observed to enhance 14,15 or inhibit 16 viral replication and is a required step in the apoptosis induced by certain chemotherapeutic agents 17,18 and stress stimuli 19-22. Thus, it has the potential to either promote or interfere with viral replication.
To elucidate JNK’s role in oncolytic vaccinia virus replication, we compared viral replication and viral-induced apoptosis in wild-type mouse embryonic fibroblasts (MEFs) with those in JNK1- and JNK2-knockout MEFs. We found that knocking out either JNK1 or JNK2 can result in dramatically increased vaccinia virus replication and that the role of JNK in vaccinia virus replication is to activate double-stranded RNA-dependent protein kinase (PKR). We also compared the replication of oncolytic vaccinia virus in human colon cancer cells that did and did not express the dominant-negative JNK, which showed that replication increased in cells that expressed dominant-negative JNK. Our results suggest that JNK plays an important role in controlling vaccinia virus replication through PKR pathway activation.
MATERIALS AND METHODS
Cell lines and cell culture
MEFs, JNK1-knockout MEFs (MEF/JNK1-/-), and JNK2-knockout MEFs (MEF/JNK2-/-) were obtained from Dr. Anning Lin 23, which were derived from C57BL/6 mice. The PKR-deficient MEFs (MEF/PKR-/-), provided by Dr. Glen Barber (University of Miami, Miami, FL, USA), have been previously described 24. The human colon cancer cell line DLD-1, human lung cancer cell line H322, H2122, H226B, H522, and human cervical cancer cell line Hela were maintained in our laboratory. All cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% heat-inactivated fetal calf serum, 100 units/mL penicillin, and 100 mg/mL streptomycin and maintained in the presence of 5% CO2 at 37°C.
Chemicals and antibodies
JNK-specific inhibitor SP600125 was purchased from Calbiochem (La Jolla, CA), dissolved in dimethyl sulfoxide, stored at -20°C, and protected from light. An equal volume of solvent was used as a control. The following antibodies were used in western blot analysis: anti-PKR (B-10) (Santa Cruz Biotechnology, Santa Cruz, CA); anti-phospho-eIF2a (Cell Signaing Technology, Danvers, MA); anti-phospho-PKR (Upstate, Temecula, CA); and anti-β-actin and hemagglutinin (HA) (Sigma, St. Louis, MO).
Viruses
Oncolytic vaccinia virus has also been described previously 5. The vF13L+ strain is a wild-type vaccinia virus of the Western Reserve (WR) strain, and vSP is a modified virus that has deletions in two antiapoptosis serpin genes, the SPI-1 and SPI-2 5. Adenoviruses expressing the wild-type double-stranded RNA-dependent protein kinase gene (Ad/PKR wt) were provided by Dr. Abujiang Pataer (The University of Texas M. D. Anderson Cancer Center, Texes, USA). The expansion, purification, titration, and quality analysis of both vectors were performed at the Vector Core Facility at M. D. Anderson Cancer Center as previously described 25 . The titer used for vaccinia virus in this study was the infectious units (IUs) determined by the TCID50 assay 25,26.
Plasmid transfection
The plasmids pLNCX-3X HA-p46JNK1a (dnJNK1) and pLNCX-3X HA-p54JNK2a (dnJNK2), which encode a hemagglutinin-tagged, dominant-negative JNK1 and JNK2 mutant, respectively 26 were provided by Dr. L.E. Heasley (University of Colorado Health Sciences Center, Denver, CO). Plasmids were transfected using FuGENE6 reagent (Roche Diagnostics, Indianapolis, IN), and stable transfectants were selected in the presence of 500 μg/mL G418. For transient transfection, MEFs were transfected with pSRa3 HA-JNKK2-JNK1, which expresses a JNK2-JNK1 fusion protein that has constitutive JNK1 activity 27.
Cell viability assay
The viability of the cell lines was determined using the sulforhodamine B assay, as previously described 28. Briefly, after adherent cells were fixed with trichloroacetic acid in a 96-well microplate, the protein was stained with sulforhodamine B, and the optical density at 570 nm was determined, with the number of stained cells indicating cell viability. Relative cell viability was determined by setting the viability of the control cells, which were exposed only to phosphate-buffered saline (PBS), at 100% and comparing the viability of the treated cells to that of the controls. Each experiment was performed in quadruplicate and repeated at least three times.
Apoptosis assay
To detect and quantify apoptosis, fixed cells were resuspended in PBS containing 10 μg/mL propidium iodide (PI) (Roche Diagnostics) and 10 μg/mL RNase A (Sigma-Aldrich, St. Louis, MO) at 37°C for 30 min. Cell-cycle analysis was performed by using an Epics Profile II flow cytometer (Beckman Coulter, Fullerton, CA) with MULTICYCLE software (Phoenix Flow Systems, San Diego, CA). The accumulation of sub-G1 cells, a known indicator of DNA fragmentation and apoptosis, was used to quantify apoptosis. All experiments were repeated at least twice.
Western blot analysis
Cells were washed twice in cold PBS, collected, and lysed in buffer [62.5 mM Tris (pH 6.8), 2% sodium dodecyl sulfate, and 10% glycerol] containing 1x proteinase inhibitor cocktail (Roche Diagnostics). The lysates were spun at 14,000 x g in a microcentrifuge at 4°C for 10 min, and the supernatants were used as whole-cell extracts. Protein concentrations were determined using a BCA protein assay kit (Pierce, Rockford, IL). Equal amounts (30-50 μg) of proteins were subjected to electrophoresis under reducing conditions on 10-12.5% (w/v) polyacrylamide gels and then electrophoretically transferred to nitrocellulose membranes (Amersham Biosciences, Piscataway, NJ). The membranes were incubated with primary antibody followed by peroxidase-linked secondary antibody. An electrochemiluminescence western blotting system (Amersham) was used to detect secondary probes.
