Abstract
Many exotoxins of gram positive bacteria, such as superantigens (staphylococcal enterotoxins, toxic shock syndrome toxin-1 [TSST-1], and streptococcal pyrogenic exotoxins) and anthrax toxin are bioterrorism agents that cause diseases by immunostimulation or cytotoxicity. Glycerol monolaurate (GML), a fatty acid monoester found naturally in humans, has been reported to prevent synthesis of gram positive bacterial exotoxins. This study explored the ability of GML to inhibit the effects of exotoxins on mammalian cells and prevent rabbit lethality from TSS. GML (≥10 ug/ml) inhibited superantigen (5 ug/ml) immunoproliferation, as determined by inhibition of 3H-thymidine incorporation into DNA of human peripheral blood mononuclear cells (1 × 106 cells/ml) as well as phospholipase Cγ1, suggesting inhibition of signal transduction. The compound (20 ug/ml) prevented superantigen (100 ug/ml) induced cytokine secretion by human vaginal epithelial cells (HVECs) as measured by ELISA. GML (250 ug) inhibited rabbit lethality due to TSST-1 administered vaginally. GML (10 ug/ml) inhibited HVEC and macrophage cytotoxicity by anthrax toxin, prevented erythrocyte lysis by purified hemolysins (staphylococcal α and β) and culture fluids containing streptococcal and Bacillus anthracis hemolysins, and was non-toxic to mammalian cells (up to 100 ug/ml) and rabbits (250 ug). GML stabilized mammalian cell membranes, as erythrocyte lysis was reduced in the presence of hypotonic aqueous solutions (0 to 0.05 M saline) or staphylococcal α and β-hemolysins when erythrocytes were pretreated with GML. GML may be useful in management of gram positive exotoxin illnesses; its action appears to be membrane stabilization with inhibition of signal transduction.
Many gram positive bacterial pathogens produce exotoxins that are potential agents of bioterrorism and contribute to their abilities to cause human diseases (1–3). Examples include anthrax toxin made by Bacillus anthracis (2), superantigens produced by Staphylococcus aureus, including staphylococcal enterotoxins (SEs)1 and toxic shock syndrome toxin-1 (TSST-1) (1), and superantigens produced by group A streptococci, including streptococcal pyrogenic exotoxins (SPEs) (3). Other exotoxins, such as staphylococcal α and β-hemolysins and streptolysins, are not agents of bioterrorism but have adjunct roles in virulence and can be used as model toxins to study mechanisms of cellular toxicity.
All of these exotoxins interact with host cell membranes to exert their toxic effects. Some effects are immunostimulatory, such as superantigen exotoxins, which stimulate T lymphocytes and macrophages to cause toxic shock syndrome (1, 3, 4). Superantigens are defined by their capacities to induce proliferation of T cells as a function of the β chain variable region of the T cell receptor complex, that causes release of interleukin 2 (IL-2), tumor necrosis factor β (TNF-β), and interferon γ (IFN-γ) (1, 4). In concert with activation of T cells is the release of interleukin 1β (IL-1β) and tumor necrosis factor α (TNF-α) from macrophages (1, 4).
Alternatively, the interactions of exotoxins with host cell membranes may lead to cytotoxicity, such as the heptamer pore-forming exotoxin staphylococcal α-hemolysin (5); streptolysin O (6); and anthrax toxin that forms heptamer pores through polymerization of the protective antigen component, which delivers lethal factor protease and edema factor adenylate cyclase into host cells (2).
The potential for purified exotoxins to be used as agents of bioterrorism has stimulated research in specific methods to block the actions of exotoxins, such as anthrax toxin and superantigens, on mammalian cells. Dominant mutants of anthrax toxin (protective antigen) have been developed, which block its ability to facilitate translocation of lethal and edema factors into cells (7, 8). In addition, specific compounds have been designed to block the enzymatic functions of both lethal and edema factors (9–11). Finally, antibodies obtained from volunteers immunized with protective antigen may have the capacity to neutralize anthrax toxin (12). Similarly, polyclonal (commercially available intravenous immunoglobulin) and monoclonal antibody preparations against superantigens have the capacity to neutralize the immunostimulatory activity of superantigens (TSST-1, SEs, and SPEs) (13–18).
Glycerol monolaurate (GML), a surfactant compound found naturally in humans with highest concentrations in breast milk, is generally recognized as safe by the Food and Drug Administration for oral consumption in foods and for use in cosmetics (19–21). The structure of GML is comprised of a monoester of glycerol and the fatty acid lauric acid, and GML is the most commonly studied member of a class of fatty acid monoesters with antimicrobial properties. GML inhibits the production of gram positive exotoxins such as S. aureus (hemolysins, superantigens [TSST-1 and SEs], and exfoliative toxins), β-hemolytic streptococci (hemolysins and superantigens [SPEs]) (22, 23), and Bacillus anthracis (anthrax toxin [protective antigen, lethal factor, and edema factor]) at the cellular level of transcription (24). GML is considered to inhibit transcription by blocking signal transduction in bacterial cells. Recent studies demonstrated GML inhibited transcription of B. anthracis anthrax toxin and S. aureus msrA1 genes (24, 25). GML is hypothesized to interfere with signal transduction by the lauric acid moiety of GML embedding and spanning approximately one-half the lipid bilayer, causing a stabilization of the membranes.
Although GML could be used to prevent exotoxin production by gram positive bacteria, a more beneficial use would be to block the actions of exotoxins on human cells (by inhibiting signal transduction and providing mammalian cell membrane stability). The ability of GML to inhibit the immunostimulatory and cytotoxic effects of gram positive bacterial exotoxins on eukaryotic cells was explored in this study. Additionally, the ability of GML to prevent TSS in a rabbit model was examined by co-administration of TSST-1 and GML vaginally.
This study demonstrated GML’s ability to inhibit both the immunoproliferative effects of superantigens on human T lymphocytes and immunostimulatory effects of superantigens on human vaginal epithelial cells (HVECs), suggesting an inhibition of signal transduction. GML prevented the cytotoxicity of anthrax toxin for HVECs as well as cytotoxicity of hemolysins for human and rabbit erythrocytes. Finally, GML inhibited TSS and lethality in a rabbit model of TSS; was not toxic to HVECs at concentrations that were effective in preventing exotoxin action on mammalian cells and rabbits; and stabilized erythrocyte membranes to the lytic effects of both hypotonic solutions and hemolysins. We conclude that GML may be a useful compound in preventing the effects of gram positive exotoxins, potential agents of bioterrorism, on human cells without significant risk of toxicity.
MATERIALS AND METHODS
All experiments performed in these studies, except those involving animals, were replicated a minimum of two times with similar results obtained.
Bacteria, exotoxins, and chemicals
Select agents used in this study were handled in accordance with guidelines established by the University of Minnesota Institutional Biosafety Committee. In addition, the Schlievert laboratory is approved by the Centers for Disease Control for handling such agents. Staphyloocccus aureus RN4220 (pCE107) was used as the source of the superantigen TSST-1 (26). Staphylococcal strain MNJA was the source of both the superantigen SEB and α-hemolysin (27). S. aureus strain RN4220 was the source of β-hemolysin. Escherichia coli containing the gene for streptococcal pyrogenic exotoxin A (speA) in a pET28b vector was the source of the superantigen SPE A (3). The Sterne strain was the source of culture fluids containing B. anthracis hemolysins and anthrax toxin (a mixture of protective antigen, lethal factor, and edema factor) (28), and Group A streptococcal strain T18P was the source of culture fluids containing the hemolysins streptolysins O and S (29). All strains are maintained in the laboratory in the lyophilized state.
Purified superantigen exotoxins were obtained as follows. The producing organisms were grown in a pyrogen free dialyzable beef heart medium (4–1200 ml flasks) at 37°C with high aeration (200 revolutions per minute) until late stationary phase of growth (24 to 48 h) (30). Briefly, TSST-1, SEB, and SPE A were purified by ethanol precipitation (80% final concentration) from culture fluids, collection of the precipitate by centrifugation (400 × g, 10 min), resolubilization of exoproteins in pyrogen free water (80 ml), centrifugation (10,000 × g, 30 min to remove insoluble material), and thin layer isoelectric focusing in pH gradients of 3 to 10 and then 6 to 8 to purify TSST-1 (pI 7.2) (31), 7 to 9 to purify SEB (pI 8.5) (32), and 4 to 6 to purify SPE A (pI 5.0) (33). Samples of all three exotoxins (5 ug of each) were determined to be homogeneous when tested by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and silver stained. In addition, the three exotoxins, including SPE A produced in the E. coli clone, were free of contaminating gram negative endotoxin (lipopolysaccharide, LPS) (lower limit of detection 1 ng LPS/mg exotoxin). TSST-1 and SEB were free of detectable hemolysin, protease, and lipase as determined by single radial diffusion (34). Staphylococcal β-hemolysin was purified from strain RN4220 comparably as SEB. The exotoxin (5 ug sample) was homogeneous when tested by SDS-PAGE and Coomassie brilliant blue stained. All of these toxins were stored at −20°C in the laboratory until used.
