Abstract
Context
Lumbar diskectomy is the most common surgical procedure performed for back and leg symptoms in US patients, but the efficacy of the procedure relative to nonoperative care remains controversial.
Objective
To assess the efficacy of surgery for lumbar intervertebral disk herniation.
Design, Setting, and Patients
The Spine Patient Outcomes Research Trial, a randomized clinical trial enrolling patients between March 2000 and November 2004 from 13 multidisciplinary spine clinics in 11 US states. Patients were 501 surgical candidates (mean age, 42 years; 42% women) with imaging-confirmed lumbar intervertebral disk herniation and persistent signs and symptoms of radiculopathy for at least 6 weeks.
Interventions
Standard open diskectomy vs nonoperative treatment individualized to the patient.
Main Outcome Measures
Primary outcomes were changes from baseline for the Medical Outcomes Study 36-item Short-Form Health Survey bodily pain and physical function scales and the modified Oswestry Disability Index (American Academy of Orthopaedic Surgeons MODEMS version) at 6 weeks, 3 months, 6 months, and 1 and 2 years from enrollment. Secondary outcomes included sciatica severity as measured by the Sciatica Bothersomeness Index, satisfaction with symptoms, self-reported improvement, and employment status.
Results
Adherence to assigned treatment was limited: 50% of patients assigned to surgery received surgery within 3 months of enrollment, while 30% of those assigned to nonoperative treatment received surgery in the same period. Intent-to-treat analyses demonstrated substantial improvements for all primary and secondary outcomes in both treatment groups. Between-group differences in improvements were consistently in favor of surgery for all periods but were small and not statistically significant for the primary outcomes.
Conclusions
Patients in both the surgery and the nonoperative treatment groups improved substantially over a 2-year period. Because of the large numbers of patients who crossed over in both directions, conclusions about the superiority or equivalence of the treatments are not warranted based on the intent-to-treat analysis.
Trial Registration
clinicaltrials.gov Identifier: NCT00000410
Lumbar diskectomy is the most common surgical procedure performed in the United States for patients having back and leg symptoms; the vast majority of the procedures are elective. However, lumbar disk herniation is often seen on imaging studies in the absence of symptoms1,2 and can regress over time without surgery.3 Up to 15-fold variation in regional diskectomy rates in the United States4 and lower rates internationally raise questions regarding the appropriateness of some of these surgeries.5,6
Several studies have compared surgical and nonoperative treatment of patients with herniated disk, but baseline differences between treatment groups, small sample sizes, or lack of validated outcome measures in these studies limit evidence-based conclusions regarding optimal treatment.7-12 The Spine Patient Outcomes Research Trial (SPORT) was initiated in March 2000 to compare the outcomes of surgical and nonoperative treatment for lumbar intervertebral disk herniation, spinal stenosis, or degenerative spondylolisthesis.13 The trial included both a randomized cohort and an observational cohort who declined to be randomized in favor of designating their own treatment but otherwise met all the other criteria for inclusion and who agreed to undergo follow-up according to the same protocol. This article reports intent-to-treat results through 2 years for the randomized cohort.
METHODS
Study Design
SPORT was conducted at 13 multidisciplinary spine practices in 11 US states (California, Georgia, Illinois, Maine, Michigan, Missouri, Nebraska, New York, New Hampshire, Ohio, Pennsylvania). The human subjects committee of each participating institution approved a standardized protocol. All patients provided written informed consent. An independent data and safety monitoring board monitored the study at 6-month intervals.13
Patient Population
Patients were considered for inclusion if they were 18 years and older and diagnosed by participating physicians during the study enrollment period as having intervertebral disk herniation and persistent symptoms despite some nonoperative treatment for at least 6 weeks. The content of preenrollment nonoperative care was not prespecified in the protocol but included education/counseling (71%), physical therapy (67%), epidural injections (42%), chiropractic therapy (32%), anti-inflammatory medications (61%), and opioid analgesics (40%).
Specific inclusion criteria at enrollment were radicular pain (below the knee for lower lumbar herniations, into the anterior thigh for upper lumbar herniations) and evidence of nerve-root irritation with a positive nerve-root tension sign (straight leg raise–positive between 30° and 70° or positive femoral tension sign) or a corresponding neurologic deficit (asymmetrical depressed reflex, decreased sensation in a dermatomal distribution, or weakness in a myotomal distribution). Additionally, all participants were surgical candidates who had undergone advanced vertebral imaging (97% magnetic resonance imaging, 3% computed tomography) showing disk herniation (protrusion, extrusion, or sequestered fragment)14 at a level and side corresponding to the clinical symptoms. Patients with multiple herniations were included if only one of the herniations was considered symptomatic (ie, if only one was planned to be operated on).
Exclusion criteria included prior lumbar surgery, cauda equina syndrome, scoliosis greater than 15°, segmental instability (>10° angular motion or >4-mm translation), vertebral fractures, spine infection or tumor, inflammatory spondyloarthropathy, pregnancy, comorbid conditions contraindicating surgery, or inability/unwillingness to have surgery within 6 months.
Study Interventions
The surgery was a standard open diskectomy with examination of the involved nerve root.15,16 The procedure agreed on by all participating centers was performed under general or local anesthesia, with patients in the prone or knee-chest position. Surgeons were encouraged to use loupe magnification or a microscope. Using a midline incision reflecting the paraspinous muscles, the interlaminar space was entered as described by Delamarter and McCullough.15 In some cases the medial border of the superior facet was removed to provide a clear view of the involved nerve root. Using a small annular incision, the fragment of disk was removed as described by Spengler.16 The canal was inspected and the foramen probed for residual disk or bony pathology. The nerve root was decompressed, leaving it freely mobile.
The nonoperative treatment group received “usual care,” with the study protocol recommending that the minimum nonsurgical treatment include at least active physical therapy, education/counseling with home exercise instruction, and nonsteroidal anti-inflammatory drugs, if tolerated. Other nonoperative treatments were listed, and physicians were encouraged to individualize treatment to the patient; all nonoperative treatments were tracked prospectively.13,17
Study Measures
The primary measures were the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) bodily pain and physical function scales18-21 and the American Academy of Orthopaedic Surgeons MODEMS version of the Oswestry Disability Index (ODI).22 As specified in the trial protocol, the primary outcomes were changes from baseline in these scales at 6 weeks, 3 months, 6 months, and 1 and 2 years from enrollment.
Secondary measures included patient self-reported improvement, work status, and satisfaction with current symptoms and with care.23 Symptom severity was measured by the Sciatica Bothersomeness Index (range, 0-24; higher scores represent worse symptoms).24,25
Recruitment, Enrollment, and Randomization
A research nurse at each site identified potential participants and verified eligibility. For recruitment and informed consent, evidence-based videotapes described the surgical and non-operative treatments and the expected benefits, risks, and uncertainties.26,27 Participants were offered enrollment in either the randomized trial or a concurrent observational cohort, the results of which are reported in a companion article.