Statistical analysis
Differences between treatment groups were assessed by ANOVA or an unpaired Student’s t-test using StatSoft statistical software (Tulsa, OK). P < 0.05 was considered statistically significant.
RESULTS
Deficiency of JNK1- or JNK2-enhanced oncolytic vaccinia virus-induced host cell killing
To identify the possible roles of JNK in vaccinia virus-mediated host cell killing, we first evaluated the viability of wild-type MEF and JNK-knockout-MEFs after infection with different doses of oncolytic vaccinia virus. Wild-type MEFs and JNK-deficient MEFs were infected with vSP at doses ranging from 0.5 to 25 multiplicity of infection (MOI). Cell viability was determined 72 h after infection.
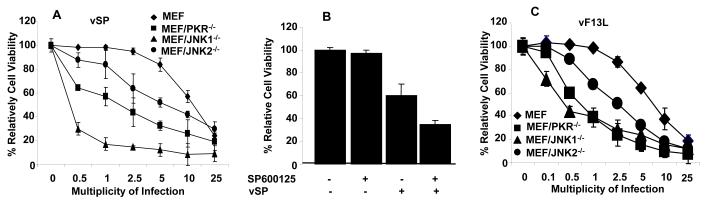
Results showed that susceptibility to oncolytic vaccinia virus increased in JNK-deficient MEFs than in wild-type MEFs. The susceptibility was more dramatically enhanced in JNK1-deficient MEFs than JNK2-deficient MEFs (Fig. 1A). Compared to control cells, at 2.5 MOI, the cell viability of the MEF/JNK1-/- and MEF/JNK2-/- cells was 60% and 10% respectively, while MEFs were not affected (P < 0.01). The enhanced cell killing in JNK1-/- MEF cells was comparable with or more dramatic than that in PKR-/- MEF cells. The host-cell-killing effect of vSP was also enhanced in wild-type MEFs after treatment with SP600125, a JNK inhibitor 29 (Fig. 1B). After treatment with 10 umol/L SP600125, MOI 10.0 vSP, or both, 98%, 60%, and 30%, respectively, of the MEFs were viable. Also, the difference in relative cell viability between cells infected with vSP alone and those infected with vSP plus SP600125 was significant (P < 0.01).
FIG. 1.
Vaccinia virus-induced host cell killing in MEFs. A) Cytotoxicity of vSP in wild-type and PKR- or JNK-deficient MEFs. Cells were treated with different doses of vSP for 72 h, and cell viability was determined using the sulforhodamine B assay. Cells treated with PBS were used as the control, and their viability was set at 100%. Each data point represents the mean ± standard deviation (SD) of three independent experiments. B) Combination effect of vSP and the JNK-specific inhibitor SP600125 in wild-type MEFs infected with 10 umol/L SP600125, MOI 10.0 vSP, or both for 72 h. Each data point represents the mean ± SD of three independent experiments. C) Cytotoxicity of vF13L in MEF and JNK-/- cells. The experiment was performed as described in A). Each data point represents the mean ± SD of three independent experiments.
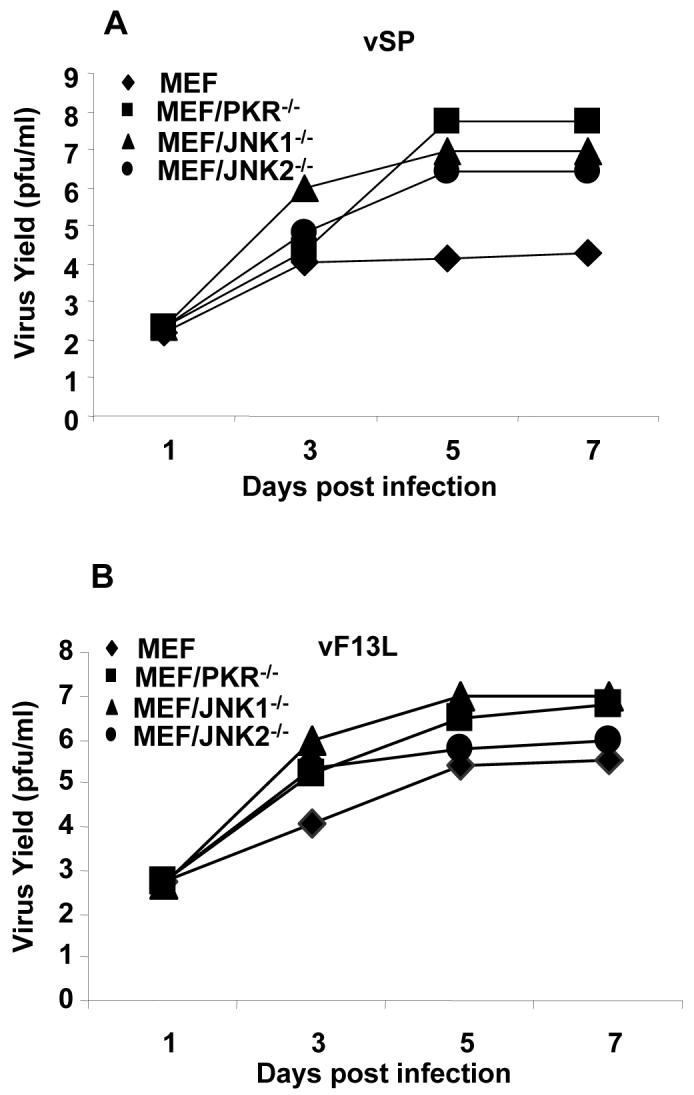
To test whether the effect of JNK in MEFs was specific to vSP, we evaluated the cell-killing effect in MEFs of a wild-type vF13L+, a Western Reserve strain virus with a LacZ gene insertion, and no viral deletions 5. A dose-response study showed that JNK-deficient MEFs were also much more susceptible to vF13L+ than were wild-type MEFs (Fig. 1C). Together, the above results demonstrated that cellular JNK status can dramatically affect the susceptibility of host cells to vaccinia virus.