α-hemolysin was purified comparably as SEB except the protein was separated from stationary phase cultures by ammonium sulfate precipitation (75% saturation), resolubilization in pyrogen free water, dialysis for 2 days to remove residual ammonium sulfate, and isoelectric focusing in pH gradients of 3 to 10 and then 6 to 8 (pI 7.5). The protein (5 ug sample) was homogeneous when tested by SDS-PAGE and stained with Coomassie brilliant blue. Other hemolysins were used directly as unpurified proteins in cell free culture fluids (0.2 um pore size filtration) of organisms grown in either dialyzable beef heart medium (streptolysins O and S) or R medium (B. anthracis hemolysins). The three B. anthracis anthrax toxin components were used as mixtures of unpurified proteins in cell free culture fluids (0.2 um pore size filtration) (24) or as purified lethal toxin (purchased as the heptamer pore-forming protective antigen and lethal factor, List Biological Laboratories, Inc, Campbell, CA). We showed that the culture fluids contained all three components of anthrax toxin (protective antigen, lethal factor, and edema factor) as determined by Western immunoblotting and real time PCR (24). In this study, the combined effects of protective antigen and lethal factor were measured.
GML (monomuls 90-L 12) was a gift from Cognis Corporation, Kankakee, IL. GML analysis determined the compound was 93.5% monoester, with the remainder being primarily diester (>5.5%) and free glycerin (0.2%). GML was dissolved in absolute ethanol at a concentration of 100 mg/ml and diluted into water, phosphate buffered saline (PBS, 0.005 NaPO4, pH 7.2, 0.15 M NaCl), or saline (0.15 M NaCl) to a maximum solubility of 100 ug/ml at 37°C for use in this study.
Inhibition of superantigen immunoproliferation
Superantigens are defined by their abilities to induce non-antigen specific T lymphocyte proliferation dependent on the composition of the variable region of the β chain of the T cell receptor (4). This stimulatory activity is also dependent on the presence of antigen presenting cells (4). On day one, human peripheral blood mononuclear cells containing T lymphocytes and antigen presenting cells (2 × 105 peripheral blood mononuclear cells/well/200 ul volume in quintuplicate in RPMI 1640 medium supplemented with 2% fetal bovine serum, penicillin/streptomycin, and L-glutamine) were incubated with superantigens (1 ug/well) for 3 days in 96 well microtiter plates (35). On day 3, T lymphocyte proliferation was assessed by addition of 1 uCi/well of 3H-thymidine (20 ul volume) to the wells for an additional 24 h, then harvesting DNA onto glass fiber filters, and finally scintillation counting on day 4. In these studies, GML (1 ug, 5 ug, 10 ug, and 20 ug in 5 ul volumes) was added to wells at designated concentrations simultaneously with superantigen on day one.
An early step in T cell receptor signal transduction leading to immunoproliferation of T lymphocytes by superantigens requires cleavage of phosphatidylinositol 4,5-bisphosphate (PIP2) into the second messengers inositol 1,4,5 triphosphate (IP3) and diacyl glycerol by phospholipase Cγ1. The ability of GML to inhibit the total cellular IP3 was used as a measure of phospholipase olil 10 s Cγl activity on PIP2. Lymphocytes and macrophages (1 × 107 cells/ml) were incubated with the superantigen TSST-1 (5.0 ug/ml) with GML (0, 0.1, 0.5, 1.0, 5, and 10.0 ug/ml) overnight at 37°C, 7% CO2 in PBS supplemented with 1% glucose and without magnesium. The cellular activity was terminated by addition of ice-cold trichloroacetic acid (15%) on ice for 20 min, followed by centrifugation (20 min, 2000 × g, 4°C). Samples were then treated 3 times with 10 volumes of water saturated ether to remove trichloracetic acid. Samples were treated with 0.1 M Tris base to bring the samples to neutral pH. Total intracellular IP3 was measured by comparison to standards in a competition radioactive IP3-receptor assay as described by the manufacturer (NEN Research Products, DuPont, Boston, MA).
Inhibition of Human Vaginal Epithelial Cell (HVEC) cytokine release
Experiments were performed to assess the ability of GML (20 ug/ml) to interfere with production of chemokines and cytokines by immortalized HVECs (2 × 107/flask) through the stimulatory actions of TSST-1 (100ug/ml), SEB (100 ug/ml), and SPE A (100 ug/ml). These superantigens were previously determined to stimulate chemokine and cytokine production from HVECs (36). The 100 ug/ml dose of all superantigens was chosen because our unpublished observations indicate that TSST-1 and SEB-positive S. aureus strains make up to 1000 ug/ml of exotoxin when cultured for 8 h in biofilms (as might be expected to occur in vivo); we used a 10-fold lower dose. HVECs were grown in keratinocyte serum free medium (Gibco, Invitrogen, Carlsbad, CA) on plastic and passaged with a 1:4 split using trypsin/EDTA. HVECs were immortalized with retroviruses expressing HPV-16 E6/E7 and the reverse transcriptase component of telomerase as described previously (37, 38). The HVECs were initially stained with a mixture of IgG monoclonal antibodies AE1 and AE7 (Chemicon International, Temecula, CA) to characterize their cytokeratin production and ensure the cells were epithelial (36). Clone AE1 detects the high molecular weight cytokeratins 10, 14, 15, and 16 and the low molecular weight cytokeratin 19. Clone AE3 detects the high molecular weight cytokeratins 1–6 and the low molecular weight cytokeratins 7 and 8.
For all HVEC experiments, confluent monolayers of HVECs in 75 cm2 flasks (BD Falcon, Bedford, MA) were co-cultured with the superantigens and with and without GML at 37°C in a 7% CO2 incubator in keratinocyte serum free medium supplemented with bovine pituitary extract and epidermal growth factor as provided by the manufacturer for 6 h.
Enzyme linked immunosorbent assays (ELISAs) were performed on cell culture supernates after the 6 h incubation periods of HVECs with exotoxins ± GML (36). ELISA kits were purchased from R and D Systems, Minneapolis, MN and included assays for chemokine ligand 20 (macrophage inflammatory protein-3α [MIP-3α]), IL-1β, IL-6, IL-8, and TNF-α. All assays including standard curve generation were performed according to the manufacturer’s specification. Absorbance values and calculated concentration of chemokines and cytokines in the supernates were derived from the linear parts of the standard curves. Controls included the supernates of HVECs incubated without superantigens or with an irrelevant foreign protein (ovalbumin 100 ug/ml) which were assayed for chemokines and cytokines. Finally, GML (20 ug/ml) was added exogenously to supernates previously determined to contain chemokines and cytokines to demonstrate that GML alone did not interfere with ELISA assays. The lower limits of detection for the ELISA kits were: MIP-3α (8 pg/ml), IL-1β (4 pg/ml), IL-6 (4 pg/ml), IL-8 (16 pg/ml), and TNF-α (8 pg/ml),
Inhibition ofTSS and lethality in rabbits
To evaluate the lethality of TSST-1 in a rabbit TSS model, the synergy between TSST-1 and LPS was exploited. TSST-1 previously has been shown to amplify the lethal effects of gram negative LPS by up to one million fold (LPS given intravenously 4 h after TSST-1 given intravaginally), as a rabbit model for staphylococcal TSS (death typically occurs in approximately 6 h following the LPS injection) (39). [Note that the lethal dose 50% end-point (LD50) of TSST-1 alone for 2 kg Dutch belted female rabbits is greater than 3 mg/animal in bolus injections, and the LD50of LPS alone intravenously is 1 mg/animal.] Rabbits (3/group, 2 kg female Dutch belted) were treated with TSST-1 (5 ug/rabbit) vaginally in 0.25 ml of PBS with and without GML (250 ug) administered in the same solution. In these experiments, the rabbits were initially anesthetized with ketamine (0.4 ml of 20 mg/ml) and xylazine (1 ml of 20 mg/ml) and then catheters placed into the vaginas through which the TSST-1-GML samples were administered (40). The animals were allowed to recover from anesthesia for 4 h, then were injected intravenously with LPS from Salmonella typhimurium (5 ug in 1 ml PBS), and were monitored for lethality for 24 h. Rabbits that failed to exhibit escape behavior and right themselves were considered in irreversible shock and were euthanized prematurely in accordance with agreements with the University of Minnesota Institutional Animal Care and Use Committee. Control animals in these experiments received GML alone (250 ug/0.25 ml) vaginally (n=2) and PBS (250 ug/0.25 ml) (n=1) vaginally. Surviving animals were euthanized at 24 h with Beuthanasia D, and vaginal tissue was examined for gross evidence of tissue inflammation.
Inhibition of cytotoxic effects of exotoxins
These experiments used multiple representative cytotoxins, including culture fluids containing lethal toxin (protective antigen and lethal factor) from B. anthracis, protective antigen and lethal factor purchased from List Biological Laboratories, Campbell, CA, purified staphylococcal α and β-hemolysins, culture fluids containing streptolysins O and S (hereafter referred to as streptolysins), and culture fluids containing B. anthracis hemolysins.
Anthrax lethal toxin forms when protective antigen forms heptamer prepores within the plasma membranes of eukaryotic cells and binds to lethal factor (41). The internalization of this complex leads to acidification and subsequent formation of mature pores with insertion of the lethal factor into the cellular cytoplasm where it causes lethality due to protease activity (42). Studies were performed to assess the ability of GML (10 ug/ml) to inhibit the toxic effects of lethal toxin from B. anthracis culture fluids (2.5ml/assay) with non-confluent HVECs (approximately 1 × 104 cell/flask) (24). [Note that epithelial cells are thought to have receptors for anthrax toxin protective antigen primarily on their baso-lateral surface, and for this reason we investigated non-confluent HVECs]. The cultures were incubated for 18 h at 37°C with lethal toxin and with or without GML, and then HVEC viability determined by Trypan blue dye exclusion. In a second set of studies, murine RAW 264.7 macrophages (1 × 105 cells/well) in quadruplicate in 96 well microtiter plates were incubated 4 h at 37°C with 100 ng protective antigen and 50 ng lethal factor (List Biological Laboratories, Inc, Campbell, CA) with or without simultaneously added GML (10 ug/ml, 20 ug/ml, 100 ug/ml). The total incubation volume was 200 ul/well. Percent lethality was determined by Trypan blue dye exclusion. In another identical experiment, we attempted to use the more standard MTT dye assay (43), but we discovered that viable macrophages could not accumulate the dye in the presence of GML. We did use this assay to verify that the combination of protective antigen and lethal factor used in our experiments was cytotoxic.