Enrollment began in March 2000 and ended in November 2004. Baseline variables were collected prior to randomization. Patients self-reported race and ethnicity using National Institutes of Health categories.
Computer-generated random treatment assignment based on permuted blocks (randomly generated blocks of 6, 8, 10, and 12)28 within sites occurred immediately after enrollment via an automated system at each site, ensuring proper allocation concealment. Study measures were collected at baseline and at regularly scheduled follow-up visits. Short-term follow-up visits occurred at 6 weeks and 3 months. If surgery was delayed beyond 6 weeks, additional follow-up data were obtained 6 weeks and 3 months postoperatively. Longer-term follow-up visits occurred at 6 months, 1 year from enrollment, and annually thereafter.
Statistical Analyses
We originally determined a sample size of 250 patients in each treatment group to be sufficient (with a 2-sided significance level of .05 and 85% power) to detect a 10-point difference in the SF-36 bodily pain and physical functioning scales or a similar effect size in the ODI. This difference corresponded to patients' reports of being “a little better” in the Maine Lumbar Spine Study (MLSS).29 The sample size calculation allowed for up to 20% missing data but did not account for any specific levels of nonadherence.
The analyses for the primary and secondary outcomes used all available data for each period on an intent-to-treat basis. Predetermined end points for the study included results at each of 6 weeks, 3 months, 6 months, 1 year, and 2 years. To adjust for the possible effect of missing data on the study results, the analysis of mean changes for continuous outcomes was performed using maximum likelihood estimation for longitudinal mixed-effects models under “missing at random” assumptions and including a term for treatment center. Comparative analyses were performed using the single imputation methods of baseline value carried forward and last value carried forward, as well as a longitudinal mixed model controlling for covariates associated with missed visits.30
For binary secondary outcomes, longitudinal logistic regression models were fitted using generalized estimating equations31 as implemented in the PROC GENMOD program of SAS version 9.1 (SAS Institute Inc, Cary, NC). Treatment effects were estimated as differences in the estimated proportions in the 2 treatment groups.
P<.05 (2-sided) was used to establish statistical significance. For the primary outcomes, 95% confidence intervals (CIs) for mean treatment effects were calculated at each designated time point. Global tests of the joint hypothesis of no treatment effect at any of the designated periods were performed using Wald tests32 as implemented in SAS. These tests account for the intraindividual correlation due to repeated measurements over time.32
Nonadherence to randomly assigned treatment may mean that the intention-to-treat analysis underestimates the real benefit of the treatment.33,34 As a preplanned sensitivity analysis, we also estimated an “as-treated” longitudinal analysis based on comparisons of those actually treated surgically and nonoperatively. Repeated measures of outcomes were used as the dependent variables, and treatment received was included as a time-varying covariate. Adjustments were made for the time of surgery with respect to the original enrollment date to approximate the designated follow-up times. Baseline variables that were individually found to predict missing data or treatment received at 1 year were included to adjust for possible confounding.
RESULTS
SPORT achieved full enrollment, with 501 (25%) of 1991 eligible patients enrolled in the randomized trial. A total of 472 participants (94%) completed at least 1 follow-up visit and were included in the analysis. Data were available for between 86% and 73% of patients at each of the designated follow-up times (Figure 1).
Figure 1. Flow Diagram of the SPORT Randomized Controlled Trial of Disk Herniation: Exclusion, Enrollment, Randomization, and Follow-up.
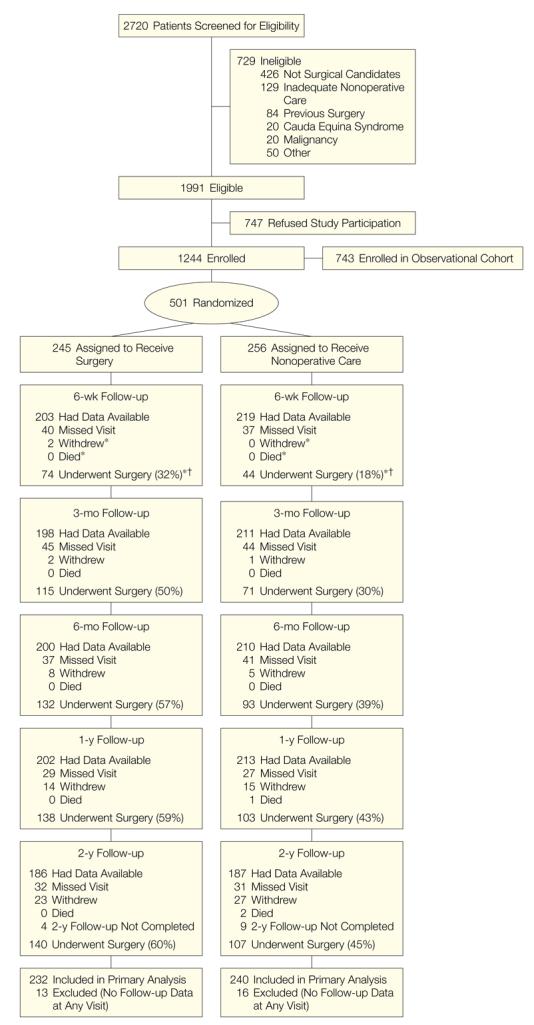
SPORT indicates Spine Patient Outcomes Research Trial.
*Cumulative over time.
†Percentages of patients undergoing surgery at each time point were calculated using the number included in the primary analysis as denominator (n=232 for surgery; n=240 for nonoperative care).
Patient Characteristics
Baseline patient characteristics are shown in Table 1. Overall, the study population had a mean age of 42 years, with majorities being male, white, employed, and having attended at least some college; 16% were receiving disability compensation. All patients had radicular leg pain, 97% in a classic dermatomal distribution. Most of the herniations were at L5-S1, posterolateral, and were extrusions by imaging criteria.14 The 2 randomized groups were similar at baseline.
Table 1.