JNK-deficiency-enhanced vaccinia virus-induced apoptosis
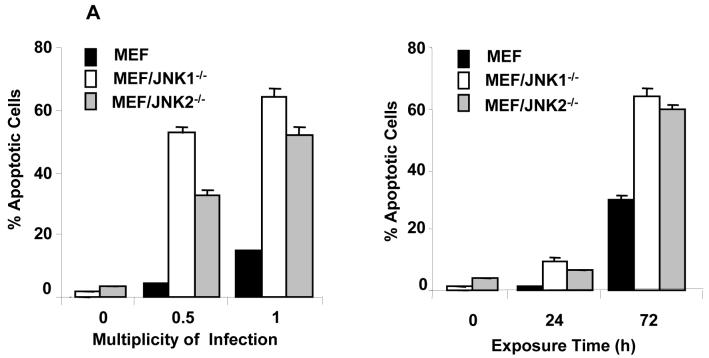
To test whether the susceptibility of MEFs was related to oncolytic vaccinia virus-induced apoptosis, we infected the MEFs with vSP at MOIs of 0, 0.5, and 1. Seventy-two h later, the cells were harvested, and the percentage of apoptotic cells was determined by fluorescence-activated cell-sorting (FACS) analysis.
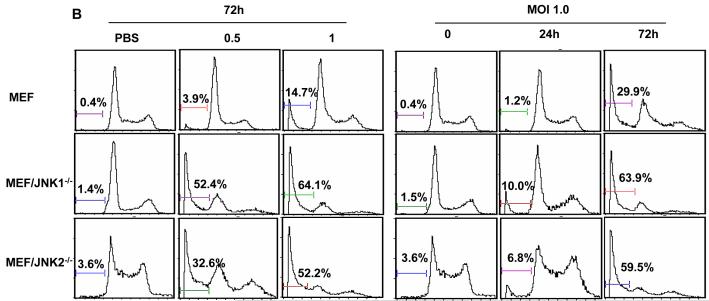
We found that at the same MOIs, the proportion of JNK-deficient MEFs that were apoptotic was greater than that of wild-type MEFs. For example, at 0.5 MOI, 52% and 33% of the MEF/JNK1-/- and MEF/JNK2-/- cells, respectively, were apoptotic, while only 4% of the MEFs were apoptotic (P < 0.01; Fig. 2A). We also compared apoptosis induction in the three MEF cell lines 24 and 72 h after infection with 1 MOI of vSP. At both time points, a greater percentage of the JNK-deficient MEFs were apoptotic than in the wild-type MEFs, although the increase was more pronounced at 72 h than at 24 h (Fig. 2B).
FIG. 2.
Apoptosis induction by vSP in wild-type and JNK-deficient MEFs. A) Cells were treated with different doses of vSP for 72 h (left panel), or with MOI 1.0 of vSP at the indicated time point (right panel), and apoptosis was determined using the PI staining by FACS. Each data point represents the mean ± SD of three independent experiments. B) Histograms derived from flow cytometric analysis. Data from one of two experiments with similar results.
Increased vaccinia virus replication in JNK-deficient MEF
To test whether the virus’ enhanced host-cell-killing and apoptosis-inducing effects were related to enhanced viral replication, we inoculated vaccinia virus in the three MEFs lines and measured their infectious units at the different time point. First, the three MEFs lines were cultured on 6-well plates and infected with vaccinia virus at 0.1 MOI. The free viruses in the medium were removed 4 h after infection, and the cells were cultured in fresh medium for up to 7 d, after which they were harvested at different times. After three cycles of freezing and thawing, the samples were centrifuged and the supernatant collected. The IUs of vaccinia virus in each sample were then titrated in HeLa cells using the TCID50 method.
We found that viral replication was dramatically enhanced in JNK-deficient MEFs infected with either vSP or vF13L+. For vSP, the virus yield was 100-fold greater in the MEF/JNK1-/- and MEF/JNK2-/- cells than in the wild-type MEFs (P < 0.01; Fig. 3A). For vF13L+, the virus yield was 40 times greater in MEF/JNK1-/- cells than in the wild-type MEFs (P < 0.01; Fig. 3A). This result is comparable to that observed in PKR-/- MEF cells. However, in the MEF/JNK2-/- cells, vF13L+ replication was enhanced compared with replication in wild-type MEFs only at day 3 and not at days 5 or 7. These results demonstrate that JNK deficiency has a major impact on vaccinia virus replication and that enhanced vaccinia virus replication may account for enhanced cell-killing and apoptosis-inducing effects seen in JNK-deficient cells.
FIG. 3.
Replication of vaccinia virus in wild-type and JNK-deficient MEFs. Wild-type and JNK-deficient MEFs were inoculated with either A) vSP or B) vF13L+ at the 0.1 MOI. Cell lysate was harvested over time and infectious vaccinia virus in the lysate was determined by using the TCID50 method. The values in Y axis are in log scale.