α-hemolysin was incubated with rabbit red blood cells, as the mammalian cell type most susceptible to the cytolytic effects of the toxin (5). Purified α-hemolysin (0.2 ug/ml and 1 ug/ml) was mixed with rabbit red blood cells (two different concentrations) that upon complete lysis with distilled water would give an absorbance at 410 nm wavelength of approximately 1 and 2, respectively, α-hemolysin at these concentrations was determined to lyse the rabbit red blood cells completely in less than 15 min. Varying concentrations of GML (0.1 ug/ml, 1.0 ug/ml, and 10 ug/ml) were simultaneously added to rabbit red blood cells with α-hemolysin in PBS to determine inhibition of lysis. All samples were incubated at 37°C, 7% CO2. Additionally, α-hemolysin (0.1 ug/ml which completely lysed the rabbit red blood cells in 1 h) was added to rabbit red blood cells in PBS and the samples incubated until 50% lysis had occurred (30 min). At that point, GML (1.0 ug/ml) was added to the cultures in attempt to prevent further lysis. In all of these studies the samples were centrifuged (400 × g, 10 min) after incubation periods, and the absorbances at 410 nm were determined as a measure of lysis. Controls consisted of rabbit red blood cells added to PBS and incubated without toxin and without GML and with the various concentrations of GML alone.
Studies were performed in quadruplicate to assess whether or not GML would prevent α-hemolysin insertion into rabbit red blood cell membranes. Prior observations determined that α-hemolysin heptamerizes in solution, and the heptamers then form pores in rabbit red blood cell membranes, causing lysis (44). Fresh heparinized blood (10 ml) was drawn from a rabbit. The rabbit red blood cells were washed 3 times with 15 ml PBS (centrifugation 400 × g, 10 min). Finally, the packed cell volume (obtained by centrifugation 400 × g, 10 min) was diluted ½ with PBS, and 0.5 ml volumes were transferred into separate tubes, α-hemolysin (100 ug) alone and with GML (100 ug) was mixed with rabbit red blood cells (to create final volumes of 1 ml) at 37°C until complete lysis occurred (less that 1 h) in the samples with α-hemolysin and rabbit red blood cells alone. This step was performed with the hypothesis that α-hemolysin would be removed from the solution by embedding into the membranes of rabbit red blood cells in the absence of GML, but remain in solution if GML prevented its insertion into the rabbit red blood cell membranes. Supernate samples were collected by centrifugation (10,000 × g, 5 min) and were treated with 4 volumes absolute ethanol at 4°C to precipitate α-hemolysin and retain GML in solution. The precipitates were re-suspended in the same volume of PBS, and 20 ul volumes were added to agarose slides containing rabbit red blood cells. Briefly slides were made as follows: Agarose (0.75%) was melted in PBS, cooled to 50°C, rRBCs added (0.1% final concentration), and 4 ml volumes of the mixture used to coat the surface of microscope slides. After the agarose slides solidified, 4 mm wells were cut in the agarose and samples (20 ul) applied. The slides were then incubated for 6 h at 37°C in a humidified 7% CO2 incubator. Zones of lysis were determined as measures of α-hemolysin presence in the supernates (not absorbed out by prior treatment with rabbit red blood cells), and concentration of α-hemolysin determined by comparison to lysis zones with known amounts of purified α-hemolysin treated comparably. The concentration of α-hemolysin was proportional to the area of lysis (34). Control samples contained rabbit red blood cells (1.0 ml final volumes) and rabbit red blood cells with GML (1.0 ml final volumes) only.
The ability of GML (1 ug/ml, 5 ug/ml, and 10 ug/ml) to inhibit the lytic effects of streptolysins and B. anthracis hemolysins were performed on rabbit red blood cells with culture fluids (100 ul/ml red blood cells) containing hemolysins, using essentially the same procedures as for α-hemolysin. The only differences in the assays were that culture fluids containing hemolysins were used rather than purified toxins, and the streptococcal culture fluids were reduced with dithiothreitol (5 mM) to insure that streptolysin O was active. In addition, the lytic effects of B. anthracis hemolysins were tested similarly to those of α-hemolysin, however, both rabbit and human red blood cells were studied. [Note: B. anthracis hemolysins in sterile culture fluids were self-inactivated 48 h after production and secretion. Experiments using these culture fluids were performed on the same day as the culture fluids were obtained.]
Staphylococcal β-hemolysin is a sphingomyelinase that prepares red blood cells for lysis by its enzymatic activity at 37°C (45). At 37°C, the red blood cells do not lyse, but upon shifting the temperature to 4°C, the red blood cells lyse, making the β-hemolysin a hot-cold hemolysin. The activity of β-hemolysin was utilized to evaluate the mechanism of action of GML on red blood cell membranes. Rabbit red blood cells (absorbance 600 nm wavelength = 2.4) in PBS were incubated with purified β-hemolysin (3 ug/ml, 7.5 ug/ml, and 15 ug/ml) at 37°C for 1 h. At that time the red blood cell samples were centrifuged (400 × g, 10 min), and the supernates were discarded. Subsequently, the samples were restored to the original volumes (5 ml) with PBS, and each sample was split in half. One half received 10 ug/ml of GML (final concentration), and one half received PBS. The samples were then immediately incubated at 4°C for 4 h and absorbance at 600 nm wavelength determined. All assays were performed in duplicate.
GML stabilization of cells in hypotonic aqueous solutions
Human red blood cells were obtained from heparinized venous blood and processed by dilution 10 fold in normal saline and then washing 3 times by centrifugation (400 × g, 10 min). The human red blood cells (5 ul) were then diluted with to 2 ml saline to give an absorbance at 600 nm wavelength of approximately 0.8. Human red blood cell stability measurements were conducted by adding diluted human red blood cells (5 ul into 2 ml) to solutions of GML (20 ug/ml, 50 ug/ml and 100 ug/ml) in distilled water, 0.025 M NaCl, and 0.05 M NaCl, 0.075 M NaCl, and 0.15 M NaCl and then measuring absorbance 600 nm wavelength continuously for 300 sec (5 min). At room temperature where measurements were made, GML (50 ug/ml and 100 ug/ml) solutions were slightly turbid, indicating incomplete solubility at that temperature.
Lack of GML cytotoxicity for HVECs
GML (20 ug/ml and 100 ug/ml) was added to partially confluent HVECs (approximately 5 × 106/flask) growing in 75 cm2 flasks (BD Falcon) and incubated for two weeks with changes of keratinocyte serum free medium and GML daily. Each day, flasks from GML treated and untreated cells were examined by Trypan blue dye exclusion for direct HVEC toxicity due to GML. On the last experimental day (day 14), the media from one flask of HVECs from each treatment group (treated or not treated with GML) were changed to fresh keratinocyte serum free medium without added GML, and the HVECs were monitored for one week for ability to grow normally.
HVEC microarray studies with GML
Affymetrix GeneChip® Human Genome U133A (Santa Clara, CA) was used to analyze HVEC gene up- or down-regulation in response to incubation in keratinocyte serum free medium (without antibiotics) with GML (20 ug/ml) for 6 h (36). Control HVEC flasks contained keratinocyte serum free medium (without antibiotics) alone and were comparably incubated for 6 h. The protocol entailed culture of the HVECs in 75 cm2 flasks (BD Falcon, Bedford, MA) until confluent (determined to be approximately 2 × 107 HVECs/flask). Prior to initiation of the experiments, the media was then changed to include keratinocyte serum free medium alone (without antibiotics) or keratinocyte serum free medium (without antibiotics) plus GML.
The HVECs were removed from the flasks by trypsinization (5 min to 8 min at 37°C) and collected by centrifugation (250 × g for 5 min). The cell pellets were used for RNA extraction, which was accomplished using an RNeasy Mini kit from QIAGEN (Valencia, CA). RNA samples were processed for hybridization assays as described by Affymetrix, Inc. (Santa Clara, CA). Hybridization analyses were performed at the University of Minnesota Biomedical Genomics Center. The data obtained were analyzed by programs provided by both Affymetrix Microarray Suite 4.0 and Microsoft (Excel). Only those genes that were definitively present and statistically determined to be up- or down-regulated by 2-fold or more were considered.
