Abstract
One-third of botanical remedies from southern Italy are used to treat skin and soft tissue infection (SSTI). Staphylococcus aureus, a common cause of SSTI, has generated increasing concern due to drug resistance. Many plants possess antimicrobial agents and provide effective remedies for SSTI. Our aim was to investigate plants from different ethnobotanical usage groups for inhibition of growth and biofilms in methicillin-resistant S. aureus (MRSA).
Three groups were assessed: plant remedies for SSTI, plant remedies not involving the skin, and plants with no ethnomedical application. We screened 168 extracts, representing 104 botanical species, for activity against MRSA (ATCC 33593). We employed broth dilution methods to determine the MIC after 18 hours growth using an optical density (OD600nm) reading. Anti-biofilm effects were assessed by growing biofilms for 40 hours, then fixing and staining with crystal violet. After washing, 10% Tween 80 was added and OD570nm readings were taken.
Extracts from 10 plants exhibited an IC50 ≤32 μg/ml for biofilm inhibition: Lonicera alpigena, Castanea sativa, Juglans regia, Ballota nigra, Rosmarinus officinalis, Leopoldia comosa, Malva sylvestris, Cyclamen hederifolium, Rosa canina, and Rubus ulmifolius. Limited bacteriostatic activity was evident. The anti-biofilm activity of medicinal plants was significantly greater than plants without any ethnomedical applications.
Keywords: Italy, medicinal plants, biofilms, methicillin-resistant Staphylococcus aureus
1. Introduction
The increase of microbial resistance to antibiotics threatens public health on a global scale as it reduces the effectiveness of treatments and increases morbidity, mortality, and health care costs (Coast et al., 1996). Evolution of highly resistant bacterial strains has compromised the use of newer generations of antibiotics (Levy, 2002). Although the active constituents may occur in lower concentrations, plant extracts may be a better source of antimicrobial compounds than synthetic drugs (Cox and Balick, 1994). The phenomenon of additive or synergistic effects is often crucial to bioactivity (Aqil et al., 2006a; b; Kamatou et al., 2006) in plant extracts and in some cases, the activity is lost in purified fractions (Cos et al., 2006). Development of bacterial resistance to synergistic drug combinations, such as those found in plants, may be slower than for single drug therapies.
Only a small percent of plants have been investigated for their bioactivity (Cox and Balick, 1994). Furthermore, most studies on the antimicrobial activity of plant extracts have been restricted to analysis of their bacteriostatic and bactericidal properties. New assays investigating other potential roles, such as the mediation of pathogenicity via quorum sensing inhibition, have recently emerged (Rasmussen and Givskov, 2006). Investigations into the anti-pathogenic potential of natural products may open new avenues for drug development in the control of antibiotic resistant pathogens.
The use of plants against skin disease is a common practice in the popular medicine of most cultures, although the precise causation of disease and mechanism of cure is not always understood (Grierson and Afolayan, 1999; Srinivasan et al., 2001). While 1–3% of pharmaceutical drugs are used to treat skin disorders and wounds, 33% of botanical therapies in non-Western societies are used to this end (Mantle and Gok, 2001). The topical application of many plant remedies for the treatment of various skin conditions has proven effective due to the antimicrobial and anti-inflammatory properties of plant compounds.
In the Vulture-Alto Bradano area of Basilicata Province, southern Italy (Figure 1), wild plants are regularly gathered as sources of food and medicine, making up an integral facet of home healthcare (Pieroni et al., 2002b; Pieroni et al., 2002c; Pieroni and Quave, 2005; Quave et al., 2008). At least one-third of all medicinal plants utilized in the folk-pharmacopoeia of this area are applied towards the treatment of skin and soft tissue infection (SSTI) (Quave et al., 2008). Vulture-Alto Bradano is characterized by small scale agriculture and its inhabitants maintain strong ties with the land. The demographic and socioeconomic aspects of this area are described in previous works (Pieroni et al., 2002c; Quave and Pieroni, 2005; Quave et al., 2008).
Figure 1.
Map of the study area: Vulture-Alto Bradano, Basilicata Province, Italy.
Staphylococcus aureus, a gram-positive species, is a ubiquitous colonizer of the skin and mucous membranes of humans and animals (Fauci et al., 1998). It is the most prominent etiological agent for SSTI and is a common target for natural product drug screenings. Many strains of S. aureus carry resistance genes for penicillin antibiotics, tetracyclines, methicillin and now, vancomycin. Methicillin-resistant S. aureus (MRSA) presents a significant threat to public health in the USA. A surveillance study on MRSA found that an estimated 94,360 patients in the USA had invasive MRSA infections in 2005, resulting in an estimated 18,650 deaths (Klevens et al., 2007), surpassing the mortality estimates for AIDS in the USA.
Biofilm-related infections caused by staphylococci are among the leading causes of nosocomial infection in the USA (Kong et al., 2006). Biofilms comprise sessile microbial communities that embed themselves within a self-made extracellular polymeric matrix. This phenotype confers some protection to bacteria against host defenses and impedes the delivery of certain large molecule antimicrobials (Lewis, 2001). Moreover, bacteria associated with biofilms grow slowly, and this restricts the efficacy of many antibiotic classes, including the β-lactams (Lewis, 2005). The first stages of staphylococcal biofilm formation involve adherence of planktonic bacterial cells to cellular or prosthetic surfaces, such as surgically implanted medical devices, including catheters, plates, screws, artificial joints and cardiac valves (Costerton et al., 1999). Biofilms present significant therapeutic barriers for many antibiotics and the discovery of agents which could prevent their formation or adherence would be of great use.
The objectives of this study were to evaluate extracts of south Italian plants for effects on growth, biofilm formation and adherence in MRSA. Our hypothesis was that plants used specifically for the treatment of SSTI would demonstrate higher mean activity for growth and biofilm inhibition than plant remedies not involving the skin or plants with no ethnomedical application.
2. Materials and Methods
2.1 Plant material and extraction
Plants from three ethnobotanical usage categories were selected for analysis: Group 1: medicinal plants for skin and soft tissue infection (SSTI) (n=25), Group 2: medicinal plants not involved in skin therapies (n=28), and Group 3: plants with no ethnomedical application (n=51). Plants were categorized based on previous ethnobotanical surveys conducted in the Vulture-Alto Bradano area of southern Italy (Pieroni et al., 2002b; Pieroni et al., 2002c; Pieroni and Quave, 2005; Pieroni and Quave, 2006; Quave et al., 2008). Parameters for collecting a sample of non-medicinal species of Vulture-Alto Bradano were that they grew in abundance and were flowering at the time of collection. Plants were field-collected in the Vulture-Alto Bradano area of southern Italy and identified using the standard botanic work Flora d’Italia (Pignatti, 2002). Familial nomenclature follows the current Angiosperm Phylogeny Group (Stevens, 2001 onwards). Bulk samples were separated by part (e.g., leaves, stems, flowers, and roots), cut into small pieces and dried in a plant drier using a heat and air flow source (35°C) for 48–72 h. In the case of herbaceous species, efforts were made to collect all parts of the plant, including both above-ground and below-ground parts. Only the above-ground parts were typically collected from trees and large shrubs. Bulk specimens were composed only of the targeted plant species – parts from other plants and soil were removed prior to drying and vacuum storage. Cross-contamination was avoided by using clean drying nets and storage containers. After drying, plant materials were packed into plastic bags with several silica packets and vacuum sealed to prevent mold growth. Voucher specimens were deposited at the Herbarium Lucanum (HLUC) in Potenza, Italy and Fairchild Tropical Botanic Gardens (FTG) in Miami, FL, USA. Bulk specimens were shipped to the US under USDA permit #DP63438 for bioassays.