Patient Baseline Demographic Characteristics, Comorbid Conditions, Clinical Findings, and Health Status Measures*
| Patients, No. (%) |
||
|---|---|---|
| Surgery (n = 232) | Nonoperative Treatment (n = 240) | |
| Age, mean (SD), y | 41.7 (11.8) | 43 (11.3) |
| Women | 101 (44) | 93 (39) |
| Race/ethnicity | ||
| Non-Hispanic | 223 (96) | 225 (94) |
| White | 197 (85) | 202 (84) |
| At least some college education | 171 (74) | 184 (77) |
| Income <$50 000 | 93 (40) | 114 (48) |
| Married | 161 (69) | 171 (71) |
| Employment status | ||
| Full-time or part-time | 142 (61) | 148 (62) |
| Disabled | 27 (12) | 31 (13) |
| Other | 63 (27) | 61 (25) |
| Compensation† | 36 (16) | 40 (17) |
| Body mass index, mean (SD)‡ | 27.8 (5.6) | 28.2 (5.4) |
| Current smoker | 47 (20) | 61 (25) |
| Comorbid conditions | ||
| Depression | 30 (13) | 32 (13) |
| Joint problem | 50 (22) | 47 (20) |
| Other§ | 101 (44) | 120 (50) |
| <6 mo since recent episode | 189 (81) | 183 (76) |
| Dermatomal pain radiation | 223 (96) | 234 (98) |
| Pain with straight-leg raise | ||
| Ipsilateral | 143 (62) | 147 (61) |
| Contralateral/both | 32 (14) | 35 (15) |
| Any neurologic deficit | 170 (73) | 177 (74) |
| Reflexes—asymmetrical depressed | 96 (41) | 96 (40) |
| Sensory—asymmetrical decrease | 104 (45) | 118 (49) |
| Motor—asymmetric weakness | 97 (42) | 93 (39) |
| Herniation level∥ | ||
| L2-3/L3-4 | 16 (7) | 16 (7) |
| L4-5 | 80 (34) | 85 (35) |
| L5-S1 | 136 (59) | 138 (57) |
| Herniation type | ||
| Protruding | 59 (25) | 67 (28) |
| Extruded | 156 (67) | 157 (65) |
| Sequestered | 17 (7) | 15 (6) |
| Posterolateral herniation | 182 (78) | 195 (81) |
| SF-36 score, mean (SD)¶ | ||
| Bodily pain | 27.1 (18.5) | 26.7 (17.4) |
| Physical function | 39.7 (24.9) | 39.2 (25.7) |
| Mental component summary | 46.3 (12.1) | 45.5 (11.9) |
| Oswestry Disability Index, mean (SD)# | 47.5 (21.4) | 46.3 (20.6) |
| Sciatica indices, mean (SD)# | ||
| Frequency | 15.8 (5.5) | 15.4 (5.5) |
| Bothersomeness | 15.4 (5.1) | 15 (5.3) |
| Satisfaction with symptoms: very dissatisfied | 184 (79) | 185 (77) |
| Patient self-assessed health trend | ||
| Problem getting better | 42 (18) | 48 (20) |
| Problem staying about the same | 108 (47) | 112 (47) |
| Problem getting worse | 82 (35) | 79 (33) |
Abbreviation: SF-36, Medical Outcomes Study 36-Item Short-Form Health Survey.
All between-group differences nonsignificant at the ≥.05 level.
Receiving workers' compensation, social security compensation, or other compensation, or application(s) pending.
Calculated as weight in kilograms divided by the square of height in meters.
Indicates problems related to stroke, diabetes, osteoporosis, cancer, fibromyalgia, chronic fatigue syndrome, posttraumatic stress disorder, alcohol, drug dependency, heart, lung, liver, kidney, blood vessel, nervous system, hypertension, migraine, anxiety, stomach, bowel.
The diagnoses for approximately 97% of patients were evaluated with magnetic resonance imaging and 3% with computed tomography.
Higher score indicates less severe symptoms. Range, 0-100.
Lower score indicates less severe symptoms. Range for Oswestry Disability Index, 0-100; for sciatica indices, 0-24.
Nonoperative Treatments
A variety of nonoperative treatments were used during the study (Table 2). Most patients received education/counseling (93%) and anti-inflammatory medications (61%) (nonsteroidal anti-inflammatory drugs, cyclooxygenase 2 inhibitors, or oral steroids); 46% received opiates; more than 50% received injections (eg, epidural steroids); and 29% were prescribed activity restriction. Forty-four percent received active physical therapy during the trial; however, 67% had received it prior to enrollment.
Table 2.
Nonoperative Treatments
| No. (%) (n = 323)* |
|
|---|---|
| Clinicians/services | |
| Education/counseling | 299 (93) |
| Emergency department | 52 (16) |
| Surgeon | 119 (37) |
| Chiropractor | 36 (11) |
| Internist/neurologist/other physician | 195 (60) |
| Physical therapist | 142 (44) |
| Acupuncturist | 13 (4) |
| Injections | 180 (56) |
| Other | 102 (32) |
| Medications | |
| NSAIDs | 193 (60) |
| COX-2 inhibitors | 101 (31) |
| Oral steroids | 15 (5) |
| Narcotics | 147 (46) |
| Muscle relaxants | 66 (20) |
| Other | 172 (53) |
| Devices | |
| Brace | 18 (6) |
| Corset | 9 (3) |
| Magnets | 12 (4) |
| Orthopedic pillow | 38 (12) |
| Shoe inserts | 25 (8) |
| TENS device | 12 (4) |
| Other medical devices | 27 (8) |
| None | 216 (68) |
Abbreviations: COX-2, cyclooxygenase 2; NSAIDS, nonsteroidal anti-inflammatory drugs; TENS, transcutaneous electrical nerve stimulation.
Patients who had used clinicians, treatments, medications, and devices within 1 year following enrollment or until the time of surgery; 323 patients either had no surgery in the first year of enrollment or had at least 1 regularly scheduled follow-up visit prior to surgery at which nonoperative treatment information could be assessed.
Surgical Treatment and Complications
Table 3 gives the characteristics of surgical treatment and complications. The median surgical time was 75 minutes (interquartile range, 58-90), with a median blood loss of 49.5 mL (interquar-tile range, 25-75). Only 2% required transfusions. There were no perioperative deaths; 1 patient died from complications of childbirth 11 months after enrollment. The most common intraoperative complication was dural tear (4%). There were no postoperative complications in 95% of patients. Reoperation occurred in 4% of patients within 1 year of the initial surgery; more than 50% of the reoperations were for recurrent herniations at the same level.
Table 3.
Operative Treatments, Complications, and Events
| No. (%) (n = 243)* |
|
|---|---|
| Diskectomy level | |
| L2-3/L3-4 | 9 (4) |
| L4-5 | 89 (37) |
| L5-S1 | 145 (61) |
| Operation time, mean (SD), min | 79.1 (36.3) |
| Blood loss, mean (SD), mL | 64.7 (88.4) |
| Blood replacement | 4 (2) |
| Length of stay | |
| Same day | 65 (27) |
| 1 Night | 137 (57) |
| ≥2 Nights | 37 (15) |
| Intraoperative complications† | |
| Dural tear/spinal fluid leak | 10 (4) |
| Vascular injury | 1 (0) |
| Other | 2 (1) |
| None | 230 (95) |
| Postoperative complications/events‡ |
|
| Wound infection, superficial | 4 (2) |
| Other | 9 (4) |
| None | 226 (95) |
| Postsurgical reoperation, | |
| No. (rate)§ | |
| 1 y | |
| Additional surgery | 9 (4) |
| Recurrent herniation | 5 (2) |
| Complication or other | 4 (2) |
| New condition | 0 |
| 2 y | |
| Additional surgery | 13 (5) |
| Recurrent herniation | 8 (3) |
| Complication or other | 4 (2) |
| New condition | 0 |
Data on surgical level, blood loss, length of stay, and complications were not available for 7 surgical patients. Detailed surgical information was available for 243 of 247 patients who had surgery.