RESULTS
GML inhibits T lymphocyte immunoproliferation
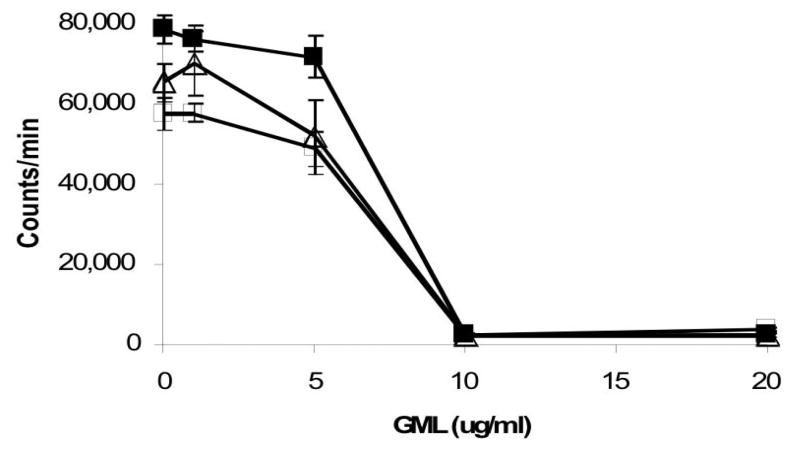
Superantigens are defined by their capacities to induce proliferation of T cells after crosslinking with antigen presenting cells, as a function of the β chain variable region of the T cell receptor complex, that causes release of IL-2, TNF-β, and IFN-γ (1, 4). In concert with activation of T cells is the release of IL-1β and TNF-α from macrophages (1, 4). GML (10 ug/ml and 20 ug/ml) effectively interfered with the T cell proliferative effect induced by three superantigens, TSST-1, SEB, and SPE A (Figure 1). Partial inhibition of T cell proliferation was observed with GML doses of 5 ug/ml.
Figure 1.
Effect of GML on superantigenicity of TSST-1 (1 ug/ml, □), SPE A (1 ug/ml, ■), and SEB (1 ug/ml, △). Superantigens plus GML (1 ug, 5 ug, 10 ug, and 20 ug) in 5 ul volumes were added simultaneously to 1 × 106/ml human peripheral blood mononuclear cells in 200 ul total volumes in replicates of 5 in 96 well microtiter plates. The plates were incubated at 37°C in the presence of 7% CO2 for 3 days, and then the wells were pulsed for 24 h with 1 uCi of 3H-thymidine for incorporation into DNA. DNA was harvested on day 4 with use of a mechanical sample harvester onto glass fiber filters and total counts determined by scintillation counting.
GML at concentrations of 0.5–10 ug/ml caused significant and dose dependent inhibition of formation of IP3 from PIP2 in lymphocytes incubated overnight with TSST-1 (5 ug/ml) compared to the positive control consisting of lymphocytes incubated with TSST-1 in the absence of GML (Table 1). The reduction in generation of IP3 in these assays is a reflection of reduced activity of phospholipase Cγl in the presence of GML, and consequently early events in T cell receptor signal transduction.
Table 1.
GML inhibition of generation of inositol 1,4,5-triphosphate (IP3) by lymphocytes stimulated with toxic shock syndrome toxin-1 (TSST-1)
| Treatment | IP3 Generateda (pmol/0.1ml ± SE) |
|---|---|
| Untreated Cells | 0.1 ± 0.05 |
| TSST-1b | 8.5 ± 0.6 |
| TSST-1 + GML (0. 1 ug/ml) | 8.0 ± 0.5 |
| TSST-1 + GML (0.5 ug/ml) | 5.0 ± 0.6 |
| TSST-1 + GML (1.0ug/ml) | 2.0 ± 0.1 |
| TSST-1 + GML (10.0 ug/ml) | 1.0 ± 0.05 |
IP3 generated by phospholipase Cγ1 cleavage of phosphatidylinositol 4,5-bisphosphate
TSST-1 concentration (5 ug/ml)
GML inhibits cytokine release by HVECs
HVECs have been determined previously to up-regulate production of chemokines and cytokines in response to stimulation with the superantigens TSST-1, SEB, and SPE A for 6 h (36). We explored whether or not GML could inhibit chemokine and cytokine release by HVECs in response to stimulation by TSST-1, SEB, and SPE A.
Representative cytokines (IL-1 β and TNF-α) and chemokines (MIP-3α, IL-6, and IL-8) were released by HVECs in response to stimulation with TSST-1, SEB, and SPE A as determined by ELISA on culture supernates (Table 2). All superantigens stimulated release of approximately the same amounts of cytokines and chemokines, except SEB caused statistically significant release of more MIP-3α and IL-8 than the other two superantigens. GML (20 ug/ml) completely prevented superantigen induced cytokine and chemokine release from HVECs (Table 2). GML (20 ug/ml) also inhibited constitutive low-level release of cytokines and chemokines when HVECs were incubated alone in keratinocyte serum free medium (Table 2). Ovalbumin (100 ug/ml), which caused only low-level release of cytokines and chemokines from HVECs, was included as a control to indicate that the cytokines and chemokines stimulated by the three superantigens was the result of unique superantigen activity, rather than simply the presence of any foreign protein.
Table 2.
Cytokines and Chemokines produced by HVEC in response to TSST-1, SEB, and SPE A, and inhibition by GML.
| Protein Tested (100 ug/ml) (N=3) | GMLa (20 ug/ml) | IL-1β (pg/ml±SE) | TNF-α (pg/ml±SE) | MIP-3α (pg/ml ± SE) | IL-6 (pg/ml±SE) | IL-8 (pg/ml ±SE) |
|---|---|---|---|---|---|---|
| None | - | 8±1 | 16±1.5 | 9.0 ± 1.5 | 8±1.5 | 16 ± 2 |
| None | + | <4b | <8 | <8 | <4 | <8 |
| Ovalbumin | - | 11±1.5 | 16±2 | 36±1.5 | 10±1.5 | 12±2 |
| TSST-1 | - | 16±3 | 62±8 | 320 ± 4 | 18±2 | 500 ± 33 |
| TSST-1 | + | <4 | <8 | <8 | <4 | <8 |
| SEB | - | 18±4 | 56±6 | 470 ± 7 | 19±4 | 620 ± 7 |
| SEB | + | <4 | <8 | <8 | <4 | <8 |
| SPEA | - | 15±2 | 97±4 | 396 ± 8 | 19±2 | 470 ± 10 |
| SPEA | + | <4 | <8 | <8 | <4 | <8 |
GML did not interfere with ELISAs since the compound did not affect cytokine and chemokine detection when added to supernates that already contained chemokines.
<refers to samples where quantities of chemokines and cytokines were below detection by the ELISA kits.
GML inhibits TSS and lethality in a rabbit model
The ability of GML to protect rabbits from TSS by administration of GML and TSST-1 vaginally was also determined. GML (250 ug/0.25 ml) when co-administered vaginally to rabbits with TSST-1, completely prevented TSST-1 induced TSS after 24 h (0/3 animals succumbed compared to 2/3 animals treated with TSST-1 alone). Minor visible edema and angiogenesis were observed in the vaginal tissue of surviving rabbits that had been treated with TSST-1 plus GML (data not shown). The one surviving rabbit that had been treated with TSST-1 alone had small amounts of pus present vaginally and there was significant edema, angiogenesis, neutrophil influx, apoptotic cells present, and epithelial sloughing (data not shown). GML alone (250 ug/0.25 ml administered volume) was not toxic (0/2) when administered vaginally to rabbits with a lack of observable pathologic effects on vaginal mucosal tissue.
GML inhibits the effects of cytotoxins
Many heptamer pore-forming exotoxins have been described, including staphylococcal α-hemolysin and Group A streptolysin O (6, 46, 47). In addition, the protective antigen component of anthrax toxin is a heptamer pore forming toxin that delivers lethal factor and edema factor into susceptible mammalian cells, leading to anthrax disease (2). Finally, B. anthracis also makes hemolysins (48–50), and group A streptococci make another hemolysin, streptolysin S (51).
GML (1 ug/ml and 10 ug/ml) effectively prevented the lytic activity of staphylococcal α-hemolysin for rabbit red blood cells in duplicate experiments containing two different α-hemolysin (0.2 ug/ml and 1 ug/ml) and two different rabbit red blood cell concentrations (complete lysis with distilled water yielded absorbances at 410 nm wavelength of 0.97 and 2.2) (Table 3). GML partially prevented hemolysis at a concentration of 0.1 ug/ml. In addition, GML (1 ug/ml) immediately stopped additional lysis due to staphylococcal α-hemolysin (0.1 ug/ml) when administered approximately 30 min into the 1 h total incubation period (Table 3).
Table 3.
GML inhibits staphylococcal α-hemolysin lysis of rabbit red blood cells (RBC).
| Experiment Number (Incubation time) | Treatment Groupa | Absorbance 410 nma |
|---|---|---|
| 1 (1h) | RBC in distilled waterb | 0.97 |
| 1 (1h) | RBC in PBS | 0 |
| 1 (1h) | RBC + GML (10 ug/ml) | 0 |
| 1 (1h) | RBC + 0.2 ug α-hemolysin | 0.97 |
| 1 (1h) | RBC + 0.2 ug α-hemolysin + GML (0.1 ug/ml) | 0.44 |
| 1 (1h) | RBC + 0.2 ug α-hemolysin + GML (1.0 ug/ml) | 0 |
| 1 (1h) | RBC + 0.2 ug α-hemolysin + GML (10 ug/ml) | 0 |
|
| ||
| 2 (1h) | RBC in distilled water | 2.2 |
| 2 (1h) | RBC + 1.0 ug α-hemolysin | 2.2 |
| 2 (1h) | RBC + 1.0 ug α-hemolysin + GML (1.0 ug/ml) | 0 |
| 2 (1h) | RBC + 1.0 ug α-hemolysin + GML (10 ug/ml) | 0 |
|
| ||
| 2 (1h) | RBC in distilled water | 2.2 |
| 2 (1h) | RBC + 0. 1 ug α-hemolysin | 2.2 |
|
| ||
| 2 (30 min incubation) | RBC + 0. 1 ug α-hemolysin | 0.9 |
| 2 (30 min incubation with α-hemolysin and then GML added; total incubation 1 h) | RBC + 0.1 ug α-hemolysin + GML (1.0 ug/ml) | 1.2 |
Rabbit red blood cells were incubated with the designated agents in PBS for the indicated time periods at 37°C and then centrifuged at 400 × g for 10 min to pellet red blood cells. The absorbances of the supernates at 410 nm were determined.