Dry plant materials were ground into a fine powder using a homogenizer. Ethanolic extracts of all plant samples were made by soaking in 95% denatured EtOH using a ratio of 1g (plant material):10 ml (EtOH) for 72 h. Flasks were agitated daily. Water extracts were made by boiling 1g (plant material): 50 ml (dH2O) for 30 minutes. Extracts were vacuum-filtered and rotary-evaporated, then frozen and lyophilized. Stock concentrations of 10 mg/ml of dry extract in the excipient (DMSO or dH2O) were prepared, sterile filtered (0.2 μm) and stored in the dark at 4°C. The excipient (DMSO or dH2O) made up less than 5.1% of the final test solution for MIC assays and less than 2.5% for biofilm assays.
2.2 Bacteria and culture conditions
Methicillin-resistant Staphylococcus aureus (ATCC 33593) was grown in Tryptic Soy Broth (TSB) or on agar plates for 18 h at 37°C. A 0.5 McFarland Standard was used to create inoculum densities of 1.5 × 108 cfu/ml in PBS using the direct suspension method (Isenberg, 2004) for MIC and biofilm assays.
2.3 Determination of minimum inhibitory concentrations (MICs)
MICs were determined by the microtiter broth method (Amsterdam, 1996) in sterile flat-bottom 96-well polystyrene plates. Serial dilution techniques were used to determine the MIC50 and MIC90 of extracts at concentrations of 8–512 μg/ml after 18 h growth. Negative controls (cells + TSB), positive controls (cells + TSB + antibiotics – vancomycin, ampicillin, and trimethroprim-sulfamethoxazole), vehicle controls (cells + TSB + DMSO), and media controls (TSB) were included. Positive controls for antibiotics were prepared at 8–512 μg/ml via serial dilution techniques. All tests were performed in triplicate. Optical density readings were taken using a KC4 microplate reader at 600 nm at 0 and 18 hours post-inoculation. Results are reported as the MIC for growth at 18 hours post-inoculation. To account for the effect of extract color on the OD600nm reading, a formula for calculating percent inhibition was used. The mean % inhibition of replicate tests was used to determine the final MIC values.
ODt18 = optical density (600 nm) of the test well at 18 hours post-inoculation
ODt0 = optical density (600 nm) of the test well at 0 hours post-inoculation
ODgc18 = optical density (600 nm) of the growth control well at 18 hours post-inoculation
ODgc0 = optical density (600 nm) of the growth control well at 0 hours post-inoculation
2.4 Biofilm formation and adherence
A modified microtiter dish system (Christensen et al., 1985; Schadow et al., 1988; Yarwood et al., 2004) was employed to test the effect of plant extracts on biofilm formation and adherence. Assays were performed in sterile flat-bottom 96-well polystyrene plates. Serial dilution techniques were employed to determine the IC50 of extracts at sub-inhibitory (for growth) concentrations of 4–128 μg/ml after 40 h growth. Negative controls (cells + TSB), positive controls (cells + TSB + antibiotics – vancomycin, ampicillin, and trimethroprim-sulfamethoxazole), vehicle controls (cells + TSB + DMSO), and media controls (TSB) were included. Positive controls for antibiotics were prepared at 4–128 μg/ml via serial dilution techniques. All tests were performed in triplicate.
To test for the effects of extracts on biofilm formation, the appropriate concentration of extract was added to the test wells prior to inoculation. Plates were placed in a 37°C incubator and bacteria were shaken for 40 h. The contents of the wells were then aspirated, rinsed 3 times with PBS, and fixed by drying for 1 h in the 37°C incubator. Once the wells were fully dry, we added 200 μl of 0.1% crystal violet stain to wells to stain for 15 m. The excess stain was rinsed off with tap water and 200 μl of 10% Tween 80 was added to wells. We pulled off the stain adhering to the biofilm biomass with the Tween 80 and transferred to new 96-well plates for spectrophotometric analysis (OD570nm).
To test for the effects of extracts on biofilm adherence, biofilms were established in the 96-well plates by growing shaking for 20 h at 37°C. At 20 h post-inoculation, planktonic cells and TSB were aspirated and fresh TSB was added with the appropriate concentration of test extract. Plates were then placed back into the 37°C incubator on a shaker for 20 h. The staining methods were the same as those described above.
Replicate absorbance readings for each concentration tested were averaged and the average of the media control (TSB + extract) was subtracted. This value was then divided by the mean absorbance of the vehicle control and multiplied by 100. The concentration at which the extract depleted the biofilm biomass by at least 50% was labeled as the IC50.
2.5 Statistical analyses
The mean values and standard deviations of all replicates described in the above tests were calculated using Excel software. Differences between means were analyzed with a One-Way ANOVA, followed by multiple comparison analysis using the Bonferroni method on SPLUS software. Differences were considered significant with p-values < 0.05.
3. Results
3.1 Effects on planktonic growth of MRSA
Plant extracts demonstrated limited bacteriostatic activity (Table 1). Roughly 18% of the extracts tested demonstrated an IC50 of 256 or 512 μg/ml. Of those demonstrating activity, the majority were not associated with any particular ethnobotanical application. The mean percent inhibition of growth for each ethnobotanical usage group when screened at 512 μg/ml ranged from 15.93–19.46% (Group 1=19.46%, Group 2=15.93%, and Group 3=18.43%). Based on results from a One-Way ANOVA (p>0.05), we found that there was no significant difference in the mean bacteriostatic activity of extracts from plants in each ethnobotanical usage group (Table 2a).
Table 1.
Effect of plant extracts on growth and biofilm formation in MRSA (ATCC 33593). The MIC50 for growth was tested in the range of 8–512 μg/ml, whereas the IC50 for biofilm formation was tested from 4–128 μg/ml due to issues with the percent excipient (DMSO) in the test solution. A dash (−) represents that no IC50 was identified within the concentration range tested.
