Abstract
Background
Birth size, perhaps a proxy for prenatal environment, might be a correlate of subsequent breast cancer risk, but findings from epidemiological studies have been inconsistent. We re-analysed individual participant data from published and unpublished studies to obtain more precise estimates of the magnitude and shape of the birth size–breast cancer association.
Methods and Findings
Studies were identified through computer-assisted and manual searches, and personal communication with investigators. Individual participant data from 32 studies, comprising 22,058 breast cancer cases, were obtained. Random effect models were used, if appropriate, to combine study-specific estimates of effect. Birth weight was positively associated with breast cancer risk in studies based on birth records (pooled relative risk [RR] per one standard deviation [SD] [= 0.5 kg] increment in birth weight: 1.06; 95% confidence interval [CI] 1.02–1.09) and parental recall when the participants were children (1.02; 95% CI 0.99–1.05), but not in those based on adult self-reports, or maternal recall during the woman's adulthood (0.98; 95% CI 0.95–1.01) (p for heterogeneity between data sources = 0.003). Relative to women who weighed 3.000–3.499 kg, the risk was 0.96 (CI 0.80–1.16) in those who weighed < 2.500 kg, and 1.12 (95% CI 1.00–1.25) in those who weighed ≥ 4.000 kg (p for linear trend = 0.001) in birth record data. Birth length and head circumference from birth records were also positively associated with breast cancer risk (pooled RR per one SD increment: 1.06 [95% CI 1.03–1.10] and 1.09 [95% CI 1.03–1.15], respectively). Simultaneous adjustment for these three birth size variables showed that length was the strongest independent predictor of risk. The birth size effects did not appear to be confounded or mediated by established breast cancer risk factors and were not modified by age or menopausal status. The cumulative incidence of breast cancer per 100 women by age 80 y in the study populations was estimated to be 10.0, 10.0, 10.4, and 11.5 in those who were, respectively, in the bottom, second, third, and top fourths of the birth length distribution.
Conclusions
This pooled analysis of individual participant data is consistent with birth size, and in particular birth length, being an independent correlate of breast cancer risk in adulthood.
Editors' Summary
Background.
Last year, more than one million women discovered that they had breast cancer. In the US, nearly 200,000 women will face the same diagnosis this year and 40,000 will die because of breast cancer. Put another way, about one in eight US women will have breast cancer during her lifetime. Like all cancers, breast cancer begins when cells acquire genetic changes that allow them to divide uncontrollably and to move around the body (metastasize). This uncontrolled division leads to the formation of a lump that can be detected by mammography (a breast X-ray) or by manual examination of the breasts. Breast cancer is treated by surgical removal of the lump or, if the cancer has started to spread, by removal of the whole breast (mastectomy). Surgery is usually followed by radiotherapy, chemotherapy, and other treatments designed to kill any remaining cancer cells. Unlike some cancers, the outlook for women with breast cancer is good. In the US, for example, nearly 90% of affected women are still alive five years after their diagnosis.
Why Was This Study Done?
Scientists have identified several factors that increase a woman's risk of developing breast cancer by comparing the characteristics of populations of women with and without breast cancer. Well-established risk factors include increasing age, not having children, and having a late menopause, but another potential risk factor for breast cancer is birth size. A baby's weight, length, and head circumference at birth (three related measures of birth size) depend on the levels of hormones (including estrogen, a hormone that often affects breast cancer growth) and other biological factors to which the baby is exposed during pregnancy—its prenatal environment. The idea that prenatal environment might also affect breast cancer risk in later life was first proposed in 1990, but the findings of studies that have tried to investigate this possibility have been inconsistent. Here, the researchers re-analyze individual participant data from a large number of studies into women's health conducted in Europe, Northern America, and China to get more precise information about the association between birth size and breast cancer risk.
What Did the Researchers Do and Find?
The researchers identified 32 published and unpublished studies that had collected information on birth size and on the occurrence of breast cancer. They then obtained the individual participant data from these studies, which involved more than 22,000 women who had developed breast cancer and more than 600,000 women who had not. Their analyses of these data show that birth weight was positively associated with breast cancer risk in those studies where this measurement was recorded at birth or based on parental recall during the study participant's childhood (but not in those studies in which birth weight was self-reported or maternally recalled during the participant's adulthood). For example, women with recorded birth weights of more than 4 kg or more had a 12% higher chance of developing breast cancer than women who weighed 3–3.5 kg at birth. Birth length and head circumference were also positively associated with breast cancer risk, but birth length was the strongest single predictor of risk. Finally, the amount by which birth size affected breast cancer risk was not affected by allowing for other established risk factors.
What Do These Findings Mean?
These findings provide strong evidence that birth size—in particular, birth length—is a marker of a woman's breast cancer risk in adulthood although the mechanisms underlying this association are unclear. The researchers note that the observed effect of birth size on breast cancer risk is of a similar magnitude to that of other more established risk factors and estimate that 5% of all breast cancers in developed countries could be caused by a high birth size. Because practically all the studies included in this pooled analysis were done in developed countries, these findings may not hold for developing countries. Further investigations into how the prenatal environment may affect breast cancer risk might identify new ways to prevent this increasingly common cancer.
Additional Information.
Please access these Web sites via the online version of this summary at http://dx.doi.org/10.1371/journal.pmed.0050193.
This study is further discussed in a PLoS Medicine Perspective by Trichopoulos and Lagiou
The US National Cancer Institute provides detailed information for patients and health professionals on all aspects of breast cancer, including information on risk factors for breast cancer (in English and Spanish)
The MedlinePlus Encyclopedia provides information for patients about breast cancer; Medline Plus also provides links to many other breast cancer resources (in English and Spanish)
The UK charity Cancerbackup also provides detailed information about breast cancer
Cancer Research UK is the UK's leading charity dedicated to cancer research
Introduction
In 1990 Trichopoulos [1] suggested that prenatal exposure to high levels of pregnancy oestrogens might affect the risk of breast cancer. This hypothesis, which has since evolved to include other in utero hormonal and biological factors [2], sparked a considerable amount of research on the prenatal origins of breast cancer, relying mainly on birth size measures as indirect markers of the in utero environment. Published estimates of the strength of the association between birth size and breast cancer, however, have been far from consistent [3–33], and several unanswered questions remain, including uncertainty regarding the magnitude and shape of the association as well as the extent to which it may be mediated, confounded, and/or modified by known breast cancer risk factors.
We set up a collaborative group to bring together and re-analyse the original individual participant data from published and unpublished studies on pre- and perinatal factors and subsequent risk of breast cancer. This paper reports on the birth size–breast cancer association. This re-analysis provides several scientific advantages over previously published overviews [34–36]. First, it is large and comprehensive, comprising published and unpublished information on over 22,000 breast cancer cases from 32 studies, many of which have been enlarged since their original publications. Second, the availability of primary data from each individual participant provided a unique opportunity to estimate study-specific effects using similar definitions and adjustments across studies. Third, it allowed a detailed investigation of between-study heterogeneity and its possible sources. Fourth, study-specific data could be combined, if appropriate, to produce far more precise estimates of the association of birth size with breast cancer risk than those obtained from any single study.
Methods
Identification of Studies and Data Extraction
We attempted to identify studies that collected information on at least one measure of birth size and were based on incident breast cancer cases. Studies were identified by computer-assisted searches (including PubMed and Embase) up to the end of June 2007, manual searches of reference lists, personal communication with investigators, and publicity regarding our collaboration in international conferences. The search strategy used the term “breast cancer” in combination with “birth weight,” “birth size,” “birth length,” “head circumference,” “ponderal index [PI],” and “gestational age” (details of search strategy available on request). A total of 27 published and seven unpublished cohort and case-control studies [3–33] were identified, including two twin studies [10,11] and a cohort of premature or very low birth weight babies [9,21]. One study [32] was excluded because most of its participants contributed to a larger unpublished study (Swedish Young Female Breast Cancer [SYFBC], Table S1) and another [33] because its original individual participant data could not be retrieved. The included studies refer to independent study populations, with the exception of two (Seattle Breast Cancer in Young Women [BCYW] [4] and Seattle Perinatal Factors and Breast Cancer [PFBC] [7], Table S1) that were conducted in the same population but used different sources of birth size information. Data from the smallest one (Seattle PFBC; 442 cases and 393 non-cases) were excluded from the analyses whenever appropriate (as indicated in Figures 1, 2, S1, and S2). Each participating study had previously obtained all relevant ethics approvals; only nonidentifiable data were sent to the London School of Hygiene & Tropical Medicine (LSHTM).
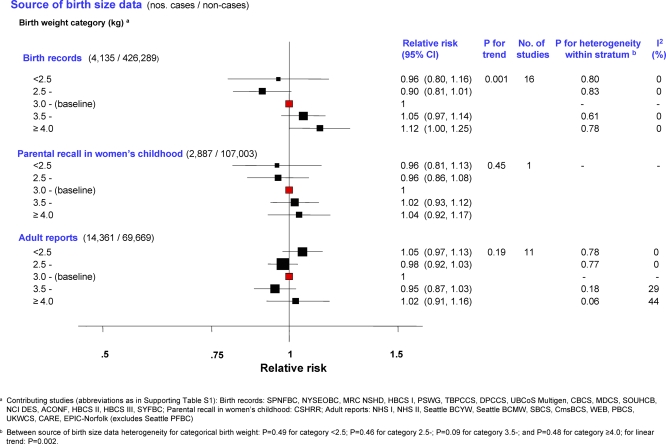
Figure 1. Minimally Adjusted Pooled Breast Cancer RRs Stratified by Source of Birth Size Data in Relation to Categorical Birth Weight (Singleton Studies Only).