All experiments were conducted in PBS except where noted (in distilled water).
GML also blocked the cytolytic effect of streptolysin O and S and anthrax hemolysins (Table 4). GML (1 ug/ml, 5 ug/ml, and 10 ug/ml) completely inhibited rabbit red blood cell lysis by 100 ul of group A streptococcal supernate. Interestingly, a higher concentration of GML (10 ug/ml) was required to inhibit the lytic effects of anthrax supernate hemolysins than either α-hemolysin (1 ug/ml) or the streptococcal hemolysins (1 ug/ml) (Table 4). Finally, GML (10 ug/ml) prevented anthrax hemolysin lysis of human red blood cells (data not shown).
Table 4.
GML prevents group A streptococcal and B. anthracis culture fluid lysis of rabbit red blood cells (RBC).
| Treatment Group | GML (ug/ml) | Absorbance 410 nm (1h)a |
|---|---|---|
| RBC in distilled waterb | 0 | 2.4 |
| RBC in PBS | 0 | 0 |
| RBC in PBS + GML | 10 | 0 |
|
| ||
| RBCs + 100 ul streptococcal supernatea | 0 | 2.4 |
| RBCs + 100 ul streptococcal supernate | 1 | 0 |
| RBCs + 100 ul streptococcal supernate | 5 | 0 |
| RBCs + 100 ul streptococcal supernate | 10 | 0 |
|
| ||
| RBC + 100 ul B. anthracis supernate | 0 | 2.4 |
| RBC + 100 ul B. anthracis supernate | 1 | 2.4 |
| RBC + 100 ul B. anthracis supernate | 5 | 1.4 |
| RBC + 100 ul B. anthracis supernate | 10 | 0 |
Rabbit red blood cells were incubated with the designated agents in PBS at 37°C and the centrifuged at 400 × g for 10 min to pellet red blood cells. The absorbance of the supernates at 410 nm were determined. Streptococcal and B. anthracis supernates were filter sterilized (0.2 um pore size) prior to use.
All experiments were conducted in PBS except where noted (in distilled water).
Anthrax toxin heptamerizes upon insertion into susceptible plasma membranes, forming what is referred to as the pre-pore (41). The heptamerized protective antigen has the capacity to bind the protease lethal factor to form lethal toxin (52). Endocytosis and acidification leads to formation of a mature pore that inserts lethal factor into the cells (41). GML (10 ug/ml) completely prevented the lethal action of B. anthracis culture fluids containing lethal toxin for HVECs (Table 5). These data suggest the effect of GML was either to interfere with protective antigen insertion into HVEC membranes or to interfere with the endocytic process. In a separate experiment, GML was 100 ug/ml was highly effective in preventing purified anthrax lethal toxin killing of RAW 264.7 macrophages (Table 5) compared to control macrophages treated only with lethal toxin. Lower concentrations of GML provided partial protection from lethality.
Table 5.
GML prevents cytotoxic activities of B. anthracis supernates and purified lethal toxin (mixture of protective antigen [PA] and lethal factor [LF]).
| Treatment Groupa | % Killing of HVEC + SE |
|---|---|
| HVEC + 2.5 ml supernate | 45±4 |
| HVEC + 2.5 supernate + 10 ug/ml GML | 0 |
| Macrophages + lethal toxin | 85±2 |
| Macrophages + lethal toxin +10 ug/ml GML | 30±8 |
| Macrophages + lethal toxin + 20 ug/ml GML | 10±5 |
| Macrophages + lethal toxin + 100 ug/ml GML | 0 |
HVEC (1 × 10 ) were treated with 2.5 ml of B. anthracis supernate, and lethality determined by Trypan blue dye exclusion after incubation 18 h at 37°C. B. anthracis supernates were filter sterilized (0.2 um pore size) prior to use. Raw 264.7 macrophages (1 × 105) were treated with protective antigen (PA) (100 ng) and lethal factor (LF) (50 ng) purchased from List Biological Laboratories, Inc for 4 h. This combination of proteins comprises lethal toxin. Cell viability was determined by Trypan blue dye exclusion.
GML prevents insertion of staphylococcal α-hemolysin into rabbit red blood membranes
GML effectively prevented the insertion of heptameric α-toxin into the eukaryotic membrane (Table 6). Prior incubation of rabbit red blood cells with GML (100 ug/ml) and staphylococcal α-hemolysin (100 ug/ml) prevented α-hemolysin embedding into rabbit red blood cell membranes and consequently removal from supernates. In contrast, prior incubation of rabbit red blood cells alone with α-hemolysin (100 ug/ml) resulted in nearly complete removal of the exotoxin from the supernates.
Table 6.
GML prevents staphylococcal α-hemolysin insertion into rabbit red blood cell (RBC) membranes.
| Treatment Group | α-hemolysin Remaining in Supernate (ug)a |
|---|---|
| RBCs alone | 0 |
| RBCs + GML(100 ug) | 0 |
| RBCs + α-hemolysin (100 ug) | 5±1 |
| RBCs + α-hemolysin (100 ug) + GML (100 ug) | 90±1 |
α-hemolysin (100 ug) alone and with GML (100 ug) were mixed with rabbit red blood cells in final volumes of 1 ml of PBS at 37°C until complete lysis occurred in the samples with α-hemolysin and rabbit red blood cells alone. Supernate samples were collected by centrifugation, treated with 4 volumes absolute ethanol, re-suspended in 1 ml amounts of PBS, and 20 ul volumes added to agarose slides containing rabbit red blood cells. The slides were incubated for 6 h at 37°C in a humidified 7% CO2 incubator. Zones of lysis were determined as compared to lysis zones with known amounts of purified α-hemolysin treated comparably. The concentration of α-hemolysin was proportional to the area of lysis.
GML prevents the lytic effects of staphylococcal β-hemolysin on rabbit red blood cells
The ability of GML to prevent the hot: cold hemolytic activity of β-hemolysin was determined. β-hemolysin is a sphingomyelinase that sensitizes red blood cells at 37°C to subsequent lysis at 4°C (45). Pre-incubation of rabbit red blood cells with β-hemolysin resulted in partial (3 ug/ml β-hemolysin) or complete (7.5 ug/ml and 15 ug/ml β-hemolysin) lysis when the red blood cells were subsequently shifted to a temperature of 4 °C in the absence of additional β-hemolysin (Table 7). In contrast, the same pre-treatment and temperature shift to 4 °C in the presence of GML (10 ug/ml) completely (3 ug/ml and 7.5 ug/ml β-hemolysin) or partially (15 ug/ml β-hemolysin) protected the red blood cells from lysis. These data suggest GML (10 ug/ml) stabilized the red blood cell membranes to the β-hemolysin induced membrane lipid conformation change that leads to lysis of red blood cells at 4 °C.
Table 7.
GML inhibits the hemolytic effects of staphylococcal β-hemolysin.
| β-hemolysina (ug/ml) | GML (ug/ml) | Absorbance (600 nm) |
|---|---|---|
| None | 0 | 2.4 |
| None | 10 | 2.4 |
|
| ||
| 3 | 0 | 1.0 |
| 3 | 10 | 2.2 |
|
| ||
| 7.5 | 0 | 0 |
| 7.5 | 10 | 2.2 |
|
| ||
| 15 | 0 | 0 |
| 15 | 10 | 0.5 |
β hemolysin was added to rabbit red blood cells in PBS at time 0 h. The samples were incubated at 37°C for 1 h, centrifuged (400 × g, 10 min), and supernates discarded. The samples were restored to the original volumes with PBS, and each sample was split in half. One half received 10 ug/ml of GML (final concentration), and one half received PBS. The samples were then immediately incubated for 4 h at 4°C and absorbance at 600 nm wavelength determined.
GML stabilizes human red blood cells to lysis by hypotonic aqueous solutions
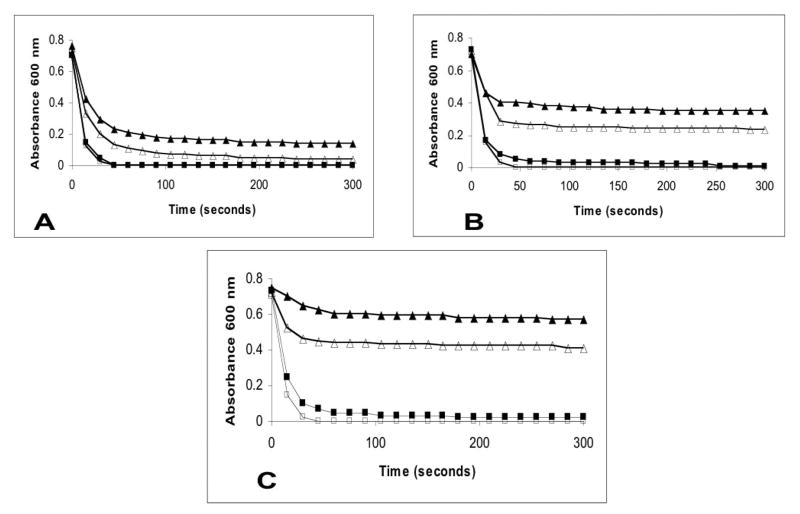
From the above experiments, we hypothesized that GML may exert its effects by stabilizing membranes and preventing signal transduction. GML was tested for ability to stabilize human red blood cells against lysis by distilled water (Figure 2A) and hypotonic solutions (0.025 M NaCl Figure 2B and 0.05 M NaCl Figure 2C) at concentrations of GML 20 ug/ml, 50 ug/ml and 100 ug/ml. Interestingly, GML stabilized and prevented lysis of human red blood cells, minimally by distilled water (Figure 2A) but significantly by hypotonic saline solutions (Figure 2B and 2C), in a concentration dependent manner, with GML (100 ug/ml) providing the greatest stabilization. Hypotonic saline solutions containing 0.075 M NaCl and normal saline containing 0.15 M NaCl did not result on lysis of human red blood cells in the presence or absence of GML (20 ug/ml, 50 ug/ml, and 100 ug/ml).