Enhancement of cell killing and virus replication in cancer cells with reduced JNK activity
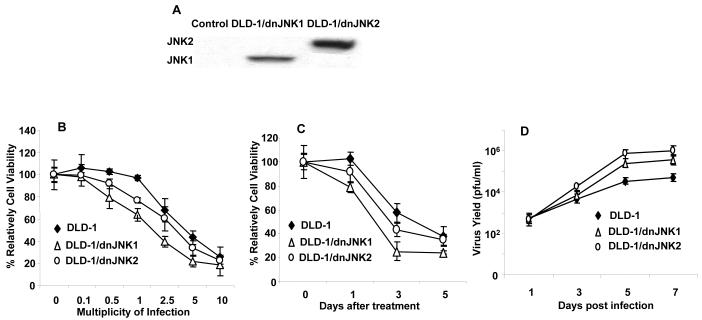
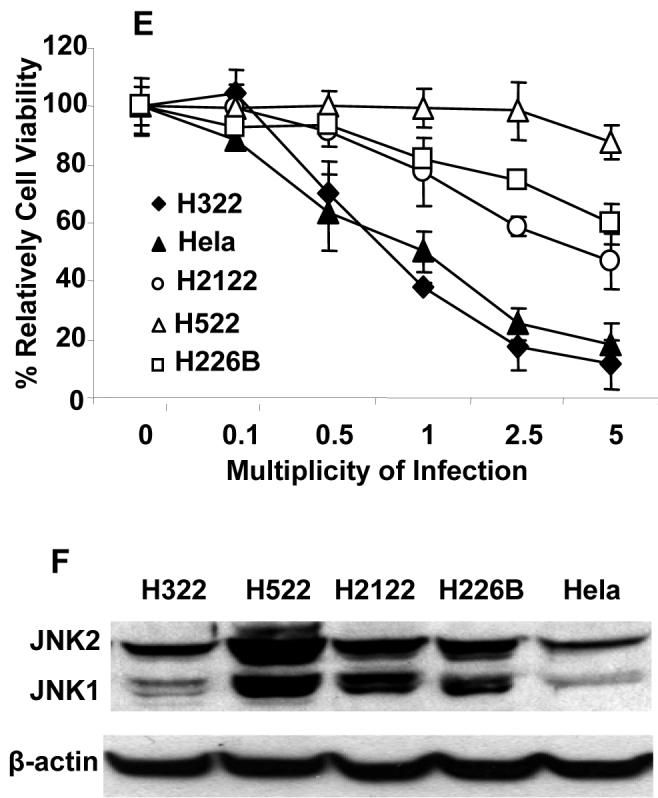
To investigate whether JNK plays a role in the cell killing induced by replication of oncolytic vaccinia virus in human cancer cells that is similar to its role in mouse embryonic cells, we evaluated vSP-mediated cell killing and vSP replication in parental DLD-1 human colon cancer cells and in DLD1 cells stably transfected with either dnJNK1 or dnJNK2 30. Each of the three types of DLD-1 cells were treated with doses of vSP ranging from 0.1 to 10 MOI. The results showed that, like the JNK deficiencies in MEFs, the expression of dnJNK1 or dnJNK2 increased the cell-killing effect of vaccinia virus at various doses, but not as much as in the JNK-deficient MEFs, possibly because of the incomplete suppression of JNKs in DLD-1 cells stably transfected with either dnJNK1 or dnJNK2. A time-course study showed that the expression of dnJNK1 in DLD-1 cells markedly enhanced vaccinia virus induced cell growth inhibition at 24 h (P < 0.05) and 72 h (P < 0.05). This effect was mild in the dnJNK2-expressing DLD-1 cells (Fig. 4B, C), consistent with the observation in JNK1- and JNK2-deficient MEFs.
FIG. 4.
Effect of vSP on parental DLD-1, DLD-1/dnJNK1, and DLD-1/dnJNK2 cells. A) DLD-1 cells transfected with dnJNK1 or dnJNK2 plasmids were analyzed by Western blot with antihemagglutinin antibody. B and C) Cytotoxicity of vSP in DLD-1, DLD-1/dnJNK1, DLD-1/dnJNK2 cells. Cells were treated with different doses of vSP for 72 h, and cell viability was determined using the sulforhodamine B assay. Cells treated with PBS were used as controls, and their viability was set at 100%. Each data point represents the mean ± SD of three independent experiments. D) The replication of vSP in DLD-1, DLD-1/dnJNK1, DLD-1/dnJNK2 cells. Viral replication was measured as described in Fig. 3 Each data point represents the mean ± SD of two independent experiments. The virus yield in DLD-1/dnJNK1 and DLD-1/dnJNK2 groups was much higher than in DLD-1 group (P < 0.05) at day 5 to day 7. E) Cytotoxicity of vSP in four lung cancer cell lines and in HeLa cells. Cells were treated with different MOIs of vSP for 72 h, and cell viability was determined using the sulforhodamine B assay. Cells treated with PBS were used as the control, and their viability was set at 100%. Each data point represents the mean ± standard deviation (SD) of three independent experiments. F) JNK expression determined by Western blot analysis in the same cell lines as E.
We also examined vaccinia virus replication in parental, dnJNK1-transfected, and dnJNK2-transfected DLD-1 cells by treating the cells with vaccinia virus at 0.1 MOI. The free virus in the medium was removed 4 h after infection, and the cells were cultured in fresh medium for up to 7 d. Cell lysates harvested at different times were titrated for viral IUs. We found that the expression of dnJNK1 and dnJNK2 in DLD-1 cells resulted in approximately 20 times greater than the yield of vaccinia virus progeny in the parental DLD-1 cells (Fig. 4D). These results indicate that the JNK status in human cancer cells may also affect vaccinia virus replication and vaccinia virus-induced cell killing.
We then investigated levels of JNK expression and susceptibility to oncolytic vaccinia virus in various lung cancer cell lines and in HeLa cells. Levels of JNK expression in human lung cancer cell lines H322, H522, H2122 and H226B, and in HeLa cells were determined by Western blot analysis. The susceptibility to oncolytic vaccinia virus vSP was determined by cell viability assay at 72 h after infection of vSP at different MOIs. The result showed that H322 and HeLa cells, which had the lowest JNK expressions, were also the most sensitive cells to vSP among the cell lines tested. In contrast, H522, which had the highest level of JNK expression, was also the most resistant cell line (Fig. 4E, F). This result indicted that cancer cells’ susceptibility to oncolytic vaccinia virus inversely correlate with their JNK expression levels and that JNK statues in cancer cells may be a useful biomarker for predicting their response to oncolytic vaccinia virotherapy.
