Abstract
Surface and interface properties are important in controlling the yield and efficiency of the photochemically initiated immobilization. Using a silane-functionalized perfluorophenylazide (PFPA-silane) as the photoactive crosslinker, the immobilization of polymers was studied by adjusting the density of the surface azido groups. Dilution of the photolinker resulted in a gradual decrease in the density of surface azido groups as well as the thickness of the immobilized film. When a non-photoactive silane was added to PFPA-silane, the film thickness decreased more rapidly, suggesting that the additive competed with PFPA-silane and effectively reduced the density of the surface azido groups. The effect of surface topography was studied by adding a non-photoactive silane with either a shorter (n-propyltrimethoxysilane (PTMS)) or a longer spacer (n-octadecyltrimethoxysilane (ODTMS)). In most cases the long chain ODTMS shielded the surface azido groups resulting in more rapid decrease in film thickness as compared to PTMS treated under the same conditions. As the density of the surface azido groups decreased, the immobilized polymer changed from smooth films to patched structures, and eventually single polymer molecules.
Keywords: photochemical immobilization, surface density, surface topography, interface control, polymer thin films
1. Introduction
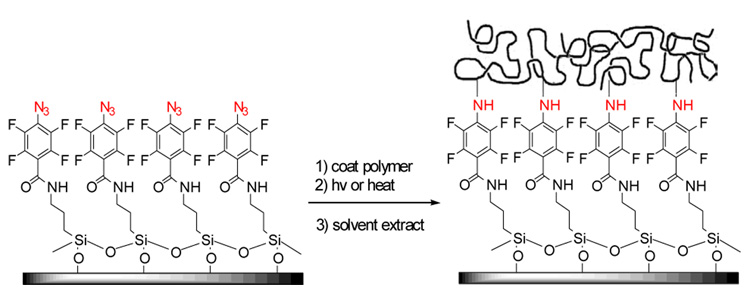
Immobilization via a photoactive heterobifunctional crosslinker has become a popular method for the covalent attachment of polymers and biomolecules on surfaces due to its simplicity and versatility.1–11 The crosslinker acts as a ‘molecular glue’, covalently attaching the molecules and materials on a substrate surface. Figure 1 is the schematic representation of the immobilization process where a silane-functionalized perfluorophenylazide (PFPA-silane) is used as the crosslinker to covalently attach macromolecules on the silicon oxide surface. The approach is based on the photochemistry of perfluorophenylazide. Upon irradiation, perfluorophenylnitrenes are generated and undergo efficient C-H and /or N-H insertion reactions with the neighboring molecules. Because the insertion reaction occurs at the substrate-polymer interface, only a “monolayer” of the polymer is covalently immobilized after the un-attached polymer is removed by solvent extraction.12
Figure 1.
Covalent immobilization of polymer on PFPA-functionalized surface.
This immobilization technique is especially effective in applications where only a thin layer of material is necessary to change the surface property of the substrate. The process is relatively simple to perform. It involves coating the material on the photolinker-functionalized surface followed by UV irradiation. Because the immobilization chemistry is based on the C-H/N-H insertion reaction of the reactive intermediate, there is no need to functionalize the molecule to be immobilized so long as it possesses C-H and/or N-H bonds. Therefore the technique is inherently versatile and should be applicable to a variety of molecules and materials especially those that do not possess reactive functional groups. Additional benefits include selective modification where the desired areas can be specifically modified by a focused UV light or beam of electrons, or by using a photomask.
We have employed PFPAs to immobilize a variety of polymers and biomolecules on silicon oxide and gold substrates. During the course of the studies, it was found that although the method should be versatile, it was not always the case. For example, isotactic polypropylene or low molecular weight poly(ethylene glycol) could not be immobilized using our standard spin coating and photoactivation procedure. Both polymers are highly crystalline and the spin-coated films had poor contact with the functionalized surface. Successful immobilization was achieved when the films were heated at above the polymer’s glass transition temperature to promote the contact with the functionalized surface.13 In a recent work by Joester and coworkers,14 polystyrene beads were functionalized with PFPA and were subsequently used to immobilize hyaluronan. Direct irradiation of the functionalized PS beads in hyaluronan solution did not yield any immobilized polymer due to the low concentration of hyaluronan in contact with the bead surface. To enhance the immobilization yield, lanthanide cations were added to precipitate hyaluronan and thus increased the local concentration of hyaluronan on the functionalized beads.
These observations demonstrated the importance of surface and interface properties. The compatibility of the functionalized surface with the materials to be immobilized, controlled by the chemical property and topography of the functionalized surfaces, directly affects the outcome of the immobilization. The density of photoactive groups on the surface also dictates the immobilization yield and efficiency.
We are interested in how the surface and interface properties would affect the photochemically induced polymer immobilization. The goal is to develop strategies for tailor-made surfaces and interfaces in order to achieve enhanced immobilization efficiencies for a greater variety of materials and biomolecules, thus making this method truly versatile. Herein, we studied the immobilization chemistry with regard to the density and topography of the functionalized surfaces. The mixed monolayer strategy was employed to control the density of functional groups, and to tailor the physical and chemical properties of the surface.