The area of the black squares is inversely proportional to the variance (on the log scale).
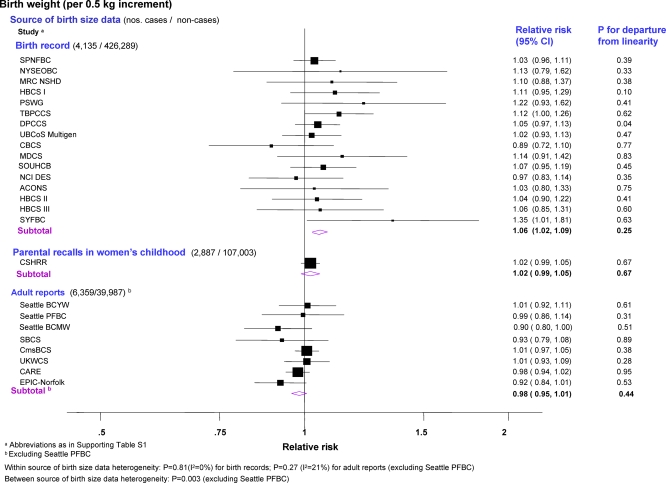
Figure 2. Minimally Adjusted Study-Specific and Pooled Breast Cancer RRs Stratified by Source of Birth Size Data in Relation to Continuous Birth Weight (Singleton Studies Only).
The area of the black squares is inversely proportional to the variance (on the log scale).
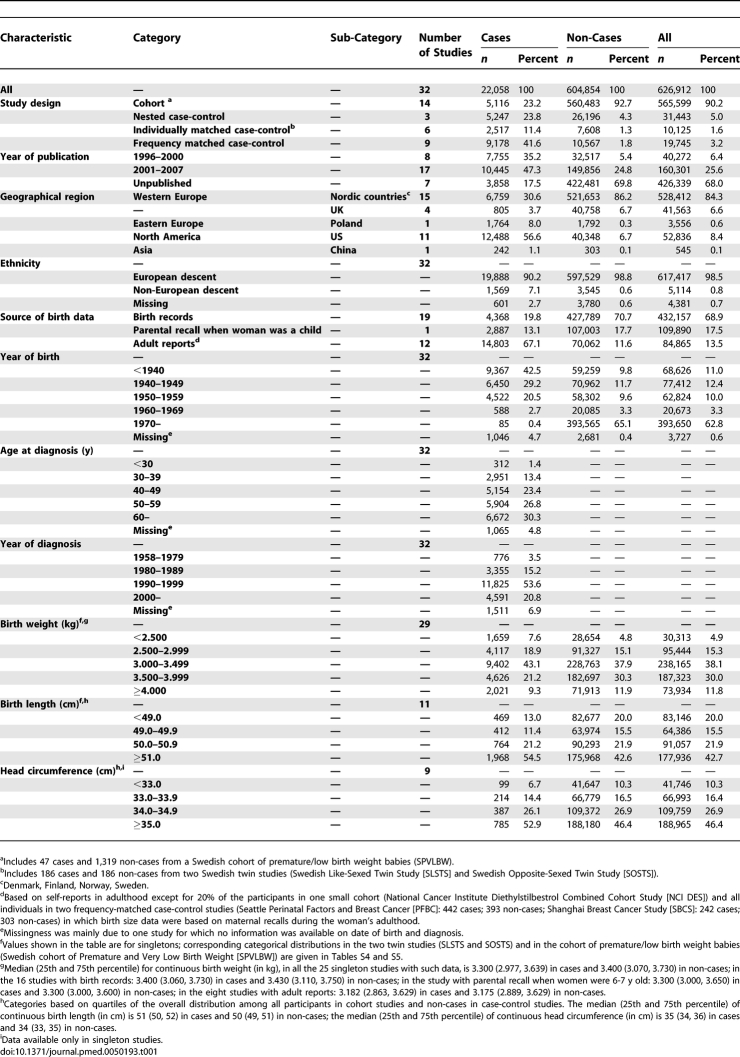
Data on individual participants were obtained in a standardised format. They included measures of birth size (i.e., weight, length, and head circumference) and, if available, data on potential confounding factors, mediators, and effect modifiers (Tables 1, S1–S3). These individual-level data were centrally collated and crosschecked at LSHTM, with data quality queries clarified by the principal investigators. As the birth size distributions were very different in the twin studies [10,11] and in the cohort study of premature/low birth weight babies [9,21] (Tables S4–S6), these were examined separately. Analyses were restricted to singletons in the remaining studies and will hereafter be referred to as singleton studies. Participants were further excluded from all studies if they had a known history of cancer other than nonmelanoma skin cancer at entry into the study (i.e., at recruitment/start of follow-up), and if all birth size data were missing. For the two Nurses Health Studies [31], only nested case-control data were provided for the pooled analyses. Because of these exclusions and updated follow-up/recruitment in some studies [22,23,27,31], study sizes may differ from those reported in the original study-specific publications.
Table 1.
Summary Characteristics of the 32 Participating Studies
Statistical Methods
The primary exposure of interest was birth size as measured by weight (kg), length (cm), head circumference (cm), and PI (defined as weight [kg]/height [m]3) at birth. These measures were examined as quantitative (for increments of approximately one standard deviation [SD], i.e., 0.5 kg for weight, 2 cm for length, 1.5 cm for head circumference, and 2.5 kg/m3 for PI) and as categorical variables. In the analyses of singleton studies, birth weight was categorised according to commonly used categories (<2.500, 2.500–2.999, 3.000–3.499 [baseline], 3.500–3.999, and ≥4.000 kg); for four studies [29–31] birth weight data were only available as predefined categories equivalent to these except in one study [30] in which the three middle categories were collapsed into a single one (Table S4). Categories for birth length, head circumference, and PI were defined by quartiles of their overall distributions among all participants in cohort studies and non-cases in case-control studies. Study-specific quartiles for birth weight, length, and PI (no data were available for head circumference) were similarly generated in each twin study [10,11] and in the cohort of premature/low birth weight babies [21].
Assessment of birth size–breast cancer associations was performed primarily using a two-stage approach [37,38]. Study-specific effects were first estimated and, if appropriate, pooled using a random effects model under the assumption that individual studies estimate different exposure effects because of potential heterogeneities in populations and data quality, but with the interest focused on their mean value. These pooled effects will hereafter be referred to as “two-stage,” with the standard errors calculated from the inverse of the sum of the adjusted weights [38]. Study-specific effects were estimated as rate ratios in cohort studies and odds ratios in case-control studies (hereafter referred to as relative risks [RRs]) using models appropriate for each study design (i.e., Cox proportional hazard or Poisson regression for cohort studies; conditional logistic regression for nested [all based on risk-set sampling] and individually matched case-control studies; and logistic regression for frequency-matched case-control studies) [39].
The analytical time scale for cohort studies was age, with the beginning of the follow-up defined as the age at recruitment into the study or the age when outcome ascertainment became possible (e.g., through linkage to cancer registries). Follow-up ended at the age of breast cancer diagnosis, death, emigration, or last follow-up, whichever occurred earlier. RRs for cohort studies were thus adjusted for age at diagnosis. The proportional hazards assumption was checked in Cox models graphically, by comparing stratum-specific cumulative incidence curves before fitting the models, and formally via the test of proportionality based on Schoenfeld residuals [40]. In Poisson models the assumption of time-constant effects (i.e., proportionality) was assessed by testing the significance of interactions between birth size measures and age. RRs for cohort studies were additionally adjusted for calendar year by stratification. The matching variables specified for each case-control study (e.g., year of birth, calendar period, recruitment centre, area of residence, or ethnicity) were accounted for in the estimation of RRs either through matched analyses (for individually matched studies) or adjustment (for frequency-matched studies). The RRs quoted in the text refer to these minimally adjusted RRs unless otherwise specified. The statistical significance of each birth size–breast cancer association, and of quadratic departures from the assumption of linearity of effects, were assessed within each study by likelihood ratio tests and for pooled estimates by Wald tests. Possible sources of between-study heterogeneity were investigated and formally tested using the Cochran Q statistic and the I2 quantity based on standard cut-off points [41,42]. Two-stage pooled estimates of groups of RRs were only calculated if there was no statistically significant evidence of systematic heterogeneity. The influence of individual studies was assessed by sequentially dropping each one before pooling study-specific estimates.
To increase statistical precision, one-stage pooled analyses, in which overall pooled estimates are derived from a single model, were also conducted on the subset of cohort studies of singleton women with birth records information. Random effects multivariable Cox regression (frailty) models [43], which account for within study clustering, were fitted to assess exposure-response relationships for each birth size variable and to estimate their joint associations with breast cancer risk. These models were also used to estimate the breast cancer cumulative incidence curve corresponding to the baseline birth length category (i.e., 49.0–49.9 cm). Cumulative incidence curves for the other birth length categories were obtained by multiplying the baseline curve by the corresponding category-specific RRs. A similar approach was used to calculate cumulative incidence curves for the five birth weight categories (taking the 3.000–3.999 kg category as the baseline).
As the availability and classification of potential confounders varied from study to study, adjustment for confounding was performed separately for each variable, or group of variables, within each study and then pooled using the two-stage procedure. Consequently, the number of cases and non-cases involved in each analysis varied accordingly. To assess whether the birth size associations were modified by age, analyses were stratified by age at breast cancer diagnosis (<45 y; 45–54 y, ≥55 y) for case-control studies and by a time-changing indicator of current age for cohort studies. Analyses were similarly stratified by menopausal status at diagnosis in the subset of studies with this information.
