Abstract
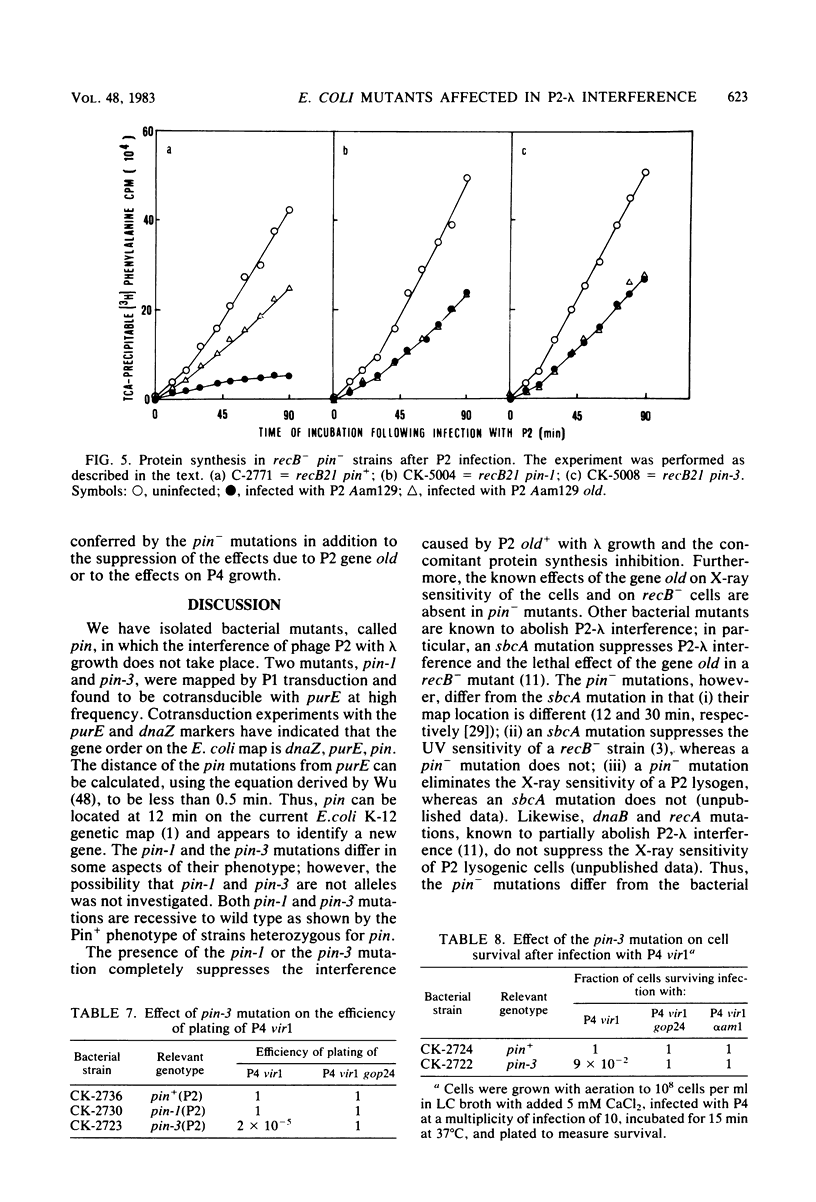
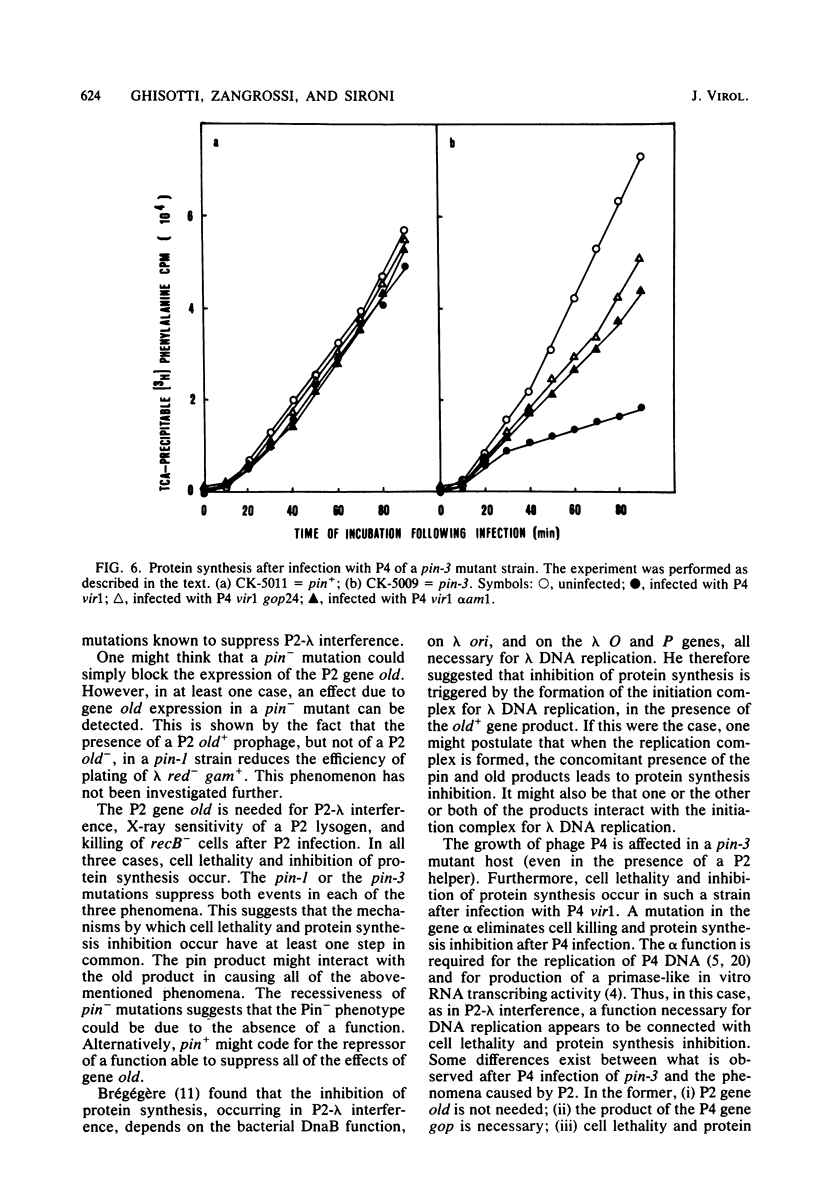
The gene old of bacteriophage P2 is known to (i) cause interference with phage lambda growth; (ii) kill recB- mutants of Escherichia coli after P2 infection; and (iii) determine increased sensitivity of P2 lysogenic cells to X-ray irradiation. In all of these phenomena, inhibition of protein synthesis occurs. We have isolated bacterial mutants, named pin (P2 interference), able to suppress all of the above-mentioned phenomena caused by the old+ gene product and the concurrent protein synthesis inhibition. Pin mutations are recessive, map at 12 min on the E. coli map, and identify a new gene. Satellite bacteriophage P4 does not plate on pin-3 mutant strains and causes cell lethality and protein synthesis inhibition in such mutants. P4 mutants able to grow on pin-3 strains have been isolated.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour S. D., Clark A. J. Biochemical and genetic studies of recombination proficiency in Escherichia coli. I. Enzymatic activity associated with recB+ and recC+ genes. Proc Natl Acad Sci U S A. 1970 Apr;65(4):955–961. doi: 10.1073/pnas.65.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour S. D., Nagaishi H., Templin A., Clark A. J. Biochemical and genetic studies of recombination proficiency in Escherichia coli. II. Rec+ revertants caused by indirect suppression of rec- mutations. Proc Natl Acad Sci U S A. 1970 Sep;67(1):128–135. doi: 10.1073/pnas.67.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett K. J., Gibbs W., Calendar R. A transcribing activity induced by satellite phage P4. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2986–2990. doi: 10.1073/pnas.69.10.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett K. J., Marsh M. L., Calendar R. Interactions between a satellite bacteriophage and its helper. J Mol Biol. 1976 Sep 25;106(3):683–707. doi: 10.1016/0022-2836(76)90259-x. [DOI] [PubMed] [Google Scholar]
- Berg C. M., Curtiss R., 3rd Transposition derivatives of an Hfr strain of Escherichia coli K-12. Genetics. 1967 Jul;56(3):503–525. doi: 10.1093/genetics/56.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertani G., Choe B. K., Lindahl G. Calcium sensitive and other mutants of bacteriophage P2. J Gen Virol. 1969 Jul;5(1):97–104. doi: 10.1099/0022-1317-5-1-97. [DOI] [PubMed] [Google Scholar]
- Bregegere F. Bacteriophage P2-lambda interference: inhibition of protein synthesis involves transfer RNA inactivation. J Mol Biol. 1974 Dec 15;90(3):459–467. doi: 10.1016/0022-2836(74)90228-9. [DOI] [PubMed] [Google Scholar]
