Abstract
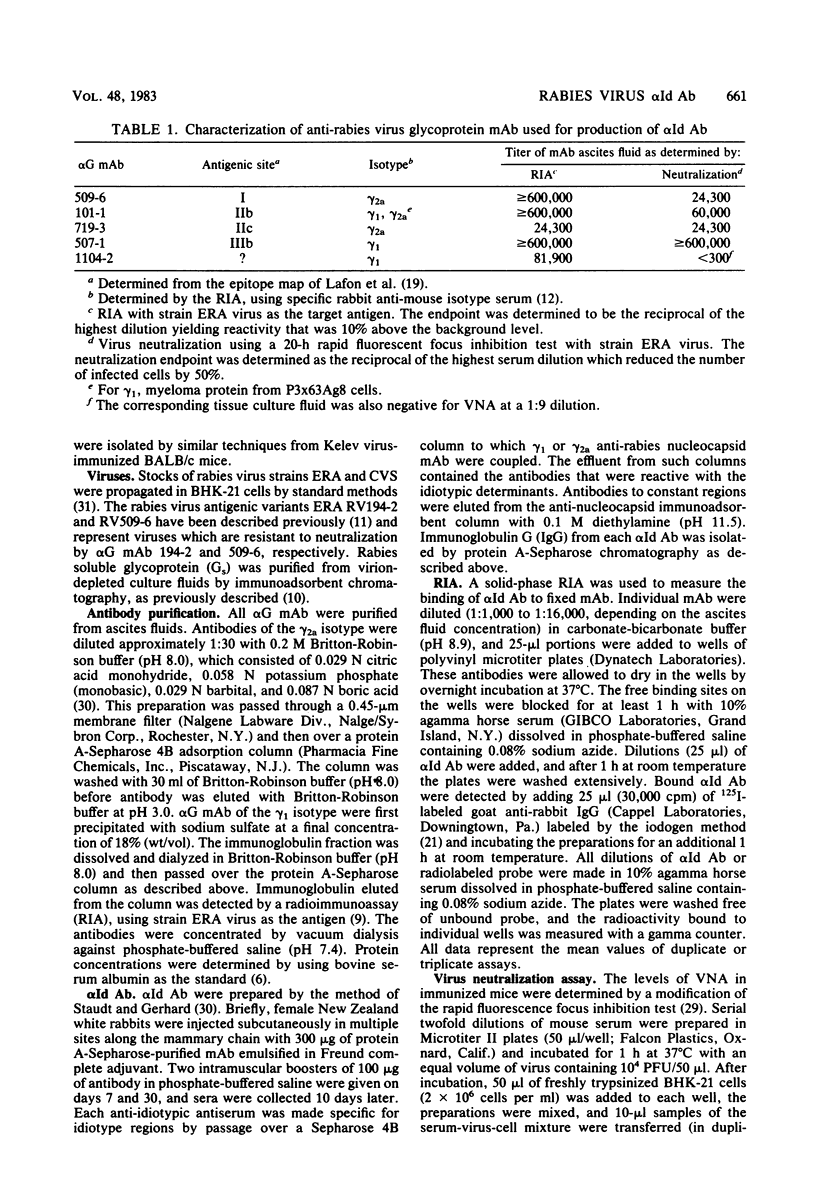
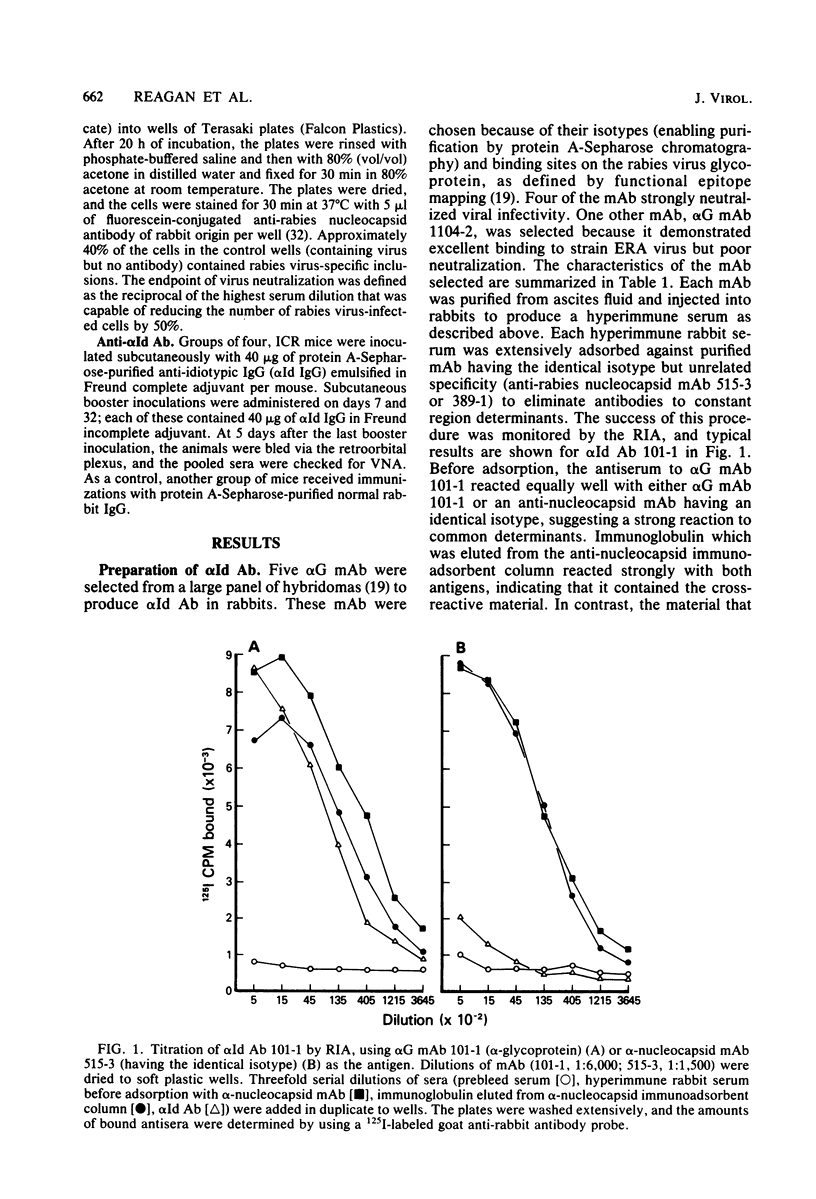
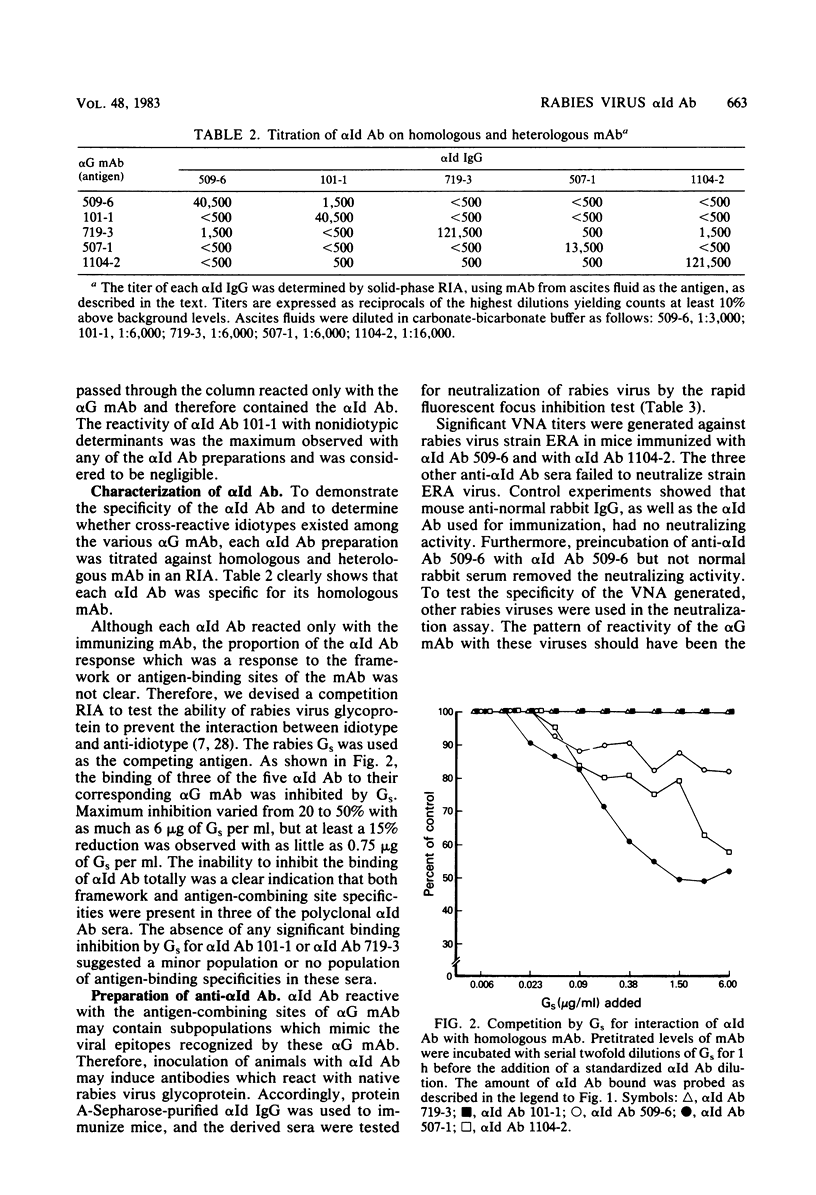
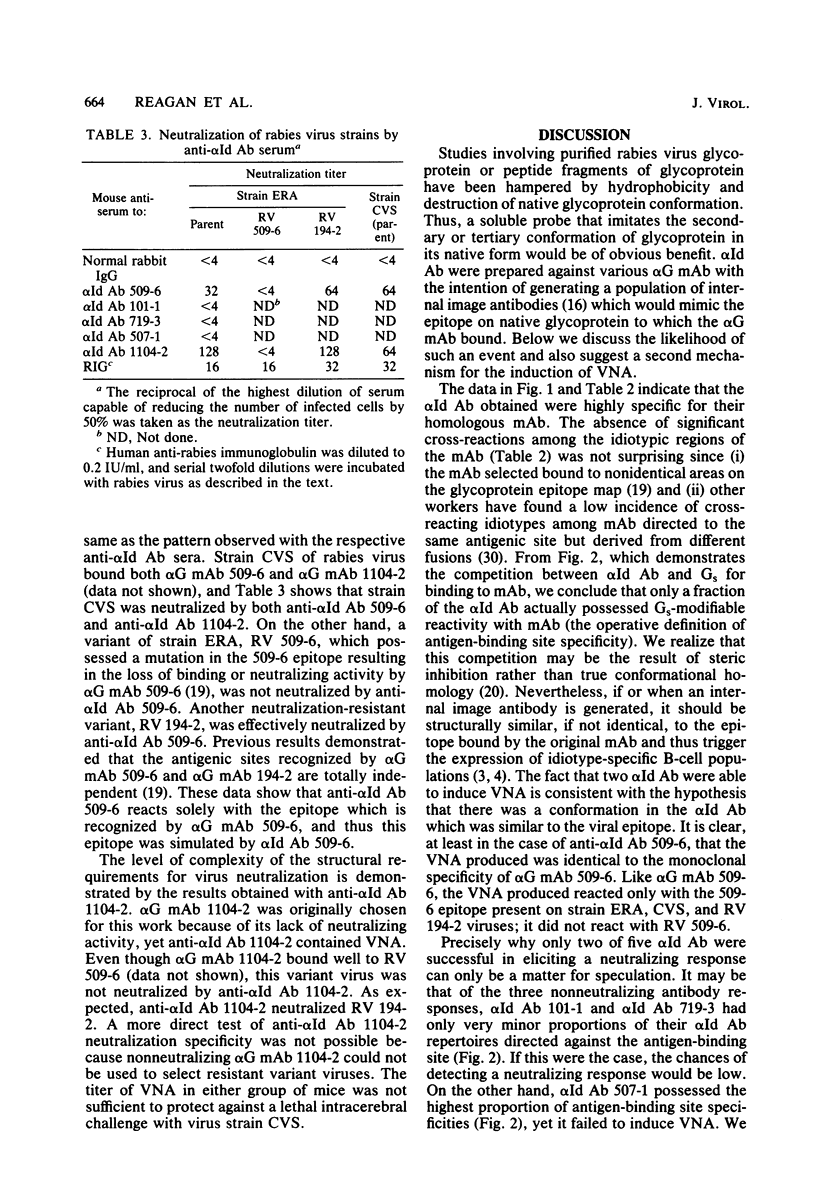
Rabbit anti-idiotypic antibodies (alpha Id Ab) were prepared against five murine monoclonal antibodies (mAb) specific for the rabies virus glycoprotein. Four of the mAb were directed against three known, type-specific, neutralizing sites on the glycoprotein, and the other mAb was directed against a topographically uncharacterized, nonneutralizing epitope. An absence of significant cross-reactivity among the alpha Id Ab for heterologous mAb suggested that the alpha Id Ab were highly specific for unique variable region determinants. The binding of three of the five alpha Id Ab to their homologous mAb could be inhibited by rabies virus-soluble glycoprotein, suggesting that the alpha Id Ab possessed subpopulations similar or adjacent to the antigen-binding site of the mAb. Two of the five alpha Id Ab injected into mice elicited a specific virus-neutralizing antibody response. Mechanisms to account for the induction of the virus-neutralizing antibody by alpha Id Ab are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ada G. L. Vaccines for the future? Aust J Exp Biol Med Sci. 1982 Dec;60(6):549–569. doi: 10.1038/icb.1982.59. [DOI] [PubMed] [Google Scholar]
- Baird L. G., Tachovsky T. G., Sandberg-Wollheim M., Koprowski H., Nisonoff A. Identification of a unique idiotype in cerebrospinal fluid and serum of a patient with multiple sclerosis. J Immunol. 1980 May;124(5):2324–2328. [PubMed] [Google Scholar]