None of the following were reported: aspiration, operation at wrong level.
Any reported complications up to 8 weeks postoperation. None of the following were reported: blood transfusion, cerebrospinal fluid leak, deep wound infection, nerve-root injury, paralysis, cauda equina injury, wound dehiscence, wound hematoma.
Rates are Kaplan-Meier estimates.
Nonadherence
Nonadherence to treatment assignment affected both groups, ie, some patients in the surgery group chose to delay or decline surgery, and some in the nonoperative treatment group crossed over to receive surgery (Figure 1). The characteristics of crossover patients that were statistically different from patients who did not cross over are shown in Table 4. Those more likely to cross over to receive surgery tended to have lower incomes, worse baseline symptoms, more baseline disability on the ODI, and were more likely to rate their symptoms as getting worse at enrollment than the other patients receiving nonoperative treatment. Those more likely to cross over to receive nonoperative care were older, had higher incomes, were more likely to have an upper lumbar disk herniation, less likely to have a positive straight leg–raising test result, had less pain, better physical function, less disability on the ODI, and were more likely to rate their symptoms as getting better at enrollment than the other surgery patients.
Table 4.
Statistically Significant Baseline Demographic Characteristics, Comorbid Conditions, Clinical Findings, and Health Status Measures, by Adherence With Treatment Assignment
| Assigned to Surgery |
Assigned to Nonoperative Treatment |
|||||
|---|---|---|---|---|---|---|
| No. (%) |
No. (%) |
|||||
| Surgery (n = 140) |
No Surgery (n = 92) |
P Value* |
Surgery (n = 107) |
No Surgery (n = 133) |
P Value* |
|
| Age, mean (SD), y | 40.1 (11.0) | 44 (12.6) | .01 | 41.9 (10.0) | 43.8 (12.3) | .21 |
| Annual income <$50 000 | 66 (47) | 27 (29) | .01 | 60 (56) | 54 (41) | .02 |
| Pain with straight leg raise (ipsilateral) | 94 (67) | 49 (53) | .05 | 72 (67) | 75 (56) | .11 |
| Herniation level‡ | ||||||
| L2-3/L3-4 | 4 (3) | 12 (13) | .01 | 5 (5) | 11 (8) | .45 |
| L4-5 | 50 (36) | 30 (33) | 41 (38) | 44 (33) | ||
| L5-S1 | 86 (61) | 50 (54) | 61 (57) | 77 (58) | ||
| SF-36 score, mean (SD)† | ||||||
| Bodily pain | 24.1 (16.7) | 31.7 (20.2) | .002 | 24.1 (16.8) | 28.9 (17.7) | .03 |
| Physical function | 35.9 (24.0) | 45.6 (25.3) | .003 | 33 (22.9) | 44.1 (26.9) | <.001 |
| Oswestry Disability Index, mean (SD)‡ | 51.7 (20.9) | 41.1 (20.7) | <.001 | 52.1 (19.2) | 41.6 (20.6) | <.001 |
| Sciatica indices, mean (SD)‡ | ||||||
| Frequency | 16.2 (5.2) | 15.1 (6) | .14 | 16.5 (5.5) | 14.6 (5.4) | .009 |
| Bothersomeness | 15.9 (4.8) | 14.8 (5.5) | .10 | 16.2 (5.0) | 14 (5.3) | .001 |
| Satisfaction with symptoms: very dissatisfied | 125 (89) | 59 (64) | <.001 | 92 (86) | 93 (70) | .005 |
| Patient self-assessed health trend: getting worse | 58 (41) | 24 (26) | .02 | 44 (41) | 35 (26) | .02 |
Abbreviation: SF-36, Medical Outcomes Study 36-Item Short Form Health Survey.
All other characteristics shown in Table 1 were not statistically significant.
Higher score indicates less severe symptoms. Range, 0-100.
Lower score indicates less severe symptoms. Range for Oswestry Disability Index, 0-100; for sciatica indices, 0-24.
Missing Data
The rates of missing data were equivalent between the groups at each time point, with no evidence of differential dropout according to assigned treatment. Characteristics of patients with missed visits were very similar to those of the rest of the cohort except that patients with missing data were less likely to be married, more likely to be receiving disability compensation, more likely to smoke, more likely to display baseline motor weakness, and had lower baseline mental component summary scores on the SF-36.
Intent-to-Treat Analyses
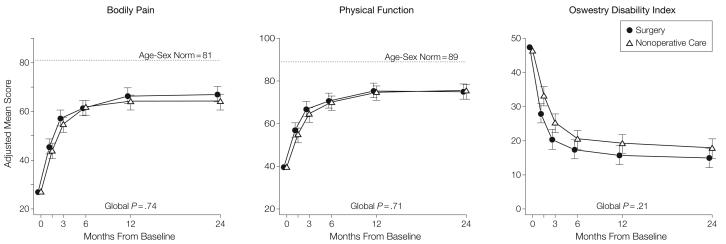
Table 5 shows estimated mean changes from baseline and the treatment effects (differences in changes from baseline between treatment groups) for 3 months, 1 year, and 2 years. For each measure and at each point, the treatment effect favors surgery. The treatment effects for the primary outcomes were small and not statistically significant at any of the points. As shown in Figure 2, both treatment groups showed strong improvements at each of the designated follow-up times, with small advantages for surgery. However, for each primary outcome the combined global test for any difference at any period was not statistically significant. This test accounts for intraindividual correlations as described in the “Methods” section.
Table 5.