- Brégégère F. Bacteriophage P2-lambda interference. II. Effects on the host under the control of lambda genes O and P. J Mol Biol. 1976 Jun 25;104(2):411–420. doi: 10.1016/0022-2836(76)90279-5. [DOI] [PubMed] [Google Scholar]
- Brégégère F. Bacteriophage P2-lambda interference. III. Essential role of an early step in the initiation of lambda replication. J Mol Biol. 1978 Jun 25;122(2):113–125. doi: 10.1016/0022-2836(78)90029-3. [DOI] [PubMed] [Google Scholar]
- COHEN D. A variant of phage P2 originating in Escherichia coli, strain B. Virology. 1959 Jan;7(1):112–126. doi: 10.1016/0042-6822(59)90180-1. [DOI] [PubMed] [Google Scholar]
- Calendar R., Lindqvist B., Sironi G., Clark A. J. Characterization of REP- mutants and their interaction with P2 phage. Virology. 1970 Jan;40(1):72–83. doi: 10.1016/0042-6822(70)90380-6. [DOI] [PubMed] [Google Scholar]
- Calendar R., Ljungquist E., Deho G., Usher D. C., Goldstein R., Youderian P., Sironi G., Six E. W. Lysogenization by satellite phage P4. Virology. 1981 Aug;113(1):20–38. doi: 10.1016/0042-6822(81)90133-1. [DOI] [PubMed] [Google Scholar]
- Dehò G. Circular genetic map of satellite bacteriophage P4. Virology. 1983 Apr 15;126(1):267–278. doi: 10.1016/0042-6822(83)90478-6. [DOI] [PubMed] [Google Scholar]
- Emmerson P. T. Recombination deficient mutants of Escherichia coli K12 that map between thy A and argA. Genetics. 1968 Sep;60(1):19–30. doi: 10.1093/genetics/60.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip C. C., Allen J. S., Gustafson R. A., Allen R. G., Walker J. R. Bacterial cell division regulation: characterization of the dnaH locus of Escherichia coli. J Bacteriol. 1974 Aug;119(2):443–449. doi: 10.1128/jb.119.2.443-449.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghisotti D., Zangrossi S., Sironi G. X-ray sensitivity of Escherichia coli lysogenic for bacteriophage P2. Mol Gen Genet. 1979 Feb 1;169(3):229–235. doi: 10.1007/BF00382268. [DOI] [PubMed] [Google Scholar]
- Gibbs W., Goldstein R. N., Wiener R., Lindqvist B., Calendar R. Satellite bacteriophage P4: characterization of mutants in two essential genes. Virology. 1973 May;53(1):24–39. doi: 10.1016/0042-6822(73)90462-5. [DOI] [PubMed] [Google Scholar]
- Herbert A. A., Guest J. R. Biochemical and genetic studies with lysine+methionine mutants of Escherichia coli: lipoic acid and alpha-ketoglutarate dehydrogenase-less mutants. J Gen Microbiol. 1968 Oct;53(3):363–381. doi: 10.1099/00221287-53-3-363. [DOI] [PubMed] [Google Scholar]
- KELLY B. Localization of P2 prophage in two strains of Escherichia coli. Virology. 1963 Jan;19:32–39. doi: 10.1016/0042-6822(63)90021-7. [DOI] [PubMed] [Google Scholar]
- Kahn M., Hopkins A. Restriction endonuclease cleavage map of bacteriophage P4 DNA. Virology. 1978 Apr;85(2):359–363. doi: 10.1016/0042-6822(78)90444-0. [DOI] [PubMed] [Google Scholar]