Figure 2.
GML added to hypotonic solutions stabilizes human red blood cells. A. Absorbance changes at 600 nm wavelength with GML (control 0 ug/ml, □ 20 ug/ml, ■ 50 ug/ml, △ and 100 ug/ml, ▲) added to human red blood cells in distilled water; B. Absorbance changes at 600 nm wavelength with the same GML concentrations added to human red blood cells in 0.025 M NaCl; C. Absorbance changes at 600 nm wavelength with the same GML concentrations added to human red blood cells in 0.05 M NaCl. Human red blood cells added to 0.075 M and 0.15 M NaCl did not lead to red blood cell lysis. GML (10 to 100 ug/ml) added to human red blood cells in 0.075 M and 0.15 M saline did not lead to red blood cell lysis.
GML is not toxic to eukaryotic cells
Initial studies examined the toxicity of GML for HVECs over a two week period. HVECs at approximately 50% confluence (5 × 106/flask) were incubated with 10 ml of keratinocyte serum free medium containing 20 ug/ml and 100 ug/ml amounts of GML for two weeks with daily exchanges of GML. By Trypan blue dye exclusion, no toxicity was observed at the end of two weeks in GML treated groups versus controls (data not shown). However, HVECs treated with GML (100 ug/ml) were observed to grow more slowly compared to control non-GML treated HVECs. On day 14, remaining GML in the supernates was removed from one flask of each treatment group by changing the media to KSFM without GML, and cell growth was monitored. Cells in all three flasks (untreated control, GML 20 ug/ml, and GML 100 ug/ml) grew normally, indicating that even though GML delayed the growth of HVECs treated with GML 100 ug/ml, the delayed growth effect was not permanent. After changing the media to KSFM without GML and allowing the cells to grow to confluence, the HVECs could be restimulated with superantigens to release cytokines at the expected levels.
Additionally, GML (20 ug/ml) was evaluated for effects on the gene expression of HVECs following 6 h incubation compared to untreated controls for 6 h. Significant alteration of normal gene expression was observed in a small number of genes [160 compared to 2386 for TSST-1 (100 ug/ml)] (36), consistent with the low toxicity of the compound [a complete listing of genes that were affected by GML (20 ug/ml) can be found at http://www.micab.umn.edu/faculty/Schlievert.html]. Examples of genes that were significantly up-regulated by GML (20 ug/ml) included insulin induced gene (13 fold), DNaJ (Hsp40) homolog, subfamily B, member 9 (13 fold) and corneodesmosin (12 fold). Only 21 genes were significantly down-regulated, with all less than 3 fold (the most down-regulated was the gene for carbonic anhydrase XII by 2.8 fold). GML was not pro-inflammatory to HVECs, as evidenced by lack of induction of proinflammatory cytokines and chemokine genes.
DISCUSSION
Gram positive bacteria produce a large number of human diseases, with TSS and necrotizing pneumonia caused by S. aureus (1, 53), pharyngitis and TSS with necrotizing fasciitis induced by group A streptococci (3, 54, 55), and cutaneous, gastrointestinal, and inhalation anthrax caused by B. anthracis (56). Many of these diseases initiate at mucosal surfaces and occur due to the action of exotoxins on the cells of human hosts. Because many of the diseases are associated with high morbidity and mortality, specific exotoxins, such as the superantigen exotoxins SEs, TSST-1, and SPEs, and anthrax toxin have been classified as select agents of bioterrorism.
This study was designed to investigate the preventation of toxicity of exotoxins on host cells by GML, a molecule from a class of fatty acid monoesters that are likely to affect globally cell membranes and signal transduction in both bacteria and eukaryotes. GML is generally recognized as safe by the Food and Drug Administration for oral use because of past safety experience and is used as an additive to foods and cosmetics (19–21). GML is a surfactant comprised of glycerol and a 12 carbon fatty acid which is found naturally in human breast milk. GML in aqueous solutions has a solubility limit of approximately 100 ug/ml at 37°C, and for this reason, we investigated the inhibitory effects of GML up to that concentration. However, the concentrations of GML that are used in foods may reach 10 mg/ml (20), or 100 times the concentrations used in this study. Other members of this general class of compounds include mono and diglycerides with longer or shorter fatty acid chain length. We chose GML as the representative compound to study because previous research suggested that GML inhibited exotoxin production by gram positive bacteria, more effectively than other members of the class with longer or shorter fatty acid chain lengths (23).
In general, the most significant findings of this study were the abilities of GML to interfere with the toxic effects of gram positive exotoxins on mammalian cells. Exotoxins typically have two types of effects on human cells: 1) direct and indirect cytotoxic capabilities through the formation of pores, functioning as surfactants, or delivering enzymes or factors that disrupt normal cellular functions; and 2) immunostimulatory, particularly as superantigens or other exotoxins stimulating cytokine and chemokine release from immune cells. Typically, patients infected with gram positive organisms present with clinical signs that are due in part or completely to the effects of exotoxins on human cells (54–60). Our studies suggest that GML may be able to inhibit both the initiation of interaction of exotoxins with human cells, and also block further toxicity even when cytotoxicity has begun. Thus, GML inhibited the immuno stimulatory effects of superantigens on mammalian cells in vitro and in vivo, including TSS-inducing and lethal effects of TSST-1 vaginally in rabbits. In addition, GML blocked the cytotoxic effects of several hemolysins for red blood cells and anthrax toxin for HVECs and macrophages. Notably, different concentrations of GML were required to interfere with the effects of hemolysins, with lower concentrations (1 ug/ml) inhibiting α-hemolysin activity but higher concentrations (10 ug/ml) required to inhibit B. anthracis hemolysins. The capacity of GML and related compounds to interfere with exotoxin effects may also depend on fatty acid chain lengths and whether or not the molecule is a mono or diester; these effects were not tested in this study.
Previous research suggested that GML interfered with signal transduction in gram positive bacteria leading to inhibition of transcription of exotoxins (22, 24, 25). Prior research has also suggested that GML inhibits T lymphocyte proliferation stimulated with nonspecific mitogenic agents (61). In this study we demonstrated that GML inhibits the lymphocyte proliferative effects of three representative superantigens, TSST-1, SEB, and SPE A. TSST-1 is the principal cause of menstrual TSS and one-half of non-menstrual TSS (62); SEB is a classical staphylococcal enterotoxin that causes food poisoning, is a significant cause of nonmenstrual TSS, and is associated with necrotizing pneumonia caused by S. aureus (53, 62, 63); and SPE A is a major superantigen associated with streptococcal TSS with necrotizing fasciitis (55). The effects of GML appear to be to interfere with the early T cell receptor signal transduction events as evidenced by GML inhibition of IPs formation from membrane PIP2, as catalyzed by phospholipase Cγ1.
Our prior studies suggested that TSS is initiated by low-level inflammation produced by S. aureus and exotoxin activation of HVECs to release chemokines and cytokines through inflammation which facilitates superantigen transport across the mucosal barrier (36). GML was highly effective in preventing the release of chemokines and cytokines by HVECs in response to stimulation with TSST-1, SEB, and SPE A, probably through inhibition of signal transduction through an unknown HVEC receptor.
The cytotoxic exotoxins studied included hemolysins that function as heptamer pore-forming agents, including staphylococcal α-hemolysin, streptolysin O and the protective antigen component of anthrax toxin. Staphylococcal α-hemolysin forms heptamers in solution with the oligomeric forms embedding in the host red blood cell membrane to cause lysis (5). Our studies demonstrated that α-hemolysin was unable to bind to red blood cells in the presence of GML, suggesting that the primary effect of GML was to stabilize red blood cell membranes and block the binding of α-hemolysin to red blood cell membranes. This likely also explains the ability of GML to block toxicity associated with other heptamer pore-forming exotoxins. Our observation was confirmed by studies that showed that staphylococcal β-hemolysin, a sphingomyelinase and hot:cold hemolysin (45), was unable to lyse red blood cells when the temperature was shifted to 4°C in the presence of GML. These data indicate that GML prevented the conformation change in sphingolipids that appear to mediate β-hemolysin lytic activity at 4°C. Finally, GML also stabilized red blood cells to the lytic effects of hypotonic aqueous solutions. The predominant effect of GML inserted into the membrane may be to “freeze” signal transduction mechanisms, such as host cell receptor signaling systems usurped by exotoxins.