JNK is required for the vaccinia virus-induced phosphorylation of double-stranded PKR and the alpha subunit of translation initiation factor 2
To investigate the molecular mechanisms of the JNK-mediated suppression of viral replication and host-cell-killing, we measured the phosphorylation of PKR and the alpha subunit of translation initiation factor 2 (eIF2a) in vaccinia virus-infected wild-type and JNK-deficient MEFs. PKR is transcriptionally induced by interferon and activated by dsRNA in virus-infected cells, which in turn inhibits viral protein synthesis by phosphorylating eIF2a 31,32. The MEFs were treated with 1.0 MOI vSP for 48 h before being harvested, and western blotting was performed with the cell lysates to determine the phosphorylation status of PKR and eIF2a (Fig. 5A).
FIG. 5.
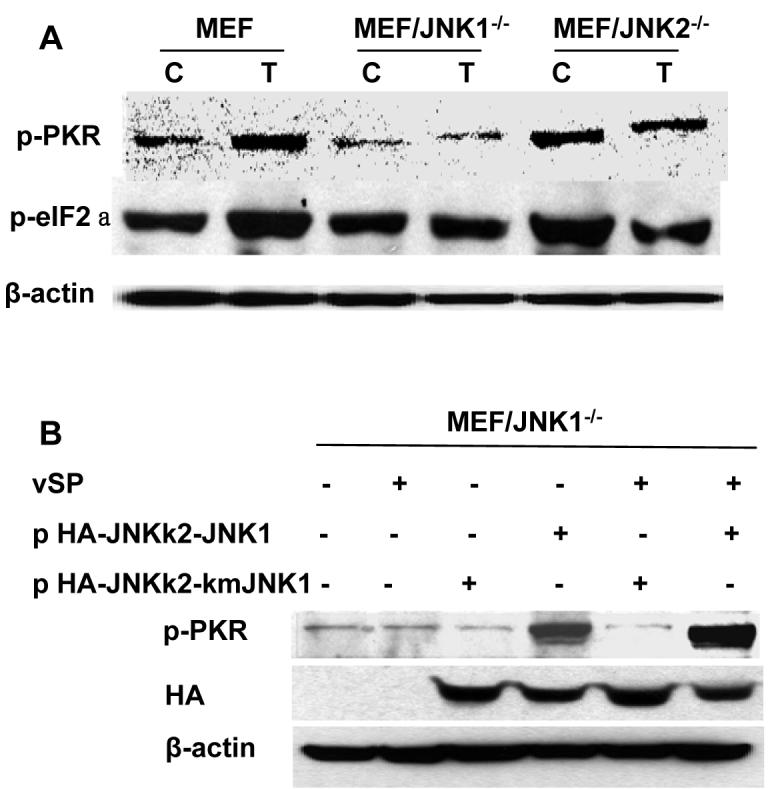
Phosphorylation of PKR and eIF2 in vSP-infected MEF and MEF/JNK-/- cells. A) Alteration in p-PKR and p-eIF2a expression after vSP treatment in MEF and MEF/JNK-/- cells. Cells were treated with PBS (C) or vSP at 1.0 MOI (T) for 72 h. Cell lysates were subjected to western blot assay for phosphorylated PKR and eIF2a. B) Level of PKR phosphorylation after vSP treatment in MEF/JNK1-/- cells transfected with pSRa3 HA-JNKK2-JNK1p SRa3-HA-JNKk2-kmJNK1 plasmids. Cells were treated with vSP at 1.0 MOI 1.0 for 72 h, and a western blot assay was performed on the lysates. β-actin expression acted as reference control.
Infection with vaccinia virus markedly increased the level of phosphorylated PKR and eIF2a in the wild-type MEFs, but it had little effect in the JNK-deficient MEFs, indicating that JNK may play a crucial role in the vaccinia virus-induced activation of PKR and phosphorylation of eIF2a.
To further investigate the role of JNK in PKR activation, we transfected MEF/JNK1-/- cells with the plasmid p3HA-JNKK2-JNK1, which expresses constitutively active JNK1. The expression of the JNKK2-JNK1 fusion protein was confirmed by western blotting using anti-HA antibody (Fig. 5B). The plasmid pHA-JNKK2-kmJNK1, which contains a mutation in its kinase domain, acted as a plasmid control. The expression of the JNKK2-JNK1 fusion protein restored vaccinia virus-induced activation of PKR in MEF/JNK1-/- cells, further demonstrating that activated JNK is required for PKR activation in vaccinia virus-infected cells.