- Kushner S. R., Nagaishi H., Clark A. J. Isolation of exonuclease VIII: the enzyme associated with sbcA indirect suppressor. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3593–3597. doi: 10.1073/pnas.71.9.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam S. T., Stahl M. M., McMilin K. D., Stahl F. W. Rec-mediated recombinational hot spot activity in bacteriophage lambda. II. A mutation which causes hot spot activity. Genetics. 1974 Jul;77(3):425–433. doi: 10.1093/genetics/77.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl G., Sironi G., Bialy H., Calendar R. Bacteriophage lambda; abortive infection of bacteria lysogenic for phage P2. Proc Natl Acad Sci U S A. 1970 Jul;66(3):587–594. doi: 10.1073/pnas.66.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist B. H., Six E. W. Replication of bacteriophage P4 DNA in a nonlysogenic host. Virology. 1971 Jan;43(1):1–7. doi: 10.1016/0042-6822(71)90218-2. [DOI] [PubMed] [Google Scholar]
- Lloyd R. G., Barbour S. D. The genetic location of the sbcA gene of Escherichia coli. Mol Gen Genet. 1974;134(2):157–171. doi: 10.1007/BF00268417. [DOI] [PubMed] [Google Scholar]
- Malone R. E., Chattoraj D. K. The role of Chi mutations in the Spi- phenotype of phage lambda: lack of evidence for a gene delta. Mol Gen Genet. 1975 Dec 30;143(1):35–41. doi: 10.1007/BF00269418. [DOI] [PubMed] [Google Scholar]
- Manly K. F., Signer E. R., Radding C. M. Nonessential functions of bacteriophage lambda. Virology. 1969 Feb;37(2):177–188. doi: 10.1016/0042-6822(69)90197-4. [DOI] [PubMed] [Google Scholar]
- OKADA T., YANAGISAWA K., RYAN F. J. A method for securing thymineless mutants from strains of E. coli. Z Vererbungsl. 1961;92:403–412. doi: 10.1007/BF00890061. [DOI] [PubMed] [Google Scholar]
- SUSSMAN R., JACOB F. [On a thermosensitive repression system in the Escherichia coli lambda bacteriophage]. C R Hebd Seances Acad Sci. 1962 Feb 19;254:1517–1519. [PubMed] [Google Scholar]
- Sakaki Y., Karu A. E., Linn S., Echols H. Purification and properties of the gamma-protein specified by bacteriophage lambda: an inhibitor of the host RecBC recombination enzyme. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2215–2219. doi: 10.1073/pnas.70.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki I., Bertani G. Growth abnormalities in Hfr derivatives of Escherichia coli strain C. J Gen Microbiol. 1965 Sep;40(3):365–376. doi: 10.1099/00221287-40-3-365. [DOI] [PubMed] [Google Scholar]
- Shinomiya S., Sakaki Y. Prophage P2 does not kill recB bacteria. Biochem Biophys Res Commun. 1979 Jan 15;86(1):167–172. doi: 10.1016/0006-291x(79)90396-6. [DOI] [PubMed] [Google Scholar]