- Bluestone J. A., Auchincloss H., Jr, Cazenave P. A., Ozato K., Sachs D. H. Anti-idiotypes to monoclonal anti-H-2 antibodies. III. Syngeneic anti-idiotypes on antibodies induced by in vivo administration of xenogeneic anti-idiotypes. J Immunol. 1982 Nov;129(5):2066–2068. [PubMed] [Google Scholar]
- Bluestone J. A., Krutzsch H. C., Auchincloss H., Jr, Cazenave P. A., Kindt T. J., Sachs D. H. Anti-idiotypes against anti-H-2 monoclonal antibodies: structural analysis of the molecules induced by in vivo anti-idiotype treatment. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7847–7851. doi: 10.1073/pnas.79.24.7847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bona C. A., Heber-Katz E., Paul W. E. Idiotype-anti-idiotype regulation. I. Immunization with a levan-binding myeloma protein leads to the appearance of auto-anti-(anti-idiotype) antibodies and to the activation of silent clones. J Exp Med. 1981 Apr 1;153(4):951–967. doi: 10.1084/jem.153.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhall S., Noack N., Wu M., Loewenberg J. R. A simple colorimetric method for determination of protein. Anal Biochem. 1969 Oct 1;31(1):146–148. doi: 10.1016/0003-2697(69)90251-6. [DOI] [PubMed] [Google Scholar]
- Claflin J. L., Lieberman R., Davie J. M. Clonal nature of the immune response to phosphorylcholine. II. Idiotypic specificity and binding characteristics of anti-phosphorylcholine antibodies. J Immunol. 1974 May;112(5):1747–1756. [PubMed] [Google Scholar]
- Cox J. H., Dietzschold B., Schneider L. G. Rabies virus glycoprotein. II. Biological and serological characterization. Infect Immun. 1977 Jun;16(3):754–759. doi: 10.1128/iai.16.3.754-759.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzschold B., Wiktor T. J., Macfarlan R., Varrichio A. Antigenic structure of rabies virus glycoprotein: ordering and immunological characterization of the large CNBr cleavage fragments. J Virol. 