2. Experimental Section
2.1 Materials
3-Aminopropyltrimethoxysilane was purchased from United Chemical Technologies (Bristol, PA). n- Propyltrimethoxysilane and n-octadecyltrimethoxysilane were purchased from Gelest (Morrisville, PA) and were checked by 1H NMR before use. Pentafluorobenzoyl chloride and N-hydroxysuccinimide were used as received from Aldrich. Monodisperse polystyrene standard of molecular weight 223,200 was obtained from Scientific Polymer Products Inc. (Ontario, NY). Water used in the contact angle measurements was obtained from a Millipore Milli-Q system with at least 18.2 MΩ resistivity. Toluene, chloroform and methanol were used as received from Fisher. Deuterated solvents were used as received from Cambridge Isotope Labs (Andover, MA). Silicon wafers with a ~ 70 nm thermally grown oxide layer (from Silicon Valley Microelectronics Inc.) were cut with a diamond pen and cleaned in the piranha solution (7:3 v/v concentrated H2SO4/35 wt% H2O2) for 1 h at 80–90 □, washed thoroughly with boiling water for 1h, and dried under a stream of nitrogen. Caution! Piranha solution reacts violently with many organic compounds; use extreme care when handling it. UV glass filters were purchased from Schott Glass Technologies Inc. (Fullerton, CA).
2.2 Instrument
Spin coating was performed using a P6204 spin-coater (Specialty Coating Systems, Indianapolis, IN). Irradiation of the polymer films was executed at ambient temperature with a medium-pressure Hg lamp (450W, Hanovia). The lamp reached its full power after ~2 min warm-up to an intensity of 5.0 mW/cm2 as measured by a model UVX radiometer and UVX-36 sensor manufactured by UVX Inc. (Upland, CA). Contact angels were determined with 2 µL of distilled water and measured using a homemade apparatus with an Intel QX3 200×microscope. A digital camera was used to record images, and contact angles were determined graphically. Film thickness measurements were made on a Gaertner model L116A analog ellipsometer with He/Ne laser (2mW, Melles Griot) at an incident angle of 70° in the manual mode. The following refractive indices: SiO2 1.465, PFPA-silane 1.503, PS 1.592 were used to determine the thickness of various film layers. A refractive index of 1.5 was used to calculate the thickness of organic layers formed from mixed silanes.15 1H NMR spectra were acquired on a Nicolet NT-500 MHz FT-NMR spectrometer equipped with a 5-mm 1H probe. Chemical shifts were reported in delta units referenced to the internal standard Si(CH3)4 at 0.00 ppm. 19F NMR spectra were recorded on a 90 MHz spectrometer with the chemical shifts reported in delta units internally referenced to Freon 11 (CCl3F) as δ 0.0 ppm. Infrared spectra were obtained using a Perkin-Elmer 2000 series FT-IR spectrometer. Elemental analysis was performed by Quantitative Technologies Inc. (Whitehouse, NJ). Atomic force microscopy images were collected on a Nanoscope III (Veeco, Santa Barbara, CA) using a silicon tip in the tapping mode at an oscillating frequency of ~300 kHz. XPS measurements were performed on a Physical Electronics Quantum 2000 Scanning ESCA Microprobe equipped with a focused monochromatic Al Kα x-ray source at 1486.7 eV for excitation and a spherical section analyzer with a 16 element multichannel detector system. The X-ray beam used was a 101.5 W, 100 µm diameter beam that was rastered over a 1.4 mm by 0.2 mm rectangle on the sample. The X-ray beam was incident normal to the sample while the photoelectron detector was set at an angle of 45° from the normal. High energy resolution data was collected with an analyzer pass energy of 46.95 eV. For the Ag 3d 5/2 line, these conditions produced FWHM of better than 0.98 eV. The binding energy (BE) scale was calibrated using the Cu 2p 3/2 feature at 932.62 ± 0.05 eV and Au 4f at 83.96 ± 0.05 eV for known standards.
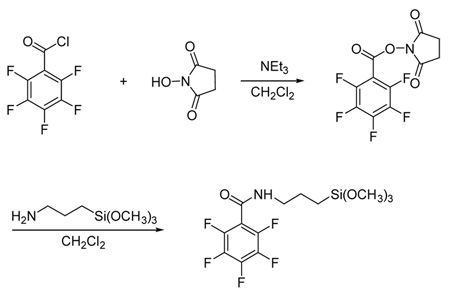
2.3 Synthesis of N-(3-trimethoxysilylpropyl)-2,3,4,5,6-pentafluorobenzamide (PFB-silane)
N-Succinimidyl pentafluorobenzoate was synthesized by the dropwise addition of pentafluorobenzoyl chloride (138 µL, 1.00 mmol) to a stirred solution of N-hydroxysuccinimide (121 mg, 1.05 mmol) in dichloromethane (5 mL) and triethylamine (146 µL, 1.05 mmol). The reaction mixture was stirred at room temperature for 2 h. The crude product was purified by column chromatography on silica gel, eluting with 1:3 v/v chloroform/hexanes with 2% methanol to yield the product as a white solid (299 mg, 96%).