Treatment Effects for Primary and Secondary Outcomes Based on Intent-to-Treat Analyses*
| 3 Months |
1 Year |
2 Years |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Surgery (n = 198) |
Nonoperative (n = 211) |
Treatment Effect (95% CI) |
Surgery (n = 202) |
Nonoperative (n = 213) |
Treatment Effect (95% CI) |
Surgery (n = 186) |
Nonoperative (n = 187) |
Treatment Effect (95% CI) |
|
| Primary outcomes | |||||||||
| SF-36 score, mean (SE)† | |||||||||
| Bodily pain | 30.5 (1.9) |
27.6 (1.8) |
2.9 (−2.2 to 8.0) |
39.7 (1.8) |
36.9 (1.8) |
2.8 (−2.3 to 7.8) |
40.3 (1.9) |
37.1 (1.9) |
3.2 (−2.0 to 8.4) |
| Physical function | 27.7 (1.9) |
24.9 (1.9) |
2.8 (−2.5 to 8.1) |
36.4 (1.9) |
35.2 (1.9) |
1.2 (−4.1 to 6.5) |
35.9 (2.0) |
35.9 (1.9) |
0 (−5.4 to 5.5) |
| Oswestry Disability Index, mean (SE)‡ | −26 (1.7) |
−21.3 (1.6) |
−4.7 (−9.3 to −0.2) |
−30.6 (1.7) |
−27.4 (1.6) |
−3.2 (−7.8 to 1.3) |
−31.4 (1.7) |
−28.7 (1.7) |
−2.7 (−7.4 to 1.9) |
| Secondary outcomes | |||||||||
| Sciatica Bothersomeness Index, mean (SE)‡ | −9.0 (0.46) |
−6.8 (0.45) |
−2.1 (−3.4 to −0.9) |
−10.3 (0.46) |
−8.7 (0.45) |
−1.6 (−2.9 to −0.4) |
−10.1 (0.48) |
−8.5 (0.47) |
−1.6 (−2.9 to −0.3) |
| Working full or part time, % (SE) | 63.8 (3.3) |
69.4 (3.1) |
−5.6 (−14.5 to 3.4) |
76.4 (2.9) |
77.0 (2.8) |
−0.6 (−8.6 to 7.3) |
74.2 (3.1) |
76.4 (3.0) |
−2.2 (−10.6 to 6.2) |
| Satisfaction with symptoms: very/somewhat satisfied, % (SE) | 54.3 (3.5) |
43.0 (3.4) |
11.3 (1.6 to 20.9) |
64.7 (3.4) |
58.5 (3.4) |
6.1 (−3.3 to 15.5) |
68.3 (3.4) |
64.4 (3.5) |
4.0 (−5.6 to 13.5) |
| Satisfaction with care: very/somewhat satisfied, % (SE) | 86.8 (2.4) |
83.3 (2.6) |
3.5 (−3.3 to 10.3) |
90.4 (2.0) |
87.1 (2.3) |
3.3 (−2.6 to 9.2) |
86.4 (2.5) |
83.1 (2.7) |
3.2 (−4.0 to 10.4) |
| Self-rated progress since enrollment: major improvement, % (SE) | 66.3 (3.3) |
62.1 (3.4) |
4.2 (−5.1 to 13.5) |
75.7 (3.0) |
66.7 (3.2) |
9.0 (0.3 to 17.6) |
76.3 (3.1) |
69.3 (3.3) |
7.0 (−1.9 to 15.9) |
Abbreviation: SF-36, Medical Outcomes Study 36-Item Short Form Health Survey.
Means, percentages, and treatment effects are derived from longitudinal regression models controlling for center, a blocking factor in the randomization. Treatment indicator was assigned on an intent-to-treat basis and results are close to but not exactly the same as the unadjusted means and proportions. SEs are derived from the same models.
Higher score indicates less severe symptoms. Range, 0-100.
Lower score indicates less severe symptoms. Range for Oswestry Disability Index, 0-100; for Sciatica Bothersomeness Index, 0-24.
Figure 2. Mean Scores Over Time for SF-36 Bodily Pain and Physical Function Scales and Oswestry Disability Index.
Age- and sex-weighted population normative scores are plotted for Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) scales. To enhance readability, the plot symbols and error bars for the surgical group are slightly offset. Error bars indicate 95% confidence intervals.
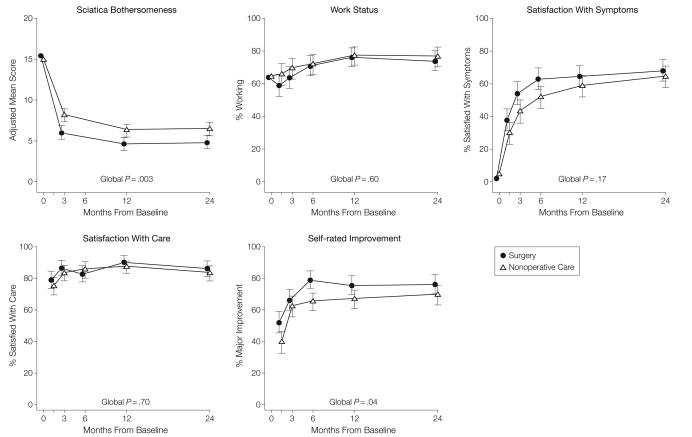
For the secondary outcome of sciatica bothersomeness, Table 5 and Figure 3 show that there were greater improvements in the Sciatica Bothersomeness Index in the surgery group at all designated follow-up times: 3 months (treatment effect, −2.1; 95% CI, −3.4 to −0.9), 1 year (treatment effect, −1.6; 95% CI, −2.9 to −0.4), and 2 years (treatment effect, −1.6; 95% CI, −2.9 to −0.3), with results of the global hypothesis test being statistically significant (P=.003). Patient satisfaction with symptoms and treatment showed small effects in favor of surgery while employment status showed small effects in favor of nonoperative care, but none of these changes was statistically significant. Self-rated progress showed a small statistically significant advantage for surgery (P=.04).
Figure 3. Measures Over Time for Sciatica Bothersomeness Index, Employment Status, Satisfaction With Symptoms, Satisfaction With Care, and Self-rated Improvement.
To enhance readability, the plot symbols and error bars for the surgical group are slightly offset. Error bars indicate 95% confidence intervals.
As-treated analyses based on treatment received were performed with adjustments for the time of surgery and factors affecting treatment crossover and missing data. These yielded far different results than the intent-to-treat analysis, with strong, statistically significant advantages seen for surgery at all follow-up times through 2 years. For example, at 1 year the estimated treatment effects for the SF-36 bodily pain and physical function scales, the ODI, and the sciatica measures were 15.0 (95% CI, 10.9 to 19.2), 17.5 (95% CI, 13.6 to 21.5), −15.0 (95% CI, −18.3 to −11.7), and −3.2 (95% CI, −4.3 to −2.1), respectively.
Sensitivity analysis was performed for 4 different analytic methods of dealing with the missing data. One method was based on simple mean changes for all patients with data at a given time point with no special adjustment for missing data. Two methods used single imputation methods—baseline value carried forward and last value carried forward.32 The latter method used the same mixed-models approach for estimating mean changes as given in Table 5 but also adjusted for factors affecting the likelihood of missing data. Treatment effect estimates at 1 year ranged from 1.6 to 2.9 for the SF-36 bodily pain scale, 0.74 to 1.4 for the physical function scale, −2.2 to −3.3 for the ODI, and −1.1 to −1.6 for the sciatica measures. Given these ranges, there appear to be no substantial differences between any of these methods.
COMMENT
Both operated and nonoperated patients with intervertebral disk herniation improved substantially over a 2-year period. The intent-to-treat analysis in this trial showed no statistically significant treatment effects for the primary outcomes; the secondary measures of sciatica severity and self-reported progress did show statistically significant advantages for surgery. These results must be viewed in the context of the substantial rates of nonadherence to assigned treatment. The pattern of nonadherence is striking because, unlike many surgical studies, both the surgical and nonoperative treatment groups were affected.35 The most comparable previous trial8 had 26% crossover into surgery at 1 year, but only 2% crossover out of surgery. The mixing of treatments due to crossover can be expected to create a bias toward the null.34 The large effects seen in the as-treated analysis and the characteristics of the crossover patients suggest that the intent-to-treat analysis underestimates the true effect of surgery.