Two-stage and one-stage pooled analyses were repeated after excluding extreme birth size observations (i.e., values outside the singleton/twins/premature-specific means ± 4 SDs). Small study bias was assessed via the Egger funnel plot asymmetry test [44] and other forms of publication bias by meta-regression. All statistical analyses were performed in Stata [45]. All tests of significance are two-sided.
Results
Characteristics of the Study Participants
A total of 32 studies contributed to these analyses, including 22,058 women with newly diagnosed invasive or in situ breast cancer and 604,854 non-cases. The characteristics of the participating studies are summarised in Table 1 (further details in Tables S1–S3). Information on birth weight was based on birth records, parental recall when the women were 6–7 y old, mother's recall during the woman's adulthood, and on self-reports in adulthood. In analyses by source of birth weight data, the two last categories produced similar effect estimates and thus were combined into one single category of adult reports. Data on categorical birth weight were available for all 32 studies, whereas data on continuous birth weight and on other measures of birth size were available for a smaller number of studies (Tables 1 and S2).
Birth Size and Breast Cancer Risk
Two-stage pooled analyses of RRs, stratified by source of birth size information, showed that the risk of breast cancer in singletons increased with increasing birth weight categories in studies based on birth records or on parental recalls in childhood (although significant only for the first, p for linear trend (pt) = 0.001), but not in those based on adult reports (Figure 1; study-specific estimates available in Table S4). Continuous analysis of birth weight (restricted to 25 studies; Figure 2) revealed a similar pattern. A 0.5-kg increment (about one SD) in birth weight was associated with a statistically significant increase in risk in studies based on birth records (pooled RR = 1.06 [95% confidence interval (CI) 1.02–1.09]; p = 0.002) and a borderline significant increase in those based on parental recalls when the women were children, but not in studies based on adult reports, with statistical evidence of heterogeneity between birth weight data sources (p = 0.003). Categorical and continuous analyses of birth weight stratified by study design revealed a positive trend in risk with increasing birth weight categories in data from cohort, nested case-control, and individually matched case-control studies (albeit only statistically significantly for the latter), but not in data from frequency-matched case-control studies (Figures S1 and S2). There was evidence of between study-design heterogeneity of the continuous birth weight effect (p = 0.03), but it was accounted for by differences in birth data sources when examined via meta-regression of the birth weight RRs on both study design and birth data sources (p-value for study-type heterogeneity = 0.67; for adult reports versus other sources = 0.08).
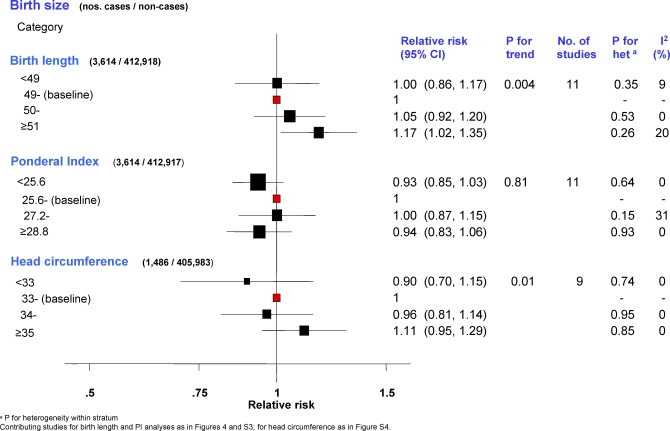
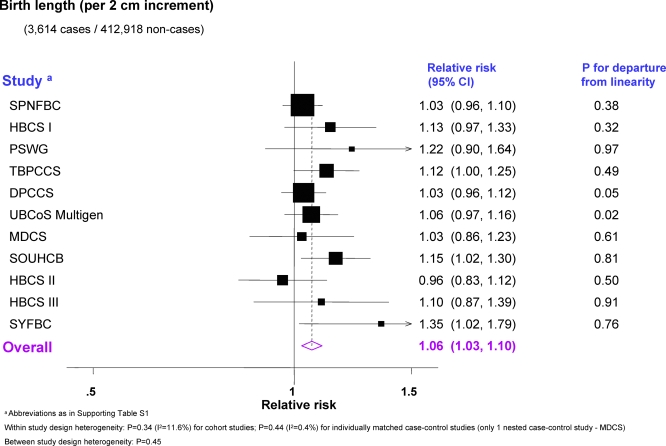
Data on birth length and head circumference were available, respectively, for 11 and nine singleton studies, all derived from birth records. Two-stage pooled analyses stratified by study design showed no statistical evidence of heterogeneity within or between strata in either categorical or continuous analyses (Figures 3, 4, and S4; study-specific estimates available in Tables S5 and S7) and, thus, overall pooled RRs were estimated. Breast cancer risk increased with increasing birth length (pt = 0.004; Figure 3), with women ≥ 51 cm long at birth having 17% (95% CI 2%–35%) higher risk of developing breast cancer relative to those in the baseline category. Women with a head circumference ≥35 cm had an 11% increase (95% CI −5% to 29%) in risk relative to those in the baseline category, whereas those with a head circumference <33 cm had a 10% decrease (95% CI −30% to 15%) (pt = 0.01) (Figure 3). These estimates corresponded to pooled RRs of 1.06 (95% CI 1.03–1.10) per one SD (= 2 cm) increment in birth length (Figure 4) and 1.09 (1.03–1.15) per one SD (= 1.5 cm) increment in head circumference (Figure S4). In contrast, there was no association with categorical (Figure 3) or continuous PI (pooled RR per 2.5 kg/m3 increment = 1.01; 0.97–1.04; Figure S3; study-specific estimates available in Table S6).
Figure 3. Minimally Adjusted Pooled Breast Cancer RRs in Relation to Categorical Birth Length, PI, and Head Circumference (Singleton Studies Only).
The area of the black squares is inversely proportional to the variance (on the log scale).
Figure 4. Minimally Adjusted Study-Specific and Pooled Breast Cancer RRs in Relation to Continuous Birth Length (Singleton Studies Only).
The area of the black squares is inversely proportional to the variance (on the log scale).
The two twin case-control studies, both based on birth records, showed stronger associations of breast cancer risk with continuous birth weight (pooled RR per one SD increment = 1.57; 95% CI 1.20–2.07) and continuous birth length (1.23; 1.01–1.49) than those found among singleton studies, and a positive association with PI (1.36; 1.06–1.75) that was not observed in the latter (Figure S5). No association between any of these birth size measures and breast cancer risk was found in the cohort study of premature/low birth weight babies, which was also based on birth records (Figure S5; study-specific estimates available in Tables S4–S6).
Shape of the Birth Size–Breast Cancer Association in Singletons
In the subset of singletons in cohort studies based on birth records, all with continuous birth size data, the one-stage pooled dose-response plot for birth weight suggested a nonlinear (quadratic) relationship, with RR for women at the extremes of the distribution being slightly lower than predicted by the linear model. However, the test for deviation from linearity was not statistically significant (p = 0.20; Figure S6). The plots for birth length and head circumference were more consistent with a linear association (p-value for departure from linearity: 0.39 and 0.58, respectively; Figure S6).
Independence of Effects of the Various Birth Size Measures in Singletons
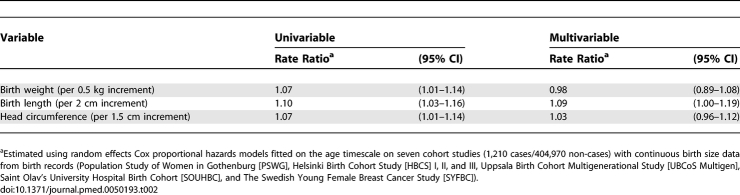
Birth weight and birth length were strongly correlated with each other (r = 0.79, p < 0.001, in the subset of cohorts with birth record data), and both were correlated with head circumference (r = 0.61 and r = 0.51, respectively; p < 0.001 for both). Simultaneous one-stage pooled analysis of these three variables in the subset of cohort studies of singletons based on birth records showed that birth length was the measure with the strongest independent association with breast cancer risk (Table 2). The association with birth weight disappeared after adjustment for birth length and head circumference, while the association with birth length persisted, and remained of borderline significance, after adjustment for birth weight and head circumference.
Table 2.
Separate (Univariable) and Mutually Adjusted (Multivariable) Breast Cancer Incidence Rate Ratios for Continuous Weight, Length, and Head Circumference at Birth in Singletons
In this subset of cohort studies, all from developed countries, the cumulative incidence of breast cancer by age 80 y is estimated to be 10.0 per 100 singleton women in those who were shorter than 49 cm at birth and 10.0, 10.4, 11.5, respectively, per 100 singleton women who were 49.0–49.9, 50.0–50.9, and ≥51.0 cm at birth. Similarly, and as data on birth weight are more widely available, the cumulative incidence is estimated to change from 10.0 per 100 singleton women in those who weighed less than 2.500 kg at birth to 9.4, 10.4, 10.9, and 11.6, respectively, per 100 singleton women who weighed 2.500–2.999, 3.000–3.499, 3.500–3.999, and ≥4.000 kg at birth. About 45%–50% of women in these cohorts were ≥ 50 cm long, or ≥ 3.5 kg, at birth. If the observed effect estimates are valid, and assuming that birth size reflects some underlying process that is causally related to breast cancer, it is estimated that about 4.5%–5.0% of breast cancers by age 80 y in these study populations are attributable to length ≥ 50 cm, or weight ≥ 3.5 kg, at birth.