A solution of N-succinimidyl pentafluorobenzoate (184 mg, 0.59 mmol), 3- aminopropyltrimethoxysilane (129 µL, 0.74 mmol), and anhydrous dichloromethane (4.0 mL) was capped in argon and stirred at room temperature for 6 h. After evaporation of the solvent, the residue was purified by flash column chromatography on silica gel using 1:3 v/v chloroform/hexane with 2% methanol as an eluant to give N-(3-trimethoxysilylpropyl)-2,3,4,5,6-pentafluorobenzamide as a colorless viscous liquid (164 mg, 74%). FT-IR (film): 3296, 2949, 1660, 1517, 1340, 1189, 1091, 992, 823 cm−1. 1H NMR (CDCl3) δ 6.46 (br, 1H), 3.57 (s, 1H), 3.46 (q, J = 12 Hz, 2H), 1.75 (m, 2H), 0.71 (t, J = 16 Hz, 2H). 19F NMR (CDCl3) δ −140.9 (br, 2F), −151.2 (br, 1F), −160.6 (br, 2F). Anal. calcd. for C13H16NF5O4Si: C, 41.82; H, 4.32; N, 3.75. Found: C, 41.83; H, 4.24; N, 3.74.
2.4 Immobilization of polystyrene on silicon wafers
N-(3-Trimethoxysilylpropyl)-4-azido-2,3,5,6-tetrafluorobenzamide (PFPA-silane) was synthesized following a previously reported procedure.12 Cleaned wafers were soaked in a solution of either PFPA-silane alone or a solution of mixed silanes in toluene for 4 h at room temperature. The total concentration of the mixed silanes was kept at 12.6 mM. This process was carried out in sealed vials to minimize contact with moisture in the air. The treated wafers were rinsed with a gentle stream of toluene, dried under nitrogen, and then allowed to cure at room temperature for at least 24 h.
The functionalized wafers were spin-coated at 2000 rpm for 60 seconds with a polystyrene solution (10 mg/mL) prepared by dissolving monodisperse PS of Mw 223,200 in toluene. The samples were then irradiated for 5 minutes with the medium-pressure Hg lamp. A 280-nm optical filter was placed on the film surface during irradiation. The unbound polymer was removed by sonication in toluene for 5 minutes. The resulting film was dried under a stream of nitrogen.
3. Results and discussion
An advantage of using polymers as the surface modifier is that polymer chains can entangle to form an interpenetrated network. High density of the polymer is therefore unnecessary to produce uniform films that cover the substrate surface. This is of advantage to small molecule-based films such as self-assembled monolayers where dense and ordered arrays are required to avoid structural defects.
In principle, only one attachment point is necessary to tether a polymer molecule to the surface. Because the larger size of the polymer in comparison to that of the photolinker, the concentration of the surface azido groups can be diluted while still maintaining the covalent attachment of the polymer to the surface. For PS of molecular weight 223,200 for example, assuming a random coil conformation of the polymer, a single polymer coil would occupy a surface area of ~ 5.3×104 Å2 (radius of gyration Rg ~ 13 nm16). Assuming that every Si-OH group on the wafer surface is available for reacting with the silane, using the surface area of 20 Å2 for Si-OH,17 the theoretical maximum dilution of the azide would be ~ 2600 while still ensuring covalent attachment of the polymer.
To test this hypothesis, we systematically decreased the density of the surface azido groups and studied the resulting immobilized polymer films. The density of the surface azido groups was altered by either changing the solution concentration of PFPA-silane, or by co-depositing a non-photoactive silane together with PFPA-silane using the mixed monolayer approach.18–23
3.1 Density control by solution concentration
The first approach to density control is to adjust the concentration of the photolinker used for surface functionalization. Experiments were carried out by treating the cleaned wafers with PFPA-silane at various concentrations. The 12.6 mM solution of PFPA-silane in toluene has been used in our previous work for the immobilization of various polymers. In this study, a series of PFPA-silane solutions were prepared by diluting the 12.6 mM stock solution with toluene. Silicon wafers were then treated with these solutions and polystyrene subsequently immobilized by photoactivation.