SPORT findings are consistent with clinical experience in that relief of leg pain was the most striking and consistent improvement with surgery. Importantly, all patients in this trial had leg pain with physical examination and imaging findings that confirmed a disk herniation. There was little evidence of harm from either treatment. No patients in either group developed cauda equina syndrome; 95% of surgical patients had no intraoperative complications. The most common complication, dural tear, occurred in 4% of patients, similar to the 2% to 7% noted in the meta-analysis by Hoffman et al,7 2.2% seen in the MLSS,29 and 4% in the recent series from Stanford.36
One limitation is the potential lack of representativeness of patients agreeing to be randomized to surgery or nonoperative care; however, the characteristics of patients agreeing to participate in SPORT were very similar to those in other studies.29,36 The mean age of 42 years was similar to the mean ages in the MLSS,29 the series of Spangfort,37 and the randomized trial by Weber,8 and only slightly older than those in the recent series from Stanford (37.5 years).36 The proportion of patients receiving workers' compensation in SPORT (16%) was similar to the proportion in the Stanford population (19%) but lower than that in the MLSS population (35%), which specifically oversampled patients receiving compensation. Baseline functional status was also similar, with a mean baseline ODI of 46.9 in SPORT vs 47.2 in the Stanford series, and a mean baseline SF-36 physical function score of 39 in SPORT vs 37 in the MLSS.
The strict eligibility criteria, however, may limit the generalizability of these results. Patients unable to tolerate symptoms for 6 weeks and demanding earlier surgical intervention were not included, nor were patients without clear signs and symptoms of radiculopathy with confirmatory imaging. We can draw no conclusions regarding the efficacy of surgery in these other groups. However, our entry criteria followed published guidelines for patient selection for elective diskectomy, and our results should apply to the majority of patients facing a surgical decision.38,39
To fully understand the treatment effect of surgery compared with nonoperative treatment, it is worth noting how each group fared. The improvements with surgery in SPORT were similar to those of prior series at 1 year: for the ODI, 31 points vs 34 points in the Stanford series; for the bodily pain scale, 40 points vs 44 in the MLSS; and for sciatica bothersomeness, 10 points vs 11 in the MLSS. Similarly, Weber8 reported 66% “good” results in the surgery group, compared with the 76% reporting “major improvement” and 65% satisfied with their symptoms in SPORT.
The observed improvements with nonoperative treatment in SPORT were greater than those in the MLSS, resulting in the small estimated treatment effect. The nonoperative improvement of 37, 35, and 9 points in bodily pain, physical function, and sciatica bothersomeness, respectively, were much greater than the improvements of 20, 18, and 3 points reported in the MLSS. The greater improvement with nonoperative treatment in SPORT may be related to the large proportion of patients (43%) who underwent surgery in this group.
The major limitation of SPORT is the degree of nonadherence with randomized treatment. Given this degree of crossover, it is unlikely that the intent-to-treat analysis can form the basis of a valid estimate of the true treatment effect of surgery. The “as-treated” analysis with adjustments for possible confounders showed much larger effects in favor of surgical treatment. However, this approach does not have the strong protection against confounding that is afforded by randomization. We cannot exclude the possibility that baseline differences between the as-treated groups, or the selective choice of some but not other patients to cross over into surgery, may have affected these results, even after controlling for important covariates. Due to practical and ethical constraints, this study was not masked through the use of sham procedures. Therefore, any improvements seen with surgery may include some degree of “placebo effect.”
Another potential limitation is that the choice of nonoperative treatments was at the discretion of the treating physician and patient. However, given the limited evidence regarding efficacy for most nonoperative treatments for lumbar disk herniation and individual variability in response, creating a limited, fixed protocol for nonoperative treatment was neither clinically feasible nor generalizable. The nonoperative treatments used were consistent with published guidelines.17,38,39 Compared with the MLSS, SPORT had lower use of activity restriction, spinal manipulation, transcutaneous electrical nerve stimulation, and braces and corsets, and higher rates of epidural steroid injections and use of narcotic analgesics. This flexible nonoperative protocol had the advantages of individualization that considered patient preferences in the choice of nonoperative treatment and of reflecting current practice among multidisciplinary spine practices. However, we cannot make any conclusion regarding the effect of surgery vs any specific nonoperative treatment. Similarly, we cannot adequately assess the relative efficacy of any differences in surgical technique.
CONCLUSION
Patients in both the surgery and nonoperative treatment groups improved substantially over the first 2 years. Between-group differences in improvements were consistently in favor of surgery for all outcomes and at all time periods but were small and not statistically significant except for the secondary measures of sciatica severity and self-rated improvement. Because of the high numbers of patients who crossed over in both directions, conclusions about the superiority or equivalence of the treatments are not warranted based on the intent-to-treat analysis alone.
Acknowledgment
We thank Tamara S. Morgan, MA, Department of Orthopaedic Surgery, Dartmouth Medical School, for graphic design and production, journal interfacing, and shepherding the SPORT study from its original submission and into the foreseeable future. Ms Morgan is funded through the department and partially by SPORT. This study is dedicated to the memory of Brieanna Weinstein.
Funding/Support: This study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) (U01-AR45444-01A1) and the Office of Research on Women's Health, National Institutes of Health; and by the National Institute of Occupational Safety and Health, US Centers for Disease Control and Prevention. The Multidisciplinary Clinical Research Center in Musculoskeletal Diseases is funded by NIAMS (P60-AR048094-01A1). Dr Lurie is supported by a Research Career Award from NIAMS (1 K23 AR 048138-01).
Role of the Sponsors: The funding sources had no role in design or conduct of the study; the collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript.
Project Coordinator, Principal Investigators, Participating Institutions, and Nurse Coordinators (in order by enrollment): Judi L. Forman, MPH (Project Coordinator), William A. Abdu, MD, MS, Barbara Butler-Schmidt, RN, MSN, J. J. Hebb, RN, BSN (Dartmouth-Hitchcock Medical Center); Harry Herkowitz, MD, Gloria Bradley, BSN, Melissa Lurie, RN (William Beaumont Hospital); Todd Albert, MD, Alan Hilibrand, MD, Carole Simon, RN, MS (Rothman Institute at Thomas Jefferson Hospital); Frank Cammisa, MD, Brenda Green, RN, BSN (Hospital for Special Surgery); Michael Longley, MD, Nancy Fullmer, RN, Ann Marie Fredericks, RN, MSN, CPNP (Nebraska Foundation for Spinal Research); Scott Boden, MD, Sally Lashley, BSN, MSA (Emory University–The Emory Clinic); Lawrence Lenke, MD, Georgia Stobbs, RN, BA (Washington University); Sanford Emery, MD, Chris Furey, MD, Kathy Higgins, RN, PhD, CNS (University Hospitals of Cleveland/Case Western Reserve); Thomas Errico, MD, Alex Lee, RN, BSN (Hospital for Joint Diseases); Harley Goldberg, DO, Pat Malone, RN, MSN, ANP (Kaiser Permanente); Serena Hu, MD, Pat Malone, RN, MSN, ANP (University of California–San Francisco); Gunnar Andersson, MD, PhD, Howard An, MD, Margaret Hickey, RN, MS (Rush-Presbyterian-St Luke's Medical Center); Robert Keller, MD (Maine Spine and Rehabilitation).