Consistency of the Findings
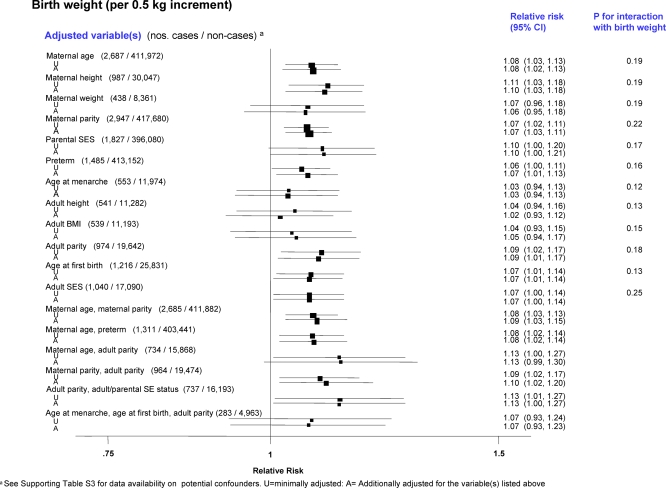
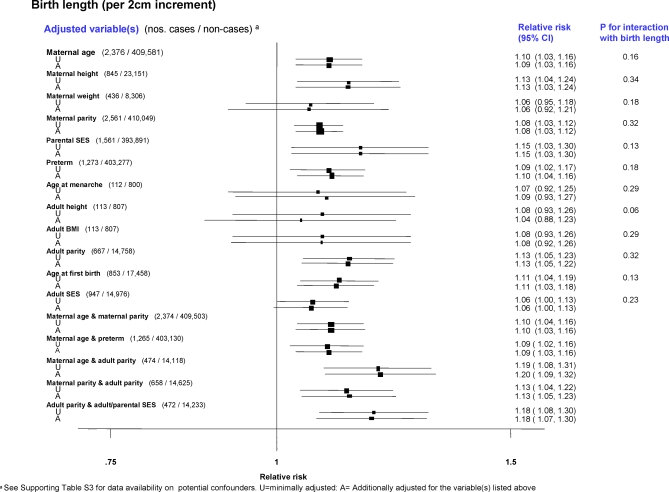
There was little evidence that the associations between the various birth size variables and breast cancer risk in singletons were confounded or modified by other peri- and postnatal factors in data from birth records (Figures 5 and 6), or adult reports (Figure S7). Even variables that were significantly associated with birth size, such as maternal height and maternal parity (e.g., correlation coefficients with birth length: r = 0.24 and r = 0.07, respectively; p < 0.001 for both), woman's adult height (r = 0.29, p < 0.001) and parental and woman's adulthood measures of socioeconomic status (SES) (p < 0.01 and p < 0.05, respectively), did not explain the positive association with breast cancer risk. In particular, adjustment for maternal height or the woman's adult height attenuated only slightly the effects of birth weight and length (Figures 5 and 6). Similarly, adjustment for the woman's adult body mass index (BMI) did not affect the birth size effects (Figures 5 and 6). Neither continuous gestational age (pooled RR per 1 wk increment = 0.99; 95% CI 0.96–1.03) or being preterm (unpublished data) was associated with breast cancer risk. Few studies collected data on the woman's use of oral contraceptives (OC) or hormone replacement therapy (HRT) (Table S3), but their findings indicate that the birth size–breast cancer associations reported here are unlikely to have been confounded by ever use of these exogenous hormones (minimally adjusted and OC-adjusted pooled RRs per 2 cm increment in birth length: 1.14 (95% CI 0.82–1.61) and 1.14 (0.82–1.61), respectively; similarly, minimally adjusted and HRT-adjusted pooled RRs: 1.08 (0.92–1.25) and 1.07 (0.86–1.34), respectively).
Figure 5. Pooled Breast Cancer RRs, Minimally Adjusted and Further Adjusted for Various Potential Confounding Factors in Relation to Continuous Birth Weight (Restricted to Singleton Studies Based on Birth Records).
Adjustments for maternal age (continuous), maternal height (continuous), maternal weight (continuous), maternal parity (categorical: 0, 1, 2, ≥3), parental SES (study-specific categories: paternal SES for Medical Research Council National Survey of Health and Development [MRC NSHD]; maternal SES for Saint Olav's University Hospital Birth Cohort [SOUHCB], Trondheim & Bergen Population-based Case-Control Study [TBPCCS], and Swedish study on Pre-Natal Factors and Breast Cancer [SPNFBC]; and parental/paternal occupation for Carolina Breast Cancer Study [CBCS]), preterm (binary), age at menarche (categorical: <12 y, 12.0–12.9 y, ≥13 y), adult height (continuous), adult BMI (continuous), adult parity (categorical: 0, 1, 2, ≥3), age at first birth (categorical: nulliparous, <20 y, 20–29 y, ≥30 y), and adult SES (study-specific categories: adult SES for MRC NSHD, Helsinki Birth Cohort Study [HBCS] I, Population Study of Women in Gothenburg [PSWG]; and occupation for Malmö Diet and Cancer Study [MDCS]).
Figure 6. Pooled Breast Cancer RRs, Minimally Adjusted and Further Adjusted for Various Potential Confounding Factors in Relation to Continuous Birth Length (Restricted to Singleton Studies Based on Birth Records).
Adjustments for maternal age (continuous), maternal height (continuous), maternal weight (continuous), maternal parity (categorical: 0, 1, 2, ≥3), parental SES (study-specific categories: paternal SES for Medical Research Council National Survey of Health and Development [MRC NSHD]; maternal SES for Saint Olav's University Hospital Birth Cohort [SOUHCB], Trondheim & Bergen Population-based Case-Control Study [TBPCCS] and Swedish study on Pre-Natal Factors and Breast Cancer [SPNFBC]; and parental/paternal occupation for Carolina Breast Cancer Study [CBCS]), preterm (binary), age at menarche (categorical: <12 y, 12.0–12.9 y, ≥13 y), adult height (continuous), adult BMI (continuous), adult parity (categorical: 0, 1, 2, ≥3), age at first birth (categorical: nulliparous, <20 y, 20–29 y, ≥30 y), and adult SES (study-specific categories: adult SES for MRC NSHD, Helsinki Birth Cohort Study [HBCS] I, Population Study of Women in Gothenburg [PSWG]; and occupation for Malmö Diet and Cancer Study [MDCS]).
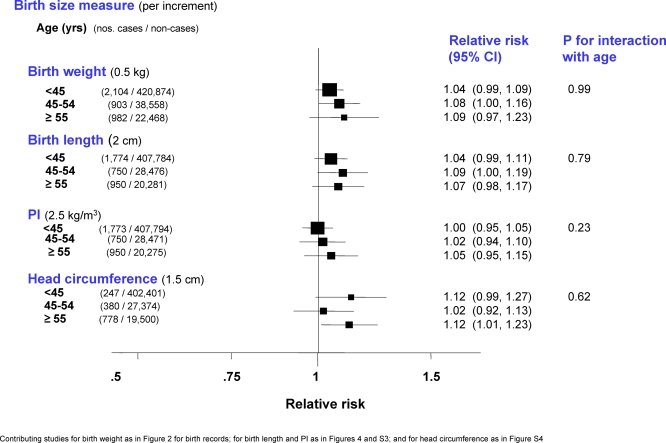
The effect of categorical (Figure S8; study-specific estimates available in Table S8) or continuous birth weight (Figure 7) was not modified by age (defined as current age for cohort studies and age at diagnosis for case-control studies). There was also no evidence of any interaction between age and continuous birth length, PI, or head circumference (Figure 7). Menopausal status was known for 33% of the cases, but analysis restricted to the subset of studies with this information showed no difference in the birth size effects between pre- and postmenopausal women.
Figure 7. Minimally Adjusted Pooled Breast Cancer RRs in Relation to Continuous Weight, Length, PI, and Head Circumference at Birth Stratified by Age (Restricted to Singleton Studies Based on Birth Records).
Sensitivity analysis showed that none of the categorical or continuous pooled birth size associations reported here was dominated by any single study. The association of birth size with breast cancer risk in birth record data persisted after exclusion of 1,510 in situ tumours among the case-control studies and censoring 28 diagnoses of in situ tumours in cohort studies (6% of all cases). Birth cohort (defined by the median year of birth in each study) and geographical area (North America, Western Europe, Eastern Europe, and Asia) did not explain any further between-study heterogeneity beyond that accounted for by source of birth size data.
The findings did not appear to be affected by study size bias (Egger funnel plot asymmetry test: p = 0.20). There was no statistical evidence of publication bias among the studies included in these analyses (Table S9).
Discussion
We analysed individual participant data on over 22,000 women with breast cancer from 32 epidemiological studies of the association between birth size and breast cancer. This pooled analysis provided evidence of moderate positive trends in the risk of breast cancer among studies based on birth records, with risk increasing with increasing birth weight, length, and head circumference. Source of birth size data was identified as the main source of between-study heterogeneity, with positive associations of birth size with breast cancer risk found only in data from birth records and, to a lesser extent, in data from parental recalls when the women were aged 6–7 y, but not in data from self-reports or maternal recalls when the women were adults.
Simultaneous adjustment for weight, length and head circumference at birth showed that length, perhaps as a measure of linear growth, was the strongest predictor of risk despite the fact that the latter tends to be more poorly measured than weight or head circumference [46,47]. Such finding should not however be overinterpreted because of the strong collinearities among these variables.
The birth size effect did not appear to be confounded or modified by known breast cancer risk factors. In particular, and contrary to previous reports [18,23,31], there was no evidence that the birth size effect was stronger for premenopausal breast cancer. The association between birth size and breast cancer risk was observed consistently in women born over a period of several decades, and in different geographical areas.