| Family | Botanic Name | Voucher ID | Plant Part | Ethno-botanical Use* | Extract Solvent | ** MIC50 (Growth) | ** IC50 (Biofilm Formation) |
|---|---|---|---|---|---|---|---|
| Adoxaceae | Sambucus ebulus L. | CQ-168 | inflorescence | N | EtOH | - | - |
| leaves | S | EtOH | - | - | |||
| stems | N | EtOH | - | - | |||
| Sambucus nigra L. | CQ-151 | woody parts | R | EtOH | - | - | |
| leaves | S | EtOH | 512 | - | |||
| dH2O | - | - | |||||
| inflorescence | S; R | EtOH | - | - | |||
| dH2O | - | - | |||||
| infructescence | F | EtOH | - | - | |||
| Alliaceae | Allium cepa L. | CQ-206 | leaves; bulbs; roots | S; M; F | EtOH | - | - |
| Apiaceae | Daucus carota L. | CQ-215 | leaves; stems | N | EtOH | - | - |
| inflorescence; infructescence | N | EtOH | - | - | |||
| Foeniculum vulgare ssp. piperitum (Ucria) Coutinho | CQ-192 | leaves; stems | M; F | EtOH | - | - | |
| Foeniculum vulgare ssp. vulgare Mill. | CQ-196 | leaves; stems | M | EtOH | - | - | |
| Tordylium apulum L. | CQ-101 | flowers; leaves; roots; stems | N | EtOH | - | - | |
| Apocynaceae | Vinca major L. | CQ-117 | flowers; leaves; roots; stems | M | EtOH | - | - |
| Aracaeae | Arum italicum Mill. | CQ-175 | stems | N | EtOH | - | - |
| fruits | N | EtOH | - | - | |||
| stalks | N | EtOH | - | - | |||
| leaves | S | EtOH | - | - | |||
| Asphodelaceae | Asphodelus microcarpus Salzm. & Viv. | CQ-109 | inflorescence | N | EtOH | - | - |
| leaves | N | EtOH | 512 | - | |||
| Asteraceae | Achillea ageratum L. | CQ-219 | leaves; stems; flowers | M | EtOH | 256 | - |
| Achillea millefolium L. | CQ-176 | inflorescence | M | EtOH | - | - | |
| leaves; stems | M | EtOH | 512 | - | |||
| leaves; stems; flowers | M | EtOH | - | - | |||
| Anacyclus tomentosus DC. | CQ-167 | leaves; stems; flowers | N | EtOH | - | - | |
| Cichorium intybus L. | CQ-106 | basal leaves; roots | F | EtOH | 512 | - | |
| dH2O | - | - | |||||
| leaves; stems; flowers | F | EtOH | - | - | |||
| Matricaria recutita L. | CQ-118 | flowers; leaves; roots; stems | S; M | EtOH | 512 | - | |
| dH2O | - | - | |||||
| Scolymus hispanicus L. | CQ-199 | leaves; stems; flowers | N | EtOH | - | - | |
| Tussilago farfara L. | CQ-202 | leaves; stems; roots | S | EtOH | - | - | |
| Urospermum dalechampii (L.) Scop. | CQ-134 | flowers; leaves; roots; stems | N | EtOH | - | - | |
| Boraginaceae | Anchusa officinalis L. (CQ-128) | CQ-128 | leaves; stems; flowers | N | EtOH | - | - |
| Borago officinalis L. | CQ-100 | flowers; leaves; roots; stems | M | EtOH | - | - | |
| dH2O | - | - | |||||
| Cerinthe major L. | CQ-110 | flowers; leaves; roots; stems | N | EtOH | - | - | |
| Echium italicum L. | CQ-162 | leaves; stems; flowers | N | EtOH | - | - | |
| Brassicaceae | Brassica rapa subsp. rapa | CQ-104 | flowers; leaves; roots; stems | F | EtOH | - | - |
| Cardaria draba (L.) Desv. | CQ-140 | flowers; leaves; roots; stems | N | EtOH | 512 | - | |
| Eruca sativa Mill. | CQ-102 | flowers; leaves; roots; stems | N | EtOH | - | - | |
| Sisymbrium officinale (L.) Scop. | CQ-131 | flowers; leaves; roots; stems | N | EtOH | - | - | |
| Caprifoliaceae | Lonicera alpigena L. | CQ-213 | woody parts | N | EtOH | - | 32 |
| leaves | N | EtOH | - | - | |||
| Caryophyllaceae | Saponaria officinalis L. | CQ-210 | leaves; stems; flowers | N | EtOH | - | - |
| Silene alba (Mill.) E.H.L. Krause | CQ-123 | leaves; stems; flowers | N | EtOH | - | - | |
| Silene nutans L. | CQ-125 | leaves; stems; flowers | N | EtOH | - | - | |
| Cucurbitaceae | Ecballium elaterium (L.) A. Richard | CQ-169 | leaves; stems; flowers | S | EtOH | 512 | - |
| Dennstaedtiaceae | Pteridium aquilinium (L.) Kuhn | CQ-211 | leaves | N | EtOH | - | - |
| stems | N | EtOH | - | - | |||
| Dipsacaceae | Dipsacus fullonum L. | CQ-201 | leaves; stems | N | EtOH | - | - |
| flowers | N | EtOH | - | - | |||
| Knautia arvensis Coult. | CQ-190 | leaves; stems; flowers | N | EtOH | - | - | |
| Knautia lucana Lacaita & Szabo | CQ-166 | leaves; stems; flowers | N | EtOH | - | - | |
| Equisetaceae | Equisetum arvense L. | CQ-226 | stems; leaves | N | EtOH | - | - |
| Fabaceae | Acacia dealbata Link | CQ-115 | inflorescence | O | EtOH | - | - |
| stems | O | EtOH | - | - | |||
| leaves; stems | O | EtOH | - | - | |||
| Anthyllis vulneraria L. | CQ-147 | leaves; stems; flowers | N | EtOH | - | - | |
| Astragalus monspessulanus L. | CQ-112 | leaves; stems; flowers; roots | N | EtOH | - | - | |
| Coronilla emerus L. | CQ-137 | leaves; flowers | N | EtOH | - | - | |
| woody stems | N | EtOH | - | - | |||
| Melilotus alba Medik. | CQ-193 | leaves; stems; flowers | N | EtOH | 512 | - | |
| Robinia pseudoacacia L. | CQ-155 | woody parts | N | EtOH | - | - | |
| leaves | N | EtOH | 512 | - | |||
| inflorescence | N | EtOH | - | - | |||
| Spartium junceum L. | CQ-144 | leaves; stems; flowers | A | EtOH | - | - | |
| Trifolium repens L. | CQ-138 | leaves; stems; flowers; roots | N | EtOH | - | - | |
| Vicia craca L. | CQ-149 | leaves; stems; flowers; roots | N | EtOH | 512 | - | |
| Vicia faba L. | CQ-103 | leaves; stems; flowers; roots | F | EtOH | - | - | |
| Vicia sativa subsp. angustifolio | CQ-124 | leaves; stems; flowers | N | EtOH | 512 | - | |
| Vicia sativa subsp. sativa | CQ-119 | leaves; stems; flowers | N | EtOH | - | - | |
| Wisteria sinensis (Sims) Sweet | CQ-126 | inflorescence | O | EtOH | 512 | - | |
| stems | O | EtOH | - | - | |||
| leaves | O | EtOH | 512 | - | |||
| Fagaceae | Castanea sativa Mill. | CQ-191 | inflorescence | N | EtOH | 256 | 16 |
| leaves | N | EtOH | 256 | - | |||
| woody parts | A | EtOH | 512 | - | |||
| Quercus cerris L. | CQ-228 | leaves | N | EtOH | - | - | |
| stems; fruits | N | EtOH | - | - | |||
| Gentianaceae | Centaurium pulchellum (Sw.) Druce | CQ-217 | leaves; stems; flowers; roots | N | EtOH | - | - |
| Geraniaceae | Erodium ciconium (L.) L’Hér. | CQ-142 | leaves; stems; flowers; roots | N | EtOH | - | - |