- Shore D., Dehò G., Tsipis J., Goldstein R. Determination of capsid size by satellite bacteriophage P4. Proc Natl Acad Sci U S A. 1978 Jan;75(1):400–404. doi: 10.1073/pnas.75.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sironi G., Bialy H., Lozeron H. A., Calendar R. Bacteriophage P2: interaction with phage lambda and with recombination-deficient bacteria. Virology. 1971 Nov;46(2):387–396. doi: 10.1016/0042-6822(71)90040-7. [DOI] [PubMed] [Google Scholar]
- Sironi G. Mutants of Escherichia coli unable to be lysogenized by the temperate bacteriophage P2. Virology. 1969 Feb;37(2):163–176. doi: 10.1016/0042-6822(69)90196-2. [DOI] [PubMed] [Google Scholar]
- Six E. W. The helper dependence of satellite bacteriophage P4: which gene functions of bacteriophage P2 are needed by P4? Virology. 1975 Sep;67(1):249–263. doi: 10.1016/0042-6822(75)90422-5. [DOI] [PubMed] [Google Scholar]
- Six E. PROPHAGE SUBSTITUTION AND CURING IN LYSOGENIC CELLS SUPERINFECTED WITH HETERO-IMMUNE PHAGE. J Bacteriol. 1960 Nov;80(5):728–729. doi: 10.1128/jb.80.5.728-729.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza L., Geisselsoder J., Hopkins A., Calender R. Physical mapping of the satellite phage P4 genome. Virology. 1978 Apr;85(2):335–342. doi: 10.1016/0042-6822(78)90442-7. [DOI] [PubMed] [Google Scholar]
- Sunshine M. G., Thorn M., Gibbs W., Calendar R., Kelly B. P2 phage amber mutants: characterization by use of a polarity suppressor. Virology. 1971 Dec;46(3):691–702. doi: 10.1016/0042-6822(71)90071-7. [DOI] [PubMed] [Google Scholar]
- Szybalski E. H., Szybalski W. Physical mapping of the att-N region of coliphage lambda: apparent oversaturation of coding capacity in the gam-ral segment. Biochimie. 1974;56(11-12):1497–1503. doi: 10.1016/s0300-9084(75)80272-0. [DOI] [PubMed] [Google Scholar]
- THOMAS R., BERTANI L. E. ON THE CONTROL OF THE REPLICATION OF TEMPERATE BACTERIOPHAGES SUPERINFECTING IMMUNE HOSTS. Virology. 1964 Nov;24:241–253. doi: 10.1016/0042-6822(64)90163-1. [DOI] [PubMed] [Google Scholar]
- Unger R. C., Clark A. J. Interaction of the recombination pathways of bacteriophage lambda and its host Escherichia coli K12: effects on exonuclease V activity. J Mol Biol. 1972 Oct 14;70(3):539–548. doi: 10.1016/0022-2836(72)90558-x. [DOI] [PubMed] [Google Scholar]
- Wiman M., Bertani G., Kelly B., Sasaki I. Genetic map of Escherichia coli strain C. Mol Gen Genet. 1970;107(1):1–31. doi: 10.1007/BF00433220. [DOI] [PubMed] [Google Scholar]
- Wu T. T. A model for three-point analysis of random general transduction. Genetics. 1966 Aug;54(2):405–410. doi: 10.1093/genetics/54.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]