Strengths and Limitations
Because of its large size this pooled analysis provided greater statistical power than any of the contributing individual studies and, therefore, more precise estimates than those previously published. It was also possible to standardise the way in which the exposure and confounding variables were defined and coded, the choice of which variables to control for, and the type of analysis conducted, thereby removing these potential sources of heterogeneity across studies. The possible influence of bias needs to be considered. Publication bias is a general problem for pooled analyses. Because inclusion in this pooled analysis was not dependent on publication, this re-analysis is likely to have been less affected by publication bias than meta-analyses of the published literature. The two nonparticipating studies [32,33] showed no association between birth weight and breast cancer, but they were based on small numbers of cases (12 and 74, respectively). We found no evidence of publication bias when examining the effect of study size, or year and type of publication.
Bias within studies, such as information or selection bias, might also have influenced the results. Exposure measurement error could have been a problem as we found evidence of statistical heterogeneity of effects by source of birth size data. Reports of birth weight by the participants themselves in adulthood, or by their mothers when the participants were adults, are likely to be more prone to measurement error than those based on birth records or on parental recall when the participants were children. This remark is consistent with the clear digit preference patterns found in the birth weight data reported by the women themselves, or their parents, but not in those from birth records (unpublished data). These errors are, however, likely to be mainly nondifferential and so likely to impose an attenuating bias in univariable analyses that use these sources of data as exposure measurements. Although differential misclassification is possible in studies in which exposure information was collected after diagnosis, it is unlikely that participants would have been aware of a possible link of birth size with breast cancer risk. Thus, the variability in results across the various sources of birth weight data might simply reflect different degrees of attenuation of the true birth weight effect due to different levels of random exposure misclassification. Selection bias could have been a problem, particularly in case-control studies. Although all case-control studies in this re-analysis were population-based, selection bias might still have occurred in studies with relatively low participation. We did not find evidence that studies with low participation levels provided systematically discrepant results (p for heterogeneity = 0.88). Bias due to incomplete follow-up is unlikely because all cohorts had high degrees of completeness.
Finally, the impact of potential confounding factors was evaluated by comparing effect estimates unadjusted and adjusted for single or multiple potential confounders. The results showed little variation. The availability of information on many potential confounding variables is a major strength of our pooled analysis. One drawback is that information for many of them was restricted to a few studies and therefore we could only assess the impact of each potential confounder separately, or only of groups with few of them at a time, when pooling data. Moreover, some of these factors were probably measured with some error. Thus, we cannot exclude residual or unmeasured confounding by these or other factors.
Biological Plausibility and New Perspectives
These results provide no direct evidence about possible mechanisms underlying the birth size–breast cancer association. Trichopoulos's initial assumption [1] was that birth size was a correlate of foetal oestrogen exposure. Oestriol represents 90% of the oestrogens produced during pregnancy [48]. Birth size indicators have been found to be correlated with maternal oestriol levels [49] but, not with foetal levels [50]. Maternal and/or foetal levels of other growth factors, such as insulin-like growth factors [51,52], leptin and adiponectin [53–55], and alpha-phetoprotein [56] have also been reported to be associated with birth size. The maternal and/or foetal hormonal environment associated with large birth size may alter programming of the breast, making it more susceptible to cancer initiation by endogenous hormone levels and other carcinogens later in life [57]. This altered programming may involve epigenetic changes in the expression of genes linked to cell proliferation, survival, and differentiation; these changes are likely to occur in the foetal mammary stem cells that give rise to all mammary epithelial structures and/or in cells that influence stem cell self-renewal and fate [58]. If pregnancy hormones are the real exposure of interest the use of a surrogate measure, such as birth size, may lead to considerable exposure misclassification with likely attenuation of the true effect. A moderate correlation of birth size with pregnancy hormone levels of about r = 0.35, as found with maternal oestriol [49,59], implies that the observed RR of 1.06 per one SD increment in birth weight would correspond to a RR as large as 1.17 for one SD increase in the underlying true exposure (although the corresponding 95% CI would be wider). If, however, the in utero origins of breast cancer result from a complex interplay of several hormonal and nonhormonal processes [60], birth size may, in fact, be a better cumulative summary measure of all relevant exposures than measured levels of any single hormone.
Foetal growth is a predictor of a woman's growth and development during childhood and early adult life, and both age at menarche and adult height [61] are associated with breast cancer risk. Thus, the observed association between foetal growth and breast cancer may be partly mediated through postnatal growth. This pathway would be consistent with our finding of a stronger association of breast cancer with birth length than birth weight, as birth length for gestational age has been shown to be a stronger predictor of adult height than birth weight for gestational age [62,63]. However the magnitude of the birth size effect was only slightly reduced after adjustment for adulthood height (but on the basis of a small number of cases, Figures 5 and 6), suggesting that the effect of birth size on risk may be only partly mediated through childhood growth [22,23]. Similarly, the woman's BMI in adulthood did not confound the birth size–breast cancer associations. This was true even at premenopausal ages when adult BMI was inversely associated with breast cancer risk and thus any potential confounding by this variable would have lead to an underestimation of the true birth size effects.
Conclusions
This pooled analysis of individual participant data provides a comprehensive and detailed description of the association between birth size and breast cancer risk. Its findings are consistent with positive associations at both pre- and postmenopausal ages, and are largely independent of postnatal risk factors including adult body size. This study is an important addition to previous meta-analyses of published results [34–36] as it offers a comprehensive assessment of possible sources of between-study heterogeneity, and it clarifies the role of several potential confounders, mediators, and effect modifiers. The magnitude of the observed effect, although modest, is similar to those reported for other more established breast cancer risk factors. The RR per one SD increment in birth length of 9% is of similar magnitude to that associated with one SD increase in adult height in our data, and similar to an increase of about 7% for each additional 10 g of alcohol consumed on a daily basis [64]. Assuming causality, we estimated that about 5% of all breast cancers in developed countries could be attributable to high birth size (length ≥ 50 cm or weight ≥ 3.5 kg). The prevalence of high birth weight has been increasing in many countries [65,66], consequent to rises in maternal prepregnancy BMI and maternal weight gain during pregnancy [67–69], but as this increase appears to reflect mainly rises in PI rather than birth length [70] it may not necessarily translate into an increase in the population attributable fraction. Even if real, the positive association of birth size with breast cancer would have to be interpreted in the context of U-shaped inverse associations of birth size with all-cause mortality [71], particularly mortality from circulatory diseases [71]. Nevertheless, continued investigation of the pathways through which prenatal factors may affect breast cancer risk, and the extent to which their effects may be mediated or modified by later life risk factors, may identify new targets for prevention of this disease in the future.
Supporting Information
(25 KB DOC)
(27 KB DOC)
The area of the black squares is inversely proportional to the variance (on the log scale).
(51 KB PDF)
The area of the black squares is inversely proportional to the variance (on the log scale).
(15 KB PDF)
The area of the black squares is inversely proportional to the variance (on the log scale).
(13 KB PDF)
The area of the black squares is inversely proportional to the variance (on the log scale).
(13 KB PDF)
(12 KB PDF)
Analyses restricted to the subset of cohort studies with birth record data: 1,210 cases / 404,970 non-cases (see Table 2). Small circle, estimated category-specific minimally adjusted RRs (reference category: 3.000–3.499 kg for birth weight; 49.0–49.9 cm for birth length; and 33.0–33.9 cm for head circumference); whiskers: 95% CIs for category-specific RRs. Continuous line, estimated linear effect centred on reference category; dotted line, estimated quadratic effect centred on reference category.
(32 KB PDF)
Adjustments for maternal age (continuous); maternal parity (categorical: 0, 1, 2, ≥3); age at menarche (categorical: <12 y, 12.0–12.9 y, ≥13 y); adult height (continuous); adult BMI (continuous); adult parity (categorical: 0, 1, 2, ≥3); age at first birth (categorical: nulliparous, <20 y, 20–29 y, ≥30 y); adult SES (study-specific categories: adult SES for European Prospective Investigation of Cancer [EPIC]-Norfolk, Nurses' Health Study [NHS] I and II, and Seattle Breast Cancer in Young Women [BCYW], Seattle Breast Cancer in Middle-Aged Women [BCMW], and Seattle Perinatal Factors and Breast Cancer [PFBC]; occupation for Shanghai Breast Cancer Study [SBCS]; and education for UK Women's Cohort Study [UKWCS] and Women's Contraceptive and Reproductive Experiences [CARE] study); oral contraceptive use (categorical: ever, never); and hormone replacement therapy use (categorical: ever, never).
(15 KB PDF)
(51 KB PDF)
(164 KB DOC)
(64 KB DOC)
(96 KB DOC)
(249 KB DOC)
(97 KB DOC)
(97 KB DOC)
(68 KB DOC)
(454 KB DOC)
(125 KB DOC)
Acknowledgments
We would like to thank the following colleagues and institutions: A.L. Herbst (University of Chicago, US), K. Noller (New England Medical Center, US), R. Kaufman (Obstetrics and Gynecology Physicians Organization, Houston Methodist Hospital, US), and R.N. Hoover (Division of Epidemiology and Genetics, National Cancer Institute, US) for their contribution to the National Cancer Institute Diethylstilbestrol Combined Cohort Study (NCI DES); K. Corsano for her help with data extraction for the Nurses' Health Studies (NHS) I and II; B. Peplonska and N. Szeszenia-Dąbrowska (Nofer Institute of Occupational Medicine, Poland), W. Zatonski (Department of Cancer Control and Epidemiology Cancer Center and M. Sklodowska-Curie Institute of Oncology, Poland) and M. Sherman (National Cancer Institute, US) for their contribution to the Polish Breast Cancer Study (PBCS), which was supported in part by Intramural Funds of the National Cancer Institute, National Institutes of Health (US); R. Mohsen for data management and extraction for the Uppsala Birth Cohort Multigenerational Study (UBCoS Multigen), which is supported by the Swedish Research Council and the Swedish Council for Working Life and Social Research; T. Byers (Department of Preventive Medicine and Biometrics, University of Colorado Comprehensive Cancer Center, US) for his contribution to New York Study of Early Onset Breast Cancer (NYSEOBC); M. de Silva and C. Abello (Cancer Research UK Epidemiology and Genetics Group, Department of Epidemiology and Population Health, London School of Hygiene & Tropical Medicine, UK) for clerical and administrative support.