| Erodium malacoides (L.) L’Hér. ex Aiton | CQ-121 | leaves; stems; flowers | N | EtOH | 128 | - | |
| Geranium columbinum L. | CQ-129 | leaves; stems; flowers | N | EtOH | 512 | - | |
| Hyacinthaceae | Leopoldia comosa (L.) Parl. | CQ-105 | bulbs | M; F | EtOH | - | 16 |
| dH2O | - | 8 | |||||
| leaves; inflorescence | N | EtOH | - | - | |||
| Hypericaceae | Hypericum perforatum L. | CQ-183 | leaves; stems; flowers | S | EtOH | 256 | 128 |
| Juglandaceae | Juglans regia L. | CQ-181 | immature fruits | S; C | EtOH | - | 16 |
| leaves | R | EtOH | - | - | |||
| woody parts | N | EtOH | 512 | - | |||
| Juncaceae | Juncus articulatus L. | CQ-216 | leaves; fruits | N | EtOH | - | - |
| Lamiaceae | Ballota nigra L. | CQ-160 | stems | S; M | EtOH | - | - |
| roots | N | EtOH | - | - | |||
| leaves | S; M | EtOH | - | - | |||
| leaves; stems; flowers | S; M | EtOH | - | - | |||
| dH2O | - | 8 | |||||
| Clinopodium vulgare L. | CQ-182 | leaves; stems; flowers | N | EtOH | - | - | |
| Marrubium vulgare L. | CQ-170 | leaves; stems; flowers | S; M | EtOH | - | - | |
| dH2O | - | - | |||||
| roots | N | EtOH | - | - | |||
| Mentha pulegium L. | CQ-200 | leaves; stems; flowers; roots | F | EtOH | - | - | |
| Mentha spicata L. | CQ-224 | leaves; stems; flowers | F | EtOH | - | - | |
| Origanum heracleoticum L. | CQ-207 | leaves; stems; flowers | F | EtOH | - | - | |
| Phlomis herba-venti L. | CQ-168 | leaves; stems; flowers | N | EtOH | - | - | |
| Rosmarinus officinalis L. | CQ-113 | leaves; stems; flowers | F; S | EtOH | 512 | 16 | |
| Salvia pratensis L. | CQ-165 | leaves; stems | N | EtOH | 512 | - | |
| Salvia virgata Jacq. | CQ-127 | leaves; stems; flowers | N | EtOH | - | - | |
| Stachys tymphaea Hausskn. | CQ-189 | leaves; stems; flowers | N | EtOH | - | - | |
| Liliaceae | Lilium candidum L. | CQ-174 | leaves; stems | N | EtOH | - | - |
| inflorescence | N | EtOH | - | - | |||
| Malvaceae | Alcea rosea L. | CQ-205 | leaves; stems; flowers; roots | O | EtOH | - | - |
| Malva sylvestris L. | CQ-156 | stems | S; M | EtOH | - | - | |
| dH2O | - | 32 | |||||
| flowers | S; M | EtOH | - | - | |||
| leaves | S; M | EtOH | 256 | - | |||
| dH2O | - | 32 | |||||
| Moraceae | Ficus carica L. | CQ-173 | leaves | N | EtOH | - | - |
| woody parts | N | EtOH | - | - | |||
| immature fruits | S; F | EtOH | - | - | |||
| Myrsinaceae | Cyclamen hederifolium Aiton | CQ-186 | tubers | M | EtOH | - | 8 |
| Nyctaginaceae | Mirabilis jalapa L. | CQ-222 | leaves; flowers; fruits | N | EtOH | - | - |
| Oleaceae | Olea europaea L. | CQ-197 | leaves | N | EtOH | - | - |
| woody parts | A | EtOH | - | - | |||
| Orchidaceae | Aceras anthropophora R. Br. | CQ-153 | leaves; stems; flowers; roots | N | EtOH | - | - |
| Orchis italica Poir. | CQ-133 | inflorescence; leaves; stems | N | EtOH | - | - | |
| Orchis purpurea Huds. | CQ-132 | inflorescence; leaves; stems | N | EtOH | - | - | |
| Papaveraceae | Fumaria officinalis L. | CQ-107 | leaves; stems; flowers; roots | N | EtOH | - | - |
| Papaver rhoeas subsp. rhoeas | CQ-145 | leaves; stems; flowers; roots | F | EtOH | - | - | |
| Papaver somniferum L. | CQ-178 | leaves; stems; flowers; roots | M; R | EtOH | - | - | |
| Plantaginaceae | Digitalis ferruginea L. | CQ-227 | leaves; stems; flowers | N | EtOH | - | - |
| Linaria vulgaris Hill | CQ-223 | leaves; stems; flowers; roots | N | EtOH | - | - | |
| Plantago major L. | CQ-225 | leaves; stems; flowers; roots | S; M | EtOH | - | - | |
| Poaceae | Agropyron repens (L.) P. Beauv. | CQ-208 | leaves; stems; roots | M | EtOH | - | - |
| Arundo donax L. | CQ-146 | stem internodes | A; R | EtOH | - | - | |
| stem nodes | S | EtOH | - | - | |||
| dH2O | - | 128 | |||||
| leaves; stems | A; R | EtOH | - | - | |||
| Polygonaceae | Rumex crispus L. | CQ-171 | leaves; stems; fruits | S | EtOH | 512 | - |
| Pottiaceae | Syntrichia ruralis (Hedw.) Web. & Mohr | CQ-229 | whole plant | N | EtOH | - | - |
| Ranunculaceae | Delphinium fissum Waldst. & Kit. | CQ-187 | leaves; stems; flowers; fruits | N | EtOH | - | - |
| Ranunculus acris L. | CQ-135 | leaves; stems; flowers | N | EtOH | - | - | |
| Rosaceae | Crataegus monogyna Jacq. | CQ-116 | leaves; stems; flowers | M | EtOH | - | - |
| Prunus spinosa L. | CQ-163 | woody parts; leaves | M | EtOH | - | - | |
| fruits | N | EtOH | 512 | - | |||
| Rosa canina var. canina | CQ-152 | fruits | N | EtOH | - | 32 | |
| woody parts | N | EtOH | - | - | |||
| leaves; stems | N | EtOH | 512 | - | |||
| Rubus ulmifolius Schott | CQ-164 | leaves; stems; flowers | S | EtOH | - | - | |
| leaves | S | EtOH | - | - | |||
| roots | M | EtOH | 512 | 8 | |||
| woody stems | N | EtOH | 512 | - | |||
| Rubiaceae | Galium verum L. | CQ-177 | leaves; stems; flowers | N | EtOH | - | - |
| Scrophulariaceae | Verbascum sinuatum L. | CQ-218 | leaves; stems; flowers | N | EtOH | - | - |
| Verbascum thapsus L. | CQ-172 | stems | M | EtOH | - | - | |
| leaves | M | EtOH | - | - | |||
| inflorescence | M | EtOH | - | - | |||
| Ulmaceae | Ulmus minor L. | CQ-195 | leaves | N | EtOH | - | - |
| woody parts | M | EtOH | - | - | |||
| Urticaceae | Parietaria diffusa Mert. & Koch | CQ-212 | leaves; stems; fruits; roots | M | EtOH | - | - |
| Urtica dioica L. | CQ-179 | leaves; stems; flowers | S; M; F | EtOH | - | - | |
| Valerianaceae | Centranthus ruber (L.) DC. | CQ-143 | leaves; stems; inflorescence | M | EtOH | 256 | - |
| Vitaceae | Vitis vinifera var. aglianico | CQ-209 | wine | S; F | - | 64 | |
| stems | N | EtOH | - | - | |||
| fruits | F | EtOH | - | - | |||
| leaves | N | EtOH | - | - |
Ethnobotanical use of specific plant part(s) in the study region: S = medicinal application to skin; M = medicinal application not involving the skin; C = cosmetic applications; A = agricultural tool; O = ornamental; R = ritual or spiritual use; F = food; N = no reported use.