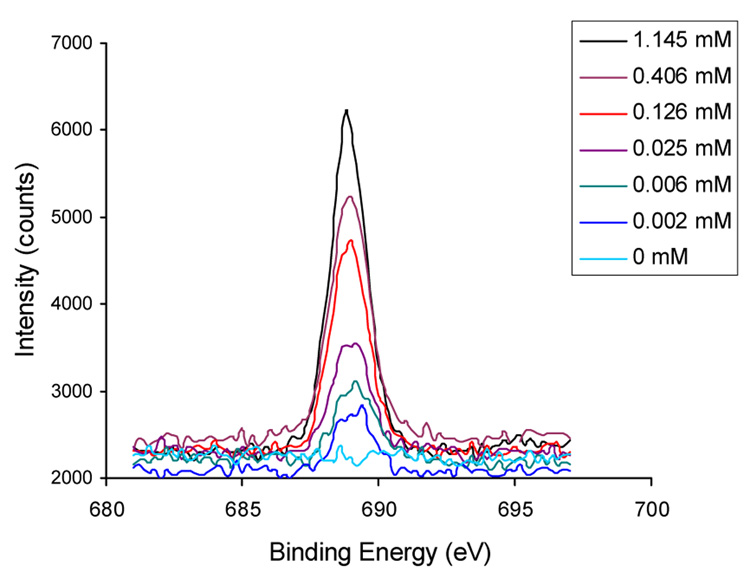
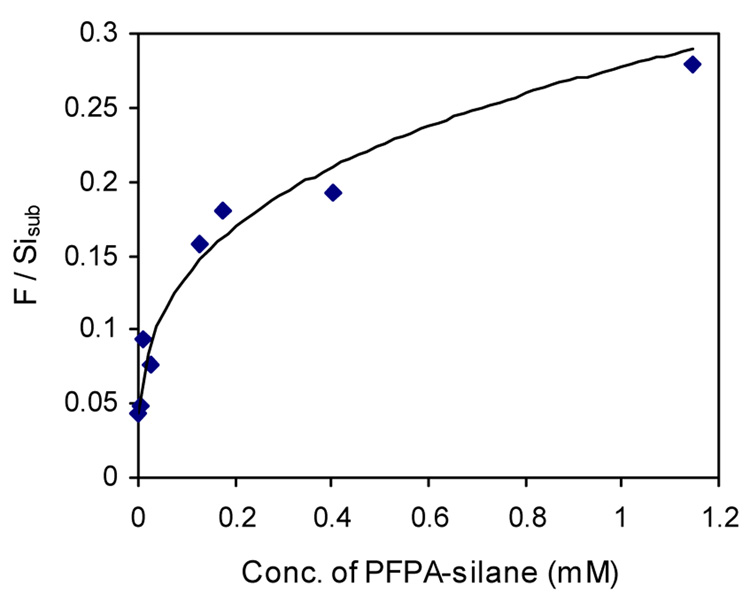
The functionalized surfaces were evaluated using X-ray photoelectron spectroscopy (XPS). As expected, the intensity of the F 1s peak, reflecting the density of azido groups on the surface, decreased with the dilution of PFPA-silane (Fig. 2). Figure 3 shows the intensity ratio F/Sisub (Sisub = Sitotal−SiPFPA) as a function of the solution concentration of PFPA-silane. The results confirmed that the dilution did not factor into the surface density of PFPA-silane in a linear fashion due to a large excess of silane in the solution during silanization.
Figure 2.
XPS F 1s peaks of functionalized surfaces.
Figure 3.
Surface density of the photolinker vs. solution concentration of PFPA-silane.
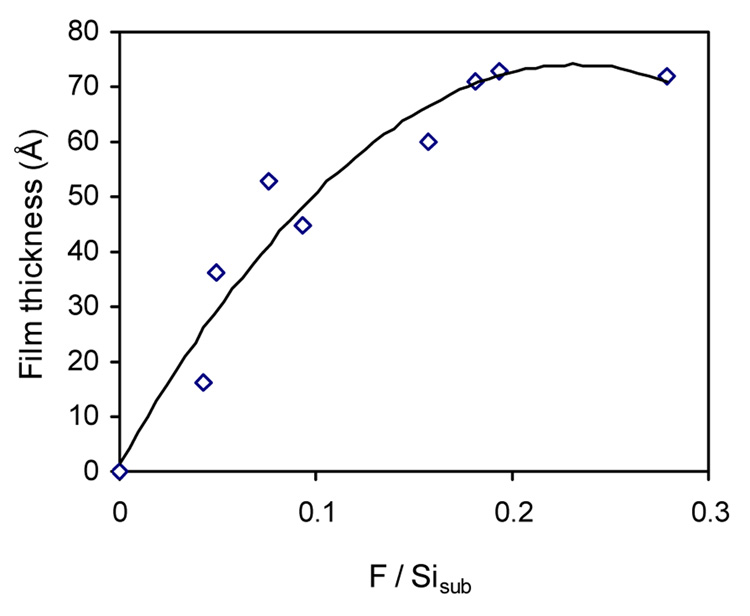
The immobilized polymer film thickness was subsequently plotted against the surface density of the photolinker (Figure 4). At higher photolinker concentrations, the film thicknesses were at the maximum and remained unchanged. The film became thinner as the concentration of the photolinker decreased. This result is consistent with our observation that it is possible to control the immobilized film thickness by adjusting the PFPA-silane concentration in solution. At higher photolinker concentrations, the polymer was immobilized through multi-point attachments via surface azido groups and the polymer chains were densely packed. As the PFPA-silane solution was diluted, the density of the surface azido groups decreased and thus the number of attachment points as well as the amount of polymer immobilized. When the attached polymer was no longer densely packed, after the polymer was soaked in toluene, the chains could collapse and flatten resulting in reduced film thickness.
Figure 4.
Polymer film thickness as a function of photolinker density on surface.