Writing Committee: Isabel dos Santos Silva, Bianca De Stavola, Valerie McCormack. (Department of Epidemiology and Population Health, London School of Hygiene & Tropical Medicine, UK).
Aberdeen Children of the 1950s (ACONF): D. Leon (Department of Epidemiology and Population Health, London School of Hygiene & Tropical Medicine, UK), S. Macintyre (Medical Research Council [MRC] Social and Public Health Sciences Unit, University of Glasgow, UK); Carolina Breast Cancer Study (CBCS): M.E. Hodgson (Department of Epidemiology, School of Public Health, University of North Carolina at Chapel Hill, US), B. Newman (School of Public Health, Queensland University of Technology, Australia); Copenhagen School Health Record Register (CSHRR): T.I.A. Sorensen, L.W. Olsen, J.L. Baker (Institute of Preventive Medicine, Copenhagen University Hospital, Centre for Health and Society, Denmark); Collaborative multi-state Breast Cancer Study (CmsBCS): J.A. Baron (Dartmouth Medical School, US), P.A. Newcomb (Cancer Prevention Program, Fred Hutchinson Cancer Research Center, US), L. Titus-Ernstoff (Dartmouth Medical School, US), K.M. Egan (Moffitt Cancer Center & Research Institute, US), A. Trentham-Dietz (University of Wisconsin Department of Population Health Sciences and Paul P. Carbone Comprehensive Cancer Center, US); Danish Population-Based Case-Control Study (DPCCS): L. Mellemkjaer (Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark), H.T. Sørensen (Department of Clinical Epidemiology, Aarhus University Hospital, Denmark and Department of Epidemiology, Boston University, US); European Prospective Investigation of Cancer (EPIC)-Norfolk: M.S. Sandhu (Department of Public Health and Primary Care, Institute of Public Health, University of Cambridge, UK), S. Bingham (MRC Dunn Human Nutrition Unit, UK), K. Tee-Khan (Department of Public Health and Primary Care, University of Cambridge, UK); Helsinki Birth Cohort Study I, II, and III (HBCS I, II, III): L. Hilakivi-Clarke (Lombardi Cancer Center, Georgetown University, US), J. Eriksson (Department of Epidemiology and Health Promotion, National Public Health Institute, Finland), C. Osmond (University of Southampton, Southampton General Hospital, UK); Malmö Diet and Cancer Study (MDCS)-EPIC Sweden: P.H. Lahmann (School of Population Health, University of Queensland, Australia and Department of Clinical Sciences, Lund University, Malmö University Hospital, Sweden), G. Berglund (Department of Clinical Sciences, Lund University, Malmö University Hospital, Sweden); Medical Research Council National Survey of Health and Development (MRC NSHD): D. Kuh, R. Hardy, G. Mishra (Department of Epidemiology and Public Health, Royal Free and University College Medical School, UK); National Cancer Institute Diethylstilbestrol Combined Cohort Study (NCI DES): R. Troisi (Division of Cancer Epidemiology and Genetics, National Cancer Institute, US), L. Titus-Ernstoff (Department of Community and Family Medicine, Dartmouth Medical School, US), J. Palmer (Slone Epidemiology Center, Boston University, US), E.E. Hatch (Department of Epidemiology and Statistics, School of Public Health, Boston University, US); New York Study of Early Onset Breast Cancer (NYSEOBC): K. Innes (Center for the Study of Complementary and Alternative Therapies, University of Virginia Health System, US); Nurses' Health Study I, II (NHS I, II): K.B. Michels (Obstetrics and Gynecology Epidemiology Center, Department of Obstetrics, Gynecology and Reproductive Biology, Brigham and Women's Hospital, Harvard Medical School; Channing Laboratory, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School; and Department of Epidemiology, Harvard School of Public Health, US); Polish Breast Cancer Study (PBCS): S.K. Park (Seoul National University College of Medicine, Korea), L.A. Brinton, M. Garcia-Closas (Division of Cancer Epidemiology and Genetics, National Cancer Institute, US), J. Lissowska (Department of Cancer Control and Epidemiology Cancer Center and M. Sklodowska-Curie Institute of Oncology, Poland); Population Study of Women in Gothenburg (PSWG): L. Lissner (Department of Public Health and Community Medicine, Gothenburg University, Sweden), L. Hulthén (Department of Clinical Nutrition, Gothenburg University, Sweden); Seattle Breast Cancer in Young Women (BCYW) study: M. Sanderson (Department of Obstetrics and Gynecology, Meharry Medical College, US), K. Malone, J. Daling (Fred Hutchinson Cancer Research Center, US); Seattle Perinatal Factors and Breast Cancer (PFBC) study: M. Sanderson (Department of Obstetrics and Gynecology, Meharry Medical College, US); Seattle Breast Cancer in Middle-Aged Women (BCMW) study: M. Sanderson (Department of Obstetrics and Gynecology, Meharry Medical College, US), J. Stanford (Fred Hutchinson Cancer Research Center, US); Shanghai Breast Cancer Study (SBCS): W. Zheng, X.O. Shu (Vanderbilt Epidemiology Center, Institute of Medicine & Public Health, Vanderbilt University Medical Center, US); Saint Olav's University Hospital Birth Cohort (SOUHBC): L.J. Vatten, T.I.L. Nilsen (Department of Public Health and General Practice, Norwegian University of Science and Technology, Norway); Swedish study on Pre-Natal Factors and Breast Cancer (SPNFBC): A. Ekbom (Unit of Clinical Epidemiology, Department of Medicine, Karolinska Institute, Sweden); Swedish cohort of Premature and Very Low Birth Weight (SPVLBW) babies: M. Kaijser (Clinical Epidemiology Unit, Department of Medicine, Karolinska Hospital, Sweden); Swedish Like-Sexed Twin Study (SLSTS): S. Cnattingius (Department of Medical Epidemiology and Biostatistics, Karolinska Institute, Sweden); Swedish Opposite-Sexed Twin Study (SOSTS): M. Kaijser (Clinical Epidemiology Unit, Department of Medicine, Karolinska Hospital, Sweden); Swedish Young Female Breast Cancer (SYFBC) study: F. Rasmussen (Department of Public Health Sciences, Karolinska Institute, Sweden); Trondheim & Bergen Population-based Case-Control (TBPCCS) study: L.J. Vatten, T.I.L. Nilsen (Department of Public Health and General Practice, Norwegian University of Science and Technology, Norway); UK Women's Cohort Study (UKWCS): J.E. Cade, V.J. Burley, D.C. Greenwood (Centre for Epidemiology and Biostatistics, University of Leeds, UK); Uppsala Birth Cohort Multigenerational Study (UBCoS Multigen): I. Koupil (Centre for Health Equity Studies [CHESS], Stockholm University/Karolinska Institute, Sweden); Western New York Exposures and Breast cancer (WEB) study: J.L. Freudenheim, J. Nie (Department of Social and Preventive Medicine, School of Public Health and Health Professions, University at Buffalo, US), M. Barba (Department of Epidemiology, Regina Elena Cancer Institute, Italy); Women's Contraceptive and Reproductive Experiences (CARE) study: J.M. Liff, D. Christensen (Rollins School of Public Health at Emory University, US)
Abbreviations
- BMI
body mass index
- CI
confidence interval
- PI
ponderal index
- RR
relative risk
- SD
standard deviation
- SES
socioeconomic status
Footnotes
Author contributions. IdSS, BDS, and VM designed the study. All members of the Collaborative Group contributed to data acquisition, with final data checking and standardisation by BDS and VM. BDS performed statistical analysis of data. IdSS, BDS, VM, and all members of the Collaborative Group interpreted the findings. IdSS and BDS had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. IdSS prepared the first draft of the manuscript. All authors provided comments in writing the paper.
Funding: Funding was obtained from Cancer Research UK (CR-UK) programme grant (C150/A5660) to IdSS. CR-UK Training Fellowship (C14292/A5609) to VM. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing Interests: The authors have declared that no competing interests exist.