Inhibiting viral replication in JNK-deficient MEFs by PKR
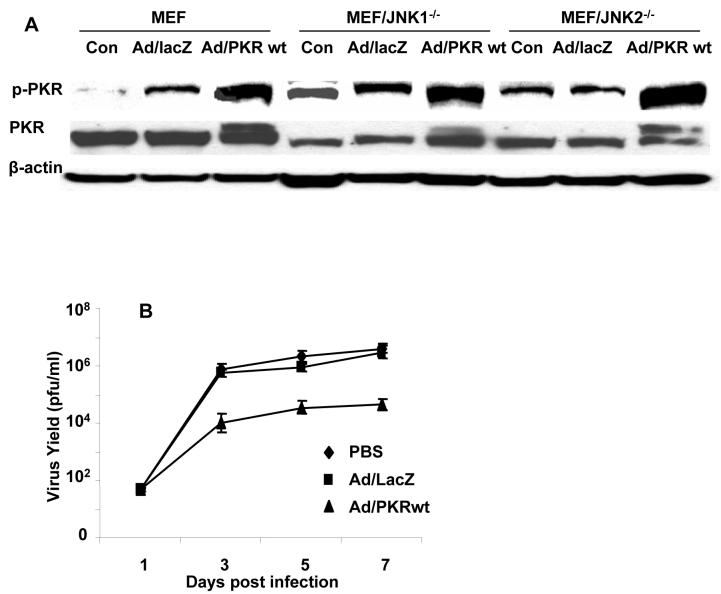
To test whether an absence of PKR activation accounts for enhanced viral replication in JNK-deficient MEFs, we first tested ability to restore PKR activation in JNK-deficient MEFs by infecting the cells with an adenovector-expressing wild-type PKR. Parental and JNK-deficient MEFs were treated with either PBS or adenovectors expressing either lacZ (Ad/lacZ) or PKR (Ad/PKR) at 2000 MOI. Cells were harvested at 36 h, and then cell lysates were subjected to western blot analysis to determine the levels of PKR and phosphorylated PKR.
Treatment with Ad/PKR but not Ad/lacZ resulted in the expression of exogenous PKR and an increase in phosphorylated PKR (Fig. 6A), suggesting that treatment with adenovectors expressing wild-type PKR results in the expression and activation of PKR.
FIG. 6.
Effect of Ad/PKR on vSP replication in MEF/JNK1-/- cells. A) Wild-type MEF and MEF/JNK-/- cells were treated with PBS (control), Ad/lacZ, and Ad/PKRwt at 2000 MOI, followed by vaccinia virus at 0.1 MOI. Cell lysates were harvested at 72 h and subjected to western blot analysis using PKR and p-PKR antibody. B) Replication of vSP in MEF/JNK1-/- after infection of Ad/PKR. Ad/lacZ and PBS treatment acted as the controls. MEF/JNK1-/- cells were treated with PBS, Ad/lacZ, or Ad/PKRwt at 2000 MOI, followed by vaccinia virus at 0.1 MOI. Cell lysates were harvested at the indicated time points and titrated using the TCID50 method. Each data point represents the mean ± SD of two independent experiments. The virus yield in Ad/ PKRwt group is much lower than in PBS and Ad/lacZ group (P < 0.01) at day 3 to day 7.
We then tested whether treating JNK deficient MEFs with Ad/PKR could restore the inhibition of vaccinia virus replication that had been observed in wild-type MEFs when their replication levels were compared to those of JNK-deficient MEFs. To do so, MEF/JNK1-/- cells were treated with PBS, Ad/lacZ, or Ad/PKR at 2000 MOI, followed by vaccinia virus at 0.1 MOI. Uninfected viruses were removed 4 h after infection, and the cells were cultured in fresh medium for up to 7 d. Cell lysates harvested at different time points were titrated for vaccinia virus progeny. The results showed that treatment with Ad/PKR but not Ad/lacZ dramatically suppressed vaccinia virus replication in JNK1-deficient MEFs (Fig. 6B). The degree of inhibition was comparable to that observed in wild-type MEFs, suggesting that the absence of PKR activation accounts for the enhanced vaccinia virus replication in JNK-deficient MEFs.
DISCUSSION
We found that oncolytic vaccinia virus replication was as much as 100 times greater in MEFs with either the JNK1 or JNK2 genes knocked out than in parental MEFs. The enhanced viral replication and host cell killing observed in JNK-deficient MEFs occurred with both vSP, which contain deletions for viral SPI-1 and SPI-2 genes, and with wild-type vF13L+. Similar results were observed in wild-type MEFs that had been treated with a JNK inhibitor or in human colon cancer cells that had been transfected with dnJNK genes. These findings suggested that a deficiency of JNK activation can promote oncolytic vaccinia virus replication and enhance virus-mediated cell-killing. We also found that PKR activation and eIF2a phosphorylation are blocked in JNK-deficient cells and that the absence of PKR activation may account for enhanced viral replication in JNK-deficient MEFs, because the restoration of active PKR in those cells reduced vaccinia virus replication to the levels observed in wild-type MEFs. Thus, our results indicate that JNK is required for PKR activation in oncolytic vaccinia virus-infected cells.
PKR is a ubiquitously expressed serine/threonine protein kinase that can be further induced by interferon. The binding of its N-terminal dsRNA binding domains with dsRNA is an intermediate product during the life cycle of many viruses 33 that results in the autophosphorylation and activation of PKR 34. In addition to dsRNA, bacterial lipopolysaccharides; cytokines, such as tumor necrosis factor α and interleukin-1; activating proteins, such as PACT 35,36; and the stress signaling pathways 37 can also activate PKR. Activated PKR phosphorylates eIF2a, which leads to the halting of protein synthesis and the inhibition of viral replication. Activated PKR can also phosphorylate protein phosphatase 2A, mediate NFκB activation, and stimulate the activities of JNK- and p38 mitogen-activated protein kinases.
However, signal transduction between JNK and PKR is not well-characterized. It is reported that PKR is required for the activation of JNK by LPS 38, but PKR is not required for JNK activation by dsRNA or viral infection 39. On the other hand, while it has not been reported whether JNK is required for PKR activation by dsRNA or viral infection, the defective or reduced induction of interferons at transcriptional levels after treatment with dsRNA or vesicular stomatitis virus has been reported in JNK deficient cells 39. Our results showed that JNK is required for PKR activation. However, the underlying mechanisms or signal transduction between JNK and PKR must be further characterized.