MIC50 and IC50 values expressed as μg/ml.
Table 2.
Statistical tests for differences in the inhibitory activity of the three ethnobotanical usage groups: Group 1 includes plants used for SSTI (n=25); Group 2 includes plants used medicinally, but not on the skin (n=28); and Group includes plants with no ethnomedical application (n=51). (a) One-way ANOVA tests were performed. These results indicate that there was no significant difference in the inhibition of planktonic growth (p > 0.05) between ethnobotanical usage groups. There was, however, a significant difference in the inhibition of biofilm formation (p< 0.05) between groups. (b) Multiple comparison analysis using the Bonferroni method was performed on the data for anti-biofilm activity. A significant difference in activity of Groups 1 and 3 is evident.
| Df | Sum of Sq | Mean Sq | F Value | P Value | |
|---|---|---|---|---|---|
| Planktonic Growth | 2 | 960.1 | 480.0347 | 0.7320253 | 0.48121 |
| Biofilm formation | 2 | 2073.34 | 1036.669 | 7.159144 | 0.00104 |
|
| |||||
| (a) | |||||
|
| |||||
| Groups Compared | Estimate | Standard Error | Lower Bound | Upper Bound | |
|
| |||||
| 1–2 | 2.13 | 3.25 | −5.730 | 9.98 | |
| 1–3 **** | 8.15 | 2.36 | 2.460 | 13.90 | |
| 2–3 | 6.03 | 2.75 | −0.634 | 12.70 | |
|
(b) | |||||
3.2 Effects on MRSA biofilm formation
Extracts from 10 plants exhibited an IC50 ≤32 μg/ml for inhibition of biofilm formation: Lonicera alpigena, Castanea sativa, Juglans regia, Ballota nigra, Rosmarinus officinalis, Leopoldia comosa, Malva sylvestris, Cyclamen hederifolium, Rosa canina var. canina, and Rubus ulmifolius (Table 1). The mean percent inhibition of biofilm formation for each ethnobotanical usage group when screened at 64 μg/ml ranged from 0.58–8.73% (Group 1=8.73%, Group 2=6.6%, and Group 3=0.58%). Plants used in folk remedies for SSTI made up 38% of plants exhibiting an IC50 of 8–128 μg/ml for MRSA biofilm formation.
Base on the results from a One-Way ANOVA test (p<0.05), the mean anti-biofilm activity of extracts from plants in each ethnobotanical usage group was not the same (Table 2a). Multiple comparison analysis using the Bonferroni method revealed a significant difference between groups 1 and 3. The anti-biofilm activity of plants categorized for medicinal applications to the skin was significantly greater than activity from plants without any ethnomedical applications (Table 2b).
4. Discussion
A significant dose-dependent response in biofilm inhibition was evident in 5 of the 168 extracts tested: Arundo donax, Ballota nigra, Juglans regia, Leopoldia comosa, Marrubium vulgare, and Rubus ulmifolius (Figure 2). Here, we offer a discussion of the ethnomedicinal applications of these plants in southern Italy and review the literature for other reports of anti-staphylococcal activity.
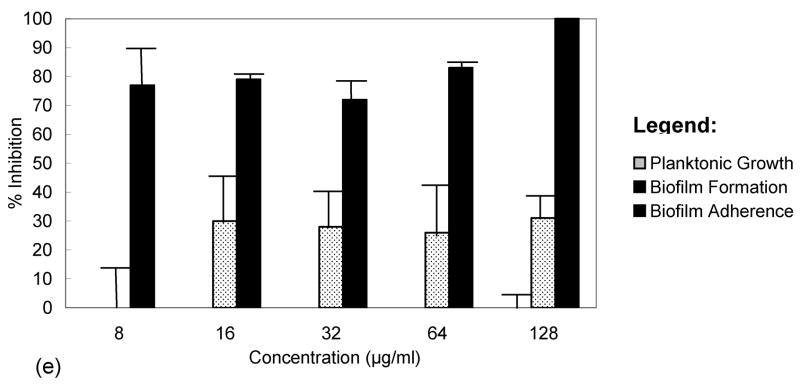
Figure 2.
The percent inhibition of extracts for planktonic growth, biofilm formation and adherence in MRSA. (a) Arundo donax, aqueous extract of nodes; (b) Ballota nigra, aqueous extract of aerial parts; (c) Juglans regia, ethanolic extract of immature fruits; (d) Leopoldia comosa, ethanolic extract of bulbs; (e) Marrubium vulgare, ethanolic extract of roots.
4.1 Arundo donax (Poaceae) - canna
The giant reed is commonly used in the construction of musical instruments and the production of industrial cellulose. It is also used for several other purposes in southern Italy, especially to support grape vines. This use was so important to local agriculture in the past that land was often divided along the border lines of reed colonies. The white hemicellulose membrane located at the nodes of the reed is inserted into fresh lacerations as a haemostatic agent (Passalacqua et al., 2007; Pieroni et al., 2002c) (Figure 3). Small tooth-sized pieces of the reed are also cut up and used in the ritual treatment of toothache (Quave and Pieroni, 2005). Antiproliferative activity against several human cancer cell lines has been reported for a lectin isolated from the rhizomes of this plant (Kaur et al., 2005). A. donax is rich in alkaloids, including bufotenidine and gramine. Small amounts of DMT have also been isolated from the flowers (Duke, 2008).
Figure 3.

The white hemicellulose membrane found at the node of the giant reed (Arundo donax L. [Poaceae]) is used as a haemostatic agent for minor lacerations.
We found that aqueous extracts of the reed nodes (which contain the white hemicellulose membrane) demonstrated a marked dose-dependent response for anti-biofilm activity, both in preventing MRSA biofilm formation and disrupting established biofilms (Figure 2a). These results may suggest that the traditional application of the reed membrane to fresh lacerations may be useful as a prophylactic for biofilm-related infection. The effect of giant reed extract on biofilm formation and adherence in MRSA has not been previously reported.
4.2 Ballota nigra (Lamiaceae) – erba cane
Infusions of the aerial parts of black horehound are used in the south Italian pharmacopoeia as a rinse for skin rashes and are also drunk to promote circulation. Extensive phytochemical studies (Bertrand et al., 2000; Bruno et al., 1986; Seidel et al., 1997; Seidel et al., 2000) have been performed on black horehound, and several phenylpropanoid glycosides have been identified and linked to moderate growth inhibition in Staphylococcus aureus (Didry et al., 1999). Black horehound also contains diterpenes, such as marubiin, as well as caffeic and ferulic acid derivatives (Gruenwald et al., 1998). Potent antioxidant activity has reported in infusions of the aerial parts (Citoglu et al., 2004; Vrchovska et al., 2007). We prepared several types of extractions of the different parts of the plant, and found that an aqueous extraction of the aerial parts, similar to that prepared in the folk medical tradition, was the most effective at inhibiting both biofilm growth and adherence. Although this extract actually promoted planktonic growth of this MRSA strain by almost twofold, significant dose-dependent responses for the inhibition of both biofilm formation and adherence were evident (Figure 2b). The effect of black horehound extract on biofilm formation and adherence in MRSA has not been previously reported.