References
- Trichopoulos D. Hypothesis: does breast cancer originate in utero. Lancet. 1990;335:939–940. doi: 10.1016/0140-6736(90)91000-z. [DOI] [PubMed] [Google Scholar]
- Trichopoulos D. Intrauterine environment, mammary gland mass and breast cancer risk. Br Cancer Res. 2003;5:42–44. doi: 10.1186/bcr555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekbom A, Trichopoulos D, Adami H-O, Hsieh C-C, Lan S-J. Evidence of prenatal influences on breast cancer risk. Lancet. 1992;340:1015–1018. doi: 10.1016/0140-6736(92)93019-j. [DOI] [PubMed] [Google Scholar]
- Sanderson M, Williams MA, Malone KE, Stanford JL, Emanuel I, et al. Perinatal factors and risk of breast cancer. Epidemiology. 1996;7:34–37. doi: 10.1097/00001648-199601000-00007. [DOI] [PubMed] [Google Scholar]
- Michels KB, Trichopoulos D, Robins JM, Rosner BA, Manson JAE, et al. Birth weight as a risk factor for breast cancer. Lancet. 1996;348:1542–1546. doi: 10.1016/S0140-6736(96)03102-9. [DOI] [PubMed] [Google Scholar]
- Ekbom A, Hsieh C-C, Lipworth L, Adami H-O, Trichopoulos D. Intrauterine environment and breast cancer risk in women: a population-based study. J Natl Cancer Inst. 1997;88:71–76. doi: 10.1093/jnci/89.1.71. [DOI] [PubMed] [Google Scholar]
- Sanderson M, Williams MA, Daling JR, Malone KE, Stanford JL, et al. Maternal factors and breast cancer risk among young women. Pediatr Perinat Epidemiol. 1998;12:397–407. doi: 10.1046/j.1365-3016.1998.00133.x. [DOI] [PubMed] [Google Scholar]
- Innes K, Byers T, Schymura M. Birth characteristics and subsequent risk for breast cancer in very young women. Am J Epidemiol. 2000;152:1121–1128. doi: 10.1093/aje/152.12.1121. [DOI] [PubMed] [Google Scholar]
- Ekbom A, Erlandsson G, Hsieh C-C, Trichopoulos D, Adami H-O, et al. Risk of breast cancer in prematurely born women. J Natl Cancer Inst. 2000;92:840–841. doi: 10.1093/jnci/92.10.840. [DOI] [PubMed] [Google Scholar]
- Hübinette A, Lichtenstein P, Ekbom A, Cnattingius S. Birth characteristics and breast cancer risk: a study among like-sexed twins. Int J Cancer. 2001;91:248–251. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1025>3.3.co;2-h. [DOI] [PubMed] [Google Scholar]
- Kaijser M, Lichtenstein P, Granath F, Erlandsson G, Cnattingius S, et al. In utero exposures and breast cancer: a study of opposite-sexed twins. J Natl Cancer Inst. 2001;93:60–62. doi: 10.1093/jnci/93.1.60. [DOI] [PubMed] [Google Scholar]
- Sanderson M, Shu XO, Jin F, Dai Q, Ruan Z, et al. Weight at birth and adolescence and premenopausal breast cancer risk in a low-risk population. Br J Cancer. 2002;86:84–88. doi: 10.1038/sj.bjc.6600009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatten LJ, Mæhle BO, Lund Nilsen TI, Tretli S, Hsieh C-c, et al. Birth weight as a predictor of breast cancer: a case-control study in Norway. Br J Cancer. 2002;86:89–91. doi: 10.1038/sj.bjc.6600011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stavola BL, Hardy R, Kuh D, dos Santos Silva I, Wadsworth M, et al. Birthweight, childhood growth and risk of breast cancer in a British cohort. Br J Cancer. 2000;83:964–968. doi: 10.1054/bjoc.2000.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson SW, Bengtsson C, Hallberg L, Lapidus L, Niklasson A, et al. Cancer risk in Swedish women: the relation to size at birth. Br J Cancer. 2001;84:1193–1198. doi: 10.1054/bjoc.2000.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilakivi-Clarke L, Forsén T, Eriksson JG, Luoto R, Tuomilehto J, et al. Tallness and overweight during childhood have opposing effects on breast cancer risk. Br J Cancer. 2001;85:1680–1684. doi: 10.1054/bjoc.2001.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus-Ernstoff L, Egan KM, Newcomb PA, Ding J, Trentheim-Dietz A, et al. Early life factors in relation to breast cancer risk in postmenopausal women. Cancer Epidemiol Biomark Prev. 2002;11:207–210. [PubMed] [Google Scholar]
- McCormack VA, dos Santos Silva I, De Stavola BL, Mohsen R, Leon DA, et al. Foetal growth and subsequent risk of breast cancer: results from long-term follow-up of Swedish cohort. Br Med J. 2003;326:248–251. doi: 10.1136/bmj.326.7383.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellemkjær L, Olsen ML, Sørensen HT, Thulstrup AM, Olsen J, et al. Birth weight and risk of early-onset breast cancer (Denmark) Cancer Causes Control. 2003;14:61–64. doi: 10.1023/a:1022570305704. [DOI] [PubMed] [Google Scholar]
- Ahlgren M, Sørensen TIA, Wohlfahrt J, Haflidadottir A, Holst C, et al. Birth weight and risk of breast cancer in a cohort of 106,504 women. Int J Cancer. 2003;107:997–1000. doi: 10.1002/ijc.11481. [DOI] [PubMed] [Google Scholar]
- Kaijser M, Akre O, Cnattingius S, Ekbom A. Preterm birth, birth weight, and subsequent risk of female breast cancer. Br J Cancer. 2003;89:1664–1666. doi: 10.1038/sj.bjc.6601357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlgren M, Melbye M, Wohlfahrt J, Sørensen TIA. Growth patterns and the risk of breast cancer. New Engl J Med. 2004;351:1619–1626. doi: 10.1056/NEJMoa040576. [DOI] [PubMed] [Google Scholar]
- dos Santos Silva I, De Stavola BL, Hardy RJ, Kuh DJ, McCormack VA, et al. Is the association of birth weight with premenopausal breast cancer mediated through childhood growth. Br J Cancer. 2004;91:519–524. doi: 10.1038/sj.bjc.6601972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahmann PH, Gullberg B, Olsson H, Boeing H, Berglund G, et al. Birth weight is associated with postmenopausal breast cancer risk in Swedish women. Br J Cancer. 2004;91:1666–1668. doi: 10.1038/sj.bjc.6602203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson ME, Newman B, Millikan RC. Birthweight, parental age, birth order and breast cancer risk in African-American and white women: a population-based case-control study. Breast Cancer Res. 2004;6:R656–R667. doi: 10.1186/bcr931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatten LJ, Lund Nilsen TI, Tretli S, Trichopoulos D, Romundstad PR. Size at birth and risk of breast cancer: prospective population-based study. Int J Cancer. 2005;114:461–464. doi: 10.1002/ijc.20726. [DOI] [PubMed] [Google Scholar]
- McCormack VA, dos Santos Silva I, Koupil I, Leon DA, Lithell HO. Birth characteristics and adult cancer incidence: Swedish cohort of 11,000 men and women. Int J Cancer. 2005;115:611–617. doi: 10.1002/ijc.20915. [DOI] [PubMed] [Google Scholar]
- Troisi R, Hatch EE, Titus-Ernstoff L, Palmer JR, Hyer M, et al. Birth weight and breast cancer risk. Br J Cancer. 2006;94:1734–1737. doi: 10.1038/sj.bjc.6603122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barba M, McCann SE, Nie J, Vito D, Stranges S, et al. Perinatal exposures and breast cancer risk in the Western New York exposures and breast cancer (WEB) study. Cancer Causes Control. 2006;17:395–401. doi: 10.1007/s10552-005-0481-5. [DOI] [PubMed] [Google Scholar]
- Park SK, Garcia-Closas M, Lissowska J, Sherman ME, McGlynn KA, et al. Intrauterine environment and breast cancer risk in a population-based case-control study in Poland. Int J Cancer. 2006;119:2136–2141. doi: 10.1002/ijc.21974. [DOI] [PubMed] [Google Scholar]
- Michels KB, Xue F, Terry KL, Willett WC. Longitudinal study of birthweight and the incidence of breast cancer in adulthood. Carcinogenesis. 2006;27:2464–2468. doi: 10.1093/carcin/bgl105. [DOI] [PubMed] [Google Scholar]
- Mogren I, Damber L, Tavelin B, Högberg U. Characteristics of pregnancy and birth and malignancy of the offspring (Sweden) Cancer Causes Control. 1999;10:85–94. doi: 10.1023/a:1008813701634. [DOI] [PubMed] [Google Scholar]
- Le Marchand L, Kolonel LN, Myers BC, Mi M-P. Birth characteristics of pre-menopausal women with breast cancer. Br J Cancer. 1988;57:437–439. doi: 10.1038/bjc.1988.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels KB, Xue F. Role of birthweight in the etiology of breast cancer. Int J Cancer. 2006;119:2007–2025. doi: 10.1002/ijc.22004. [DOI] [PubMed] [Google Scholar]
- Xue F, Michels KB. Intrauterine factors and risk of breast cancer: a systematic review and meta-analyses of current evidence. Lancet Oncol. 2007;8:1088–1100. doi: 10.1016/S1470-2045(07)70377-7. [DOI] [PubMed] [Google Scholar]
- Park SK, Kang D, McGlynn KA, Garcia-Closas M, Kim Y, et al. Intrauterine environments and breast cancer risk: meta-analysis and systematic review. Breast Cancer Res. 2008;10:R8. doi: 10.1186/bcr1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stukel TA, Demidenko E, Dykes J, Karagas MR. Two-stage methods for the analysis of pooled data. Stat Med. 2001;20:2115–2130. doi: 10.1002/sim.852. [DOI] [PubMed] [Google Scholar]
- Smith-Warner S, Spiegelman D, Ritz J, Albanes D, Beeson WL, et al. Methods for pooling results of epidemiologic studies: The Pooling Project of Prospective Studies of Diet and Cancer. Am J Epidemiol. 2006;163:1053–1064. doi: 10.1093/aje/kwj127. [DOI] [PubMed] [Google Scholar]