1982 Nov;44(2):595–602. doi: 10.1128/jvi.44.2.595-602.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzschold B., Wiktor T. J., Wunner W. H., Varrichio A. Chemical and immunological analysis of the rabies soluble glycoprotein. Virology. 1983 Jan 30;124(2):330–337. doi: 10.1016/0042-6822(83)90349-5. [DOI] [PubMed] [Google Scholar]
- Dietzschold B., Wunner W. H., Wiktor T. J., Lopes A. D., Lafon M., Smith C. L., Koprowski H. Characterization of an antigenic determinant of the glycoprotein that correlates with pathogenicity of rabies virus. Proc Natl Acad Sci U S A. 1983 Jan;80(1):70–74. doi: 10.1073/pnas.80.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichmann K. Idiotype suppression. I. Influence of the dose and of the effector functions of anti-idiotypic antibody on the production of an idiotype. Eur J Immunol. 1974 Apr;4(4):296–302. doi: 10.1002/eji.1830040413. [DOI] [PubMed] [Google Scholar]
- Epstein S. L., Masakowski V. R., Sharrow S. O., Bluestone J. A., Ozato K., Sachs D. H. Idiotypes of anti-Ia antibodies. II. Effects of in vivo treatment with xenogeneic anti-idiotype. J Immunol. 1982 Oct;129(4):1545–1552. [PubMed] [Google Scholar]
- Fields B. N., Greene M. I. Genetic and molecular mechanisms of viral pathogenesis: implications for prevention and treatment. Nature. 1982 Nov 4;300(5887):19–23. doi: 10.1038/300019a0. [DOI] [PubMed] [Google Scholar]