4.3 Juglans regia (Juglandaceae) - noce
The common walnut has been reported in the ethnobotanical literature for its use as an aromatic for cheese and to protect it from parasites (Guarrera et al., 2005a) in Italy, and as a medicine for rheumatism, fever, fungal infection, hemorrhoid, cough and eczema in Turkey (Erdemoglu et al., 2003; Kultur, 2007). The bark is prepared in a decoction and gargled for toothache and fresh leaves are applied topically to reduce the swelling associated with varicose veins (Quave et al., 2008). The immature fruits are used for cosmetic (hair dye) applications (Pieroni et al., 2004) and the flowers are employed in ritual healing ceremonies for mal d’arco (rainbow illness) (Quave and Pieroni, 2005).
An ethanolic extract of the immature fruits reduced biofilm adherence and formation. Limited growth inhibition was noted at higher doses 128–512 μg/ml (Figure 2c). Similar activity in staphylococcal growth inhibition by aqueous extracts of walnut leaves has also been reported (Pereira et al., 2007). The walnut tree is rich in phenolic compounds, including naphtoquinones and flavonoids that are likely responsible for the anti-bacterial activity of extracts derived from this plant (Pereira et al., 2007; Stampar et al., 2006). The use of walnut leaves for mild skin inflammations has been approved by the German Commission E (Blumenthal et al., 2000). This is the first report of anti-biofilm activity for MRSA by walnut extract.
4.4 Leopoldia comosa (Hyacinthaceae) - cipuldjin
In southern Italy, the tassel grape hyacinth is used in folk remedies for toothache (bulb is grated and applied topically) (Guarrera et al., 2005b) and for food (Casoria et al., 1999; Pieroni et al., 2002b). Extracts from the bulbs have demonstrated potent in vitro anti-oxidant activity (Pieroni et al., 2002a). Both the aqueous and ethanolic extracts of the bulb demonstrated potent anti-biofilm activity for this strain of MRSA. The IC50 for preventing biofilm formation and adherence was 16 and 8 μg/ml, respectively. The IC90 for biofilm adherence was 128 μg/ml (Figure 2d). This is the first report of anti-biofilm activity for this plant.
4.5 Marrubium vulgare (Lamiaceae) - marugg
White horehound is one of the most frequently quoted medicinal species applied in south Italian traditional medicine. A local rhyme is associated with this plant: “U’ marrugxh, ogni mal’ destrugg ” (the white horehound destroys every disease). It is used in the treatment of furuncle, boil, athlete’s foot, skin infection, dermatitis, foot and mouth disease (in animals), gastritis, and malaria. It is thought to be particularly good at cleansing the liver and is used as a hepatoprotectant and general panacea (Pieroni et al., 2002c; Pieroni and Quave, 2005). Aqueous decoctions of the aerial parts are either used to rinse the skin or are drunk, depending upon the illness being treated. An ethanolic extract of the roots was effective in reducing adherence of established biofilms at very low concentrations (IC50 = 8 μg/ml and IC90=128 μg/ml). This extract was not, however, very effective at preventing the formation of biofilms on plastic surfaces. The maximum percent inhibition for biofilm formation was 31% at a concentration of 128 μg/ml (Figure 2e).
Previous studies have demonstrated that white horehound extracts exhibit antispasmodic (Schlemper et al., 1996), antioxidant (Berrougui et al., 2006; Matkowski and Piotrowska, 2006; Weel et al., 1999), hypotensive (El Bardai et al., 2001), insecticidal (Pavela, 2004), and analgesic (Meyre-Silva et al., 2005) properties. Marrubium species are rich in diterpenes, caffeic acid derivatives, sterols, and flavonoids (Khanavi et al., 2005; Lazari et al., 1999). One of the principle medicinal components of horehound extract is marubiin, a furanic labdane diterpene (Blumenthal et al., 2000). It has been found to exhibit non-selective anti-inflammatory properties in mouse models, which may support its use as a folk remedy for dermatitis (Stulzer et al., 2006). The German Commission E approved the use of this herb for the treatment of loss of appetite and dyspepsia (Blumenthal et al., 2000). The effect of white horehound extract on biofilm formation and adherence in MRSA has not been previously reported.
5. Conclusion
Plant extracts demonstrated limited bacteriostatic activity, and in fact, many actually promoted planktonic growth. More significantly, extracts from five plants exhibited dose-dependent inhibition of biofilm formation and adherence. We identified a significant correlation in anti-biofilm activity with medicinal plants used for SSTI when compared with plants with no apparent ethnomedical use. These results validate the efficacy of the topical application of Arundo donax, Ballota nigra, Juglans regia, Leopoldia comosa, and Marrubium vulgare in Italian folk remedies for SSTI.
While these results are based on the analysis of crude, unfractionated extracts, they also represent the first of many steps towards the development of new anti-biofilm drugs. Inhibition of biofilms provides a method of controlling the effects of pathogenic bacteria without strong selection for drug resistance. The emergence of infections due to highly resistant MRSA capable of forming and many times involving biofilms presents a significant dilemma to medicine today. The healthcare community is ill-prepared to effectively treat such infections as the old classes of antibiotics currently in use rapidly lose their efficacy. Effective treatments for the disruption of established biofilms could save many lives and decrease healthcare costs related to the treatment and potential replacement of infected implanted prosthetic devices. Prophylactic agents for biofilms embedded in surgically implanted devices and catheters could significantly diminish morbidity and subsequently reduce healthcare costs associated with nosocomial infection.
We recommend further investigation of natural products from the following plants for anti-biofilm activity in MRSA: Lonicera alpigena, Castanea sativa, Juglans regia, Ballota nigra, Rosmarinus officinalis, Leopoldia comosa, Malva sylvestris, Cyclamen hederifolium, Rosa canina, and Rubus ulmifolius.
Acknowledgments
The project described was funded supported by Grant Number 1F31AT004288-01A1 from the National Center for Complementary and Alternative Medicine. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Center for Complementary and Alternative Medicine or the National Institutes of Health. Additional support for this project was provided by MBRS RISE - NIH/NIGMS R25GM061347, Botany in Action, Anne Chatham Fellowship in Medicinal Botany, USDA CSREES 20053842215940 and NIH/NCAAM 1T32AT01060-01.