3.2 Density control using mixed monolayer
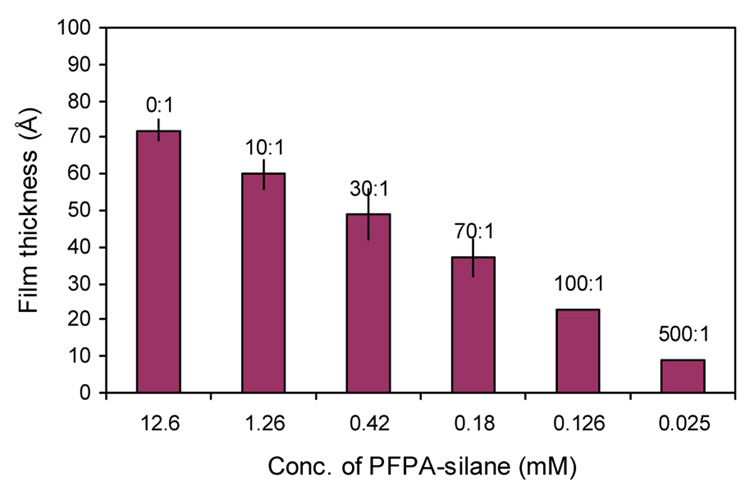
An alternative means to alter the density of surface azido groups is the mixed monolayer approach where a non-photoactive silane can be added to the PFPA-silane solution to reduce the concentration of the azido groups on the surface. In the experiments, clean wafers were treated with a solution containing PFPA-silane and a non-photoactive silane. The total concentration of the silanes was kept at 12.6 mM. The mole ratio of the non-photoactive silane to PFPA-silane was increased to produce mixed monolayers with decreasing density of the surface azido groups. The resulting functionalized surface was spin-coated with polystyrene, irradiated, and the film thickness measured after the excess polymer was removed by toluene extraction.
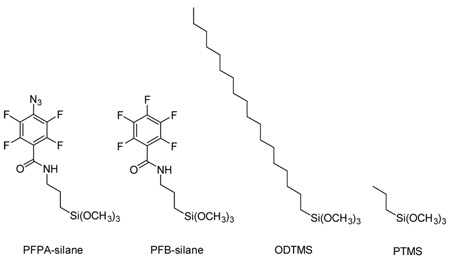
We chose N-(3-trimethoxysilylpropyl)-2,3,4,5,6-pentafluorobenzamide (PFB-silane) as the first non-photoactive silane candidate. PFB-silane resembles PFPA-silane in structure with the only difference being the lack of the photoactive azido group. When the surface was treated with the mixed silanes, the immobilized PS films were thinner than those immobilized with PFPA-silane alone at the same PFPA-silane concentrations (Figure 5). This is probably because PFB-silane competed with PFPA-silane for the sites on the wafer surface, leading to decreased azide densities and thus thinner polymer films. At the mole ratio of 500:1, the average film thickness was ~9 Å, suggesting that very little polymer remained on the surface. Indeed, the immobilized PS film was no longer uniform and patched structures were revealed by AFM (Figure 8a). The contact angle of the film was measured to be ~78°. In comparison, the contact angle of ~90° was observed for PS films immobilized on wafers treated with the mixed silanes at lower mole ratios, or the PS film immobilized with PFPA-silane alone at the same concentration (0.025 mM).
Figure 5.
Thickness of PS films immobilized on wafers treated with a mixture of PFB-silane and PFPA-silane. The mole ratios of PFB-silane:PFPA-silane are indicated in the graph.
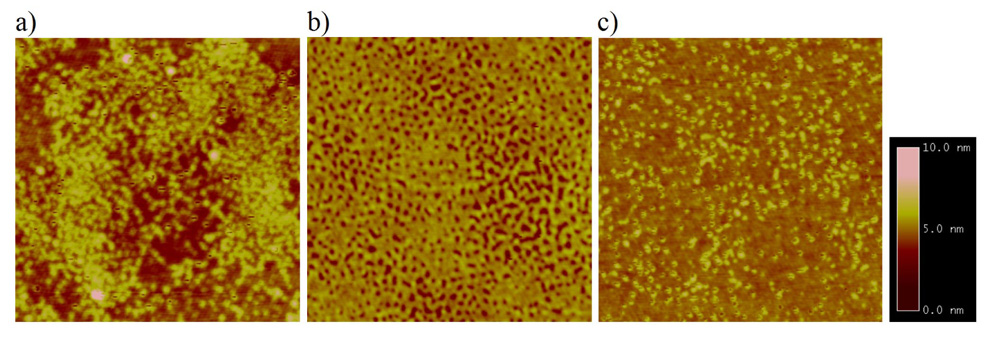
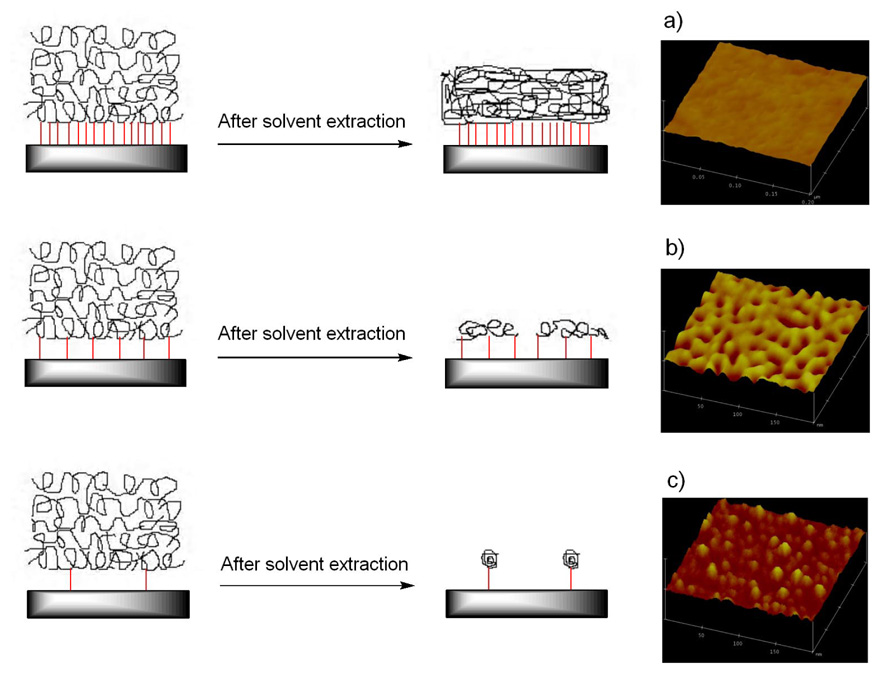
Figure 8.