- Geha R. S. Regulation of the immune response by idiotypic-antiidiotypic interactions. N Engl J Med. 1981 Jul 2;305(1):25–28. doi: 10.1056/NEJM198107023050105. [DOI] [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Kelsoe G., Reth M., Rajewsky K. Control idiotope expression by monoclonal anti-idiotope antibodies. Immunol Rev. 1980;52:75–88. doi: 10.1111/j.1600-065x.1980.tb00331.x. [DOI] [PubMed] [Google Scholar]
- Lafon M., Wiktor T. J., Macfarlan R. I. Antigenic sites on the CVS rabies virus glycoprotein: analysis with monoclonal antibodies. J Gen Virol. 1983 Apr;64(Pt 4):843–851. doi: 10.1099/0022-1317-64-4-843. [DOI] [PubMed] [Google Scholar]
- Lindenmann J. Homobodies: do they exist? Ann Immunol (Paris) 1979 Mar-Apr;130(2):311–318. [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- Marshak-Rothstein A., Benedetto J. D., Kirsch R. L., Gefter M. L. Unique determinants associated with hybridoma proteins expressing a cross-reactive idiotype: frequency among individual immune sera. J Immunol. 1980 Nov;125(5):1987–1992. [PubMed] [Google Scholar]
- Miller R. A., Maloney D. G., Warnke R., Levy R. Treatment of B-cell lymphoma with monoclonal anti-idiotype antibody. N Engl J Med. 1982 Mar 4;306(9):517–522. doi: 10.1056/NEJM198203043060906. [DOI] [PubMed] [Google Scholar]
- Nepom J. T., Weiner H. L., Dichter M. A., Tardieu M., Spriggs D. R., Gramm C. F., Powers M. L., Fields B. N., Greene M. I. Identification of a hemagglutinin-specific idiotype associated with reovirus recognition shared by lymphoid and neural cells. J Exp Med. 1982 Jan 1;155(1):155–167. doi: 10.1084/jem.155.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks D. L., Esser K. M., Sher A. Immunization of mice against African trypanosomiasis using anti-idiotypic antibodies. J Exp Med. 1982 Apr 1;155(4):1108–1119. doi: 10.1084/jem.155.