We thank Dr. Carmine Colacino and Dr. Andrea Pieroni for assisting in the taxonomic identification of plants. We also thank the Caputo family for logistical support during field research. Thanks to Dr. Roberto Perez and Prof. Steve Davis for providing bacterial isolates and training in biofilm assays. Special thanks to volunteers who assisted in laboratory work: Jana Rose, Marco Caputo and Susan Mendez. Lastly, we extend our gratitude to all of the study participants who graciously shared a wealth of knowledge regarding the traditional medical practices of their communities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amsterdam D. Susceptibility testing of antimicrobials in liquid media. In: Loman V, editor. Antibiotics in laboratory medicine. Williams and Wilkins; Baltimore, MD: 1996. pp. 52–111. [Google Scholar]
- Aqil F, Ahmad I, Owais M. Evaluation of anti-methicillin-resistant Staphylococcus aureus (MRSA) activity and synergy of some bioactive plant extracts. Journal of Biotechnology. 2006a;1:1093–1102. doi: 10.1002/biot.200600130. [DOI] [PubMed] [Google Scholar]
- Aqil F, Ahmad I, Owais M. Evaluation of anti-methicillin-resistant Staphylococcus aureus (MRSA) activity and synergy of some bioactive plant extracts. Journal of Biotechnology. 2006b;1:1093–1102. doi: 10.1002/biot.200600130. [DOI] [PubMed] [Google Scholar]
- Berrougui H, Isabelle M, Cherki M, Khalil A. Marrubium vulgare extract inhibits human-LDL oxidation and enhances HDL-mediated cholesterol efflux in THP-1 macrophage. Life Sciences. 2006;80:105–112. doi: 10.1016/j.lfs.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Bertrand MC, Tillequin F, Bailleul F. Two major flavonoids from Ballota nigra. Biochemical Systematics and Ecology. 2000;28:1031–1033. doi: 10.1016/s0305-1978(00)00015-6. [DOI] [PubMed] [Google Scholar]
- Blumenthal M, Goldberg A, Brinckmann J, editors. Herbal Medicine. Expanded Commission E Monographs. American Botanical Council. Integrative Medicine Communications; Newton, MA: 2000. [Google Scholar]
- Bruno M, Savona G, Pascual C, Rodriguez B. Preleosibirin, a prefuranic labdane diterpene from Ballota nigra subsp. foetida. Phytochemistry. 1986;25:538–539. [Google Scholar]
- Casoria P, Menale B, Muoio R. Muscari comosum, Liliaceae, in the food habits of south Italy. Economic Botany. 1999;53:113–117. [Google Scholar]
- Christensen GD, Simpson WA, Younger JJ, Baddour LM, Barrett FF, Melton DM, Beachey DH. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. Journal of Clinical Microbiology. 1985;22:996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citoglu GS, Coban T, Sever B, Iscan M. Antioxidant properties of Ballota species growing in Turkey. Journal of Ethnopharmacology. 2004;92:275–280. doi: 10.1016/j.jep.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Coast J, Smith R, Miller M. Superbugs: should antimicrobial resistance be included as a cost in economic evaluation? Health Economics. 1996;5:217–226. doi: 10.1002/(SICI)1099-1050(199605)5:3<217::AID-HEC200>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Cos P, Vlietinck AJ, Berghe DV, Maes L. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. Journal of Ethnopharmacology. 2006;106:290–302. doi: 10.1016/j.jep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infection. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- Cox PA, Balick MJ. The ethnobotanical approach to drug discovery. Scientific American. 1994;270:82–87. [PubMed] [Google Scholar]
- Didry N, Seidel V, Dubreuil L, Tillequin F, Bailleul F. Isolation and antibacterial activity of phenylpropanoid derivatives from Ballota nigra. Journal of Ethnopharmacology. 1999;67:197–202. doi: 10.1016/s0378-8741(99)00019-7. [DOI] [PubMed] [Google Scholar]
- Duke J. Dr. Duke’s Phytochemical and Ethnobotanical Databases. 2008 [Online Database] 25 October 2007., http://www.ars-grin.gov/duke/
- El Bardai s, Lyoussi B, Wibo M, Morel N. Pharmacological evidence of hypotensive activity of Marrubium vulgare and Foeniculum vulgare in spontaneously hypertensive rat. Clinical and Experimental Hypertension. 2001;23:329–343. doi: 10.1081/ceh-100102671. [DOI] [PubMed] [Google Scholar]
- Erdemoglu N, Kupeli E, Yesilada E. Anti-inflammatory and antinociceptive activity assessment of plants used as remedy in Turkish folk medicine. Journal of Ethnopharmacology. 2003;89:123–129. doi: 10.1016/s0378-8741(03)00282-4. [DOI] [PubMed] [Google Scholar]
- Fauci AS, Braunwald E, Issekbacher KJ, Wilson JD, Martin JB, Kasper DL, Hauser SL, Longo DL, editors. Harrison’s Principles of Internal Medicine. McGraw-Hill; New York: 1998. [Google Scholar]
- Grierson DS, Afolayan AJ. Antibacterial activity of some indigenous plants used for the treatment of wounds in the Eastern Cape, South Africa. Journal of Ethnopharmacology. 1999;66:103–106. doi: 10.1016/s0378-8741(98)00202-5. [DOI] [PubMed] [Google Scholar]
- Gruenwald J, Brendler T, Jaenicke C. PDR for Herbal Medicines. Medical Economics Company; Montvale, NJ: 1998. [Google Scholar]
- Guarrera PM, Forti G, Marignoli S. Ethnobotanical and ethnomedicinal uses of plants in the district of Acquapendente (Latium, Central Italy) Journal of Ethnopharmacology. 2005a;96:429–444. doi: 10.1016/j.jep.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Guarrera PM, Salerno G, Caneva G. Folk phytotherapeutical plants from Maratea area (Basilicata, Italy) Journal of Ethnopharmacology. 2005b;99:367–378. doi: 10.1016/j.jep.2005.01.039. [DOI] [PubMed] [Google Scholar]
- Isenberg HD. Clinical Microbiology Procedures Handbook. ASM Press; Washington, D.C.: 2004. [Google Scholar]
- Kamatou GPP, Viljoen AM, van Vuuren SF, van Zyl RL. In vitro evidence of antimicrobial synergy between Salvia chamelaeagnea and Leonotis leonurus. South African Journal of Botany. 2006;72:634–636. [Google Scholar]
- Kaur A, Singh J, Kamboj SS, Sexana AK, Pandita RM, Shamnugavel M. Isolation of an N-acetyl-d-glucosamine specific lectin from the rhizomes of Arundo donax with antiproliferative activity. Phytochemistry. 2005;66:1933–1940. doi: 10.1016/j.phytochem.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Khanavi M, Ghasemian L, Motlagh EH, Hadjiakhoondi A, Shafiee A. Chemical composition of the essential oils of Marrubium parviflorum Fisch. & C. A. Mey. and Marrubium vulgare L. from Iran. Flavour and Fragrance Journal. 2005;20:324–326. [Google Scholar]
- Klevens RM, Morrison M, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim G, McDougal LK, Carey RB, Fridkin SK. Invasive Methicillin-Resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- Kong KF, Vuong C, Otto M. Staphylococcus quorum sensing in biofilm formation and infection. International Journal of Medical Microbiology. 2006;296:133–139. doi: 10.1016/j.ijmm.2006.01.042. [DOI] [PubMed] [Google Scholar]
- Kultur S. Medicinal plants used in Kirklareli Province (Turkey) Journal of Ethnopharmacology. 2007;111:341–364. doi: 10.1016/j.jep.2006.11.035. [DOI] [PubMed] [Google Scholar]