- Clayton D, Hills M. Statistical models in epidemiology. Oxford: Oxford University Press; 1993. [Google Scholar]
- Marubini E, Valsecchi MG. Analysing survival data from clinical trials and observational studies. Chichester: John Wiley; 1995. [Google Scholar]
- Cochrane WG. The combination of estimates from different experiments. Biometrics. 1954;10:10129. [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JP. Semiparametric estimation of random effects using the Cox model based on the EM algorithm. Biometrics. 1992;48:795–806. [PubMed] [Google Scholar]
- Sutton AJ. Wiley Series in Probability and Statistics. New York: Wiley; 2000. Methods for meta-analysis in medical research. [Google Scholar]
- Stata. Software version 9.2. College Station (Texas): Stata Corporation; 2005. [Google Scholar]
- Johnson TS, Engstrom JL, Gelhar DK. Intra- and interexaminer reliability of anthropometric measurements of term infants. J Pediatr Gastroenterol Nutr. 1997;24:497–505. doi: 10.1097/00005176-199705000-00001. [DOI] [PubMed] [Google Scholar]
- Johnson TS, Engstrom JL, Haney SL, Mulcrone SL. Reliability of three length measurement techniques in term infants. Pediatr Nur. 1999;25:13–17. [PubMed] [Google Scholar]
- Johnson MH, Everitt BJ. Essential reproduction. Oxford: Blackwell Science; 2000. pp. 199–200. [Google Scholar]
- Kaijser M, Granath F, Jacobsen G, Cnattingius S, Ekbom A. Maternal pregnancy estriol levels in relation to anamnestic and fetal anthropometric data. Epidemiology. 2000;11:315–319. doi: 10.1097/00001648-200005000-00015. [DOI] [PubMed] [Google Scholar]
- Troisi R, Potischman N, Roberts J, Siiteri P, Daftary A, et al. Associations of maternal and umbilical cord hormone concentrations with maternal, gestational and neonatal factors (United States) Cancer Causes Control. 2003;14:347–355. doi: 10.1023/a:1023934518975. [DOI] [PubMed] [Google Scholar]
- Skalkidou A, Petridou E, Papathoma E, Salvanos H, Kedikoglou S, et al. Determinants and consequences of major insulin-like growth factor components among full-term healthy neonates. Cancer Epidemiol Biomark Prev. 2003;12:860–865. [PubMed] [Google Scholar]
- Vatten LJ, Nilsen ST, Ødegård RA, Romundstad PR, Austgulen R. Insulin-like growth factor I and leptin in umbilical cord plasma and infant birth size at term. Paediatrics. 2002;109:1131–1135. doi: 10.1542/peds.109.6.1131. [DOI] [PubMed] [Google Scholar]
- Harigaya A, Nagashima K, Nako Y, Morikawa A. Relationship between concentration of serum leptin and fetal growth. J Clin Endocrinol Metab. 1997;82:3281–3284. doi: 10.1210/jcem.82.10.4321. [DOI] [PubMed] [Google Scholar]
- Sivan E, Mazaki-Tovi S, Pariente C, Efraty Y, Schiff E, et al. Adiponectin in human cord blood: relation to foetal birth weight and gender. J Clin Endocrinol Metab. 2003;88:5656–5660. doi: 10.1210/jc.2003-031174. [DOI] [PubMed] [Google Scholar]
- Ong KKL, Ahmed ML, Sherriff A, Woods KA, Watts A, et al. Cord blood leptin is associated with size at birth and predicts infancy weight in humans. J Clin Endocrinol Metab. 1999;84:1145–1148. doi: 10.1210/jcem.84.3.5657. [DOI] [PubMed] [Google Scholar]
- Nagata C, Iwasa S, Shiraki M, Shimizu H. Estrogen and alpha-fetoprotein levels in maternal and umbilical cord blood samples in relation to birth weight. Cancer Epidemiol Biomarkers Prev. 2006;15:1469–1472. doi: 10.1158/1055-9965.EPI-06-0158. [DOI] [PubMed] [Google Scholar]
- Hilakivi-Clarke L, de Assis S. Fetal origins of breast cancer. Trends Endocrinol Metab. 2006;17:340–348. doi: 10.1016/j.tem.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Savarese TM, Strohsnitter WC, Low HP, Liu Q, Baik I, et al. Correlation of umbilical cord blood hormones and growth factors with stem cell potential: implications for the prenatal origin of breast cancer hypothesis. Breast Cancer Res. 2007;9:R29. doi: 10.1186/bcr1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck JF, Hulka BS, Savitz DA, Baird D, Poole C, et al. Accuracy of fetal growth indicators as surrogate measures of steroid levels during pregnancy. Am J Epidemiol. 2003;157:258–266. doi: 10.1093/aje/kwf183. [DOI] [PubMed] [Google Scholar]
- Trichopoulos D, Adami H-O, Ekbom A, Hsieh C-C, Lagiou P. Early life events and conditions and breast cancer risk: from epidemiology to etiology. Int J Cancer. 2008;122:481–485. doi: 10.1002/ijc.23303. [DOI] [PubMed] [Google Scholar]
- van den Brandt PA, Spiegelman D, Yaun SS, Adami H-O, Beeson L, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152:514–527. doi: 10.1093/aje/152.6.514. [DOI] [PubMed] [Google Scholar]
- Sørensen HT, Sabroe S, Rothman KJ, Gillman M, Steffensen FH, et al. Birth weight and length as predictors for adult height. Am J Epidemiol. 1999;149:726–729. doi: 10.1093/oxfordjournals.aje.a009881. [DOI] [PubMed] [Google Scholar]
- Tuvemo T, Cnattingius S, Jonsson B. Prediction of male adult stature using anthropometric data at birth: a nationwide population-based study. Pediatric Res. 1999;46:491–495. doi: 10.1203/00006450-199911000-00001. [DOI] [PubMed] [Google Scholar]
- Collaborative Group on Hormonal Factors in Breast Cancer. Alcohol, tobacco and breast cancer – collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br J Cancer. 2002;87:1234–1245. doi: 10.1038/sj.bjc.6600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anath CV, Wen SW. Trends in fetal growth among singleton gestations in the United States and Canada, 1985 through 1998. Semin Perinatol. 2002;26:260–267. doi: 10.1053/sper.2002.34772. [DOI] [PubMed] [Google Scholar]
- Orskou J, Kesmodel U, Henriksen TB, Secher NJ. An increasing proportion of infants weigh more than 4000 grams at birth. Acta Obstet Gynecol Scand. 2001;80:931–936. doi: 10.1034/j.1600-0412.2001.801010.x. [DOI] [PubMed] [Google Scholar]
- Ricart W, Lopez J, Mozas J, Pericot J, Sancho A, et al. Body mass index has a greater impact on pregnancy outcomes than gestational hyperglycaemia. Diabetologia. 2005;48:1736–1742. doi: 10.1007/s00125-005-1877-1. [DOI] [PubMed] [Google Scholar]
- Stotland NE, Hopkins LM, Caughey AB. Gestational weight gain, macrosomia, and risk of caesarean birth in nondiabetic nulliparas. Obstet Gynecol. 2004;104:671–677. doi: 10.1097/01.AOG.0000139515.97799.f6. [DOI] [PubMed] [Google Scholar]
- Surkan PJ, Hsieh CC, Johansson AL, Dickman PW, Cnattingius S. Reasons for increasing trends in large for gestational age births. Obstet Gynecol. 2004;104:720–726. doi: 10.1097/01.AOG.0000141442.59573.cd. [DOI] [PubMed] [Google Scholar]
- Schack-Nielsen L, Mølgaard C, Sørensen TIA, Greisen G, Michaelsen KF. Secular change in size at birth from 1973 to 2003: national data from Denmark. Obesity. 2006;14:1257–1263. doi: 10.1038/oby.2006.143. [DOI] [PubMed] [Google Scholar]
- Baker JL, Olsen LW, Sørensen TIA. Weight at birth and all-cause mortality in adulthood. Epidemiology. 2008;19:197–203. doi: 10.1097/EDE.0b013e31816339c6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(25 KB DOC)
(27 KB DOC)
The area of the black squares is inversely proportional to the variance (on the log scale).
(51 KB PDF)
The area of the black squares is inversely proportional to the variance (on the log scale).
(15 KB PDF)
The area of the black squares is inversely proportional to the variance (on the log scale).
(13 KB PDF)
The area of the black squares is inversely proportional to the variance (on the log scale).
(13 KB PDF)
(12 KB PDF)
Analyses restricted to the subset of cohort studies with birth record data: 1,210 cases / 404,970 non-cases (see Table 2). Small circle, estimated category-specific minimally adjusted RRs (reference category: 3.000–3.499 kg for birth weight; 49.0–49.9 cm for birth length; and 33.0–33.9 cm for head circumference); whiskers: 95% CIs for category-specific RRs. Continuous line, estimated linear effect centred on reference category; dotted line, estimated quadratic effect centred on reference category.
(32 KB PDF)
Adjustments for maternal age (continuous); maternal parity (categorical: 0, 1, 2, ≥3); age at menarche (categorical: <12 y, 12.0–12.9 y, ≥13 y); adult height (continuous); adult BMI (continuous); adult parity (categorical: 0, 1, 2, ≥3); age at first birth (categorical: nulliparous, <20 y, 20–29 y, ≥30 y); adult SES (study-specific categories: adult SES for European Prospective Investigation of Cancer [EPIC]-Norfolk, Nurses' Health Study [NHS] I and II, and Seattle Breast Cancer in Young Women [BCYW], Seattle Breast Cancer in Middle-Aged Women [BCMW], and Seattle Perinatal Factors and Breast Cancer [PFBC]; occupation for Shanghai Breast Cancer Study [SBCS]; and education for UK Women's Cohort Study [UKWCS] and Women's Contraceptive and Reproductive Experiences [CARE] study); oral contraceptive use (categorical: ever, never); and hormone replacement therapy use (categorical: ever, never).
(15 KB PDF)
(51 KB PDF)
(164 KB DOC)
(64 KB DOC)
(96 KB DOC)
(249 KB DOC)
(97 KB DOC)
(97 KB DOC)
(68 KB DOC)
(454 KB DOC)
(125 KB DOC)