Enhanced apoptosis may have different impact on oncolytic virotherapy, depending on the timeframe when apoptosis occurs. A premature apoptosis occuring at an early time point of may reduced virus replication and have negative impact on virotherapy. However, an enhanced apoptosis at a later time point, such as 72 h after viral infection, may release and spread of viral progeny among tumor cells. This may explain why enhanced apoptosis in JNK deficient cells had positive impact on vaccine virus replication. The finding that JNK status can affect oncolytic vaccinia virus replication and host cell killing may, however, have implications in oncolytic virotherapy. It is possible that JNK status in target cells can be manipulated through small compounds, dominant negative constructs, or siRNA to enhance oncolytic effects of oncolytic vaccinia virus. Indeed, our results showed that oncolytic effects of vSP virus in DLD-1 cells were enhanced by transfection with dnJNK1 or dnJNK2. Because vaccinia virus has been used as an expression vector for foreign genes, it will be interesting to test whether the therapeutic effects of an oncolytic vaccinia virus might be improved by embedding in it an expression cassette for dnJNK or siRNA for JNK. Moreover, our results result indicted that levels of JNK in cancer cells correlate inversely with their susceptibility to oncolytic vaccinia virotherapy. Thus, JNK status in cancer cells may be a selective biomarker for oncolytic vaccinia virotherapy, and useful for predicting responses to such a therapy.
Supplementary Material
ACKNOWLEDGEMENTS
We thank for Angelique Lee Siy for editorial review, and Li Wang for the preparation and quality testing of adenovectors. This work has been supported by National Cancer Institute grants CA 092487 and CA 098582 (both to B. Fang), National Institutes of Health core grant CA 16672, Lockton grant-matching funds, Homer Flower Gene Therapy Research Fund, and Charles Rogers Gene Therapy Fund.
References
- 1.Cebere I, Dorrell L, McShane H, Simmons A, McCormack S, Schmidt C, et al. Phase I clinical trial safety of DNA- and modified virus Ankara-vectored human immunodeficiency virus type 1 (HIV-1) vaccines administered alone and in a prime-boost regime to healthy HIV-1-uninfected volunteers. Vaccine. 2006;24:417–425. doi: 10.1016/j.vaccine.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 2.Moss B. Vaccinia virus: a tool for research and vaccine development. Science. 1991;252:1662–1667. doi: 10.1126/science.2047875. [DOI] [PubMed] [Google Scholar]
- 3.Harrington LE, Most RR, Whitton JL, Ahmed R. Recombinant vaccinia virus-induced T-cell immunity: quantitation of the response to the virus vector and the foreign epitope. J Virol. 2002;76:3329–3337. doi: 10.1128/JVI.76.7.3329-3337.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo ZS, Bartlett DL. Vaccinia as a vector for gene delivery. Expert Opin Biol Ther. 2004;4:901–917. doi: 10.1517/14712598.4.6.901. [DOI] [PubMed] [Google Scholar]
- 5.Guo ZS, Naik A, O’Malley ME, Popovic P, Demarco R, Hu Y, et al. The enhanced tumor selectivity of an oncolytic vaccinia lacking the host range and antiapoptosis genes SPI-1 and SPI-2. Cancer Res. 2005;65:9991–9998. doi: 10.1158/0008-5472.CAN-05-1630. [DOI] [PubMed] [Google Scholar]
- 6.Thorne SH, Hwang TH, Kirn DH. Vaccinia virus and oncolytic virotherapy of cancer. Curr Opin Mol Ther. 2005;7:359–365. [PubMed] [Google Scholar]
- 7.Hruby DE, Lynn DL, Kates JR. Vaccinia virus replication requires active participation of the host cell transcriptional apparatus. Proc Natl Acad Sci U S A. 1979;76:1887–1890. doi: 10.1073/pnas.76.4.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SB, Esteban M. The interferon-induced double-stranded RNA-activated human p68 protein kinase inhibits the replication of vaccinia virus. Virology. 1993;193:1037–1041. doi: 10.1006/viro.1993.1223. [DOI] [PubMed] [Google Scholar]
- 9.Karupiah G, Xie QW, Buller RM, Nathan C, Duarte C, MacMicking JD. Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase. Science. 1993;261:1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- 10.Melkova Z, Esteban M. Interferon-gamma severely inhibits DNA synthesis of vaccinia virus in a macrophage cell line. Virology. 1994;198:731–735. doi: 10.1006/viro.1994.1087. [DOI] [PubMed] [Google Scholar]
- 11.Diaz-Guerra M, Rivas C, Esteban M. Inducible expression of the 2-5A synthetase/RNase L system results in inhibition of vaccinia virus replication. Virology. 1997;227:220–228. doi: 10.1006/viro.1996.8294. [DOI] [PubMed] [Google Scholar]
- 12.McLean TI, Bachenheimer SL. Activation of cJUN N-terminal kinase by herpes simplex virus type 1 enhances viral replication. J Virol. 1999;73:8415–8426. doi: 10.1128/jvi.73.10.8415-8426.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holloway G, Coulson BS. Rotavirus activates JNK and p38 signaling pathways in intestinal cells, leading to AP-1-driven transcriptional responses and enhanced virus replication. J Virol. 2006;80:10624–10633. doi: 10.1128/JVI.00390-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke P, Meintzer SM, Wang Y, Moffitt LA, Richardson-Burns SM, Johnson GL, et al. JNK regulates the release of proapoptotic mitochondrial factors in reovirus-infected cells. J Virol. 2004;78:13132–13138. doi: 10.1128/JVI.78.23.13132-13138.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirasawa K, Kim A, Han HS, Han J, Jun HS, Yoon JW. Effect of p38 mitogen-activated protein kinase on the replication of encephalomyocarditis virus. J Virol. 2003;77:5649–5656. doi: 10.1128/JVI.77.10.5649-5656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahaus M, Desloges N, Wolff MH. Replication of varicella-zoster virus is influenced by the levels of JNK/SAPK and p38/MAPK activation. J Gen Virol. 2004;85:3529–3540. doi: 10.1099/vir.0.80347-0. [DOI] [PubMed] [Google Scholar]