- Lazari DM, Skaltsa HD, Constantinidis T. Essential oils of Marrubium velutinum Sm. and Marrubium peregrinum L., growing wild in Greece. Flavour and Fragrance Journal. 1999;14:290–292. [Google Scholar]
- Levy SB. The Antibiotic Paradox: How the Misuse of Antibiotics Destroys their Curative Powers. Perseus Publishing; Cambridge, MA: 2002. [Google Scholar]
- Lewis K. Riddle of biofilm resistance. Antimicrobial Agents and Chemotherapy. 2001;45:999–1007. doi: 10.1128/AAC.45.4.999-1007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis K. Persister cells and the riddle of biofilm survival. Biochemistry (Moscow) 2005;70:267–274. doi: 10.1007/s10541-005-0111-6. [DOI] [PubMed] [Google Scholar]
- Mantle D, Gok MA. Adverse and beneficial effects of plant extracts on skin and skin disorders. Adverse Drug Reactions and Toxicological Reviews. 2001;20:89–103. [PubMed] [Google Scholar]
- Matkowski A, Piotrowska M. Antioxidant and free radical scavenging activities of some medicinal plants from the Lamiaceae. Fitoterapia. 2006;77:346–353. doi: 10.1016/j.fitote.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Meyre-Silva C, Yunes RA, Schlemper V, Campos-Buzzi F, Cechinel-Filho V. Analgesic potential of marrubiin derivatives, a bioactive diterpene present in Marrubium vulgare (Lamiaceae) Il Farmaco. 2005;60:321–326. doi: 10.1016/j.farmac.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Passalacqua NG, Guarrera PM, De Fine G. Contribution to the knowledge of the folk plant medicine in Calabria region (Southern Italy) Fitoterapia. 2007;78:52–68. doi: 10.1016/j.fitote.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Pavela R. Insecticidal activity of certain medicinal plants. Fitoterapia. 2004;75:745–749. doi: 10.1016/j.fitote.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Pereira JA, Oliveira I, Sousa A, Valentao P, Andrade PB, Ferreira ICFR, Ferreres F, Bento A, Seabra R, Estevinho L. Walnut (Juglans regia L.) leaves: Phenolic compounds, antibacterial activity and antioxidant potential of different cultivars. Food and Chemical Toxicology. 2007;45:2287–2295. doi: 10.1016/j.fct.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Pieroni A, Janiak V, Dürr CM, Lüdeke S, Trachsel E, Heinrich M. In vitro antioxidant activity of non-cultivated vegetables of ethnic Albanians in southern Italy. Phytotherapy Research. 2002a;16:467–473. doi: 10.1002/ptr.1243. [DOI] [PubMed] [Google Scholar]
- Pieroni A, Nebel S, Quave C, Munz H, Heinrich M. Ethnopharmacology of liakra: traditional weedy vegetables of the Arbëreshë of the Vulture area in southern Italy. Journal of Ethnopharmacology. 2002b;81:165–185. doi: 10.1016/s0378-8741(02)00052-1. [DOI] [PubMed] [Google Scholar]
- Pieroni A, Quave C, Nebel S, Heinrich M. Ethnopharmacy of the ethnic Albanians (Arbëreshë) of northern Basilicata, Italy. Fitoterapia. 2002c;73:217–241. doi: 10.1016/s0367-326x(02)00063-1. [DOI] [PubMed] [Google Scholar]
- Pieroni A, Quave CL. Traditional pharmacopoeias and medicines among Albanians and Italians in southern Italy: A comparison. Journal of Ethnopharmacology. 2005;101:258–270. doi: 10.1016/j.jep.2005.04.028. [DOI] [PubMed] [Google Scholar]
- Pieroni A, Quave CL. Functional foods or food medicines? On the consumption of wild plants among Albanians and southern Italians in Lucania. In: Pieroni A, Price LL, editors. Eating and Healing. Traditional Food as Medicine. Haworth Press; New York: 2006. pp. 101–129. [Google Scholar]
- Pieroni A, Quave CL, Villanelli ML, Mangino P, Sabbatini G, Santini L, Boccetti T, Profili M, Ciccioli T, Rampa LG, Antonimi G, Girolamini C, Cecchi M, Tomasi M. Ethnopharmacognistic survey on the natural ingredients used in folk cosmetics, cosmeceuticals and remedies for healing skin diseases in the inland Marches, Central-Eastern Italy. Journal of Ethnopharmacology. 2004;91:331–344. doi: 10.1016/j.jep.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Pignatti S. Flora d’Italia. Edizioni Edagricole; Bologna, Italy: 2002. [Google Scholar]
- Quave CL, Pieroni A. Folk illness and healing in Arbëreshë Albanian and Italian communities of Lucania, southern Italy. Journal of Folklore Research. 2005;42:57–97. [Google Scholar]
- Quave CL, Pieroni A, Bennett BC. Dermatological remedies in the traditional pharmacopoeia of Vulture-Alto Bradano, inland southern Italy. Journal of Ethnobiology and Ethnomedicine. 2008;4 doi: 10.1186/1746-4269-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen TB, Givskov M. Quorum-sensing inhibitors as anti-pathogenic drugs. International Journal of Medical Microbiology. 2006;296:149–161. doi: 10.1016/j.ijmm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Schadow KH, Simpson WA, Christensen GD. Characteristics of adherence to plastic tissue culture plates of coagulase-negative staphylococci exposed to subinhibitory concentrations of antimicrobial agents. Journal of Infectious Diseases. 1988;157:71–77. doi: 10.1093/infdis/157.1.71. [DOI] [PubMed] [Google Scholar]
- Schlemper V, Ribas A, Nicolau M, Cechinel Fihlo V. Antispasmodic effects of hydroalcoholic extract of Marrubium vulgare on isolated tissues. Phytomedicine. 1996;3:211–216. doi: 10.1016/S0944-7113(96)80038-9. [DOI] [PubMed] [Google Scholar]
- Seidel V, Bailleul F, Libot F, Tillequin F. A phenylpropanoid glycoside from Ballota nigra. Phytochemistry. 1997;44:691–693. doi: 10.1016/s0031-9422(96)00578-x. [DOI] [PubMed] [Google Scholar]
- Seidel V, Verholle M, Malard Y, Tillequin F, Fruchart JC, Duriez P, Bailleul F, Teissier E. Phenylpropanoids from Ballota nigra L. inhibit in vitro LDL peroxidation. Phytotherapy Research. 2000;14:93–98. doi: 10.1002/(sici)1099-1573(200003)14:2<93::aid-ptr558>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Srinivasan D, Nathan S, Suresh T, Permalsamy PL. Antimicrobial activity of certain Indian medicinal plants used in folkloric medicine. Journal of Ethnopharmacology. 2001;74:217–220. doi: 10.1016/s0378-8741(00)00345-7. [DOI] [PubMed] [Google Scholar]
- Stampar F, Solar A, Hudina M, Veberic R, Colaric M. Traditional walnut liqueur -cocktail of phenolics. Food Chemistry. 2006;95:627–631. [Google Scholar]
- Stevens PF. Angiosperm Phylogeny Website. 2001 onwards. Version 8, June 2007. http://www.mobot.org/MOBOT/research/APweb/
- Stulzer HK, Tagliari MP, Zampirolo JA, Cechinel-Filho V, Schlemper V. Antioedematogenic effect of marrubiin obtained from Marrubium vulgare. Journal of Ethnopharmacology. 2006;108:379–384. doi: 10.1016/j.jep.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Vrchovska V, Spilkova J, Valentao P, Sousa C, Andrade PB, Seabra RM. Antioxidative properties and phytochemical composition of Ballota nigra infusion. Food Chemistry. 2007;105:1396–1403. [Google Scholar]
- Weel KGC, Venskutonis PR, Pukalskas A, Gruzdiene D, Linssen JPH. Antioxidant activity of horehound (Marrubium vulgare L.) grown in Lithuania. Lipid - Fett. 1999;101:395–400. [Google Scholar]
- Yarwood JM, Bartels DJ, Volper EM, Greenberg EP. Quorum sensing in Staphylococcus aureus biofilms. Journal of Bacteriology. 2004;186:1838–1850. doi: 10.1128/JB.186.6.1838-1850.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]