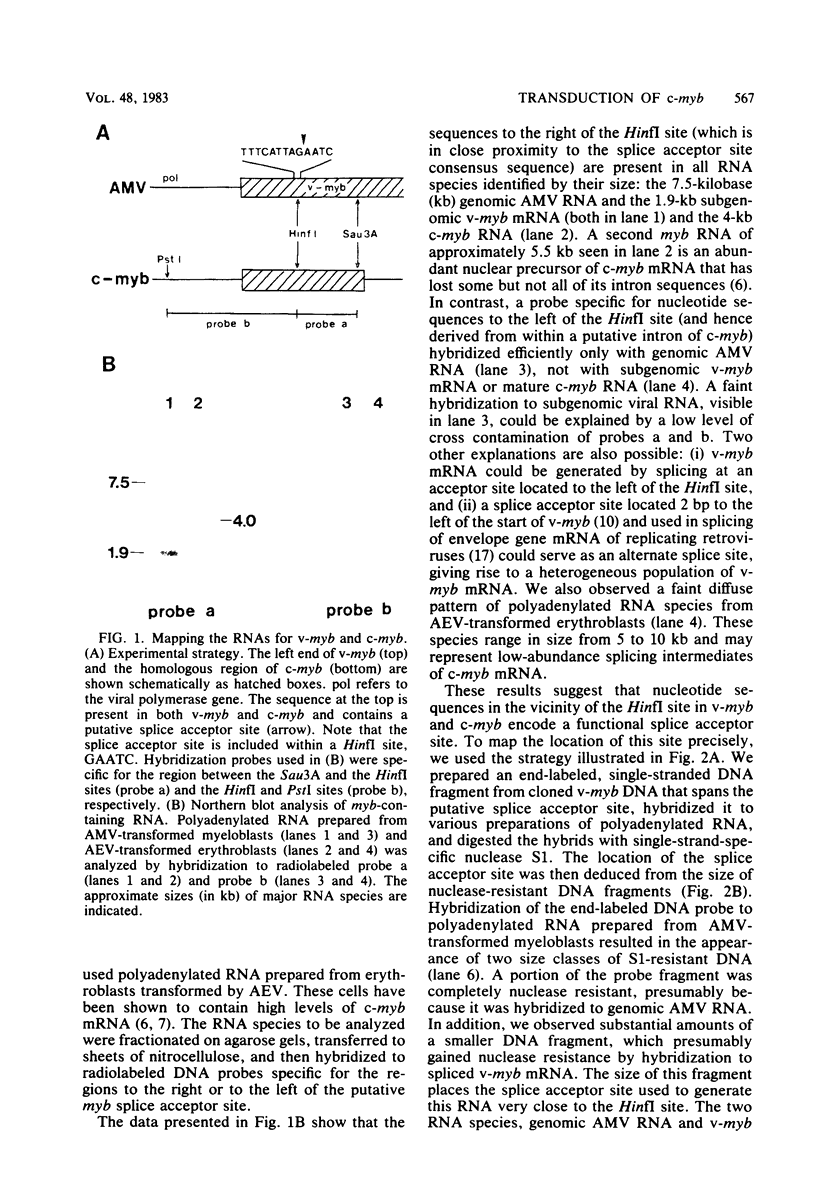
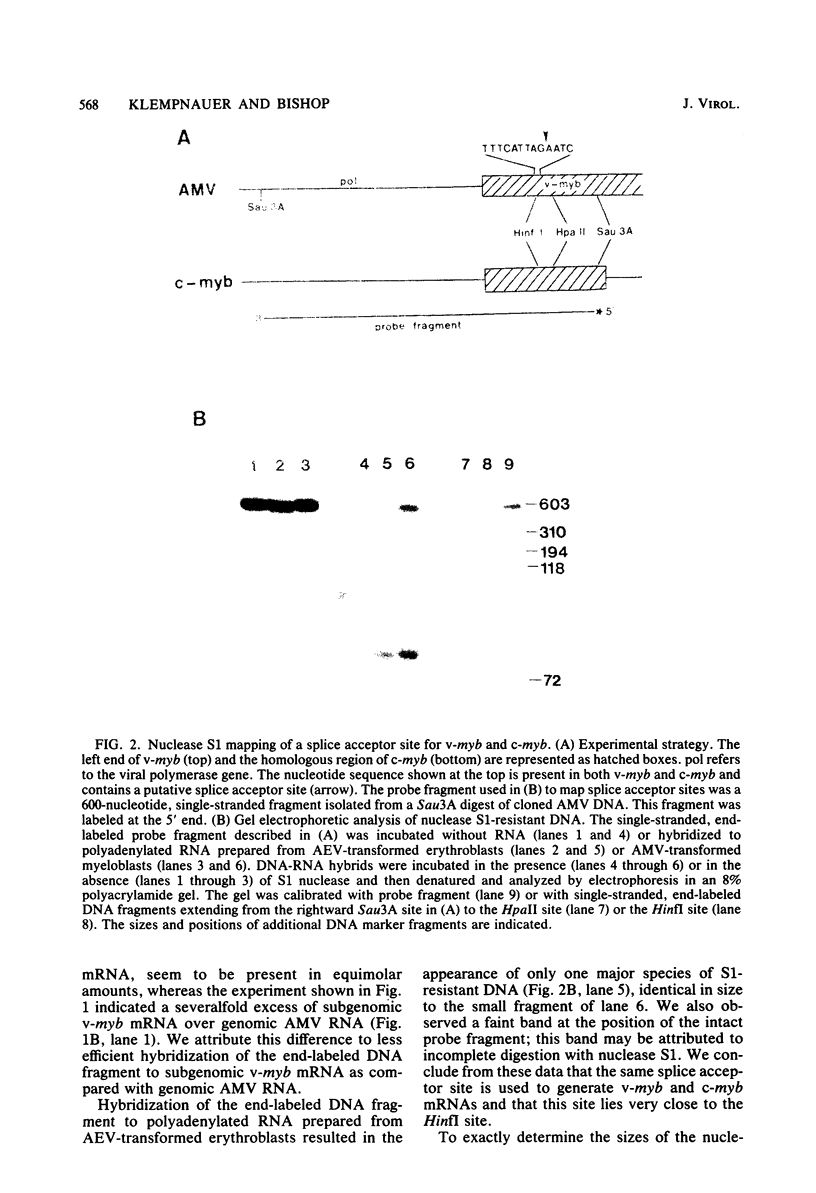
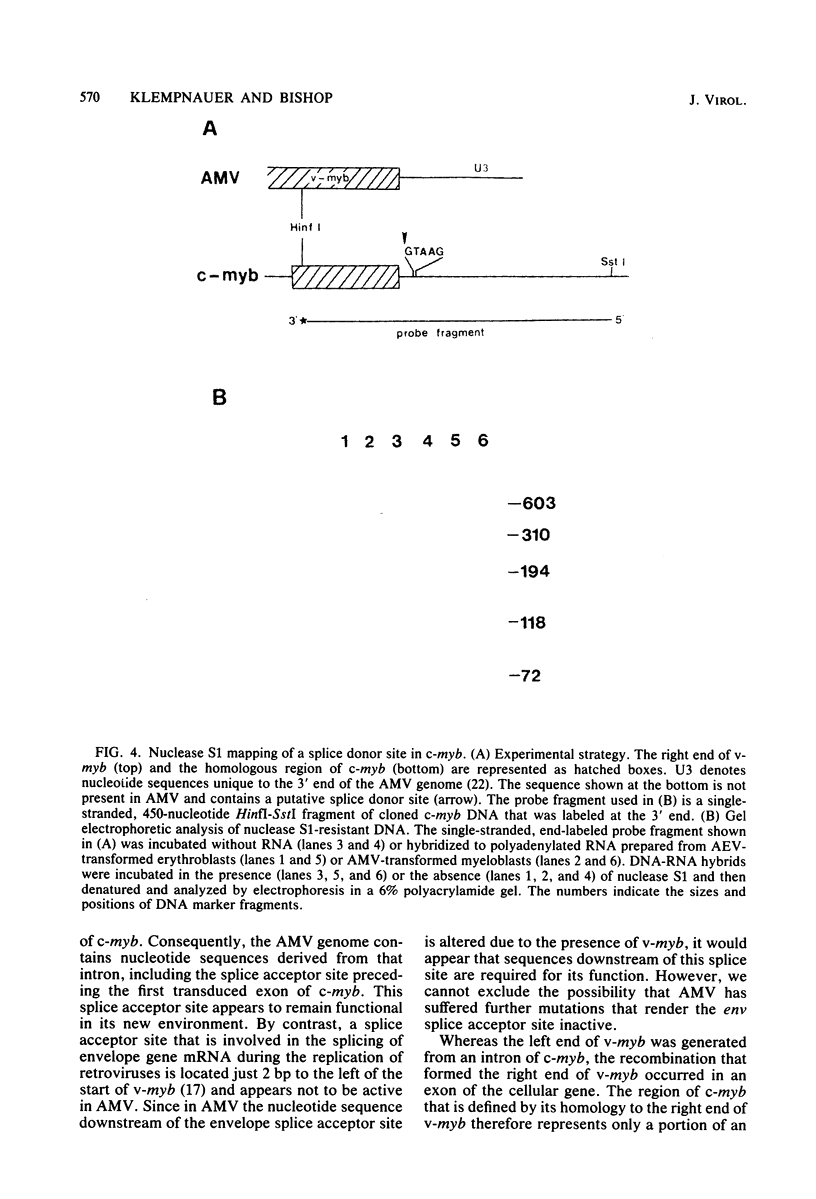
Abstract
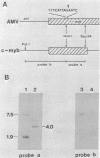
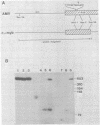
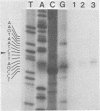
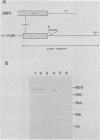
The oncogene (v-myb) of avian myeloblastosis virus apparently arose by transduction of nucleotide sequences from a cellular gene (c-myb). In c-myb the nucleotide sequences that formed v-myb exist at seven distinct regions separated by nontransduced stretches of sequence that are flanked by eucaryotic splice signals. By contrast, the sequences at the outside boundaries of the transduced region of c-myb do not resemble splice sites. We mapped the nucleotide sequences that are homologous to the ends of v-myb with respect to the exons and introns of c-myb. The results indicate that the leftward recombination between c-myb and the transducing retrovirus occurred within an intron of the cellular gene, whereas the rightward recombination took place in an exon of c-myb. Transduction of c-myb sequences may therefore have involved a DNA rearrangement.