AFM images of immobilized polystyrene on wafers treated with 500:1 non-photoactive silane: PFPA-silane. The non-photoactive silane was a) PFB-silane, b) PTMS, c) ODTMS. The scan area is 1µm × 1µm. RMS roughness values are 0.791 nm, 0.587 nm, and 0.494 nm, respectively.
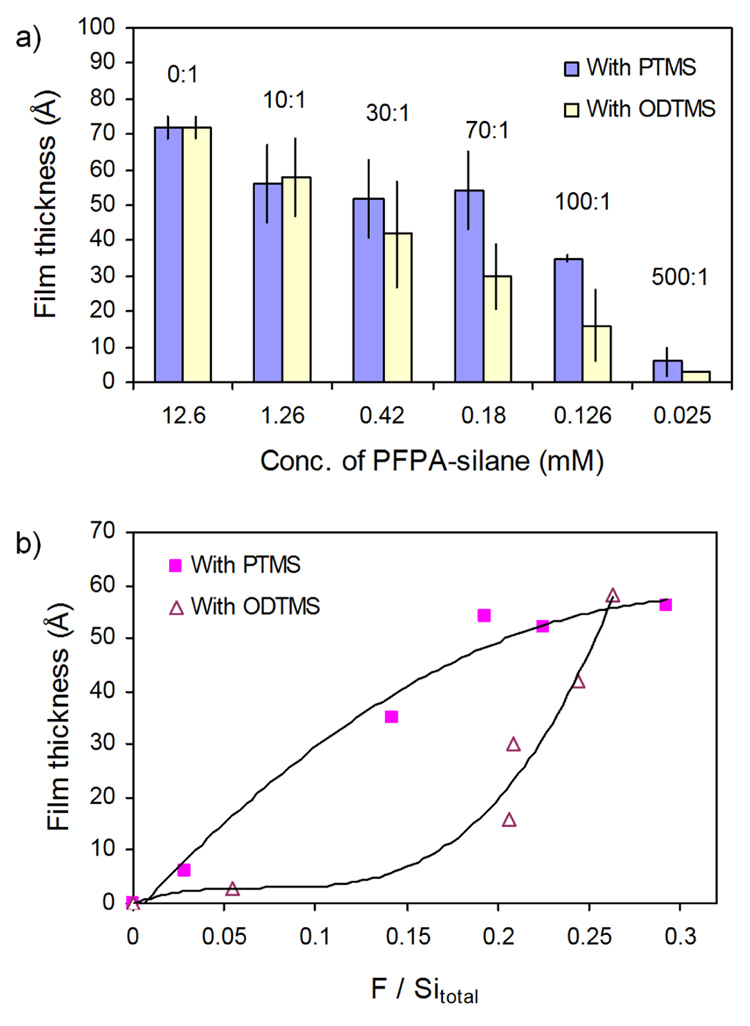
Next, n-propyltrimethoxysilane (PTMS) and n-octadecyltrimethoxysilane (ODTMS) were used as the non-photoactive silanes. PTMS and ODTMS possess 3 and 18 CH2 groups as the spacer, respectively. It was expected that the large excess of the long chain silane would bury the azide thus lowering the amount of available azido groups for insertion reactions with the polymer. A likely event would be the insertion reaction of the azido groups into the alkyl chains causing crosslinking between the molecules in the monolayer. Except for the data from the mole ratio of 10:1, experimental results supported this hypothesis (Figure 6a). At the same surface photolinker concentration, more polymer was immobilized when using PTMS as the non-photoactive silane in comparison to ODTMS (Figure 6b). This can be attributed to the shielding effect of ODTMS leading to a lower immobilization efficiency relative to PTMS.
Figure 6.
Thickness of PS films immobilized on wafers treated with a mixture of PFPA-silane and PTMS or ODTMS as a function of a) mole ratio in solution; b) photolinker density on functionalized surface.