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sege K., Peterson P. A. Use of anti-idiotypic antibodies as cell-surface receptor probes. Proc Natl Acad Sci U S A. 1978 May;75(5):2443–2447. doi: 10.1073/pnas.75.5.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher A., Cohn M. Effect of haptens on the reaction of anti-idiotype antibody with a mouse anti-phosphorylcholine plasmacytoma protein. J Immunol. 1972 Jul;109(1):176–178. [PubMed] [Google Scholar]
- Smith J. S., Yager P. A., Baer G. M. A rapid tissue culture test for determining rabies neutralizing antibody. Monogr Ser World Health Organ. 1973;(23):354–357. [PubMed] [Google Scholar]
- Staudt L. M., Gerhard W. Generation of antibody diversity in the immune response of BALB/c mice to influenza virus hemagglutinin. I. Significant variation in repertoire expression between individual mice. J Exp Med. 1983 Feb 1;157(2):687–704. doi: 10.1084/jem.157.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J., Kamo I., Koprowski H. In vitro stimulation of rabbit lymphocytes after immunization with live and inactivated rabies vaccines. J Immunol. 1974 Jun;112(6):2013–2019. [PubMed] [Google Scholar]
- Wiktor T. J., Koprowski H. Antigenic variants of rabies virus. J Exp Med. 1980 Jul 1;152(1):99–112. doi: 10.1084/jem.152.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J., Koprowski H. Monoclonal antibodies against rabies virus produced by somatic cell hybridization: detection of antigenic variants. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3938–3942. doi: 10.1073/pnas.75.8.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J. Laboratoty techniques in rabies: tissue culture methods. Monogr Ser World Health Organ. 1973;(23):101–123. [PubMed] [Google Scholar]