Enrolling Physicians: William Abdu (Dartmouth-Hitchcock Medical Center); David Montgomery, Harry Herkowitz (William Beaumont Hospital); Ted Conliffe, Alan Hilibrand (Rothman Institute at Thomas Jefferson Hospital); Perry Ball (Dartmouth); Frank Cammisa (Hospital for Special Surgery); S. Tim Yoon (Emory University–The Emory Clinic); Randall Woodward (Nebraska Foundation for Spinal Research); Brett Taylor (Washington University); Todd Albert (Rothman); Richard Schoenfeldt (Hospital for Joint Diseases); Jonathan Fuller (Nebraska Foundation for Spinal Research); Harvinder Sandhu (Hospital for Special Surgery); Scott Boden (Emory); Carolyn Murray (Dartmouth); Michael Longley (Nebraska Foundation for Spinal Research); Ronald Moskovich (Hospital for Joint Diseases); Keith Bridwell (Washington University); John McClellan (Nebraska Foundation for Spinal Research); Lawrence Lenke (Washington University); Ferdy Massimino (Kaiser Permanente); Lawrence Kurz (Beaumont); Joseph Dryer (Hospital for Joint Diseases); Sanford Emery (University Hospitals of Cleveland/Case Western Reserve); Susan Dreyer, Howard Levy (Emory); Patrick Bowman (Nebraska Foundation for Spinal Research); Thomas Errico (Hospital for Joint Diseases); Lee Thibodeau, MD (Maine Spine and Rehabilitation); Jeffrey Fischgrund (Beaumont); Mark Splaine (Dartmouth); John Bendo (Hospital for Joint Diseases); Taylor Smith (University of California–San Francisco); Eric Phillips (Nebraska Foundation for Spinal Research); Dilip Sengupta (Dartmouth); David Hubbell (Emory); Henry Schmidek (Dartmouth); Harley Goldberg (Kaiser); Robert Rose (Dartmouth); Sig Berven (University of California–San Francisco); Frank Phillips, Howard An (Rush-Presbyterian-St Luke's Medical Center); Colleen Olson (Dartmouth); Anthony Margherita, John Metzler (Washington University); Jeffrey Goldstein (Hospital for Joint Diseases); Phaedra Mcdonough (Dartmouth); James Farmer (Hospital for Special Surgery); Marsolais (Case Western); Gunnar Andersson (Rush-Presbyterian-St Luke's); Hilda Magnadottir, Jim Weinstein, Jon Lurie (Dartmouth); J. X. Yoo (Case Western); John Heller (Emory); Jeffrey Spivak (Hospital for Joint Diseases); Roland Hazard (Dartmouth); Michael Schaufele (Emory); Jeffrey Florman (Maine Spine and Rehabilitation); Philip Bernini (Dartmouth); Eeric Truumees (Beaumont); K. Daniel Riew (Washington University); Timothy Burd (Nebraska Foundation for Spinal Research); John Rhee (Emory); Henry Bohlman (Case Western); Richard Perry (Hospital for Joint Diseases); Edward Goldberg (Rush-Presbyterian-St Luke's); Christopher Furey (Case Western).
Footnotes
Financial Disclosures: Dr Weinstein reports that he is Editor-in-Chief of Spine, has been a consultant to United Health Care (proceeds are donated to the Brie Fund, a fund for children with disabilities, in the name of his daughter who passed away from leukemia), and has been a consultant to the Foundation for Informed Medical Decision Making, proceeds to the Department of Orthopaedics, Dartmouth. Dr Lurie reports that he receives grant support from St Francis Medical Technologies and American Board of Orthopaedic Surgery; has served on advisory boards for Ortho-MacNeil Pharmaceuticals, the Robert Graham Center of the American Academy of Family Practice, and Centocor; and as a consultant for Myexpertdoctor.com, Pacific Business Group on Health, and the Foundation for Informed Medical Decision Making. Dr A. Tosteson reports receiving funding from St Francis Medical Technologies. Mr Hanscom reports working for the National Spine Network and receiving funding from Medtronic. Dr Boden reports serving as a consultant for Medtronic. Dr Deyo reports receiving research program benefits as a gift to the University of Washington from Synthes, a manufacturer of surgical appliances, from which he receives no personal benefits. No other disclosures were reported.