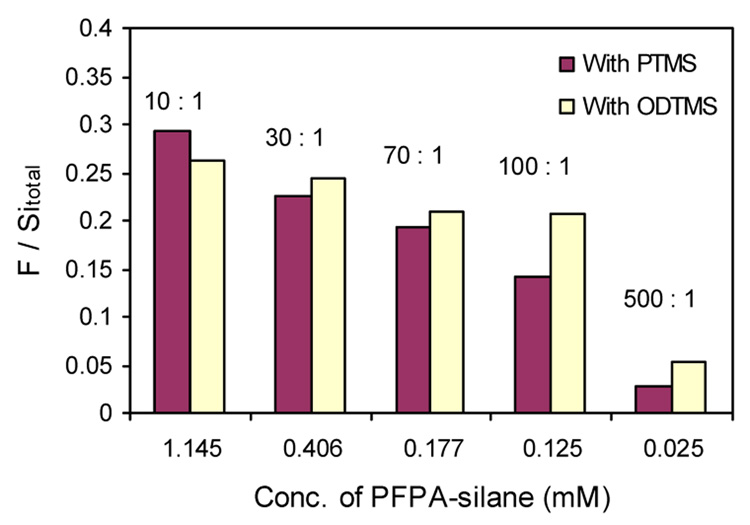
With the density of the photolinker on the surface determined by XPS, the differential reactivity of the silanes may be compared. Figure 7 correlates the azide density (as intensity ratio F/Sitotal) with its concentration in the solution. Except for the mole ratio of 10:1, dilution by ODTMS leads to a higher density of the photolinker on the surface relative to PTMS, indicating that ODTMS is of lower reactivity than PTMS.
Figure 7.
Surface density of photolinker for wafers treated with mixed silanes of PFPA-silane and PTMS or ODTMS.
An issue in mixed monolayer formation is the potential for phase separation. Microscopic phase separation on the nanometer scale is extremely difficult to characterize spectroscopically. Whether they produce homogeneous or phase-separated monolayers on the substrate has been a controversial issue. A model invoking phase separation and nanoscale domains explained data obtained from experiments with mixed thiols on gold substrates.23–24 For alkyltrichlorosilanes however, the two components in the mixed SAMs were reported to be uniformly distributed, and no phase separation was observed at least on the length scale of the instrument, e.g. ~80 nm by AFM.25–27 Work involving mixed trialkoxysilanes was seldom reported. Ejaz et al. showed that a blend of 2-(4-chloro-sulfonylphenyl) ethyltrimethoxysilane (CTS) and n-octadecyltrimethoxysilane (ODTMS) was immiscible and phase-separated into two monolayer phases.28 However their SAMs were formed by the Langmuir-Blodgett technique, not the chemisorption method employed in this work.
On the other hand, the surface morphology of the immobilized polymer could be used as an indirect means to probe the homogeneity of the mixed monolayer underneath. From the AFM image of the immobilized polymer using PFB-silane as the non-photoactive silane (Figure 8a), clusters of polymer were observed. It could therefore be speculated that phase separation occurred in the mixed monolayer at the length scale of the AFM. Polymers were immobilized in regions populated by PFPA-silane, whereas in areas rich in PFB-silane no polymer was attached. When PTMS (Figure 8b) or ODTMS (Figure 8c) was used as the non-photoactive silane however, such clusters of polymer were not observed, suggesting the absence of phase separation at the length scale of the instrument.
3.3 Polymer morphology by interface control
An interesting result of these studies was the observation of various polymer microstructures after the immobilized films collapsed. The transition from smooth films to patched structures to single molecules was evident for surfaces treated with either the diluted PFPA-silane or the mixed silanes. For example, with PTMS as the additive, at the mole ratio of 70:1, the immobilized PS film was smooth with a contact angle of 90° and the RMS roughness value of 0.355 nm, respectively (Figure 9a). At the mole ratio of 500:1, the surface revealed porous mesh morphology with a water contact angle of ~90° and RMS roughness value of 0.587 nm (Figure 9b). The film was completely depleted at the mole ratio of 2000:1, whereby single polymer molecules were instead observed (Figure 9c). The detailed analyses of these single molecules were reported elsewhere.29
Figure 9.
Schematic illustration of polymers immobilized with different concentrations of surface azido groups and the corresponding AFM images of the immobilized polymers. Wafer surfaces were treated with mixed silane solutions of PFPA-silane and PTMS, and the mole ratio used was a) 70:1, b) 500:1, c) 2000:1, respectively. The scan area is 200 nm × 200 nm. Z-scale is 10 nm.
The “patchiness” of immobilized films obtained under certain experimental conditions is an important transition structure between the thin film which is going to collapse and separated single molecules. The capture of this morphology is a strong evidence for our proposed immobilization mechanism and further supports the effectiveness of manipulating polymer microstructures by way of density control of the photolinker on the surface.
4. Conclusion
The photochemically initiated immobilization chemistry was investigated by specifically altering the density and topography of the functionalized surfaces. As the density of the surface azido group decreased, either by reducing the concentration of PFPA-silane or by adding a non-photoactive silane, the immobilized polymer became thinner, changing from uniform films to patched structures and eventually single molecules. The topography of the functionalized surface also affected the immobilization efficiency. Films immobilized on surfaces treated with long chain silane (ODTMS) as the additive were on the average thinner than those treated with short chain silane (PTMS).