Since anthrax spores were sent via post in 2001, there has been considerable effort in the United States to develop ways to interfere with the toxic effects of exotoxins of gram positive bacteria. Many of the studies have attempted specifically to interfere with their actions by competitive inhibition with small molecules or antibody neutralization of function (9–12, 18). The potential significance of the present studies is that GML has the ability to block signal transduction and stabilize membranes of a wide range of host cells without significant host cell or rabbit toxicity as applied vaginally. GML is both inexpensive and has already been used in products for human consumption. It is important to note, however, that GML inhibition of signal transduction is dose dependent, and doses higher than tested in this study may interfere with normal host functions. We have not tested GML effects when administered intravenously, but significant inhibition of signal transduction may preclude its use systemically. Finally, the inhibition of inflammatory cytokine and chemokine production as was observed in the case of inhibition of superantigen effects on T cells, macrophages, and vaginal epithelial cells, suggests GML may function also generally as a topical anti-inflammatory agent (36).
Footnotes
This work was supported in part by National Institutes of Health Grant HL36611 and AI57164. M.L.P. was supported by National Institutes of Health training grant T32-HD07381.
Abbreviations used: SEs, staphylococcal enterotoxins; TSST-1, toxic shock syndrome toxin-1; SPEs, streptococcal pyrogenic exotoxins; IL-2, interleukin 2; TNF-β, tumor necrosis factor β; IFNγ, interferon γ; TNF-α, tumor necrosis factor α; GML, glycerol monolaurate; HVECs, human vaginal epithelial cells; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis; LPS, lipopolysaccharide; PBS, phosphate buffered saline; PIP2, phosphatidylinositol 4,5 bisphosphate; IP3 inositol 1,4,5 triphosphate; ELISAs, enzyme linked immunosorbent assays; MIP-3α, macrophage inflammatory protein 3α; LD50, lethal dose 50% end point.
References
- 1.McCormick JK, Yarwood JM, Schlievert PM. Toxic shock syndrome and bacterial superantigens: an update. Annu Rev Microbiol. 2001;55:77–104. doi: 10.1146/annurev.micro.55.1.77. [DOI] [PubMed] [Google Scholar]
- 2.Lacy DB, Collier RJ. Structure and function of anthrax toxin. Curr Top Microbiol Immunol. 2002;271:61–85. doi: 10.1007/978-3-662-05767-4_4. [DOI] [PubMed] [Google Scholar]
- 3.McCormick JK, Schlievert PM. Toxins and superantigens of group A streptococci. In: Fischetti VA, editor. Gram-positive pathogens. American Society for Microbiology; 2000. pp. 43–52. [Google Scholar]
- 4.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705–11. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 5.Gouaux JE, Braha O, Hobaugh MR, Song L, Cheley S, Shustak C, Bayley H. Subunit stoichiometry of staphylococcal alpha-hemolysin in crystals and on membranes: aheptameric transmembrane pore. Proc Natl Acad Sci USA. 1994;91:12828–31. doi: 10.1073/pnas.91.26.12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhakdi S, Bayley H, Valeva A, Walev I, Walker B, Kehoe M, Palmer M. Staphylococcal alpha-toxin, streptolysin-O, and Escherichia coli hemolysin: prototypes of pore-forming bacterial cytolysins. Arch Microbiol. 1996;165:73–9. doi: 10.1007/s002030050300. [DOI] [PubMed] [Google Scholar]
- 7.Yan M, Collier RJ. Characterization of dominant-negative forms of anthrax protective antigen. Mol Med. 2003;9:46–51. [PMC free article] [PubMed] [Google Scholar]
- 8.Singh Y, Khanna H, Chopra AP, Mehra V. A dominant negative mutant of Bacillus anthracis protective antigen inhibits anthrax toxin action in vivo. J Biol Chem. 2001;276:22090–4. doi: 10.1074/jbc.M010222200. [DOI] [PubMed] [Google Scholar]
- 9.Lee YS, Bergson P, He WS, Mrksich M, Tang WJ. Discovery of a small molecule that inhibits the interaction of anthrax edema factor with its cellular activator, calmodulin. Chem Biol. 2004;11:1139–46. doi: 10.1016/j.chembiol.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 10.Sarac MS, Peinado JR, Leppla SH, Lindberg I. Protection against anthrax toxemia by hexa-D-arginine in vitro and in vivo. Infect Immun. 2004;72:602–5. doi: 10.1128/IAI.72.1.602-605.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turk BE, Wong TY, Schwarzenbacher R, Jarrell ET, Leppla SH, Collier RJ, Liddington RC, Cantley LC. The structural basis for substrate and inhibitor selectivity of the anthrax lethal factor. Nat Struct Mol Biol. 2004;11:60–6. doi: 10.1038/nsmb708. [DOI] [PubMed] [Google Scholar]
- 12.Casadevall A. Passive antibody administration (immediate immunity) as a specific defense against biological weapons. Emerg Infect Dis. 2002;8:833–41. doi: 10.3201/eid0808.010516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blomster-Hautamaa DA, Novick RP, Schlievert PM. Localization of biologic functions of toxic shock syndrome toxin-1 by use of monoclonal antibodies and cyanogen bromide-generated toxin fragments. J Immunol. 1986;137:3572–6. [PubMed] [Google Scholar]
- 14.Kaul R, McGeer A, Norrby-Teglund A, Kotb M, Schwartz B, O’Rourke K, Talbot J, Low DE. Intravenous immunoglobulin therapy for streptococcal toxic shock syndrome--a comparative observational study. The Canadian Streptococcal Study Group. Clin Infect Dis. 1999;28:800–7. doi: 10.1086/515199. [DOI] [PubMed] [Google Scholar]
- 15.Leung DY, Hauk P, Strickland I, Travers JB, Norris DA. The role of superantigens in human diseases: therapeutic implications for the treatment of skin diseases. Br J Dermatol. 1998;139(Suppl 53):17–29. doi: 10.1046/j.1365-2133.1998.1390s3017.x. [DOI] [PubMed] [Google Scholar]
- 16.Schlievert PM. Role of superantigens in human disease. J Infect Dis. 1993;167:997–1002. doi: 10.1093/infdis/167.5.997. [DOI] [PubMed] [Google Scholar]
- 17.Schlievert PM. Alteration of immune function by staphylococcal pyrogenic exotoxin type C: possible role in toxic-shock syndrome. J Infect Dis. 1983;147:391–8. doi: 10.1093/infdis/147.3.391. [DOI] [PubMed] [Google Scholar]
- 18.Schlievert PM. Use of intravenous immunoglobulin in the treatment of staphylococcal and streptococcal toxic shock syndromes and related illnesses. J Allergy Clin Immunol. 2001;108:107S–10S. doi: 10.1067/mai.2001.117820. [DOI] [PubMed] [Google Scholar]
- 19.Kabara JJ. GRAS antimicrobial agents for cosmetic products. J Soc Cosmet Chem. 1980;31:1–10. [Google Scholar]
- 20.Kabara JJ. Food-grade chemicals for use in designing food preservatives. J Food Prot. 1981;44:633–647. doi: 10.4315/0362-028X-44.8.633. [DOI] [PubMed] [Google Scholar]
- 21.Kabara JJ. Cosmetic and Drug Preservation. Marcel Dekker; New York: 1984. Fatty acids and derivatives as antimicrobial agents: a review; pp. 1–14. [Google Scholar]
- 22.Projan SJ, Brown-Skrobot S, Schlievert PM, Vandenesch F, Novick RP. Glycerol monolaurate inhibits the production of beta-lactamase, toxic shock toxin-1, and other staphylococcal exoproteins by interfering with signal transduction. J Bacterial. 1994;176:4204–9. doi: 10.1128/jb.176.14.4204-4209.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlievert PM, Deringer JR, Kim MH, Projan SJ, Novick RP. Effect of glycerol monolaurate on bacterial growth and toxin production. Antimicrob Agents Chemother. 1992;36:626–31. doi: 10.1128/aac.36.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vetter S, Tripp TJ, Schlievert PM. Glycerol monolaurate inhibits virulence factor production in Bacillus anthracis. Antimicrob Agents Chemother. 2005;49:1302–05. doi: 10.1128/AAC.49.4.1302-1305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pechous R, Ledala N, Wilkinson BJ, Jayaswal RK. Regulation of the expression of cell wall stress stimulon member gene msrA1 in methicillin-susceptible or -resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2004;48:3057–63. doi: 10.1128/AAC.48.8.3057-3063.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCormick JK, Tripp TJ, Llera AS, Sundberg EJ, Dinges MM, Mariuzza RA, Schlievert PM. Functional analysis of the TCR binding domain of toxic shock syndrome toxin-1 predicts further diversity in MHC class D/superantigen/TCR ternary complexes. J Immunol. 2003;171:1385–92. doi: 10.4049/jimmunol.171.3.1385. [DOI] [PubMed] [Google Scholar]