Full text
PDF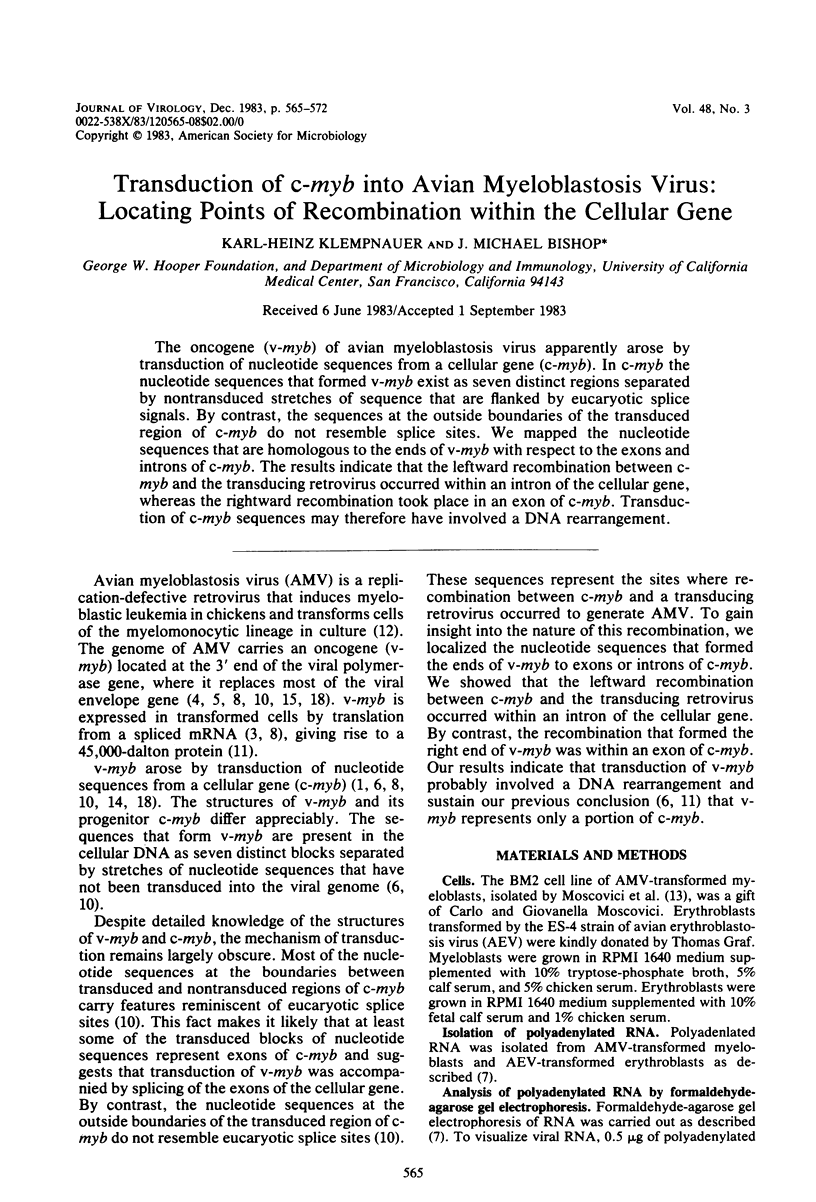




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergmann D. G., Souza L. M., Baluda M. A. Vertebrate DNAs contain nucleotide sequences related to the transforming gene of avian myeloblastosis virus. J Virol. 1981 Nov;40(2):450–455. doi: 10.1128/jvi.40.2.450-455.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Chen J. H., Hayward W. S., Moscovici C. Size and genetic content of virus-specific RNA in myeloblasts transformed by avian myeloblastosis virus (AMV). Virology. 1981 Apr 15;110(1):128–136. doi: 10.1016/0042-6822(81)90014-3. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Varmus H. E., Bishop J. M., Essex M., Hardy W. D., Jr, Martin G. S., Rosenberg N. E., Scolnick E. M., Weinberg R. A., Vogt P. K. Proposal for naming host cell-derived inserts in retrovirus genomes. J Virol. 1981 Dec;40(3):953–957. doi: 10.1128/jvi.40.3.953-957.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Bister K., Moscovici C. Genetic structure of avian myeloblastosis virus, released from transformed myeloblasts as a defective virus particle. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5120–5124. doi: 10.1073/pnas.77.9.5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Bishop J. M. Structure and transcription of the cellular homolog (c-myb) of the avian myeloblastosis virus transforming gene (v-myb). J Virol. 1983 Apr;46(1):212–220. doi: 10.1128/jvi.46.1.212-220.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Sheiness D. K., Bishop J. M. Transcripts from the cellular homologs of retroviral oncogenes: distribution among chicken tissues. Mol Cell Biol. 1982 Jun;2(6):617–624. doi: 10.1128/mcb.2.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Sheiness D. K., Fanshier L., Bishop J. M., Moscovici C., Moscovici M. G. The genome and the intracellular RNAs of avian myeloblastosis virus. Cell. 1981 Jan;23(1):279–290. doi: 10.1016/0092-8674(81)90292-0. [DOI] [PubMed] [Google Scholar]
- Green M. R., Roeder R. G. Definition of a novel promoter for the major adenovirus-associated virus mRNA. Cell. 1980 Nov;22(1 Pt 1):231–242. doi: 10.1016/0092-8674(80)90171-3. [DOI] [PubMed] [Google Scholar]