An important conclusion that can be drawn from these studies is that this surface immobilization technique is highly reproducible and defect-tolerant. Uniform films were obtained from wafers treated with PFPA-silane at concentrations of a few µM, or when more than 100 times of a non-photoactive silane was added. This feature is important in practical applications where defect-tolerance and reproducibility are critical. Secondly, the strategies presented herein allow the control of the immobilization efficiency and microstructures of immobilized materials. Films of various thickness and morphology as well as covalently immobilized single molecules can be obtained simply by adjusting the density of the photoactive groups on the surface. Thirdly, by using the mixed monolayer approach, one can control not only the surface density and topography, but also chemical functionality. The functional group on the non-photoactive molecule can be chosen, depending on the material to be immobilized, to create a chemically compatible surface for increased immobilization yield and efficiency. Work in this aspect is currently in progress.
Acknowledgment
The authors acknowledge the financial support of an NIH AREA award 1R15 GM066279-01A2.
References
- 1.Yan M. Poly. News. 2002;27:6–12. [Google Scholar]
- 2.Prucker O, Naumann C, Rühe J, Knoll W, Frank C. J. Am. Chem. Soc. 1999;121:8766–8770. [Google Scholar]
- 3.Griep-Raming N, Karger M, Menzel H. Langmuir. 2004;20:11811–11814. doi: 10.1021/la0485327. [DOI] [PubMed] [Google Scholar]
- 4.Cosnier S, Senillou A. Chem. Comm. 2003;3:414–415. doi: 10.1039/b211546h. [DOI] [PubMed] [Google Scholar]
- 5.Kado Y, Mitsuishi M, Miyashita T. Adv. Mater. 2005;17:1857–1861. [Google Scholar]
- 6.Pallandre A, de Lambert B, Attia R, Jonas A, Viovy J-L. Electrophoresis. 2006;27:584–610. doi: 10.1002/elps.200500761. [DOI] [PubMed] [Google Scholar]
- 7.Cosnier S, Molins C, Mousty C, Galland B, Lepellec A. Mat. Sci. Eng. C. 2006;26:436–441. [Google Scholar]
- 8.Carroll G, Wang D, Turro N, Koberstein J. Langmuir. 2006;22:2899–2905. doi: 10.1021/la0531042. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Mu L, Liu B, Kong J. Chem. Eur. J. 2005;11:2622–2631. doi: 10.1002/chem.200400931. [DOI] [PubMed] [Google Scholar]
- 10.Pahnke J, Ruehe J. Polym. Prepr. 2005;46:487–488. [Google Scholar]
- 11.Woerz A, Chang B, Prucker O, Biesalski M, Ruehe J. Polym. Prepr. 2005;46:114–115. [Google Scholar]
- 12.Bartlett M, Yan M. Adv. Mater. 2001;13:1449–1451. [Google Scholar]
- 13.Yan M, Ren J. Chem. Mater. 2004;16:1627–1632. [Google Scholar]
- 14.Joester D, Klein E, Geiger B, Addadi L. J. Am. Chem. Soc. 2006;128:1119–1124. doi: 10.1021/ja0537474. [DOI] [PubMed] [Google Scholar]
- 15.Allara D, Nuzzo R. Langmuir. 1985;1:45–52. [Google Scholar]
- 16.Brûlet A, Boué F, Menelle A, Cotton J. Macromolecules. 2000;33:997–1001. [Google Scholar]
- 17.Ulman A. An Introduction to Ultrathin Organic Films. Boston: Academic Press; 1991. [Google Scholar]
- 18.Lahiri J, Isaacs L, Tien J, Whitesides G. Anal. Chem. 1999;71:777–790. doi: 10.1021/ac980959t. [DOI] [PubMed] [Google Scholar]
- 19.Mrksich M, Grunwell J, Whitesides G. J. Am. Chem. Soc. 1995;117:12009–12010. [Google Scholar]
- 20.Mrksich M, Sigal G, Whitesides G. Langmuir. 1995;11:4383–4385. [Google Scholar]
- 21.Whiteside G, Laibinis P. Langmuir. 1990;6:87–96. [Google Scholar]
- 22.Sigal G, Bamdad C, Barberis A, Strominger J, Whitesides G. Anal. Chem. 1996;68:490–497. doi: 10.1021/ac9504023. [DOI] [PubMed] [Google Scholar]
- 23.Folkers J, Laibinis P, Deutch J, Whitesides G. J. Phys. Chem. 1994;98:563–571. [Google Scholar]
- 24.Tamada K, Hara M, Sasabe H, Knoll W. Langmuir. 1997;13:1558–1566. [Google Scholar]
- 25.Offord D, Griffin J. Langmuir. 1993;9:3015–3025. [Google Scholar]
- 26.Mathauser K, Frank CW. Langmuir. 1993;9:3002–3008. [Google Scholar]
- 27.Zhang Q, Archer LA. J. Phys. Chem. B. 2003;107:13123–13132. [Google Scholar]
- 28.Ejaz M, Yamamoto S, Tsujii Y, Fukuda T. Macromolecules. 2002;35:1412–1418. [Google Scholar]
- 29.Liu L, Yan M. Angew. Chem. Int. Ed. Engl. 2006 in press. [Google Scholar]