- 27.Yarwood JM, McCormick JK, Paustian ML, Kapur V, Schlievert PM. Repression of the Staphylococcus aureus accessory gene regulator in serum and in vivo. J Bacteriol. 2002;184:1095–101. doi: 10.1128/jb.184.4.1095-1101.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Brien J, Friedlander A, Dreier T, Ezzell J, Leppla S. Effects of anthrax toxin components on human neutrophils. Infect Immun. 1985;47:306–10. doi: 10.1128/iai.47.1.306-310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlievert PM, Bettin KM, Watson DW. Purification and characterization of group A streptococcal pyrogenic exotoxin type C. Infect Immun. 1977;16:673–9. doi: 10.1128/iai.16.2.673-679.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blomster-Hautamaa DA, Schlievert PM. Preparation of toxic shock syndrome toxin-1. Methods Enzymol. 1988;165:37–43. doi: 10.1016/s0076-6879(88)65009-9. [DOI] [PubMed] [Google Scholar]
- 31.Schlievert PM, Shands KN, Dan BB, Schmid GP, Nishimura RD. Identification and characterization of an exotoxin from Staphylococcus aureus associated with toxic-shock syndrome. J Infect Dis. 1981;143:509–16. doi: 10.1093/infdis/143.4.509. [DOI] [PubMed] [Google Scholar]
- 32.Jones CL, Khan SA. Nucleotide sequence of the enterotoxin B gene from Staphylococcus aureus. J Bacteriol. 1986;166:29–33. doi: 10.1128/jb.166.1.29-33.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nauciel C, Blass J, Mangalo R, Raynaud M. Evidence for two molecular forms of streptococcal erythrogenic toxin. Conversion to a single form by 2-mercaptoethanol. Eur J Biochem. 1969;11:160–4. doi: 10.1111/j.1432-1033.1969.tb00754.x. [DOI] [PubMed] [Google Scholar]
- 34.Schlievert PM, Osterholm MT, Kelly JA, Nishimura RD. Toxin and enzyme characterization of Staphylococcus aureus isolates from patients with and without toxic shock syndrome. Ann Intern Med. 1982;96:937–40. doi: 10.7326/0003-4819-96-6-937. [DOI] [PubMed] [Google Scholar]
- 35.Barsumian EL, Schlievert PM, Watson DW. Nonspecific and specific immunological mitogenicity by group A streptococcal pyrogenic exotoxins. Infect Immun. 1978;22:681–8. doi: 10.1128/iai.22.3.681-688.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peterson M, Ault K, Kremer MJ, Klingelhutz AJ, Davis CC, Squier CA, Schlievert PM. Innate immune system is activated by stimulation of vaginal epithelial cells with Staphylococcus aureus and toxic shock syndrome toxin-1. Infect Immun. 2005;73:2164–74. doi: 10.1128/IAI.73.4.2164-2174.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiyono T, Foster SA, Koop JI, McDougall JK, Galloway DA, Klingelhutz AJ. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature. 1998;396:84–8. doi: 10.1038/23962. [DOI] [PubMed] [Google Scholar]
- 38.Halbert CL, Demers GW, Galloway DA. The E6 and E7 genes of human papillomavirus type 6 have weak immortalizing activity in human epithelial cells. J Virol. 1992;66:2125–34. doi: 10.1128/jvi.66.4.2125-2134.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlievert PM. Enhancement of host susceptibility to lethal endotoxin shock by staphylococcal pyrogenic exotoxin type C. Infect Immun. 1982;36:123–8. doi: 10.1128/iai.36.1.123-128.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schlievert PM, Jablonski LM, Roggiani M, Sadler I, Callantine S, Mitchell DT, Ohlendorf DH, Bohach GA. Pyrogenic toxin superantigen site specificity in toxic shock syndrome and food poisoning in animals. Infect Immun. 2000;68:3630–34. doi: 10.1128/iai.68.6.3630-3634.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lacy DB, Wigelsworth DJ, Melnyk RA, Harrison SC, Collier RJ. Structure of heptameric protective antigen bound to an anthrax toxin receptor: a role for receptor in pH-dependent pore formation. Proc Natl Acad Sci USA. 2004;101:13147–51. doi: 10.1073/pnas.0405405101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanna PC, Kochi S, Collier RJ. Biochemical and physiological changes induced by anthrax lethal toxin in J774 macrophage-like cells. Mol Biol Cell. 1992;3:1269–77. doi: 10.1091/mbc.3.11.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hering D, Thompson W, Hewetson J, Little S, Norris S, Pace-Templeton J. Validation of the anthrax lethal toxin neutralization assay. Biologicals. 2004;32:17–27. doi: 10.1016/j.biologicals.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 44.Valeva A, Schnabel R, Walev I, Boukhallouk F, Bhakdi S, Palmer M. Membrane insertion of the heptameric staphylococcal alpha-toxin pore. A domino-like structural transition that is allosterically modulated by the target cell membrane. J Biol Chem. 2001;276:14835–41. doi: 10.1074/jbc.M100301200. [DOI] [PubMed] [Google Scholar]
- 45.Maheswaran SK, Lindorfer RK. Staphylococcal beta-hemolysin. II. Phospholipase C activity of purified beta-hemolysin. J Bacteriol. 1967;94:1313–9. doi: 10.1128/jb.94.5.1313-1319.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dinges MM, Orwin PM, Schlievert PM. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev. 2000;13:16–34. doi: 10.1128/cmr.13.1.16-34.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhakdi S, Valeva A, Walev I, Zitzer A, Palmer M. Pore-forming bacterial cytolysins. Symp Ser SocAppl Microbiol. 1998;27:15S–25S. doi: 10.1046/j.1365-2672.1998.0840s115s.x. [DOI] [PubMed] [Google Scholar]
- 48.Pomerantsev AP, Staritsin NA, Mockov Yu V, Marinin LI. Expression of cereolysine AB genes in Bacillus anthracis vaccine strain ensures protection against experimental hemolytic anthrax infection. Vaccine. 1997;15:1846–50. doi: 10.1016/s0264-410x(97)00132-1. [DOI] [PubMed] [Google Scholar]
- 49.Pruss BM, Dietrich R, Nibler B, Martlbauer E, Scherer S. The hemolytic enterotoxin HBL is broadly distributed among species of the Bacillus cereus group. Appl Environ Microbiol. 1999;65:5436–42. doi: 10.1128/aem.65.12.5436-5442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shannon JG, Ross CL, Koehler TM, Rest RF. Characterization of anthrolysin O, the Bacillus anthracis cholesterol-dependent cytolysin. Infect Immun. 2003;71:3183–9. doi: 10.1128/IAI.71.6.3183-3189.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nizet V, Beall B, Bast DJ, Datta V, Kilburn L, Low DE, De Azavedo JC. Genetic locus for streptolysin S production by group A streptococcus. Infect Immun. 2000;68:4245–54. doi: 10.1128/iai.68.7.4245-4254.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lacy DB, Mourez M, Fouassier A, Collier RJ. Mapping the anthrax protective antigen binding site on the lethal and edema factors. J Biol Chem. 2002;277:3006–10. doi: 10.1074/jbc.M109997200. [DOI] [PubMed] [Google Scholar]
- 53.Methicillin-resistant Staphylococcus aureus infections in correctional facilities-Georgia, California, and Texas, 2001–2003. MMWR Morb Mortal Wkly Rep. 2003;52:992–6. [PubMed] [Google Scholar]
- 54.Cone LA, Woodard DR, Schlievert PM, Tomory GS. Clinical and bacteriologic observations of a toxic shock-like syndrome due to Streptococcus pyogenes. N Engl J Med. 1987;317:l46–9. doi: 10.1056/NEJM198707163170305. [DOI] [PubMed] [Google Scholar]
- 55.Stevens DL, Tanner MH, Winship J, Swarts R, Ries KM, Schlievert PM, Kaplan E. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N Engl J Med. 1989;321:1–7. doi: 10.1056/NEJM198907063210101. [DOI] [PubMed] [Google Scholar]
- 56.Friedlander AM. Anthrax: clinical features, pathogenesis, and potential biological warfare threat. Curr Clin Top Infect Dis. 2000;20:335–49. [PubMed] [Google Scholar]
- 57.Fast DJ, Schlievert PM, Nelson RD. Nonpurulent response to toxic shock syndrome toxin 1-producing Staphylococcus aureus. Relationship to toxin-stimulated production of tumor necrosis factor. J Immunol. 1988;140:949–53. [PubMed] [Google Scholar]
- 58.Shands KN, Schmid GP, Dan BB, Blum D, Guidotti RJ, Hargrett NT, Anderson RL, Hill DL, Broome CV, Band JD, Fraser DW. Toxic-shock syndrome in menstruating women: association with tampon use and Staphylococcus aureus and clinical features in 52 cases. N Engl J Med. 1980;303:1436–42. doi: 10.1056/NEJM198012183032502. [DOI] [PubMed] [Google Scholar]
- 59.Todd JK, Kapral FA, Fishaut M, Welch TR. Toxic shock syndrome associated with phage group 1 staphylococci. Lancet. 1978;2:1116–1118. doi: 10.1016/s0140-6736(78)92274-2. [DOI] [PubMed] [Google Scholar]
- 60.Davis JP, Chesney PJ, Wand PJ, LaVenture M. Toxic-shock syndrome: epidemiologic features, recurrence, risk factors, and prevention. N Engl J Med. 1980;303:1429–35. doi: 10.1056/NEJM198012183032501. [DOI] [PubMed] [Google Scholar]
- 61.Witcher KJ, Novick RP, Schlievert PM. Modulation of immune cell proliferation by glycerol monolaurate. Clin Diagn Lab Immunol. 1996;3:10–3. doi: 10.1128/cdli.3.1.10-13.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schlievert PM. Staphylococcal enterotoxin B and toxic-shock syndrome toxin-1 are significantly associated with non-menstrual TSS. Lancet. 1986;1:1149–50. doi: 10.1016/s0140-6736(86)91859-3. [DOI] [PubMed] [Google Scholar]
- 63.Bergdoll MS. Monkey feeding test for Staphylococcal enterotoxin. Methods Enzymol. 1988;165:324–33. doi: 10.1016/s0076-6879(88)65048-8. [DOI] [PubMed] [Google Scholar]