- 17.Teraishi F, Zhang L, Guo W, Dong F, Davis JJ, Lin A, et al. Activation of c-Jun NH2-terminal kinase is required for gemcitabine’s cytotoxic effect in human lung cancer H1299 cells. FEBS Lett. 2005;579:6681–6687. doi: 10.1016/j.febslet.2005.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teraishi F, Wu S, Sasaki J, Zhang L, Davis JJ, Guo W, et al. JNK1-dependent antimitotic activity of thiazolidin compounds in human non-small-cell lung and colon cancer cells. Cell Mol Life Sci. 2005;62:2382–2389. doi: 10.1007/s00018-005-5365-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng Y, Ren X, Yang L, Lin Y, Wu X. A JNK-dependent pathway is required for TNFalpha-induced apoptosis. Cell. 2003;115:61–70. doi: 10.1016/s0092-8674(03)00757-8. [DOI] [PubMed] [Google Scholar]
- 20.Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, et al. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288:870–874. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- 21.Verheij M, Bose R, Lin XH, Yao B, Jarvis WD, Grant S, et al. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 22.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Minemoto Y, Lin A. c-Jun N-terminal protein kinase 1 (JNK1), but not JNK2, is essential for tumor necrosis factor alpha-induced c-Jun kinase activation and apoptosis. Molecular & Cellular Biology. 2004;24:10844–10856. doi: 10.1128/MCB.24.24.10844-10856.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang YL, Reis LF, Pavlovic J, Aguzzi A, Schafer R, Kumar A, et al. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 1995;14:6095–6106. doi: 10.1002/j.1460-2075.1995.tb00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang B, Ji L, Bouvet M, Roth JA. Evaluation of GAL4/TATA in vivo. Induction of transgene expression by adenovirally mediated gene codelivery. J Biol Chem. 1998;273:4972–4975. doi: 10.1074/jbc.273.9.4972. [DOI] [PubMed] [Google Scholar]
- 26.Wojtaszek PA, Heasley LE, Siriwardana G, Berl T. Dominant-negative c-Jun NH2-terminal kinase 2 sensitizes renal inner medullary collecting duct cells to hypertonicity-induced lethality independent of organic osmolyte transport. J Biol Chem. 1998;273:800–804. doi: 10.1074/jbc.273.2.800. [DOI] [PubMed] [Google Scholar]
- 27.Zheng C, Xiang J, Hunter T, Lin A. The JNKK2-JNK1 fusion protein acts as a constitutively active c-Jun kinase that stimulates c-Jun transcription activity. J Biol Chem. 1999;274:28966–28971. doi: 10.1074/jbc.274.41.28966. [DOI] [PubMed] [Google Scholar]
- 28.Pauwels B, Korst AE, De Pooter CM, Pattyn GG, Lambrechts HA, Baay MF, et al. Comparison of the sulforhodamine B assay and the clonogenic assay for in vitro chemoradiation studies. Cancer Chemother Pharmacol. 2003;51:221–226. doi: 10.1007/s00280-002-0557-9. [DOI] [PubMed] [Google Scholar]
- 29.Bennett BL, Sasaki DT, Murray BW, O’Leary EC, Sakata ST, Xu W, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci U S A. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teraishi F, Wu S, Zhang L, Guo W, Davis JJ, Dong F, et al. Identification of a novel synthetic thiazolidin compound capable of inducing c-Jun NH2-terminal kinase-dependent apoptosis in human colon cancer cells. Cancer Res. 2005;65:6380–6387. doi: 10.1158/0008-5472.CAN-05-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rice AP, Kerr IM. Interferon-mediated, double-stranded RNA-dependent protein kinase is inhibited in extracts from vaccinia virus-infected cells. J Virol. 1984;50:229–236. doi: 10.1128/jvi.50.1.229-236.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawagishi-Kobayashi M, Silverman JB, Ung TL, Dever TE. Regulation of the protein kinase PKR by the vaccinia virus pseudosubstrate inhibitor K3L is dependent on residues conserved between the K3L protein and the PKR substrate eIF2alpha. Mol Cell Biol. 1997;17:4146–4158. doi: 10.1128/mcb.17.7.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meurs E, Chong K, Galabru J, Thomas NS, Kerr IM, Williams BR, et al. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell. 1990;62:379–390. doi: 10.1016/0092-8674(90)90374-n. [DOI] [PubMed] [Google Scholar]
- 34.Williams BR. Signal integration via PKR. Sci STKE. 2001;2001:RE2. doi: 10.1126/stke.2001.89.re2. [DOI] [PubMed] [Google Scholar]
- 35.Li S, Peters GA, Ding K, Zhang X, Qin J, Sen GC. Molecular basis for PKR activation by PACT or dsRNA. Proc Natl Acad Sci U S A. 2006;103:10005–10010. doi: 10.1073/pnas.0602317103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel RC, Sen GC. PACT, a protein activator of the interferon-induced protein kinase, PKR. EMBO J. 1998;17:4379–4390. doi: 10.1093/emboj/17.15.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams BR. PKR; a sentinel kinase for cellular stress. Oncogene. 1999;18:6112–6120. doi: 10.1038/sj.onc.1203127. [DOI] [PubMed] [Google Scholar]
- 38.Goh KC, deVeer MJ, Williams BR. The protein kinase PKR is required for p38 MAPK activation and the innate immune response to bacterial endotoxin. EMBO J. 2000;19:4292–4297. doi: 10.1093/emboj/19.16.4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chu WM, Ostertag D, Li ZW, Chang L, Chen Y, Hu Y, et al. JNK2 and IKKbeta are required for activating the innate response to viral infection. Immunity. 1999;11:721–731. doi: 10.1016/s1074-7613(00)80146-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.