- Klempnauer K. H., Gonda T. J., Bishop J. M. Nucleotide sequence of the retroviral leukemia gene v-myb and its cellular progenitor c-myb: the architecture of a transduced oncogene. Cell. 1982 Dec;31(2 Pt 1):453–463. doi: 10.1016/0092-8674(82)90138-6. [DOI] [PubMed] [Google Scholar]
- Klempnauer K. H., Ramsay G., Bishop J. M., Moscovici M. G., Moscovici C., McGrath J. P., Levinson A. D. The product of the retroviral transforming gene v-myb is a truncated version of the protein encoded by the cellular oncogene c-myb. Cell. 1983 Jun;33(2):345–355. doi: 10.1016/0092-8674(83)90416-6. [DOI] [PubMed] [Google Scholar]
- Moscovici C. Leukemic transformation with avian myeloblastosis virus: present status. Curr Top Microbiol Immunol. 1975;71:79–101. doi: 10.1007/978-3-642-66193-8_2. [DOI] [PubMed] [Google Scholar]
- Roussel M., Saule S., Lagrou C., Rommens C., Beug H., Graf T., Stehelin D. Three new types of viral oncogene of cellular origin specific for haematopoietic cell transformation. Nature. 1979 Oct 11;281(5731):452–455. doi: 10.1038/281452a0. [DOI] [PubMed] [Google Scholar]
- Rushlow K. E., Lautenberger J. A., Papas T. S., Baluda M. A., Perbal B., Chirikjian J. G., Reddy E. P. Nucleotide sequence of the transforming gene of avian myeloblastosis virus. Science. 1982 Jun 25;216(4553):1421–1423. doi: 10.1126/science.6283631. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Souza L. M., Strommer J. N., Hillyard R. L., Komaromy M. C., Baluda M. A. Cellular sequences are present in the presumptive avian myeloblastosis virus genome. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5177–5181. doi: 10.1073/pnas.77.9.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanstrom R., Parker R. C., Varmus H. E., Bishop J. M. Transduction of a cellular oncogene: the genesis of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1983 May;80(9):2519–2523. doi: 10.1073/pnas.80.9.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya T., Hanafusa H. Structure and sequence of the cellular gene homologous to the RSV src gene and the mechanism for generating the transforming virus. Cell. 1983 Mar;32(3):881–890. doi: 10.1016/0092-8674(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E. Form and function of retroviral proviruses. Science. 1982 May 21;216(4548):812–820. doi: 10.1126/science.6177038. [DOI] [PubMed] [Google Scholar]