REFERENCES
- 1.Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects: a prospective investigation. J Bone Joint Surg Am. 1990;72:403–408. [PubMed] [Google Scholar]
- 2.Jensen MC, Brant-Zawadzki MN, Obuchowski N, Modic MT, Malkasian D, Ross JS. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331:69–73. doi: 10.1056/NEJM199407143310201. [DOI] [PubMed] [Google Scholar]
- 3.Saal JA, Saal JS. Nonoperative treatment of herniated lumbar intervertebral disc with radiculopathy. Spine. 1989;14:431–437. doi: 10.1097/00007632-198904000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Weinstein JN, Dartmouth Atlas Working Group . Dartmouth Atlas of Musculoskeletal Health Care. American Hospital Association Press; Chicago, Ill: 2000. [PubMed] [Google Scholar]
- 5.Deyo RA, Weinstein JN. Low back pain. N Engl J Med. 2001;344:363–370. doi: 10.1056/NEJM200102013440508. [DOI] [PubMed] [Google Scholar]
- 6.Weinstein JN, Bronner KK, Morgan TS, Wennberg JE. Trends and geographic variations in major surgery for degenerative diseases of the hip, knee, and spine. Health Aff (Millwood) 2004;(suppl Web exclusive):var81–89. doi: 10.1377/hlthaff.var.81. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman RM, Wheeler KJ, Deyo RA. Surgery for herniated lumbar discs: a literature synthesis. J Gen Intern Med. 1993;8:487–496. doi: 10.1007/BF02600110. [DOI] [PubMed] [Google Scholar]
- 8.Weber H. Lumbar disc herniation: a controlled, prospective study with ten years of observation. Spine. 1983;8:131–140. [PubMed] [Google Scholar]
- 9.Buttermann GR. Treatment of lumbar disc herniation: epidural steroid injection compared with discectomy: a prospective, randomized study. J Bone Joint Surg Am. 2004;86:670–679. [PubMed] [Google Scholar]
- 10.Gibson JN, Grant IC, Waddell G. The Cochrane review of surgery for lumbar disc prolapse and degenerative lumbar spondylosis. Spine. 1999;24:1820–1832. doi: 10.1097/00007632-199909010-00012. [DOI] [PubMed] [Google Scholar]
- 11.Gibson JN, Grant IC, Waddell G. Surgery for lumbar disc prolapse. Cochrane Database Syst Rev. 2000;(3):CD001350. doi: 10.1002/14651858.CD001350. [DOI] [PubMed] [Google Scholar]
- 12.Jordan J, Shawver Morgan T, Weinstein J, Konstantinou K. Herniated lumbar disc. Clin Evid. 2003 June;:1203–1215. [PubMed] [Google Scholar]
- 13.Birkmeyer NJ, Weinstein JN, Tosteson AN, et al. Design of the Spine Patient Outcomes Research Trial (SPORT) Spine. 2002;27:1361–1372. doi: 10.1097/00007632-200206150-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fardon DF, Milette PC. Nomenclature and classification of lumbar disc pathology: recommendations of the Combined Task Forces of the North American Spine Society, American Society of Spine Radiology, and American Society of Neuroradiology. Spine. 2001;26:E93–E113. doi: 10.1097/00007632-200103010-00006. [DOI] [PubMed] [Google Scholar]
- 15.Delamarter R, McCullough J. Microdiscectomy and microsurgical laminotomies. In: Frymoyer J, editor. The Adult Spine: Principles and Practice. 2nd ed. Lippincott-Raven Publishers; Philadelphia, Pa: 1996. [Google Scholar]
- 16.Spengler DM. Lumbar discectomy: results with limited disc excision and selective foraminotomy. Spine. 1982;7:604–607. [PubMed] [Google Scholar]
- 17.Cummins J, Lurie JD, Tosteson T, et al. Descriptive epidemiology and prior healthcare utilization of patients in the Spine Patient Outcomes Research Trial's (SPORT) three observational cohorts: disc herniation, spinal stenosis, and degenerative spondylolisthesis. Spine. 2006;31:806–814. doi: 10.1097/01.brs.0000207473.09030.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ware JE, Jr, Sherbourne D. The MOS 36-item short-form health survey (SF-36), I: conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 19.Ware JE., Jr . SF-36 Health Survey: Manual and Interpretation Guide. Nimrod Press; Boston, Mass: 1993. [Google Scholar]
- 20.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36), III: tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32:40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Stewart AL, Greenfield S, Hays RD, et al. Functional status and well-being of patients with chronic conditions: results from the Medical Outcomes Study. JAMA. 1989;262:907–913. [PubMed] [Google Scholar]
- 22.Daltroy LH, Cats-Baril WL, Katz JN, Fossel AH, Liang MH. The North American Spine Society lumbar spine outcome assessment instrument: reliability and validity tests. Spine. 1996;21:741–749. doi: 10.1097/00007632-199603150-00017. [DOI] [PubMed] [Google Scholar]
- 23.Deyo RA, Diehl AK. Patient satisfaction with medical care for low-back pain. Spine. 1986;11:28–30. doi: 10.1097/00007632-198601000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Atlas SJ, Deyo RA, Patrick DL, Convery K, Keller RB, Singer DE. The Quebec Task Force classification for spinal disorders and the severity, treatment, and outcomes of sciatica and lumbar spinal stenosis. Spine. 1996;21:2885–2892. doi: 10.1097/00007632-199612150-00020. [DOI] [PubMed] [Google Scholar]
- 25.Patrick DL, Deyo RA, Atlas SJ, Singer DE, Chapin A, Keller RB. Assessing health-related quality of life in patients with sciatica. Spine. 1995;20:1899–1908. doi: 10.1097/00007632-199509000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Phelan EA, Deyo RA, Cherkin DC, et al. Helping patients decide about back surgery: a randomized trial of an interactive video program. Spine. 2001;26:206–211. doi: 10.1097/00007632-200101150-00016. [DOI] [PubMed] [Google Scholar]
- 27.Weinstein JN. Partnership: doctor and patient: advocacy for informed choice vs. informed consent. Spine. 2005;30:269–272. doi: 10.1097/01.brs.0000155479.88200.32. [DOI] [PubMed] [Google Scholar]
- 28.Friedman L, Furberg C, DeMets D. Fundamentals of Clinical Trials. 3rd ed. Springer-Verlag; Cambridge, Mass: 1998. The randomization process; pp. 61–81. [Google Scholar]
- 29.Atlas SJ, Deyo RA, Keller RB, et al. The Maine Lumbar Spine Study, II: 1-year outcomes of surgical and nonsurgical management of sciatica. Spine. 1996;21:1777–1786. doi: 10.1097/00007632-199608010-00011. [DOI] [PubMed] [Google Scholar]
- 30.Little R, Rubin D. Statistical Analysis With Missing Data. 2nd ed. John Wiley & Sons; Philadelphia, Pa: 2002. [Google Scholar]
- 31.Diggle P, Haeagery P, Liang K, Zeger S. The Analysis of Longitudinal Data. 2nd ed. Oxford University Press; Oxford, England: 2002. [Google Scholar]
- 32.Fitzmaurice G, Laird N, Ware J. Applied Longitudinal Analysis. John Wiley & Sons; Philadelphia, Pa: 2004. [Google Scholar]
- 33.Altman DG, Schulz KF, Moher D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 2001;134:663–694. doi: 10.7326/0003-4819-134-8-200104170-00012. [DOI] [PubMed] [Google Scholar]
- 34.Meinert CL. Clinical Trials: Design, Conduct, and Analysis. Oxford University Press; New York, NY: 1986. [Google Scholar]
- 35.Kuppermann M, Varner RE, Summitt RL, Jr, et al. Effect of hysterectomy vs medical treatment on health-related quality of life and sexual functioning: the medicine or surgery (Ms) randomized trial. JAMA. 2004;291:1447–1455. doi: 10.1001/jama.291.12.1447. [DOI] [PubMed] [Google Scholar]
- 36.Carragee EJ, Han MY, Suen PW, Kim D. Clinical outcomes after lumbar discectomy for sciatica: the effects of fragment type and anular competence. J Bone Joint Surg Am. 2003;85:102–108. [PubMed] [Google Scholar]
- 37.Spangfort EV. The lumbar disc herniation: a computer-aided analysis of 2,504 operations. Acta Orthop Scand Suppl. 1972;142:1–95. doi: 10.3109/ort.1972.43.suppl-142.01. [DOI] [PubMed] [Google Scholar]
- 38.Agency for Health Care Policy and Research . Acute Low Back Problems in Adults. US Dept of Health & Human Services; Bethesda, Md: 1994. [Google Scholar]
- 39.North American Spine Society . North American Spine Society Phase III Clinical Guidelines for Multidisciplinary Spine Care Specialists. NASS; LaGrange, Ill: 2000. Herniated disc. [Google Scholar]